Abstract
Noninflammatory monocyte macrophages use αvβ3 integrin to selectively bind apoptotic cells, initiating their phagocytic removal. In a related process, the retinal pigment epithelium (RPE) employs αvβ5 integrin to recognize spent photoreceptor outer segment particles (OS). Here, we show that apoptotic cells and OS compete for binding to these receptors, indicating that OS and apoptotic cells expose surface signals recognizable by αvβ3 and αvβ5. Particle binding to αvβ5 required protein kinase C (PKC) activation. In RPE, αvβ5 binding was maximally activated even before any phagocytic challenge and was reduced by PKC inhibitors. In macrophages, it was dormant but became activated upon PKC stimulation. PKC-activated αvβ5-mediated binding in macrophages differed from constitutive binding to the same integrin receptor in RPE cells in that the former followed much faster kinetics, similar to particle binding mediated by αvβ3. Activation of αvβ5 for particle binding correlated with its recruitment into a detergent-insoluble fraction, a process sensitive to pharmacological modulation of PKC in both types of phagocytes. Furthermore, αvβ5 but not αvβ3 particle binding required actin microfilaments. These data constitute the first evidence that noninflammatory phagocytes actively regulate the earliest phase of phagocytic clearance, particle binding, by controlling receptor activity.
Keywords: phagocytosis, recognition, integrins, macrophages, retinal pigment epithelium
Clearance phagocytosis of apoptotic cells and debris plays a critical role in remodeling and maintenance of tissues. Noninflammatory phagocytosis prevents tissue damage that would result from leakage of cytoplasmic components 1 2. Typical examples of this macrophage activity are the efficient removal of large numbers of apoptotic cells during embryonic development 3 or of activated granulocytes undergoing programmed cell death at the end of an immune response 4. The binding and phagocytosis phases of apoptotic cell removal each involve specific receptors in the macrophage, such as the lipopolysaccharide receptor CD14 5, phosphatidylserine receptors 6, the integrin receptor αvβ3 7, and the scavenger receptors SRA1 and CD36 8 9. Binding receptors recognize phosphatidylserine, oxidized lipids, and other unidentified phagocytic signals exposed by apoptotic cells 10 11. The available data suggest that different recognition receptors initiating phagocytosis of apoptotic cells may be used independently, depending on the activation state of the macrophage population 12 13. This is especially well established for the availability of αvβ3 integrin for apoptotic cell binding. αvβ3 does not participate in particle binding by macrophages that were previously stimulated by thioglycollate, activated by cytokines, or phagocytically challenged 12 14. In contrast, αvβ3 is an important binding receptor in noninflammatory bone marrow– or monocyte-derived macrophages, as αvβ3 function-blocking antibodies or peptides containing the cognate integrin ligand motif Arg-Gly-Asp drastically reduce particle attachment 7 12. Monocyte-derived macrophages use either αvβ3 integrin or leukocyte-specific integrin receptors αMβ2 (CD11b/CD18, CR3) and αXβ2 (CD11c/CD18, CR4), presumably in parallel, to bind apoptotic cells, which have been opsonized with complement components 15. The mechanisms used by macrophages to activate or deactivate specific binding receptors for apoptotic cells are entirely unknown.
Noninflammatory receptor–mediated clearance of cell debris is also efficiently carried out by a specialized tissue in the retina, the retinal pigment epithelium (RPE).1 RPE cells phagocytose huge loads of material shed by the adjacent photoreceptor layer (∼7% of their outer segment mass, daily) as part of a circadian renewal program that compensates the damaging effects of light 16 17. RPE phagocytosis of shed outer segment particles (OS) is key for the integrity of the eye, as its failure leads to retinal degeneration 18 19. RPE cells highly prefer OS over bacteria, yeast, and opsonized red blood cells, even when they may express a population of Fc receptors 20. CD36 is expressed by the RPE and participates in the internalization phase of OS clearance 21. Indirect evidence suggests that a mannose receptor–like activity also plays a role in RPE internalization of OS 22. However, the RPE mannose receptor sequence has not yet been cloned, and its exact role in OS phagocytosis is unclear. Although only some soluble mannose receptor ligands affect OS phagocytosis 23 24, yeast and zymosan particles, which are recognized by macrophages via the mannose receptor, do not compete with OS for binding or internalization by RPE cells (20, and our unpublished results).
OS binding by RPE cells and apoptotic cell binding by macrophages use the related integrin receptors αvβ5 and αvβ3, respectively 25 26. However, the binding phases of the two processes show some important differences: (a) RPE cells bind OS slowly (over ∼2 h) only after a lag phase 20 26, whereas macrophages bind particles maximally in 30 min. (b) RPE cells bind OS only at temperatures >17°C 27, whereas a temperature requirement of macrophage binding of apoptotic cells, to our knowledge, has not been reported. However, macrophage binding of particles via Fc receptors or complement receptor β2 integrins is normal at 0°C 28. (c) RPE cells primarily use αvβ5 25 26 for particle binding (αvβ5 antibodies reduce binding by 85%), whereas macrophages may use αvβ3 and other receptors, in parallel (αvβ3 antibodies reduce binding by ∼50%). Very recent work has shown that phagocytosis of apoptotic cells by immature human blood–derived dendritic cells can be reduced to a similar extent by αvβ5 integrin–inhibiting antibodies; upon cytokine-induced maturation, the phagocytic activity of these cells decreases coincident with downregulation of β5 29.
The experiments in this report characterize the role of αvβ3 and αvβ5 integrin receptors in the initial binding phase of monocyte macrophage and RPE phagocytosis of apoptotic cells and OS. We first tested the hypothesis that the specific particle ligand (OS versus apoptotic cell) could determine the use of a particular integrin, αvβ5 by RPE and αvβ3 by macrophages. The experiments led us to discard this hypothesis as both cell types recognized apoptotic cells and OS efficiently and competitively, employing the same binding integrins they use for their respective physiologic ligands. As both phagocyte types expressed αvβ3 and αvβ5, we tested the hypothesis that the activation of a particular integrin pathway by each cell type could be regulated by specific signaling pathways. Indeed, this was observed: our experiments revealed a critical role for PKC in activating αvβ5 for particle binding. Activation of dormant αvβ5 receptors in macrophages did not alter cell-specific differences in particle binding, as these receptors bound apoptotic cells or OS with fast kinetics distinct from the characteristic slow onset of binding initiating RPE phagocytosis. Both αvβ5 and αvβ3 integrins required temperatures of at least 18°C for particle binding, in contrast to β2 integrins or Fc receptors. Remarkably, αvβ5 receptors themselves became resistant to detergent extraction upon PKC activation. Since intact actin microfilaments were required for αvβ5-mediated particle binding, interaction with the actin cytoskeleton appears to be an important feature of αvβ5 receptor activation.
Materials and Methods
Materials.
Reagents were from Sigma Chemical Co. or GIBCO BRL unless otherwise stated. Pharmacological reagents were obtained from Biomol or Sigma Chemical Co., stored as 1,000× stock solutions in DMSO at −20°C, and diluted into cell culture medium directly before use. The following antibodies were used: rat αvβ3 function-blocking β3 antibody clone F11 30 31 and mouse β3 function-blocking antibody clone 2C9.G2 32 were from PharMingen. Immunoprecipitating, function-blocking, heterodimer-selective αvβ5 antibody clone P1F6 33 was from Chemicon. P1F6 recognizes αvβ5 in many species, including rodents (26 33; and this study, see Fig. 8). For immunoblot detection, β5 antibodies were purchased from Chemicon or from Upstate Biotechnology (clone B5-IVF2 34), and β3 antibody clone 26 was from Transduction Laboratories. Myeloperoxidase antiserum was from Dako. Mouse αM function-blocking antibody 5C6 35 was a gift from Dr. W.A. Muller (Weill Medical College of Cornell University). Secondary antibodies were from Jackson ImmunoResearch Laboratories.
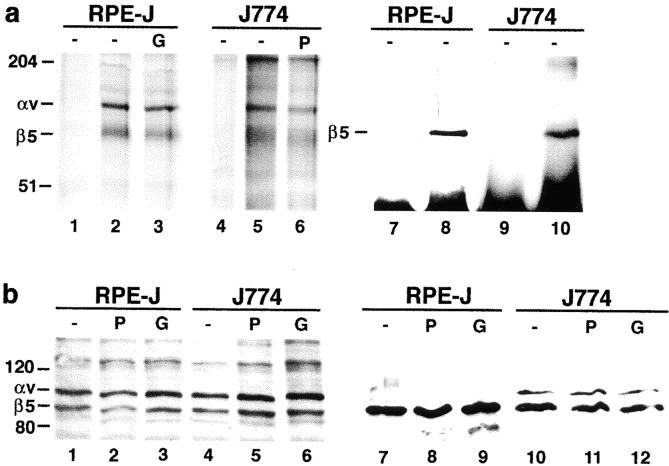
Figure 8.
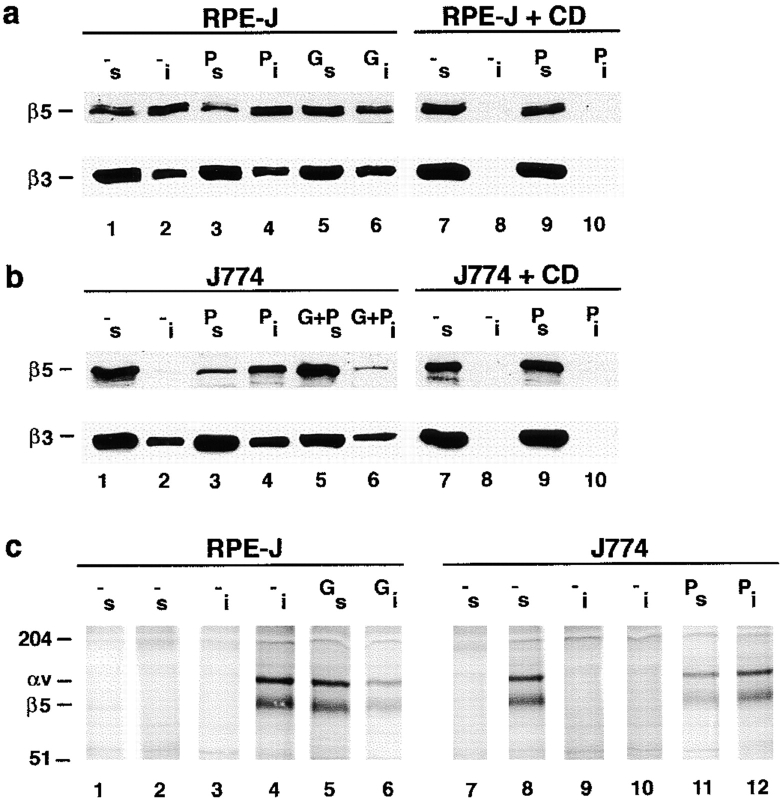
PKC activation or inhibition does not alter levels or plasma membrane localization of αvβ5 receptors. RPE-J cells were pretreated with solvent (−) or PMA for 30 min (P) or with Gö6976 for 3 h (G) before lysis. J774 cells were pretreated with solvent (−), PMA (P), or Gö6976 for 30 min (G), and lysed. (a) αvβ5 heterodimer levels. Immunoprecipitates from total IP lysate with nonimmune mouse IgG (lanes 1, 4, 7, and 9), or with αvβ5 antibody P1F6 (other lanes). Lanes 1–6 are fluorographs after immunoprecipitation from radioactively labeled cells and nonreducing SDS-PAGE. Lanes 7–10 are immunoblots after immunoprecipitation from unlabeled RPE-J and J774 lysates detected with β5 antibody B5-IVF2 after reducing SDS-PAGE. P1F6 antibody precipitated αvβ5 heterodimers from both metabolically labeled RPE-J and J774 cells, and quantification revealed ∼25% higher levels in the RPE (compare lane 2 with lane 5; relative intensities RPE 1.0/J774 0.78). Immunoblotting of β5 with antibody P1F6 gave similar quantification results and further confirmed the specificity of P1F6 for rodent αvβ5. Pharmacological activation or inhibition of PKC did not influence αvβ5 receptor levels in either cell type, and intensity differences were not significant. (b) Cell surface localization of αvβ5. Cells were pretreated and surface biotinylated before lysis. In lanes 1–6, immune complexes precipitated with P1F6 from biotinylated lysates were probed with streptavidin-peroxidase/ECL after reducing SDS-PAGE and blotting. Similar steady state levels of surface αvβ5 receptors were detected in RPE-J or J774, regardless of PKC inhibition or activation. Note that, under reducing conditions, the αv subunit appears at a lower molecular mass than under nonreducing conditions (∼110 kD reduced, ∼140 kD nonreduced). Other bands, possibly coprecipitates, were not identified. Lanes 7–12 show surface protein fractions enriched by streptavidin-agarose precipitation separated by reducing SDS-PAGE. Immunodetection with β5 antibody B5-IVF2 confirmed equal amounts of β5 protein at plasma membranes of RPE-J cells, regardless of PKC status (lanes 7–9). The same was true for J774 cells (lanes 10–12). One of three representative experiments is shown for each method. In both types of experiments, minor variations did not correspond to PKC modulation and were insignificant.
Terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) detection was performed using the Fluorescein In Situ Cell Death detection kit from Boehringer Mannheim. Surface phosphatidylserine was detected using the ApoAlert Cell Death detection kit from Clontech.
Cell Culture.
J774.A1 murine macrophages, NRK-F49 rat fibroblasts, and rat RPE-J cells were obtained from American Type Culture Collection and routinely maintained in DMEM supplemented with 10 or 4% (RPE) FCS. For binding and phagocytosis experiments, macrophages and fibroblasts were seeded at confluence on glass coverslips for 30 min or overnight before use. RPE-J cells were differentiated on semipermeable filters as described previously 36. Rat primary cells were isolated from adult Long Evans rats (Richard Harlan, Inc.) immediately after CO2 asphyxiation. Rat bone marrow–derived macrophages and rat blood–derived monocyte macrophages were prepared and maintained according to published procedures 13 37. In brief, blood-derived monocytes were isolated by plastic adherence after Ficoll gradient purification of leukocytes from anticoagulant-treated rat blood. Bone marrow cells were flushed aseptically from femur bones of Long Evans rats, washed, seeded, and used without repassaging. All primary macrophages were cultured for 6–7 d before use in IMDM, supplemented with 10% autologous serum; bone marrow–derived cell medium was supplemented with 5% L cell–conditioned medium (Sigma Chemical Co.) as a source of M-CSF 13. Primary rat neutrophils, which were isolated from fresh rat blood according to established protocols 38, underwent spontaneous apoptosis over 24 h in culture. HL-60 cells, a gift from Dr. F. Maxfield (Weill Medical College of Cornell University) were maintained in 10% FCS in RPMI. To induce differentiation and apoptosis, HL-60 cells were cultured in the dark at 2 × 105 cells/ml in growth medium containing 1 μM all-trans retinoic acid for 6 d. Before binding or phagocytosis assays, apoptotic cells were routinely tested for viability by trypan blue exclusion and phosphatidylserine surface exposure staining with FITC–annexin V. All populations used were >95% viable and >60% annexin V positive.
Preparation of Photoreceptor OS.
OS were isolated according to established protocols from bovine eyes obtained fresh from the slaughterhouse 39. OS were stored suspended in 10 mM sodium phosphate, pH 7.2, 0.1 M sodium chloride, 2.5% sucrose at −80°C. Before use, OS were thawed and labeled by addition of 20% vol of 1 mg/ml FITC or 0.2 mg/ml Texas red (both from Molecular Probes) in 0.1 M sodium bicarbonate, pH 9.0, for 1 h at room temperature in the dark, before being washed and resuspended in cell culture medium.
Opsonization of Zymosan.
Zymosan particles were opsonized with serum complement components as described previously 40. In brief, 1% zymosan particles in PBS were boiled for 30 min and opsonized in 50% bovine serum in HBSS for 1 h at 37°C. Washed particles were labeled with FITC as described for OS, and stored at 4°C.
Particle Binding and Phagocytosis Experiments.
To study particle binding, phagocytes at confluence were challenged with 10 particles per cell in growth medium (RPE) or serum-free growth medium (other cells) for the duration of the experiment, washed three times with PBS containing 1 mM MgCl2 and 0.2 mM CaCl2 (PBS-CM), and fixed in ice-cold methanol. OS were covalently labeled with FITC or Texas red, and thus could be observed directly. Apoptotic cells were visualized by TUNEL staining or by granulocyte-specific myeloperoxidase immunofluorescence staining using FITC- or Texas red–conjugated secondary antibodies. Nuclei were counterstained with DAPI or propidium iodide at 1 ng/ml in PBS-CM. For competition experiments, OS and apoptotic cells were labeled with different fluorochromes. Particle labeling with either fluorochrome yielded identical results. Unlabeled particles competed with fluorescence-labeled particles for binding and phagocytosis by all cell types. For inhibition experiments, GRGDSP or GRADSP peptides (Calbiochem) were used at 1 mg/ml. Effects of peptides and antibodies were concentration dependent as established previously 26, and maximal effective concentrations of antibodies, 20–50 μg/ml, were used throughout this study. Concentrations of pharmacological reagents were as follows: calphostin C at 100 nM (light activated), cytochalasin D (Cyt D) at 5–20 μM, Gö6976 at 10 nM, hypericin at 5 μM, and latrunculin B at 1 μM. PMA was routinely used at 50 nM; 16–160 nM gave similar results. Cells were pretreated for times indicated in the figure legends with activators or inhibitors before challenge with OS or apoptotic granulocytes in the continuous presence of reagent. Cell viability and morphology remained unchanged, and none of the cell types initiated detectable apoptosis over the course of the experiments. Since experiments were performed on confluent cells, the spreading effect of PMA on macrophages was negligible.
Phagocytosis or binding of OS was quantified by fluorescence scanning of fixed samples as described 26. Samples were scanned with a STORM 860 Imager, at 950 V (blue or red fluorescence setup; Molecular Dynamics). Areas representing the binding by 1–2 × 105 phagocytes were selected, and the fluorescent signals were quantified with ImageQuant 1.2 (Molecular Dynamics). Within one experiment, particle counts directly correlated with particle binding. To compare particle binding of different cell types, as RPE cells and macrophages, the fluorescence of propidium iodide (nuclei, red) and the particle-derived FITC fluorescence were both measured in each field. The binding plus internalization index or the binding index (bound particles at early times of phagocytic challenge) were calculated dividing particle counts by nuclei counts, thereby normalizing for phagocyte numbers. Quenching of fluorescence derived from externally bound particles using trypan blue 26 41 allowed determination of the internalization index. Phagocytosis or binding of apoptotic cells was quantified following the same procedure based on TUNEL or myeloperoxidase immunostaining fluorescence emissions; binding indices were determined dividing by nuclei counts of control fields, so as not to count apoptotic nuclei. Microscopic observation revealed that 75% of RPE-J cells and >90% of J774 cells had bound or phagocytosed an average of 5 ± 1 OS after 2 h. Using the double fluorescence scanning method on the same samples, this translated into a mean index combining binding plus internalization of 7.6 ± 0.9 for macrophages and 6.3 ± 0.9 for RPE. After 30 min, 75% of macrophages had bound multiple OS; this translated to a mean binding index of 3.8 ± 0.4. At this time point, <20% of particles had been internalized by macrophages, similar to the 2-h time point of RPE 26 41.
Immunofluorescence Microscopy.
Samples were fixed in ice-cold methanol or 4% paraformaldehyde in PBS-CM and processed as described previously 42. Samples were observed with a Nikon fluorescence microscope E600. Digital images were acquired with a back-illuminated cooled CCD camera (CCD1000 PB; Princeton Instruments), translated using MetaMorph (Universal Imaging), and recompiled in Adobe Photoshop® 4.0. Horizontal (x–y) sections were acquired at 0.5-μm steps using a z motor (Prior), and out of focus light was removed using MetaMorph.
Cell Fractionation.
Integrin cytoskeletal association was assayed by cell fractionation into detergent-soluble and insoluble fractions according to previously described protocols 43. Confluent cells in 6-cm dishes, preincubated with pharmacological reagents or vehicle as before binding or phagocytosis assays, were extracted in 800 μl 50 mM MES, 5 mM MgCl2, 3 mM EGTA, 0.5% Triton X-100, pH 6.4, for 40 s at room temperature (soluble fraction). The remaining cellular material was completely solubilized in an equal volume of RIPA (50 mM Tris-HCl, pH 7.8, 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 0.1% SDS, 1% sodium desoxicholate, 1% Triton X-100) (insoluble fraction). Equal volumes of soluble and insoluble fractions were compared by 7.5% SDS-PAGE and immunoblotting. For immunoprecipitation of integrins from the different fractions, the soluble fraction was harvested as described above, whereas the insoluble fraction was obtained by scraping the remaining cellular material in an equal volume of 50 mM Tris-HCl, pH 7.8, 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 1% Triton X-100, 1% NP-40 supplemented with 2 mM each of aprotinin, leupeptin, pepstatin, iodoacetamide, and PMSF, and 1 mM N-ethylmaleimide (IP lysis buffer), vortexing for 30 min at 4°C. This procedure completely solubilized integrin proteins. Immunoprecipitations were performed from this insoluble and the soluble fraction as follows.
Surface Protein and Immunoprecipitations, SDS-PAGE, and Immunoblotting.
For metabolic labeling, confluent cells on plastic dishes were starved for 90 min in cysteine, methionine-free DMEM, and labeled with 0.1 mCi/ml 35S Express (NEN) for 12–14 h. Preincubation with pharmacological reagents or vehicle as control was performed as before binding/phagocytosis assays. To biotinylate surface proteins, cells were incubated with Sulfo-NHS-LC biotin (Pierce Chemical Co.) at 0.5 mg/ml in PBS-CM twice for 20 min on ice. Excess reactive biotin was quenched in 50 mM NH4Cl in PBS-CM. Cells were lysed in IP lysis buffer for 30 min. Protein concentration of supernatant lysates was determined according to Bradford 44. Crude lysates were analyzed by nonreducing 7.5% SDS-PAGE followed by Western blot detection of integrins as published previously 45. Streptavidin-agarose precipitation has been described in detail elsewhere 46. Immunoprecipitates were formed incubating precleared lysate with 5 μg P1F6 IgG or nonimmune mouse IgG, with 5 μg rabbit anti–mouse IgG, and with 5 mg protein A–sepharose, each for 1 h at 4°C. Samples were washed four times in 50 mM Tris-HCl, pH 8.5, 0.5 M NaCl, 2 mM CaCl2, 1 mM MgCl2, 1 mg/ml egg albumin, 0.1% Triton X-100 eluted in nonreducing or reducing SDS sample buffer, and analyzed on 10% SDS-PAGE followed by fluorography or blotting. Blots were incubated with integrin antibodies, horseradish peroxidase–conjugated streptavidin, or secondary antibodies, followed by ECL (NEN) detection. X-ray films were scanned, and signals were quantified using NIH Image 1.61.
Results
Macrophages and RPE Cells Bind, Nonpreferentially, OS and Apoptotic Cells but Use Different Integrins for This Purpose.
Blood monocyte or bone marrow–derived macrophages use αvβ3 integrin receptors to bind apoptotic cells before phagocytosis 7, whereas RPE cells bind OS via αvβ5 integrins 25 26. We initially tested the hypothesis that the characteristic choice of different integrin receptors by macrophages and RPE cells was determined by differences in the bound particles. To this end, we exposed the phagocytes to the physiologic ligands of the other cell, i.e., macrophages to OS and RPE cells to apoptotic cells (Fig. 1). Rat bone marrow macrophages and the murine macrophage cell line J774, which retains the αvβ3 integrin–dependent apoptotic cell clearance mechanism of noninflammatory monocyte macrophages 13, efficiently bound and phagocytosed OS (Fig. 1, a and b). Primary cultures of rat RPE, and rat (RPE-J) and human (ARPE-19) RPE cell lines identified and took up apoptotic granulocytes (and other apoptotic cells; data not shown), but not their nonapoptotic counterparts (Fig. 1c–f). Most subsequent studies were performed on J774 macrophages and RPE-J cells, but were confirmed on rat bone marrow–derived macrophages and primary rat RPE cultures.
Figure 1.
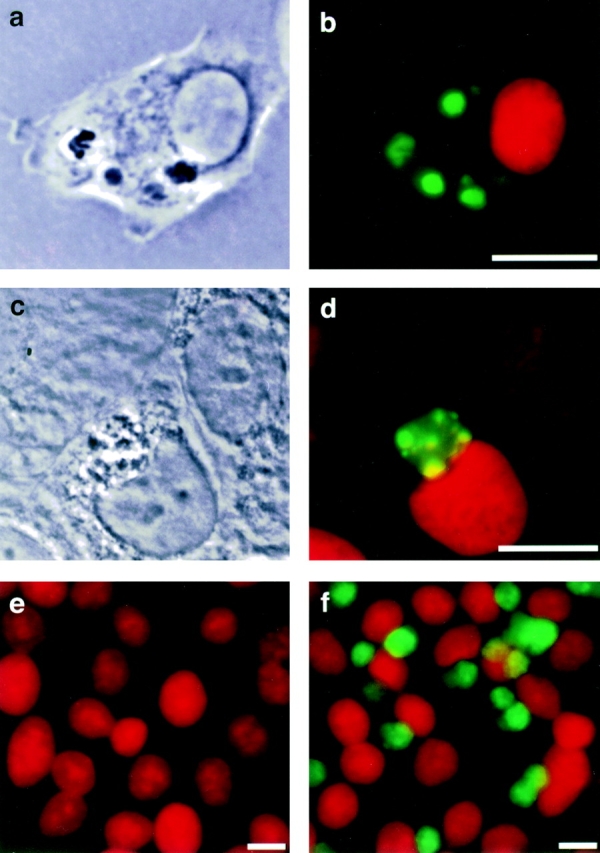
Macrophages take up OS, and RPE cells recognize and phagocytose apoptotic cells. (a) Phase-contrast and (b) merged fluorescence image of rat bone marrow–derived macrophage phagocytosing FITC-labeled OS (green) 40 min after particle challenge. Macrophage nuclei were stained with propidium iodide (red). (c) Phase-contrast and (d) merged fluorescence image of RPE-J cell after internalization of an apoptotic granulocyte, 3 h after phagocytic challenge. Apoptotic DNA was stained with TUNEL (green), nuclei with propidium iodide. (e and f) RPE-J monolayer challenged for 2 h with equal numbers of HL-60 cells, which had been induced to undergo apoptosis (f) or not (e). Samples were stained for myeloperoxidase to detect HL-60 cells (green) and counterstained with propidium iodide. RPE-J cells take up exclusively cells from the apoptotic population. Bound or phagocytosed HL-60 cells were also positive in TUNEL staining (data not shown). Nonapoptotic and apoptotic HL-60 cells expressed identical amounts of myeloperoxidase (data not shown). Scale bars, 10 μm.
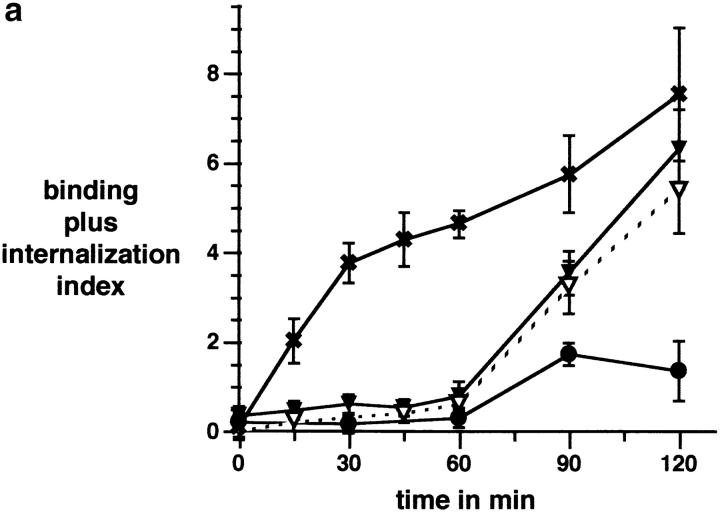
Particle binding and phagocytosis proceeded with kinetics that were characteristic for each cell type. Macrophages bound and internalized OS with the same rapid and linear kinetics with which they take up apoptotic cells (Fig. 2 a, and data not shown). The early phase of RPE clearance of OS is characterized by a lag phase following particle challenge after which OS binding occurs rapidly 26. RPE cells bound apoptotic cells as slowly as OS (Fig. 2 a). After 2 h of phagocytic challenge, the sum of bound plus internalized OS was similar in macrophages and RPE cells (Fig. 2 a). However, at this time 78 ± 9% of these OS in macrophages had been internalized, compared with only 17 ± 4% internalized OS in RPE cells. Phagocytosis of apoptotic cells bound by RPE cells followed the same slow time course as previously established for OS: we removed unbound apoptotic cells after 2 h and followed the fate of bound cells. Over the next 3 h, 85% of bound apoptotic cells were internalized by RPE cells (26; and data not shown).
Figure 2.
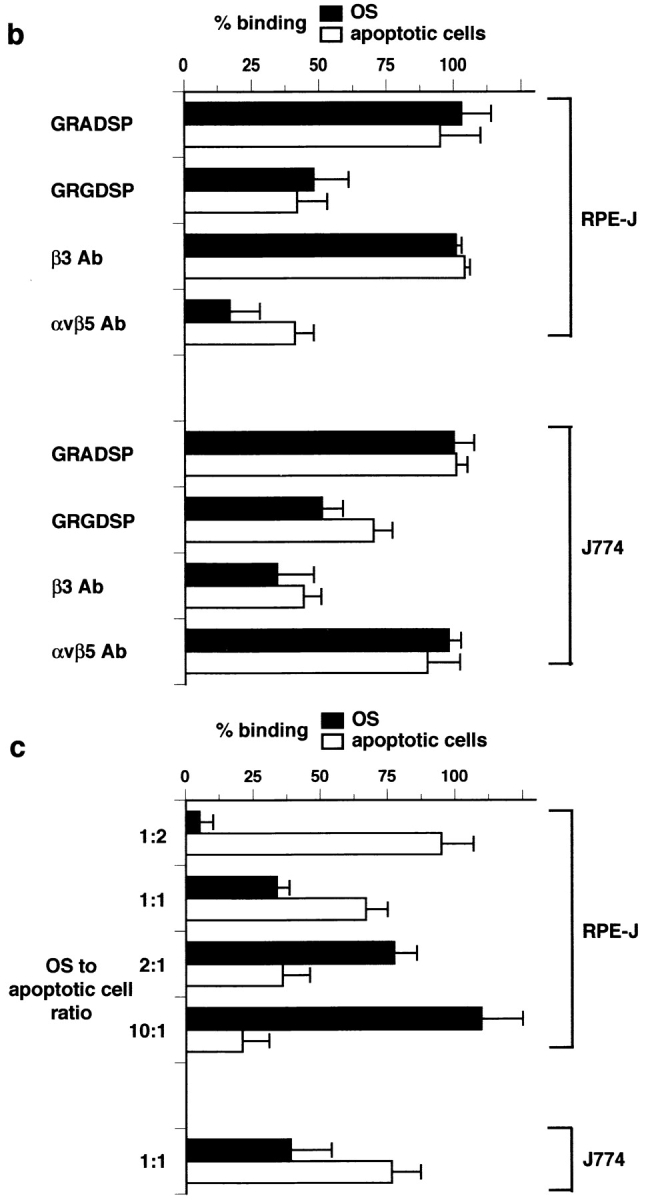
Apoptotic cells and OS compete for binding to αvβ3 of macrophages and for binding to αvβ5 of RPE cells. (a) Time course of combined OS binding plus internalization by rat bone marrow–derived macrophages (x) and NRK-F49 fibroblasts (•) compared with RPE-J cell combined binding plus internalization of OS (▾) and apoptotic cells (▿). J774 binding and internalization were indistinguishable from those of rat primary macrophages. (b) Integrin dependence and RGD peptide inhibition of binding of OS (black bars) and apoptotic cells (white bars) by RPE-J cells and by J774 macrophages. Mean OS binding indices in the absence of apoptotic cells were 6.3 ± 0.9 for RPE-J and 3.8 ± 0.4 for J774, after 2 h or 30 min of particle challenge, respectively. At these time points, particle phagocytosis accounted for <20% of total particle count. Mean apoptotic cell binding indices in the absence of OS were 5.5 ± 0.4 for RPE-J and 3.7 ± 0.5 for J774, at the same time points. (c) Quantitative competition of OS with apoptotic cells for phagocyte binding. RPE-J and J774 macrophages were challenged with different ratios of OS and apoptotic cells, for 2 h or 30 min, respectively. Particles were labeled with different fluorophores (FITC and Texas red) and quantified by fluorescence scanning. 100% represents binding of each particle in the absence of the other; for binding indices, see b. OS, black bars; apoptotic cells, white bars. Results represent means ± SEM (n = 5). When challenged with equal numbers of OS and apoptotic cells, both phagocytes seem to prefer apoptotic cells. However, this may be attributed to the larger size of apoptotic cells compared with OS (see Fig. 1).
To specifically address particle binding, we chose to study 30 min of particle challenge for macrophages and 2 h for RPE cells, both of which corresponded primarily to the recognition/binding phase of particle clearance (see above, and Materials and Methods). Peptides containing the cognate integrin-binding motif, RGD, reduced binding of both particles by either phagocyte (Fig. 2 b). In contrast, function-blocking β3 antibodies only inhibited particle binding by macrophages while αvβ5 antibody P1F6 only blocked RPE recognition (Fig. 2 b). OS and apoptotic cells competed for binding by both macrophages and RPE cells (Fig. 2 c). These experiments indicate that neither macrophage nor RPE binding receptor systems discriminate between ligands of both particles and that these systems involve αvβ3 in macrophages and αvβ5 in RPE cells.
Binding of Apoptotic Cells and OS by αvβ5 Is Dormant in Macrophages but May Be Activated by PKC.
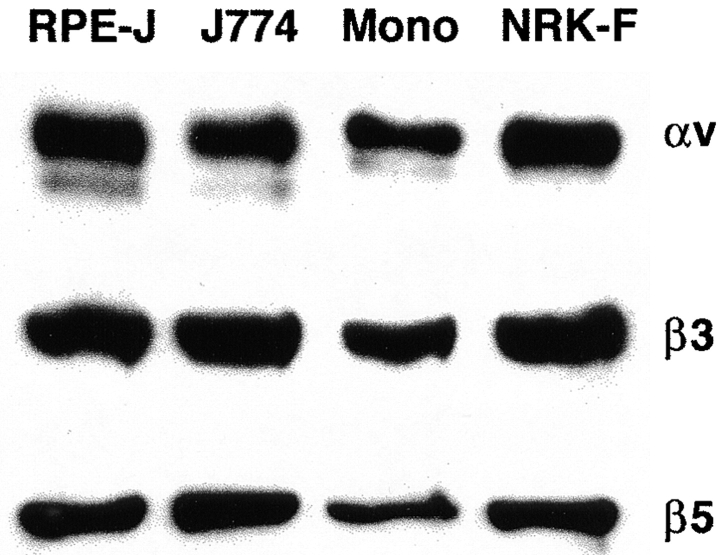
We tested three hypotheses that might account for particle binding by different integrin binding receptors in macrophages and RPE cells. Hypothesis 1 was that cell type–specific integrin protein expression determined receptor availability for particle binding. However, Fig. 3 shows that selective integrin expression was not involved, as both β3 and β5 were expressed at similar levels by J774 cells, rat bone marrow–derived macrophages, and RPE-J cells. Immunoprecipitation of αvβ5 from RPE and macrophage lysate using the antibody P1F6, which recognizes only intact heterodimers, and coimmunoprecipitation of β3 integrin with αv integrin confirmed the formation of αvβ3 (data not shown) and αvβ5 receptors (see Fig. 8). We have shown previously that the steady state distribution of β3 integrins is basolateral in the RPE 26. Although this does not exclude a temporary presence of αvβ3 at the apical phagocytic surface, this spatial segregation may render it less available for efficient apoptotic cell or OS binding by the RPE than αvβ5, which localizes apically and cytoplasmically. In contrast, double immunofluorescence staining with antibodies recognizing the β3 extracellular domain and with P1F6 antibodies specific for the extracellular face of the αvβ5 receptor complex showed that in nonpermeabilized macrophages, both antigens were localized in the same optical sections of the plasma membrane of a given cell, even if their distribution within the plane of the membrane differed. Like β3 integrins, αvβ5 receptors localized partially to basal attachment sites of macrophages but were also available at their free surface for binding to apoptotic cells or OS.
Figure 3.
J774 and rat macrophages express similar levels of αv, β3, and β5 integrin subunits as rat RPE-J cells and rat NRK-F49 fibroblasts. Proteins were detected by comparative immunoblotting after SDS-PAGE of 50 μg each of detergent lysates of RPE-J cells, J774 macrophages, primary rat monocytes (Mono), and rat NRK-F49 fibroblasts. Tested rodent primary macrophages and cell lines expressed levels of β5 integrin protein comparable to RPE cells, while an earlier study did not detect β5 in human monocyte–derived macrophages (reference 29). This may be attributed to species differences, or to differences in in vitro monocyte maturation conditions.
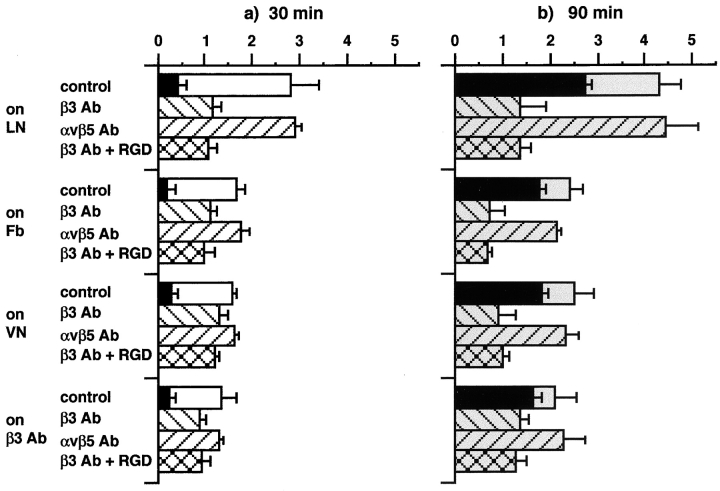
Hypothesis 2, applicable to macrophages, was that their preferred use of αvβ3 for apoptotic cell or OS binding might be based on the faster early kinetics of this pathway over αvβ5-mediated uptake (Fig. 2 a). To facilitate detection of a hidden αvβ5 particle binding activity, αvβ3 receptors were depleted from the free macrophage surface. When compared with control cells plated on laminin (which is not a substrate for either αvβ3 or αvβ5), a large fraction of αvβ3 receptors was recruited to the attached surface in macrophages plated on the specific αvβ3 substrate fibrinogen (47; compare Fig. 4, a1 and a2, with Fig. 4, b1 and b2), resulting in a reduction in particle binding of 40% after 30 min of particle challenge (Fig. 5 a), and a 44% decrease of combined binding and internalization even after 90 min (Fig. 5 b). αvβ5 receptor distribution remained unchanged on fibrinogen, as judged from the similar appearance of αvβ5 immunofluorescence signals on both attached and open cell surfaces on laminin and fibrinogen (compare Fig. 4, a3 and a4, with Fig. 4, b3 and b4). However, the remaining binding at 30 min and the combined binding plus internalization at 90 min were still reduced by β3 but not by αvβ5 function-blocking antibodies (Fig. 5). Incubation with β3 antibody in the presence of the peptide inhibitor GRGDSP did not have an additive effect (but the peptide alone reduced binding by ∼30%; data not shown, and see Fig. 2 b), indicating that αvβ3 was the only RGD-sensitive receptor, which mediates apoptotic cell or OS recognition in this system. However, ligand binding by leukocyte β2 integrins may not be inhibitable by RGD peptides 48. At the 90-min time point, at least 65% of the particles had been internalized (Fig. 5 b, black bars). Comparing total and internal particles of laminin- and fibrinogen-seeded macrophages, differences in internalized particles were less pronounced than differences in surface-bound particles (as deduced by subtracting internal from total particles), confirming that attachment to fibrinogen inhibited particle binding but not internalization (Fig. 5 b, gray and black bars). Similar results were obtained when cells were plated on β3 integrin antibodies or on vitronectin, a substrate for both αvβ3 and αvβ5 (Fig. 5). These experiments indicate that αvβ5 integrin receptors are present in macrophages but are not active in apoptotic cell or OS binding.
Figure 4.
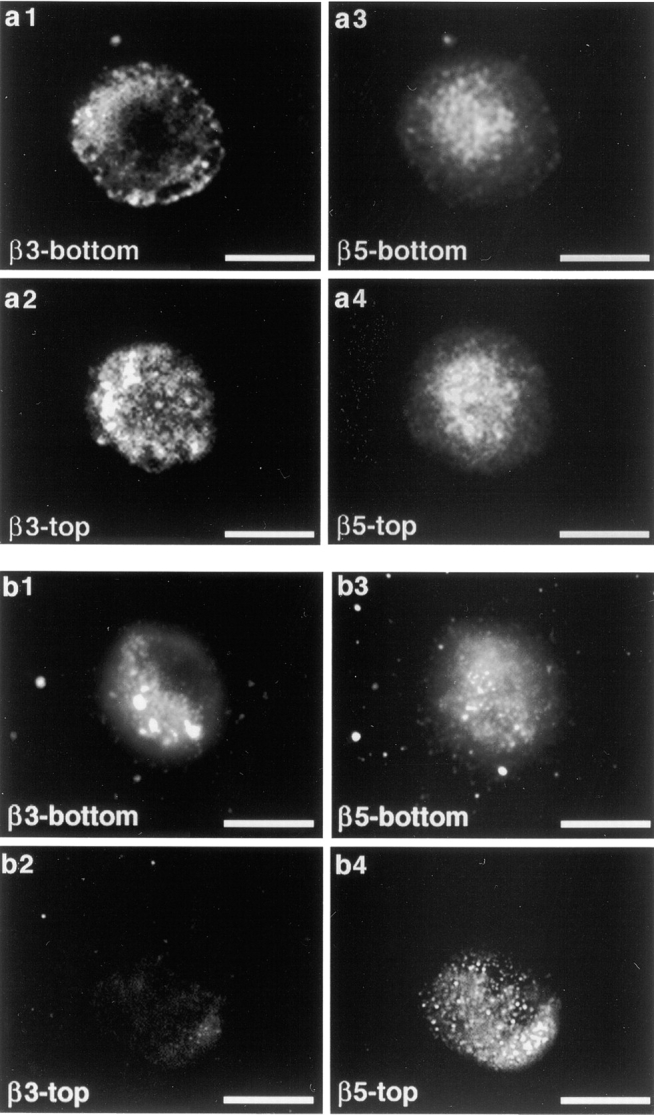
β3 integrin and αvβ5 integrin localize to attached and free macrophage plasma membrane. β3 integrins but not αvβ5 integrin redistribute to attachment sites in macrophages seeded on fibrinogen substrate. Immunodetection of surface integrins was performed on unpermeabilized cells with β3-specific or αvβ5 receptor–specific antibodies. Top and bottom panels shown are en face views of the apical and the attached cell surface, respectively. (a) β3 and αvβ5 integrin distribution in J774 macrophages attached to laminin-coated coverslips for 30 min. β3 and αvβ5 are present on both surfaces, as demonstrated by the immunofluorescence signals in horizontal optical sections at the levels of attached (a1 and a3) and free (a2 and a4) cell surfaces. (b) Same staining as in a, of J774 macrophages seeded on fibrinogen for 30 min, reveals specific relocalization of β3 integrins to attachment sites. Compare a1 with b1, a2 with b2. Scale bars, 10 μm.
Figure 5.
Effects of attachment on different extracellular substrates or β3-specific antibodies on J774 macrophage apoptotic cell or OS binding and internalization. J774 macrophages were seeded on coated coverslips in serum-free medium for 30 min. Particles were added for 30 min (a) or 90 min (b) in the presence or absence of inhibitory antibodies or peptides. White (a) and gray (b) bars, hatched or not, represent mean binding plus internalization indices ± SEM (n = 6). Black bars represent mean internalization indices ± SEM (n = 3). Note that cells seeded for 30 min before particle challenge, as studied in this experiment, bound fewer particles than cells which had attached overnight (see Fig. 2). Results represent data from OS challenges. Identical results were obtained challenging with apoptotic cells. Cell adhesion or morphology did not notably change due to attachment to the β3 antibody or to different substrates (LN, laminin; Fb, fibrinogen; VN, vitronectin). Attachment to αvβ5 or β1 antibody had no effect on particle binding (data not shown).
Hypothesis 3 was that integrin-mediated binding of apoptotic cells or OS was subject to cell type–specific regulation. Several earlier studies have reported that, unlike αvβ3 integrin, αvβ5 is sensitive to pharmacological modulation by protein kinase C (PKC) 33 49 50. Here, we compared the effects on macrophage and RPE particle binding of generic PKC activation using phorbol esters (PMA), and inhibition by calphostin C, Gö6976, and hypericin. Inhibitors were used at concentrations near IC50 for PKC. PMA treatment did not alter RPE particle binding; on the other hand, calphostin C, Gö6976, and hypericin reduced binding by RPE cells (Fig. 6 a). Significant inhibition (by 54%) by calphostin C required a preincubation of 3 h. It is possible that RPE cells take up calphostin C more slowly than other cultured cells or maintain lower cytoplasmic concentrations of this inhibitor. Hypericin and Gö6976 significantly reduced particle binding after as little as 30 min of preincubation by 39 and 22%, respectively, but both were also more effective after 3 h of preincubation (69 and 81% reduction). These experiments indicate that the apoptotic cell or OS binding activity of resting confluent RPE cells is drastically reduced by pharmacological inhibition of PKC.
Figure 6.
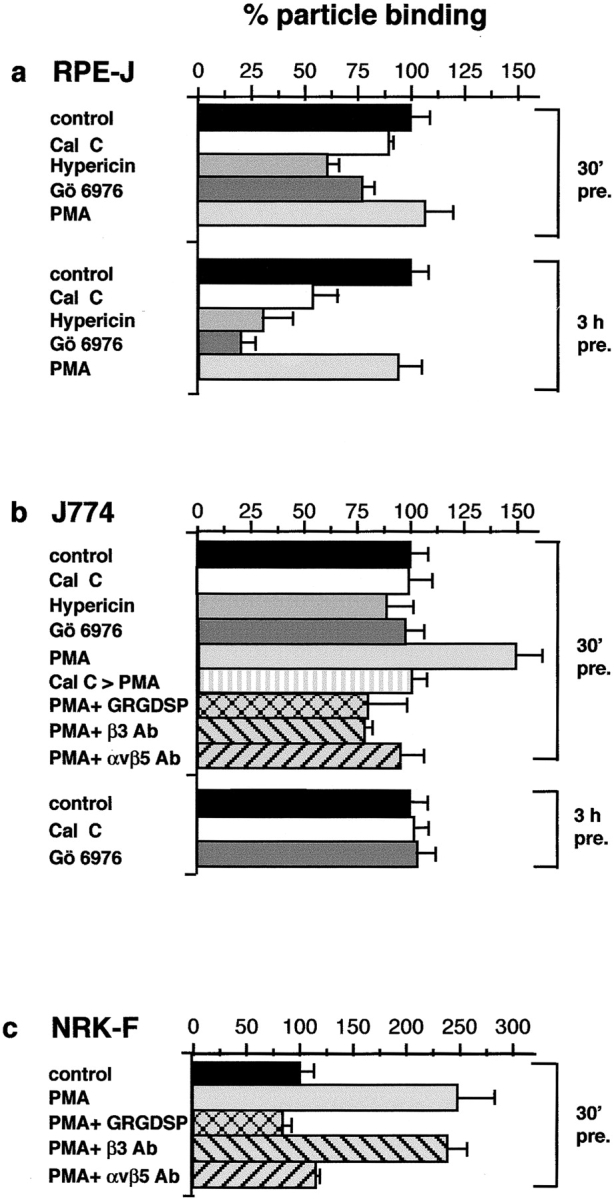
αvβ5 apoptotic cell or OS binding activity requires PKC stimulation. Endogenous PKC signaling preactivates αvβ5 binding by RPE cells, and stimulation of PKC induces a novel αvβ5-dependent binding mechanism in macrophages and fibroblasts. Cal C, calphostin C. 100% binding represents binding of DMSO controls for each phagocyte. Results are expressed as means ± SEM, of six independent experiments performed in duplicate. Using the Student's t test, P < 0.001 for all conditions discussed as significant in the text. (a) Effects of PKC activators and inhibitors on RPE-J particle binding. Longer preincubation time increased the effects of all PKC inhibitors used, suggesting that immediately before particle addition, RPE αvβ5 is already activated. Mean binding index of controls (100%) after 2 h of particle challenge was 5.39 ± 0.9 regardless of preincubation time. (b) Effect of PKC activators and inhibitors on macrophage particle binding. J774 or rat primary macrophages were preincubated for 30 min or 3 h in growth medium with pharmacological reagents or solvent, before OS challenge for 30 min. Similar results were obtained incubating with apoptotic cells, which competed with OS for binding to PMA-activated macrophages (data not shown). Binding index of controls (100%) was 4.2 ± 0.7. (c) Activation of αvβ5-mediated particle binding in NRK-F49 fibroblasts induced by PMA treatment. Cells were preincubated with reagents for 30 min before challenge with OS for 2 h, at which time point numbers of internalized apoptotic cells or OS were insignificant. Mean binding index of control fibroblasts was 1.36 ± 0.2, and of PMA-treated cells was 3.4 ± 0.5.
A strikingly different scenario was observed in the case of macrophages. PKC inhibition by calphostin C, Gö6976, or hypericin did not alter particle binding, regardless of preincubation time. On the other hand, stimulation of PKC by incubation with PMA increased particle attachment by 45%. This enhanced binding activity was sensitive to RGD-containing peptides, to β3 antibodies, and, importantly, to αvβ5 antibody Fab fragments (Fig. 6 b). Thus, PMA treatment activated αvβ5-mediated particle binding that was dormant in untreated macrophages. The effect of the PMA treatment directly involved PKC, since it was abolished by preincubation of cells with calphostin C for 20 min before PMA and particle challenge. To confirm the PKC dependence of αvβ5-mediated apoptotic cell or OS binding in a different cell context, we carried out similar experiments in NRK-F49 fibroblasts. When challenged with OS for 2 h, these cells bind (but do not internalize) few OS, equivalent to one fifth of the number bound by RPE over the same period (Fig. 2 a), although they express comparable levels of αv, β3, and β5 integrins (Fig. 3). Incubation with integrin antibodies had no effect on the basal NRK-F49 particle binding of apoptotic cells or OS (data not shown). However, addition of PMA stimulated particle binding by NRK-F49 fibroblasts by 250% to a binding index of 3.4 ± 0.5, which is an increase to approximately half-maximal binding capacity of professional phagocytes. Strikingly, this induced binding activity was completely sensitive to RGD peptides, was blocked specifically by αvβ5 antibodies, and was not sensitive to β3 antibodies (Fig. 6 c). Over the 2-h time period studied, NRK-F49 fibroblasts retained particles at the surface and did not internalize them, regardless of pharmacological stimulation. These experiments demonstrate that inhibition and activation of PKC regulate the function of αvβ5 integrin as binding receptor for apoptotic cells or OS.
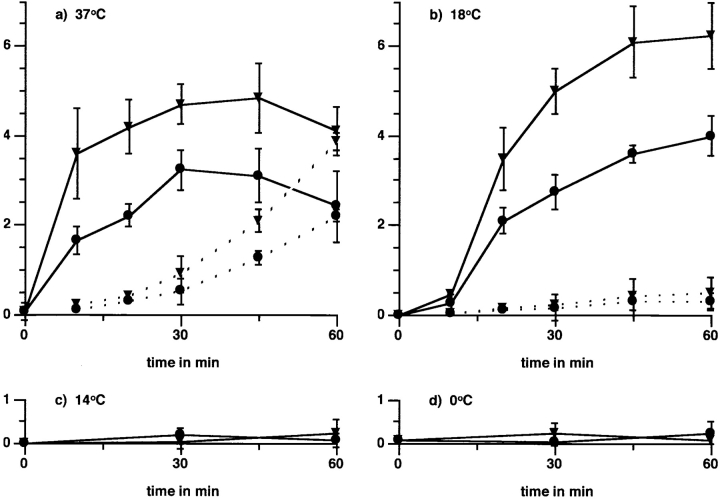
To determine if the stimulated αvβ5 integrin–mediated particle binding in macrophages exhibited typical features of OS binding by RPE cells, we compared the temperature sensitivity and kinetics of particle binding and internalization by J774 cells, in the presence or absence of PMA. Total (bound plus internalized) and internalized particles were measured to calculate binding and internalization indices plotted in Fig. 7. Onset of binding of apoptotic cells or OS to control and PMA-stimulated cells occurred with the same rapid kinetics typical of macrophages (Fig. 7 a, solid lines). When challenged with particles for 30 min or longer, both control and PMA-stimulated cells increasingly internalized bound material (Fig. 7 a, dotted lines), resulting in a decrease in particles detected bound to the cell surface. J774 cells, which were equilibrated to 18°C before particle challenge, also bound both particles with the same fast kinetics regardless of whether they had been preincubated with PMA or with solvent alone, albeit slightly delayed regarding the 37°C time course (Fig. 7 b, solid lines). Under these conditions, <10% of total particles were internalized regardless of PMA treatment and time of incubation (Fig. 7 b, dotted lines). Finally, incubation at 14°C or on ice during particle challenge abolished particle binding with or without PMA treatment (Fig. 7c and Fig. d), while allowing normal binding of complement-opsonized zymosan via macrophage αMβ2, sensitive to αM-blocking antibody 5C6 (data not shown). These experiments demonstrate that apoptotic cell or OS binding by macrophages via αvβ3 or αvβ3 plus αvβ5 integrin shows the same temperature requirement as particle binding via αvβ5 to RPE cells. On the other hand, PMA-stimulated macrophages, which possess an activated αvβ5 integrin, bind apoptotic cells or OS with the same rapid kinetics as control macrophages, which do not use αvβ5, indicating that RPE particle binding does not occur slowly due to an intrinsic property of αvβ5.
Figure 7.
Temperature sensitivity of apoptotic cell or OS binding and internalization by macrophages. Particle binding by macrophages, which use both αvβ3 and αvβ5, retains the fast macrophage binding kinetics and onset of binding. J774 cells were challenged with apoptotic cells or OS after 20 min preincubation with PMA (▾) or solvent alone (•) and preequilibration to different temperatures for 20 min. To establish binding indices for all temperatures, internalized particle counts were subtracted from counts representing bound plus internalized particles. Binding indices are plotted in solid lines for four temperatures: (a) incubation at 37°C, (b) 18°C, (c) 14°C, and on ice (at 0°C) (d). At both 37°C and 18°C, binding by PMA-treated cells exceeded that by control cells by approximately the same margin at all times. Thus, the onset of αvβ5-mediated binding by macrophages was fast and there was no lag phase as characteristic for αvβ5 binding by RPE. Reequilibration of cells to permissive temperatures after 60 min on ice restored their binding activity, and led to increased binding in the presence of PMA (data not shown), excluding nonspecific cell damage. Internalization indices are plotted in dotted lines. Internalization occurred regardless of treatment at 37°C (a). Cells at 18°C bound but did not internalize particles (b), but internalized prebound particles within 45 min after being shifted to 37°C (data not shown). Complement-opsonized zymosan binding occurred efficiently at all temperatures (data not shown). Values shown represent means ± SEM (n = 3) of results obtained with OS. Identical results were obtained challenging macrophages with apoptotic cells.
PKC-activated αvβ5 Is Linked to the Actin Cytoskeleton.
To gain insight into the mechanism of activation of αvβ5-mediated binding of apoptotic cells or OS by PKC, we searched for biochemical changes associated with PKC activation. Pharmacological activation or inhibition of PKC did not influence αvβ5 receptor levels in either cell type (Fig. 8 a, compare lane 2 with lane 3, and lane 5 with lane 6) or their steady state expression levels at the cell surface, as revealed by surface biotinylation, αvβ5 immunoprecipitation, and streptavidin blot (Fig. 8 b, lanes 1–6) or enrichment of plasma membrane fractions and β5 immunoblot (Fig. 8 b, lanes 7–12). Incomplete extraction of plasma membrane proteins with 0.5% Triton X-100 in a buffer that preserves the cortical cytoskeleton serves to distinguish integrin receptors, which are stabilized by actin cytoskeletal elements 43 51. PKC inhibition in RPE and PKC activation in macrophages correlated with decreased and increased β5 resistance to nonionic detergent, respectively, as shown in Fig. 9, a and b, suggesting a regulation of β5 association with actin cytoskeletal elements. β5 was 65% insoluble in control RPE, 58% insoluble in PMA-treated RPE, but only 33% insoluble in RPE treated with PKC inhibitor Gö6976. In contrast, β5 was mostly soluble in control macrophages (5% insoluble) but became 78% insoluble after PMA incubation. This effect was greatly inhibited in the presence of Gö6976 (23% insoluble). This effect was specific for β5, as β3 solubility was unaffected by PKC activation/inhibition (Fig. 9, a and b, lower lanes). β3 integrin was 30% insoluble in RPE and 40% insoluble in J774 cells, regardless of treatment. Pretreatment of RPE or macrophages with the actin microfilament disrupting drug, Cyt D, rendered β3 and β5 completely soluble in RPE and macrophages, confirming that integrin resistance to nonionic detergent extraction in our biochemical analysis reflected their association with actin microfilaments (Fig. 9, a and b, lanes 7 and 8). As expected, PMA treatment did not induce integrin insolubility in cells in which actin microfilaments had been disrupted by Cyt D (Fig. 9, a and b, lanes 9 and 10). We further determined the detergent solubility of intact αvβ5 receptors (Fig. 9 c). Strikingly, the effect of PKC activation or inhibition on αvβ5 heterodimer solubility was most pronounced: 90% of αvβ5 in RPE cells was insoluble but became mostly solubilized (26% insoluble) upon incubation with the PKC inhibitor Gö6976 (Fig. 9 c, lanes 1–6). In control macrophages, the entire αvβ5 receptor pool was present in the soluble fraction (Fig. 9 c, lanes 8 and 10). In contrast, PMA-treated macrophages exhibited 62% insoluble αvβ5 (Fig. 9 c, lanes 11 and 12). Attempts to visualize the change in receptor solubility by indirect immunofluorescence staining of αvβ5 or β5 in cells fixed before or after a short pulse of nonionic detergent failed, as, unfortunately, detergent pretreatment abolished all immunoreactivity. Loss of immunoreactivity after detergent treatment was independent of cell type or pharmacological treatment, suggesting that antibody–antigen binding did not tolerate detergent treatment followed by paraformaldehyde fixation. Nonetheless, the biochemical changes observed clearly demonstrate that αvβ5 function as particle binding receptor correlates with its increased resistance to nonionic detergent extraction, which was reversibly regulated by PKC signaling.
Figure 9.
PKC activation or inhibition affects the cytoskeletal stabilization of αvβ5 integrin receptors in RPE and in macrophages. (a and b) β5 but not β3 resistance to nonionic detergent extraction correlated with PKC activation in RPE and macrophages. Pretreatment conditions were as in Fig. 8. G+P represents treatment with Gö6976 for 15 min, followed by the addition of PMA to this inhibitor medium for 30 min. After pretreatment, cells were separated in detergent-soluble and insoluble fractions as described in Materials and Methods. Protein concentrations in the fractions revealed that 35% total cellular protein was present in the soluble fraction, and 65% in the insoluble fraction. This distribution was the same comparing RPE and macrophages and did not change after treatment with PKC activators or inhibitors. Integrin distribution in subcellular fractions were compared by Western blotting with β5 polyclonal antibody (upper lanes) or β3 antibody 26 (lower lanes) after nonreducing SDS-PAGE. Lanes with odd numbers are soluble fractions (labeled s), and lanes with even numbers are the corresponding insoluble fractions (labeled i), of cell types and treatment as indicated (see legend to Fig. 8). Actin microfilament disruption with 10 μM Cyt D completely solubilized β3 and β5 (a and b, lanes 7 and 8) and prevented PMA-induced recruitment of β5 into insoluble fractions (a and b, lanes 9 and 10). Shown here is one representative experiment out of a total of five experiments. Corresponding soluble and insoluble fractions were separated in duplicate on the same gels and blotted for β5 or β3. The appearance of unidentified secondary bands in some β5 blots was variable and did not correlate with pharmacological treatment. (c) PKC activation or inhibition regulated extractability of αvβ5 heterodimeric receptors. Metabolically labeled cells were pretreated and separated into soluble (lanes 1, 2, 5, 7, 8, and 11, labeled s) and insoluble fractions (lanes 3, 4, 6, 9, 10, and 12, labeled i). From each fraction, immunoprecipitates with nonimmune mouse IgG (lanes 1, 3, 7, and 9) or P1F6 antibody (all other lanes) were analyzed by nonreducing SDS-PAGE and fluorography. Note that J774 immunoprecipitates with P1F6 after separation of cells into soluble and insoluble fractions exhibited lower backgrounds than immunoprecipitates from total cell lysates (see Fig. 8 a, lanes 5 and 6), possibly due to the dilution of proteins that bound nonspecifically to P1F6 immune complexes. Quantification values given in the text represent means of four experiments, of which one representative experiment is shown.
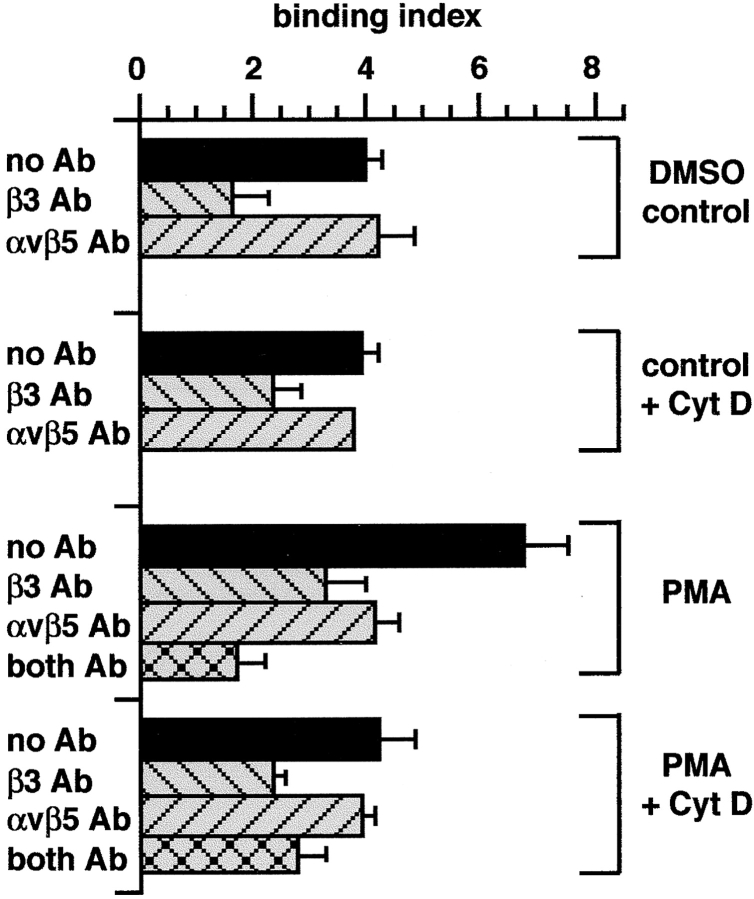
To directly test the possibility that an intact actin cytoskeleton might be required for αvβ5-mediated particle binding, we challenged J774 macrophages with OS in the presence or absence of PMA and Cyt D. Fig. 10 shows particle binding determined at 18°C to minimize phagocytosis of bound particles. OS bound efficiently to control macrophages even in the presence of Cyt D (Fig. 10), in both cases in an β3 integrin–sensitive, αvβ5-independent manner (average β3 antibody inhibition 59% in control cells, 42% in Cyt D–treated cells). Increased OS binding in the presence of PMA exhibited the dual β3- and αvβ5-dependent mechanism (51% inhibited by β3 antibody, 39% inhibited by αvβ5 antibody), and blocking of both integrins with a combination of antibodies resulted in maximal inhibition of OS binding (75%). Strikingly, the addition of Cyt D decreased OS binding to PMA-treated cells by 40%, abolishing the increase induced by PMA. Addition of αvβ5-inhibiting antibody to these cells had no effect, whereas OS binding remained sensitive to β3-inhibiting antibodies (52% inhibition). Similar effects on PMA-induced particle binding only were observed when 1 μM latrunculin B, a different actin-disruptive drug, was substituted for Cyt D (data not shown). When we tested RPE-J particle binding in the presence of 5–20 μM Cyt D, we also found their binding activity reduced. However, the morphology of the epithelium was dramatically altered after 2 h of actin disruption. These experiments demonstrate that, in macrophages and RPE-J cells, binding of apoptotic cells or OS to αvβ5 integrin receptors but not to αvβ3 required intact actin microfilaments.
Figure 10.
αvβ5 integrin, but not αvβ3 integrin, requires actin microfilaments for apoptotic cell or OS binding. J774 macrophages were preincubated with or without 10 μM Cyt D for 5 min, then PMA or solvent was added for an additional 25 min. Cells were equilibrated to 18°C for 20 min and challenged with FITC-OS or apoptotic cells for 45 min. At this time point, 18°C binding was similar in amount to binding at 37°C (see Fig. 7). Binding by cells treated with DMSO or PMA alone reproduced the integrin inhibition pattern established for 30-min challenge at 37°C (see Fig. 6 b). Both integrins bound particles independently, as dual inhibition had an additive effect on particle binding by PMA-treated macrophages (cross-hatched bars). Cyt D reduced the particle binding index of PMA-activated samples to that of vehicle controls and abolished its sensitivity to αvβ5 inhibition. Binding indices are means ± SEM (n = 3) of results obtained with OS. Similar results were obtained challenging macrophages with apoptotic cells.
Discussion
The experiments reported here demonstrate that noninflammatory phagocytosis of apoptotic cells or OS can proceed along routes gated by different integrin-binding receptors, αvβ3 and αvβ5. Several important aspects of the particle recognition functions of these integrins were unraveled.
Recognition Mechanisms of RPE Cells and Monocyte Macrophages Do Not Distinguish between Surface Signals of Photoreceptor Outer Segments and Signals Exposed during Apoptosis.
Although RPE cells lack inflammatory phagocytic mechanisms mediated in immune cells by Fc receptors, complement receptors, and glycan receptors, they possess a highly efficient clearance pathway for OS. The RPE phagocytic mechanism for OS and apoptotic cells has a capacity similar to the monocyte macrophage phagocytic mechanism. Our results confirm and extend earlier reports that described the extraordinary specificity of the RPE recognition machinery towards OS 20 and of the machinery used by noninflammatory macrophages for apoptotic over normal cells 4.OS and apoptotic cells quantitatively compete for both monocyte macrophage and RPE binding, demonstrating that the binding receptors used by either cell type to initiate phagocytosis of these types of particles do not discriminate between them. They also suggest that at least some of the local surface changes elicited by the daily photoreceptor outer segment renewal program are functionally equivalent to the recognition signals exposed during apoptosis.
RPE Cells Use αvβ5 Integrin, Whereas Macrophages Use αvβ3 Integrin to Bind Both OS and Apoptotic Cells.
Surprisingly, rat primary and murine J774 macrophages used αvβ3 integrin to bind OS, even though they expressed abundant αvβ5 integrin at the cell surface. The partial reduction in particle binding observed when using αvβ3 inhibitory antibodies and RGD peptides on macrophages confirms earlier reports that they also use nonintegrin binding receptors for apoptotic cells or OS, presumably in parallel mechanisms (for a review, see reference 11). In contrast, RPE cells likely use αvβ5 integrin as primary binding receptor for OS, as αvβ5-inhibiting antibodies abolish 85% of particle binding. RPE cells bound apoptotic cells as efficiently as OS via an αvβ5-dependent pathway, even though they express equal levels of β3 integrins as macrophages. Since β3 integrins in RPE cells are polarized at the basolateral surface of the RPE, their availability for particle binding may be limited. Although we expect this hypothesis to be difficult to test experimentally in epithelial cells, macrophages do not share similar permeability barriers between plasma membrane domains. Indeed, on appropriate immobilized substrates or specific antibodies, β3 integrins were efficiently trapped at macrophage attachment sites, leaving αvβ5 receptors exposed at the particle contact surface of the phagocytes. In spite of their favorable exposure, αvβ5 receptors failed to mediate apoptotic cell or OS binding. These experiments clearly demonstrate that neither the ligand (OS versus apoptotic cells), the exclusive expression of a given integrin receptor (αvβ3 versus αvβ5), nor its localization at the site of particle contact is sufficient to generate the characteristic receptor selectivity of RPE and macrophage particle recognition.
The Selection of a Particular Integrin Receptor by a Given Phagocyte Is Determined by the Activation of Specific Signaling Pathways.
Our results show that specific cellular context determines which integrin receptor, αvβ3 or αvβ5, will be activated for recognition and binding of OS and apoptotic cells. Pharmacological studies revealed that the PKC signaling pathway plays a key role in the activation of αvβ5 for particle binding. Interestingly, RPE cells possess a maximally activated αvβ5 integrin–gated binding mechanism that can be blocked by PKC inhibitors but cannot be enhanced by PKC stimulators. In contrast, monocyte macrophages constitutively express a functionally equivalent binding mechanism insensitive to PKC, in which αvβ3 plays an important role, but activate a dormant αvβ5 binding mechanism upon PKC stimulation that acts in parallel with their αvβ3 pathway. Although there is no evidence to date supporting a functional role for the novel αvβ5-mediated particle recognition mechanism in phagocytosis by monocyte macrophages, it is likely that this route may be important in vivo. Indeed, an active αvβ5-dependent phagocytic recognition mechanism for apoptotic cells was recently described in immature dendritic cells 29, and our results predict that PKC signaling may also be involved in the regulation of αvβ5 binding activity in these cells. Future studies should address the molecular mechanisms underlying αvβ5 integrin activation by cellular inside-out signaling mechanisms involving PKC (for a recent review, see reference 52). It is equally important to understand the unavailability of the abundant αvβ3 integrin receptors in the RPE, which, as mentioned above, may be related to their subcellular distribution, and the αvβ3 activation in macrophages.
Temperature Sensitivity (18°C) May Be an Intrinsic Property of Recognition Receptors for Apoptotic Cells and OS, Whereas the Kinetics and the Restoration of αv Integrin–mediated Particle Binding Are Determined by Cellular Context.
Particle binding involving both αvβ3 and αvβ5 integrins share a requirement of temperatures of at least 18°C, which may be a characteristic property of receptor mechanisms recognizing apoptotic cells and OS. Importantly, our results show that the activation of αvβ5-mediated particle recognition in macrophages by PKC did not result in slower binding or a lag phase after particle challenge, as would have been expected from the very slow kinetics of this process in RPE cells. Furthermore, RPE cells retain their αvβ5 integrin–dependent activity even after successive phagocytic challenges in vivo, and in vitro (Finnemann, S.C., unpublished data). In contrast, macrophages lose their αvβ3 integrin–dependent phagocytic recognition mechanism upon an initial particle challenge 2. Thus, RPE cells are permanent noninflammatory phagocytes whose clearance mechanism exhibits unique binding characteristics that cannot solely be attributed to intrinsic properties of their primary recognition receptor, αvβ5 integrin.
PKC Activation or Inhibition Controls αvβ5 Integrin Function as Binding Receptor for Apoptotic Cells or OS by Regulating Its Interaction with Cytoskeletal Elements: αvβ5 but Not αvβ3 Integrin–mediated Particle Binding Requires Intact Actin Microfilaments.
Our findings agree well with earlier investigations which demonstrated that αvβ5 but not αvβ3 integrin function is sensitive to modulation by PKC signaling 33 49 50 53. However, this is the first report to demonstrate a specific effect of PKC on the association of αvβ5 itself with the actin cytoskeleton. Our biochemical assays did not determine whether PKC induced a direct or indirect association of αvβ5 with actin. Lewis et al. 50 have shown a correlation between PMA activation of αvβ5 and a redistribution or activation of several cytoskeleton-associated molecules, e.g., α-actinin, tensin, and vinculin. These actin-associated proteins may mediate both mechanical as well as signaling roles of integrin receptors. Cytoskeleton-associated proteins and their roles in apoptotic cell or OS binding and subsequent noninflammatory phagocytosis have yet to be studied in detail. Before inflammatory phagocytosis, particle binding to surface Fc receptors or αMβ2 integrins appears to be independent of the integrity of the actin cytoskeleton 54 55. The hypothesis that binding of the same particle to a different integrin binding receptor, in response to phagocyte- rather than ligand-specified signals, induces different outside-in signaling effects, which ultimately determines properties of later phases of phagocytic clearance, is intriguing and will be the subject of further studies. The unique susceptibility of RPE phagocytes to genetic manipulation by viral or plasmid expression vectors in vitro and in vivo 42 56 will certainly be helpful in future studies designed to characterize the roles of specific actin binding and regulatory proteins in apoptotic cell or OS binding and phagocytosis.
Acknowledgments
We thank Dr. Geri Kreitzer for help with microscopic analysis, and Dr. Timothy McGraw for critical comments on the manuscript. We also thank Dr. William A. Muller for his gift of 5C6 antibody.
This work was supported by National Institutes of Health grant EY08538 and a Jules and Doris Stein Professorship awarded by the Research to Prevent Blindness Foundation to E. Rodriguez-Boulan. S.C. Finnemann was supported by a David Warfield Fellowship in Ophthalmology of The New York Community Trust and The New York Academy of Medicine, and a Norman and Rosita Winston Foundation Fellowship in Biomedical Research.
Footnotes
1used in this paper: Cyt D, cytochalasin D; OS, outer segment particle(s); PKC, protein kinase C; RPE, retinal pigment epithelium; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling
References
- Voll R.E., Herrmann M., Roth E.A., Stach C., Kalden J.R., Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M.D., Weil M., Raff M.C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Savill J.S., Wyllie A.H., Henson J.E., Walport M.J., Henson P.M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt A., Moffatt O.D., Raykundalia C., Capra J.D., Simmons D.L., Gregory C.D. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Platt N., Suzuki H., Kurihara Y., Kodama T., Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro . Proc. Natl. Acad. Sci. USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Hogg N., Ren Y., Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. Apoptosis. Phagocytic docking without shocking. Nature. 1998;392:442–443. doi: 10.1038/33025. [DOI] [PubMed] [Google Scholar]
- Platt N., da Silva R.P., Gordon S. Recognizing deaththe phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- Fadok V.A., Savill J.S., Haslett C., Bratton D.L., Doherty D.E., Campbell P.A., Henson P.M. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- Pradhan D., Krahling S., Williamson P., Schlegel R.A. Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol. Biol. Cell. 1997;8:767–778. doi: 10.1091/mbc.8.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Laszlo D.J., Noble P.W., Weinstein L., Riches D.W., Henson P.M. Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells. J. Immunol. 1993;151:4274–4285. [PubMed] [Google Scholar]
- Mevorach D., Mascarenhas J.O., Gershov D., Elkon K.B. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.W., Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.W. The renewal of photoreceptor cell outer segments. J. Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D., Hall M.O. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J. Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R.B., Szamier R.B. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science. 1977;197:1001–1003. doi: 10.1126/science.560718. [DOI] [PubMed] [Google Scholar]
- Mayerson P.L., Hall M.O. Rat retinal pigment epithelial cells show specificity of phagocytosis in vitro. J. Cell Biol. 1986;103:299–308. doi: 10.1083/jcb.103.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom S.W., Sparrow J.R., Silverstein R.L. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J. Cell Sci. 1996;109:387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- Boyle D., Tien L.F., Cooper N.G., Shepherd V., McLaughlin B.J. A mannose receptor is involved in retinal phagocytosis. Invest. Ophthalmol. Vis. Sci. 1991;32:1464–1470. [PubMed] [Google Scholar]
- Hall M.O., Abrams T.A. The phagocytosis of ROS by RPE cells is not inhibited by mannose-containing ligands. Exp. Eye Res. 1991;53:167–170. doi: 10.1016/0014-4835(91)90070-u. [DOI] [PubMed] [Google Scholar]
- Lutz D.A., Guo Y., McLaughlin B.J. Natural, high-mannose glycoproteins inhibit ROS binding and ingestion by RPE cell cultures. Exp. Eye Res. 1995;61:487–493. doi: 10.1016/s0014-4835(05)80144-7. [DOI] [PubMed] [Google Scholar]
- Miceli M.V., Newsome D.A., Tate D.J. Vitronectin is responsible for serum-stimulated uptake of rod outer segments by cultured retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1997;38:1588–1597. [PubMed] [Google Scholar]
- Finnemann S.C., Bonilha V.L., Marmorstein A.D., Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M.O., Abrams T. Kinetic studies of rod outer segment binding and ingestion by cultured rat RPE cells. Exp. Eye Res. 1987;45:907–922. doi: 10.1016/s0014-4835(87)80105-7. [DOI] [PubMed] [Google Scholar]
- Griffin F.M., Jr., Griffin J.A., Leider J.E., Silverstein S.C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J. Exp. Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Pearce S.F.A., Francisco L.M., Sauter B., Roy P., Silverstein R.L., Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich M.H., Nesbitt S.A., Horton M.A. Integrins on rat osteoclastscharacterization of two monoclonal antibodies (F4 and F11) to rat β3. J. Bone Miner. Res. 1992;7:345–351. doi: 10.1002/jbmr.5650070315. [DOI] [PubMed] [Google Scholar]
- Crippes B.A., Engleman V.W., Settle S.L., Delarco J., Ornberg R.L., Helfrich M.H., Horton M.A., Nickols G.A. Antibody to β3 integrin inhibits osteoclast-mediated bone resorption in the thyroparathyroidectomized rat. Endocrinology. 1996;137:918–924. doi: 10.1210/endo.137.3.8603604. [DOI] [PubMed] [Google Scholar]
- Schultz J.F., Armant D.R. β1- and β3-class integrins mediate fibronectin binding activity at the surface of developing mouse peri-implantation blastocysts. Regulation by ligand-induced mobilization of stored receptor. J. Biol. Chem. 1995;270:11522–11531. doi: 10.1074/jbc.270.19.11522. [DOI] [PubMed] [Google Scholar]
- Friedlander M., Brooks P.C., Shaffer R.W., Kincaid C.M., Varner J.A., Cheresh D.A. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Pasqualini R., Bodorova J., Ye S., Hemler M.E. A study of the structure, function and distribution of β5 integrins using novel anti-β5 monoclonal antibodies. J. Cell Sci. 1993;105:101–111. doi: 10.1242/jcs.105.1.101. [DOI] [PubMed] [Google Scholar]
- Rosen H., Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J. Exp. Med. 1987;166:1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi I.R., Mathews A.P., Cohen-Gould L., Gundersen D., Rodriguez-Boulan E. Immortalization of polarized rat retinal pigment epithelium. J. Cell Sci. 1993;104:37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- Musson R.A. Human serum induces maturation of human monocytes in vitro. Am. J. Pathol. 1983;111:331–340. [PMC free article] [PubMed] [Google Scholar]
- Savill J.S., Henson P.M., Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge-sensitive” recognition mechanism. J. Clin. Invest. 1989;84:1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday R.S., Hicks D., Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest. Ophthalmol. Vis. Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- Murata T., Sullivan J.A., Sawyer D.W., Mandell G.L. Influence of type and opsonization of ingested particle on intracellular free calcium distribution and superoxide production by human neutrophils. Infect. Immun. 1987;55:1784–1791. doi: 10.1128/iai.55.8.1784-1791.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hed J. Methods for distinguishing ingested from adherent particles. Methods Enzymol. 1986;132:198–204. doi: 10.1016/s0076-6879(86)32008-1. [DOI] [PubMed] [Google Scholar]
- Marmorstein A.D., Gan Y.C., Bonilha V.L., Finnemann S.C., Csaky K.G., Rodriguez-Boulan E. Apical polarity of N-CAM and EMMPRIN in retinal pigment epithelium resulting from suppression of basolateral signal recognition. J. Cell Biol. 1998;142:697–710. doi: 10.1083/jcb.142.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E.G., Wan K.M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffoldthree-dimensional organization and protein composition. J. Cell Biol. 1984;98:1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Finnemann S., Kühl M., Otto G., Wedlich D. Cadherin transfection of Xenopus XTC cells downregulates expression of substrate adhesion molecules. Mol. Cell. Biol. 1995;15:5082–5091. doi: 10.1128/mcb.15.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein A.D., Zurzolo C., Le Bivic A., Rodriguez-Boulan E. Cell surface biotinylation techniques and determination of protein polarity. In: Celis J.E., editor. Cell BiologyA Laboratory Handbook, Vol. 4. Academic Press, Inc.; New York: 1998. pp. 341–350. [Google Scholar]
- Smith J.W., Vestal D.J., Irwin S.V., Burke T.A., Cheresh D.A. Purification and functional characterization of integrin αvβ5. An adhesion receptor for vitronectin. J. Biol. Chem. 1990;265:11008–11013. [PubMed] [Google Scholar]
- Arnaout M.A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- Klemke R.L., Yebra M., Bayna E.M., Cheresh D.A. Receptor tyrosine kinase signaling required for integrin αvβ5-directed cell motility but not adhesion on vitronectin. J. Cell Biol. 1994;127:859–866. doi: 10.1083/jcb.127.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J.M., Cheresh D.A., Schwartz M.A. Protein kinase C regulates αvβ5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J. Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L.M., Messier J.M., Mercurio A.M. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the α6β1 integrin. J. Cell Biol. 1990;110:2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P.E., Pfaff M. Integrin affinity modulation. Trends Cell Biol. 1998;8:359–364. doi: 10.1016/s0962-8924(98)01339-7. [DOI] [PubMed] [Google Scholar]
- Brooks P.C., Klemke R.L., Schon S., Lewis J.M., Schwartz M.A., Cheresh D.A. Insulin-like growth factor receptor cooperates with integrin αvβ5 to promote tumor cell dissemination in vivo. J. Clin. Invest. 1997;99:1390–1398. doi: 10.1172/JCI119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S.L., Mikus L.K., Tucci M.A. Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J. Immunol. 1991;146:967–974. [PubMed] [Google Scholar]
- Allen L.A., Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor–mediated phagocytosis in macrophages. J. Exp. Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Davidson B.L. Phenotype correction in retinal pigment epithelium in murine mucopolysaccharidosis VII by adenovirus-mediated gene transfer. Proc. Natl. Acad. Sci. USA. 1995;92:7700–7704. doi: 10.1073/pnas.92.17.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]