Abstract
Intestinal intraepithelial lymphocytes (IELs) in mice include two main subsets of TCR-α/β1 cells which differ functionally and ontogenically from each other. One expresses the CD8α/α homodimer, whereas the other expresses the CD8α/β heterodimer. Although the presence of all CD8+TCR-α/β1 IELs is dependent on β2-microglobulin molecules, the nature of the major histocompatibility complex (MHC) class I molecules recognized by the CD8α/α and the CD8α/β1 subsets has remained elusive. Using mutant mice lacking the expression of both H2-Kb and H2-Db, we show that the CD8α/β1TCR-α/β1 subset is dependent on K or D molecules, whereas the CD8α/α1TCR-α/β1 subset is independent of classical MHC class I molecules. Furthermore, the CD8α/α1 cells are conserved in mice lacking expression of CD1, a nonclassical MHC class I–like molecule previously proposed to be a potential ligand for IELs. Using transporter associated with antigen processing (TAP)-deficient mice, this cell population can be further separated into a TAP-dependent and a TAP-independent subset, suggesting either the recognition of two nonclassical MHC-like molecules, only one of which is TAP dependent, or the involvement of a single nonclassical MHC-like molecule that is only partially TAP dependent. These findings demonstrate that CD8α/β1TCR-α/β1 IELs are restricted by H-2K and H-2D molecules, whereas the unusual subset of CD8α/α1TCR-α/β1 resident IELs recognize nonclassical MHC class I–like molecules that are distinct from CD1.
Keywords: major histocompatibility complex, CD1, intestinal intraepithelial lymphocytes, CD8, gene-targeted mouse
Alarge contingent of lymphocytes is located in the epithelial barriers that are exposed to the outside environment. Although a few of these lymphocytes are related to peripheral lymphocytes, most of them appear to be resident cells that are only encountered inside epithelial barriers. The ontogeny and functions of these resident cells appear to be unique. For example, sequential waves of TCR-γ/δ1 T cells expressing distinct TCR gene families seed the epithelia of the skin, reproductive tract, and intestine 1. Their antigen specificity and their function remain elusive, although it is increasingly recognized that they participate in the stress response of epithelia and are involved in the wound healing process 2 3.
Intestinal intraepithelial lymphocytes (IELs) include prominent populations of both TCR-γ/δ1 and TCR-α/β1 cells 4 5 6. Among the TCR-α/β1 IELs, there exist two subsets according to the expression of the CD8α/β heterodimer or the CD8α/α homodimer (which is also expressed on most of the TCR-γ/δ1 IELs). The CD8α/β1 subset can participate in conventional T cell responses against microbes as well as food antigens 7 8 9 10 11 12 13. It includes cells that were primed in the Peyer's patches, and have circulated back to seed the whole length of the intestinal epithelium 14 15. In contrast, the CD8α/α1 subset remains mysterious, as it appears to be a resident subset that has not yet been associated with antigen-specific responses but might instead play local functions of regulation 16 17. However, the detection of oligoclonal expansions of CD8α/α1TCR-α/β1 IELs and their activated phenotype do suggest that, like CD8α/β1 cells, they may be actively involved in ongoing immune responses 18 19 20.
Although the MHC or MHC-like molecules contributing to the selection or activation/expansion of the TCR-α/β1 IEL subsets have not been identified, they are known to be dependent on β2-microglobulin (β2m), because all CD8+TCR-α/β1 IELs were absent in β2m-deficient mice 21. In transporter associated with antigen processing (TAP)-deficient mice, the CD8α/β subset is absent while some CD8α/α cells are conserved 22, suggesting that at least some of the CD8α/α IELs might recognize nonclassical MHC-like molecules such as CD1, which is TAP-independent and has been reported to be expressed by the intestinal epithelium of mice and humans and recognized by CD8 clones derived from human IELs 23.
In this paper, we used the recently generated CD1-deficient, H-2Kb/Db double-deficient, and Kb/Db/CD1 triple-deficient mice to investigate the nature of the MHC and MHC-like ligands associated with intestinal TCR-α/β1 lymphocytes. Our results demonstrate that all CD8α/β1TCR-α/β1 IELs are dependent on the classical H-2K and H-2D MHC class I molecules, whereas CD8α/α1TCR-α/β1 IELs are independent of both the classical H-2 and the nonclassical CD1 molecules. Thus, the CD8α/α1TCR-α/β1 IELs must recognize nonclassical, non-CD1, as yet undefined MHC class I–like molecules. These results show that the phenotypic expression of CD8α/α versus CD8α/β among TCR-α/β1 IELs reflects a fundamental dichotomy with respect to antigen specificity and function.
Materials and Methods
Mice.
Mice were housed at Princeton University under specific pathogen-free conditions and were 10–14 wk old at the time of study. Mutant mice (deficient in TAP-1 24, Kb/Db 25, CD1.1 [Park, S.-H., and A. Bendelac, manuscript in preparation], CD1.1/1.2 26, or β2m 27) were generated from embryonic stem cells of 129 origin and used after 6–10 backcrosses to C57BL/6. Kb/Db/CD1-deficient mice were generated by crossing CD1.1- with Kb/Db-deficient mice. In all cases, +/+ or +/− littermates were used as controls, except for β2m- and CD1.1/CD1.2-deficient mice, which were compared with age- and sex-matched C57BL/6 mice.
Histology.
For histological study, duodenal fragments of 1 cm taken 3 cm below the pylorus were properly oriented on filter paper and fixed in Carnoy's fluid for 24–48 h. Paraffin-embedded sections were prepared and stained with periodic acid-Schiff (PAS), and the numbers of IELs per 100 epithelial cells (ECs) were counted under the microscope.
Lymphocyte Isolation.
The small intestine was separated from Peyer's patches and mesenteric lymph nodes, cut longitudinally, and washed in PBS. Fragments of 0.5–1 cm were incubated for 30 min at 37°C in RPMI 1640 (GIBCO BRL) containing 1% dialyzed FCS (Biofluids), 1.5 mM MgCl2, and 1 mM EGTA and supplemented with 1 mM dithiothreitol (Sigma Chemical Co.), under constant shaking. The IELs contained in the supernatant were collected, washed three times in PBS/5% FCS, and passed through a nylon mesh. IELs were further purified by centrifugation over Ficoll-Paque™ (Amersham Pharmacia Biotech).
Flow Cytometry.
Lymphocytes were first incubated for 30 min with 2.4G2 anti-Fc antibodies in order to block Fc receptors. For triple membrane staining with directly conjugated antibodies, cells were labeled with a combination of antibodies conjugated to PE, FITC, Cy-Chrome, or biotin. Biotinylated antibodies were revealed with Streptavidin Tricolor (Caltag Laboratories).
Anti–TCR-α/β, TCR-γ/δ, CD8β CD8α, CD4, CD19, CD44, CD69, B220, CD103, and TCR Vβ were purchased from PharMingen.
Fluorescence was analyzed on a FACScan™ (Becton Dickinson). The live gate for acquisition contained >95% CD103+ cells, no CD19+ cells, and <10% CD4+ cells in all cases.
Results
Persistence of CD8+TCR-α/β1 Cells in the Intestinal Epithelium of Kb/Db Double-deficient Mice.
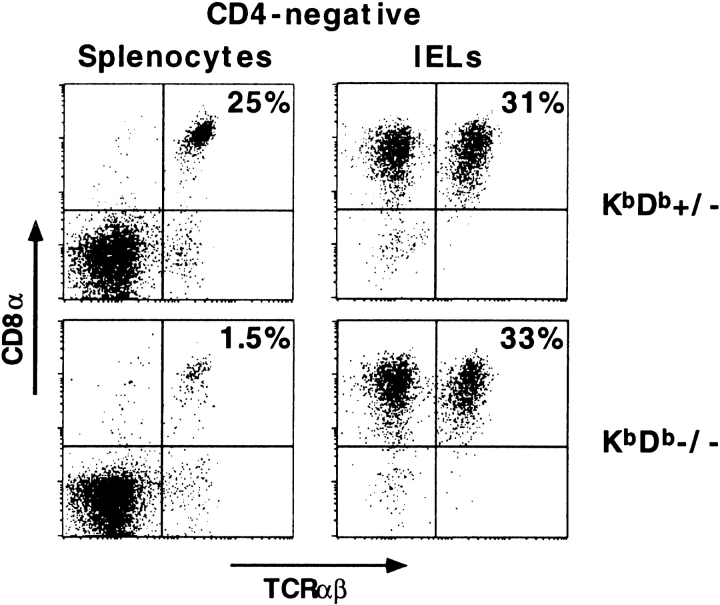
Fig. 1 shows a flow cytometry analysis of splenocytes and intestinal IELs from Kb/Db-deficient mice and littermate controls. Unlike CD8+TCR-α/β1 cells in the spleen, which were virtually all dependent on Kb or Db expression, the percentage of CD8+TCR-α/β1 IELs was unaffected by the loss of the classical MHC molecules. This striking observation was confirmed on a total of nine Kb/Db-deficient mice (33 ± 9% CD8+TCR-α/β1 IELs) and eight littermate controls (38 ± 9% CD8+TCR-α/β1 IELs). Furthermore, because cell recoveries after isolation of IELs may vary from one sample or one experiment to another, we counted the number of IELs per 100 ECs on histological sections of duodenal samples, and found that the numbers of IELs/100 ECs were comparable in Kb/Db-deficient mice (15 ± 3 IELs/100 ECs) and littermate controls (15 ± 3 IELs/100 ECs) (Table ). Thus, the absolute number of CD8+TCR-α/β1 IELs seemed to be conserved.
Figure 1.
The CD8+TCR-α/β1 IEL subset is present in Kb/Db-deficient mice. Splenocytes and IELs were triple-stained with anti–TCR-α/β, anti-CD4, and anti-CD8α. The dot plots display the CD8α versus TCR-α/β profiles of gated CD4-negative cells.
Table 1.
IEL Subsets of Normal and Mutant Mice
| Mice | No. IELs/100 ECs | TCR-α/β | TCR-γ/δ | ||
|---|---|---|---|---|---|
| CD8α/α | CD8α/β | CD4+8− | |||
| Controls (n = 27) | 14 (3) | 20 (10) | 7 (4) | 2 (2) | 59 (8) |
| KbDb KO (n = 9) | 14 (3) | 28 (10) | 0.6 (0.4) | 2 (1) | 60 (4) |
| CD1 KO (n = 6) | 13 (2) | 17 (5) | 12 (8) | 1 (1) | 51 (9) |
| TAP KO (n = 6) | 13 (3) | 10 (7) | 0.5 (0.4) | 2 (1) | 80 (7) |
| β2m KO (n = 7) | 10 (3) | 1.4 (1.1) | 0.1 (0.1) | 1.1 (0.3) | 90 (3) |
In addition, no change in the frequency of TCR-γ/δ1 or CD4+TCR-α/β1 IELs could be detected in Kb/Db-deficient mice (Fig. 1, and Table ).
CD8α/α1TCR-α/β1 IELs Are Independent of Kb/Db, Whereas CD8α/β1TCR-α/β1 IELs Are Dependent.
To further investigate the observation that CD8+TCR-α/β1 IELs seemed unaffected in Kb/Db double-deficient mice, we analyzed the CD8α/α1 and CD8α/β1 subsets of TCR-α/β1 cells separately. These two subsets differ with respect to their ontogeny, recirculation, and function, suggesting that they might exhibit different requirements for MHC ligands as well 4 5 6. Fig. 2 and Table show that the CD8α/β1 subset was virtually entirely absent in Kb/Db double-deficient mice, whereas the CD8α/α1 cells were conserved or even increased. As previously noted, there were no significant differences between the absolute numbers of IELs/100 ECs found in the various genetically modified mice used in this study and in their littermate controls. Thus, the frequency of various subsets among total IELs directly reflected their absolute number (Fig. 2, and Table ).
Figure 2.
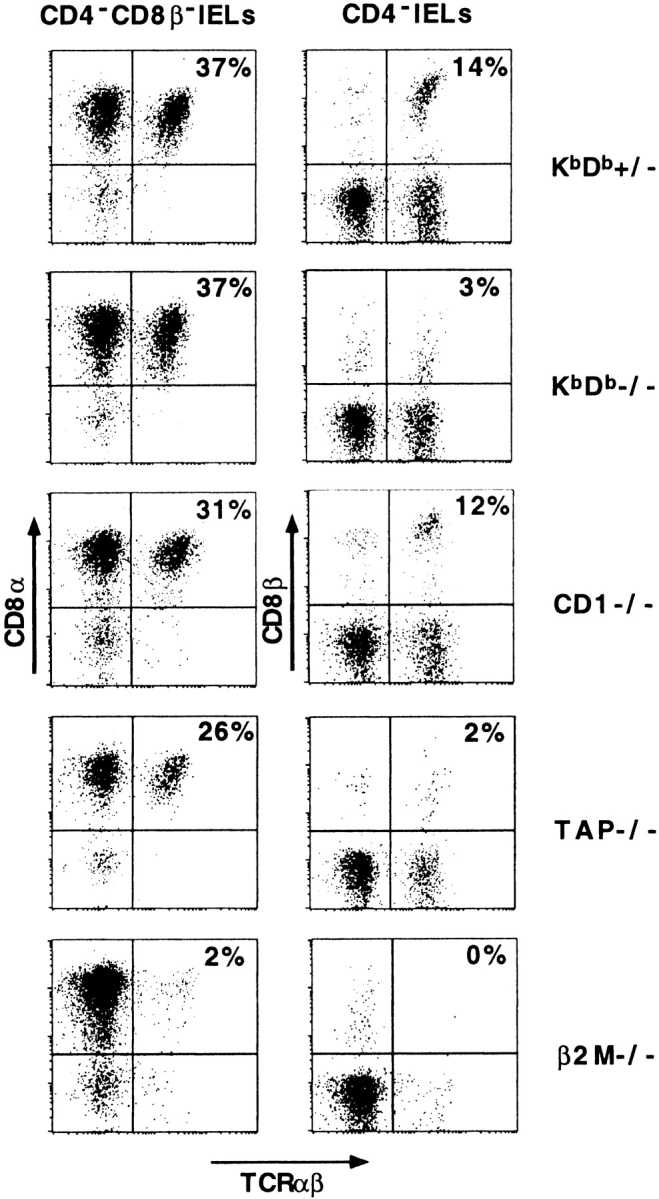
CD8α/α1 and CD8α/β1 IEL subsets in mutant mice. IELs of control and mutant mice were stained with anti–TCR-α/β, anti-CD4, anti-CD8α, and anti-CD8β. The left panels display the CD8α versus TCR-α/β profiles of gated CD4/CD8β-negative IELs, and the right panels display the CD8β versus TCR-α/β profiles of gated CD4-negative IELs. Thus, in the left panels the upper right quadrants of the dot plots correspond to the CD8α/α1TCR-α/β1 cells, whereas in the right panels they correspond to the CD8α/β1TCR-α/β1 cells. TCR-γ/δ1 cells are seen in the left quadrants as TCR-α/β–negative CD8β-negative cells (most TCR-γ/δ1 IELs are CD8α/α1).
These results demonstrate a fundamental difference in the MHC ligands used by the two subsets of CD8+TCR-α/β1 IELs. Most CD8α/α1 cells are independent of classical MHC class I molecules, whereas most CD8α/β1 cells are dependent on Kb/Db.
The CD8α/α1TCR-α/β1 Population in Kb/Db-deficient Mice Has a Normal Phenotype.
To further investigate the possibility that the development and/or expansion of a CD8α/α1TCR-α/β1 cell population was dependent in any way on classical Kb and Db molecules, we examined their surface phenotype in the Kb/Db-deficient mice. We found that they expressed the same degree of activation as their littermate controls, as judged by the expression of the activation markers CD44, CD69, and B220 (Fig. 3, and data not shown). We also examined the rare subset of CD8/CD4 double-negative TCR-α/β1 IELs, as it might include precursors of the CD8α/α1 lineage. Again, no difference could be detected between Kb/Db-deficient mice and littermate controls (data not shown).
Figure 3.
Unaltered phenotype of CD8α/α1TCR-α/β1 IELs in Kb/Db-deficient mice. IELs were stained with anti-CD4/CD8β, anti-CD44, and anti–TCR-α/β. CD44 versus TCR-α/β profiles of gated CD4/CD8β-negative IELs are displayed.
We next analyzed the Vβ repertoire of the CD8α/α1 population of Kb/Db-deficient mice. Table shows that CD8α/α1 cells expressed a diverse TCR Vβ repertoire, but that the frequency of various Vβs varied considerably from mouse to mouse. This pattern was also found in Kb/Db +/− littermates, and most likely results from the presence of oligoclonal expansions, as reported previously 19 20.
Table 2.
TCR Vβ Repertoire of CD8α/α1TCR-α/β1 IELs in KbDb +/− and KbDb −/− Mice
| Mouse | Vβ2 | Vβ3 | Vβ4 | Vβ5.1 + 5.2 | Vβ6 | Vβ7 | Vβ8.1 + 8.2 | Vβ8.3 | Vβ9 | Vβ10 | Vβ11 | Vβ12 | Vβ13 | Vβ14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KbDb +/− | 1.7 | 0.6 | 2.4 | 10.9 | 3.7 | 0.6 | 12.4 | 9.8 | 3.2 | 3.1 | 7.2 | 0.2 | 35.1 | 1.1 |
| KbDb +/− | 5.3 | ND | 4.1 | 3.6 | 8.2 | ND | 15.7 | 9.1 | ND | 15.6 | 12.2 | ND | 12.2 | 2.1 |
| KbDb −/− | 1.4 | 0.4 | 5.4 | 4.6 | 2.9 | 0.6 | 8.4 | 15.8 | 3.7 | 5.3 | 18.2 | 0.4 | 12.1 | 1.1 |
| KbDb −/− | 6.3 | ND | 4.4 | 8.2 | 0.8 | ND | 9.2 | 20.3 | ND | 1.4 | 18.9 | ND | 13.5 | 1.3 |
| KbDb −/− | 2.9 | ND | 4.5 | 4.8 | 4.4 | ND | 11.1 | 14.0 | ND | 3.1 | 9.0 | ND | 2.2 | 1.0 |
IELs of KbDb-deficient and littermate control mice were stained with anti-CD8α, anti-Vβs, and a cocktail of anti–TCR-γ/δ/CD4/CD8β. The percentages of cells expressing indicated Vβs among TCR-γ/δ/CD4/CD8β–negative, CD8α1 IELs (corresponding to the CD8α/α1TCR-α/β1 subset) are represented.
Thus, the CD8α/α1TCR-α/β1 IEL subset of Kb/Db-deficient mice appears to conserve the activated phenotype and the pattern of oligoclonal expansions that are characteristic of normal IELs, further indicating that CD8α/α1TCR-α/β1 IELs persist unaltered in Kb/Db-deficient mice.
Kb/Db-independent, CD8α/α1TCR-α/β1 IELs Can Be Divided into Subsets of TAP-dependent and TAP-independent Populations.
Although it was previously reported that all of the CD8α/β1 IELs were dependent on the presence of TAP, some CD8α/α1TCR-α/β1 IELs seemed to be TAP independent 21 22. We confirmed these results, showing that TAP-deficient mice retained about half the number of CD8α/α1TCR-α/β1 IELs in their littermate controls (10 ± 7 vs. 19 ± 10%; see Fig. 2 and Table ). In contrast, as reported previously 21, all CD8α/α1 IELs as well as CD8α/β1 IELs depended on β2m. These results suggest two possibilities. Either CD8α/α1TCR-α/β1 cells recognize two distinct nonclassical MHC class I–like molecules, only one of which is TAP dependent, or they recognize one nonclassical MHC class I–like molecule that is partially TAP dependent.
CD8α/α1TCR-α/β1 IELs Are Conserved in CD1-deficient Mice.
The conservation of the nonclassical MHC class I–like molecule CD1d in mammals, and the reports that it is expressed by intestinal epithelial cells of both mice and humans and that CD8+ T cell clones isolated from human IELs were CD1d reactive in a TAP-independent fashion, made CD1d an attractive candidate as the ligand of a subset of the CD8α/α1TCR-α/β1 IELs 23. Mice have two CD1 genes that are 95% identical, both belonging to the CD1d family 28 29. However, the CD1.2 gene has a frameshift mutation in the B6 strain that is predicted to abolish cell surface expression and in other strains CD1.2 also seems to be poorly, if at all, expressed on the cell surface 30 31. We analyzed the IEL population of CD1.1 as well as CD1.1/CD1.2 double-deficient mice. Fig. 2 shows that both the CD8α/α1 and CD8α/β1TCR-α/β1 IELs were conserved in CD1.1/CD1.2-deficient mice. Results obtained from CD1.1- and CD1.1/CD1.2-deficient mice were comparable and are pooled in Table . Altogether, these results show that neither the CD8α/α nor the CD8α/β subset is dependent on CD1, and suggest that the CD8α/α subset requires an unknown nonclassical MHC-like molecule that is β2m dependent and partially TAP independent.
CD8α/α1TCR-α/β1 IELs Are Conserved in Kb/Db/CD1 Triple-deficient Mice.
Because of the compensatory expansion of IEL subsets often observed in the various mutant mice (see Table ), there remained the possibility that CD8α/α1TCR-α/β1 IELs were a heterogeneous population made of a TAP-dependent subset restricted by Kb/Db and another TAP-independent subset restricted by CD1, but that the ablation of one subset in the corresponding mutant mouse was masked by the expansion of the other. To formally address this possibility, we generated Kb/Db/CD1 triple-deficient mice. Fig. 4 shows that the IELs isolated from Kb/Db/CD1 triple-deficient mice contained similar proportions of CD8α/α1TCR-α/β1 cells as CD1-deficient or Kb/Db-deficient control littermates. These results suggest that neither CD1 nor Kb/Db are ligands of CD8α/α1TCR-α/β1 cells, unambiguously demonstrating the existence of a large population of intestinal IELs that is dependent on a nonclassical, non-CD1 MHC class I–like molecule.
Figure 4.
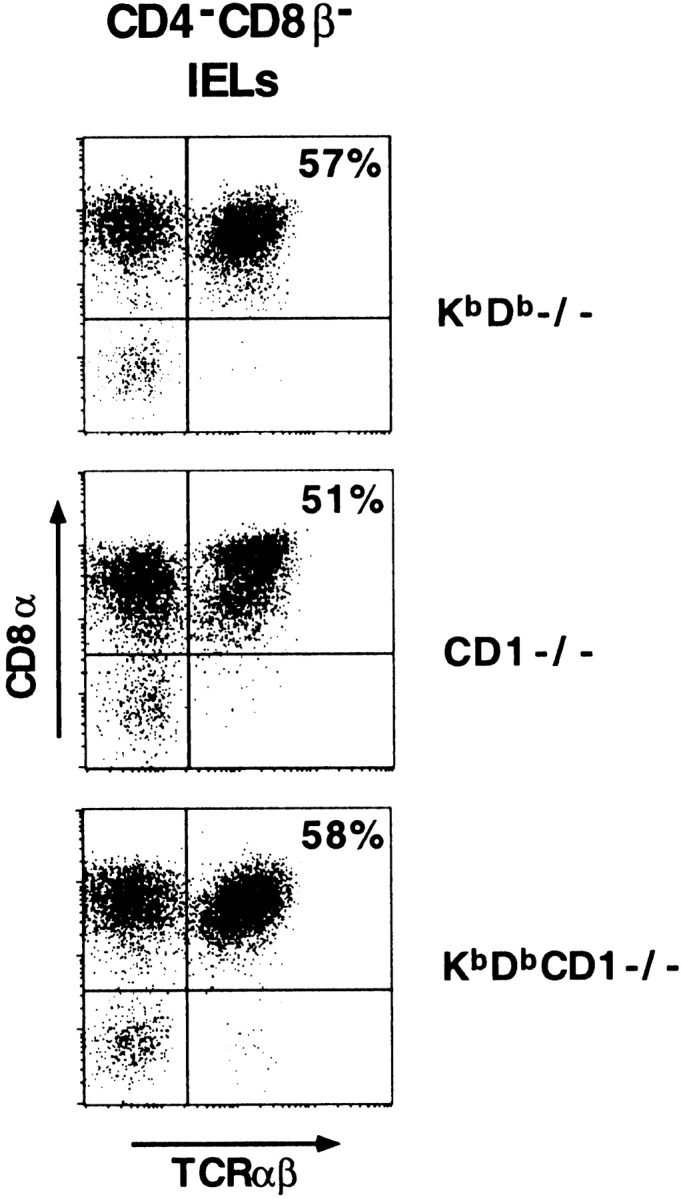
Persistence of CD8 α/α1TCR-α/β1 IELs in Kb/Db/CD1 triple-deficient mice. IELs were triple-stained with anti-CD4/CD8β, anti-CD8α, and anti–TCR-α/β. CD8α versus TCR-α/β profiles of gated CD4/CD8β-negative IELs are displayed. The upper right quadrants of the dot plots correspond to the CD8α/α1TCR-α/β1 cells.
Discussion
Although CD8α/α1TCR-α/β1 IELs could be detected in transgenic mice expressing TCR-α/β with defined MHC class I/peptide specificities 32 33 34, normal nontransgenic CD8α/α1TCR-α/β1 IELs have not been associated with antigen-specific, classical MHC class I–restricted function. The pattern of expression of CD8α/α1TCR-α/β1 IELs in β2m-, TAP-, Kb/Db-, and CD1-deficient mice, as reported in this study, clearly demonstrates that most CD8α/α1TCR-α/β1 IELs do not recognize classical MHC class I molecules, and points to nonclassical MHC class I–like molecules that are β2m dependent and partially TAP independent. Since CD1 could be ruled out by the study of CD1-deficient mice, candidate ligands include, but may not be restricted to, TL, which is expressed on intestinal epithelial cells 35, and Qa1 36 37, both nonclassical MHC class I molecules that can function in the absence of TAP.
There is increasing recognition that several body tissues, especially barrier epithelia of the skin and intestine, and the liver, have specialized immune systems that contain prominent populations of resident T lymphocytes with original, yet poorly understood, antigen specificity and functions. In recent years, two previously orphan families of nonclassical MHC class I–like molecules have become associated with such populations, including MICA/MICB for human intestinal TCR-γ/δ1 IELs and CD1 for liver NKT cells 3 31. Thus, the emerging pattern suggests that nonclassical MHC-like molecules with specialized functions are critical in specialized tissue environments. Our results now clearly demonstrate that another major subset of mouse intestinal IELs, the CD8α/α1TCR-α/β1 cells, recognize a nonclassical, non-CD1 type of MHC class I–like molecule. Further studies are warranted to identify the ligand(s) of CD8α/α1TCR-α/β1 intestinal IELs, a key for our understanding of local immunity in the intestine.
Acknowledgments
We thank P. Matzinger for reviewing the manuscript, A. Beavis for help with flow cytometry, and G. Brackee and L. Antonucci for managing the mouse colonies.
This work was supported by grants from the National Institutes of Health (RO1 AI38339) and the American Cancer Society (IM 788).
References
- Allison J.P., Asarnow D.M., Bonyhadi M., Carbone A., Havran W.L., Nandi D., Noble J. Gamma delta T cells in murine epitheliaorigin, repertoire, and function. Adv. Exp. Med. Biol. 1991;292:63–69. doi: 10.1007/978-1-4684-5943-2_8. [DOI] [PubMed] [Google Scholar]
- Boismenu R., Havran W.L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Groh V., Steinle A., Bauer S., Spies T. Recognition of stress-induced MHC molecules by intestinal gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Extrathymic differentiation of intraepithelial lymphocytesgeneration of a separate and unequal T-cell repertoire? Immunol. Today. 1991;12:436–438. doi: 10.1016/0167-5699(91)90015-L. [DOI] [PubMed] [Google Scholar]
- Poussier P., Julius M. Intestinal intraepithelial lymphocytesthe plot thickens. J. Exp. Med. 1994;180:1185–1189. doi: 10.1084/jem.180.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., Guy-Grand D., Vassalli P. Extrathymic T cell differentiation. Curr. Opin. Immunol. 1995;7:235–242. doi: 10.1016/0952-7915(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Sydora B.C., Jamieson B.D., Ahmed R., Kronenberg M. Intestinal intraepithelial lymphocytes respond to systemic lymphocytic choriomeningitis virus infection. Cell. Immunol. 1996;167:161–169. doi: 10.1006/cimm.1996.0023. [DOI] [PubMed] [Google Scholar]
- London S.D., Cebra J.H., Rubin D.H. Intraepithelial lymphocytes contain virus-specific, MHC-restricted cytotoxic precursors after gut immunization with reovirus serotype 1/Lang. Reg. Immunol. 1989;2:98–102. [PubMed] [Google Scholar]
- Chardès T., Buzoni-Gatel D., Lepage A., Bernard F., Bout D. Toxoplasma gondii oral infection induces specific cytolytic CD8α/β+ Thy-1+ gut intraepithelial lymphocytes, lytic for parasite-infected enterocytes. J. Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- Roberts S.C., Smith A.L., West A.B., Wen L., Findly R.C., Owen M.J., Hayday A.C. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc. Natl. Acad. Sci. USA. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y., Yamashiro Y., Maeda M., Oguchi S., Shimizu T., Nagata S., Yagita H., Yabuta K., Okumura K. Food antigen activates intraepithelial and lamina propria lymphocytes in food-sensitive enteropathy in mice. Pediatr. Res. 1996;39:862–866. doi: 10.1203/00006450-199605000-00020. [DOI] [PubMed] [Google Scholar]
- Buzonigatel D., Lepage A.C., Dimierpoisson I.H., Bout D.T., Kasper L.H. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii . J. Immunol. 1997;158:5883–5889. [PubMed] [Google Scholar]
- Kim S.K., Reed D.S., Heath W.R., Carbone F., Lefrancois L. Activation and migration of CD8 T cells in the intestinal mucosa. J. Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J. Exp. Med. 1978;148:1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand, D., and P. Vassalli. 1988. Origin and traffic of gut mucosal lymphocytes and mast cells. In Migration and Homing of Lymphoid Cells. A. Husband, editor. CRC Press, Boca Raton, FL. 99–111.
- Guy-Grand D., Cuenod-Jabri B., Malassis-Seris M., Selz F., Vassalli P. Complexity of the mouse gut T cell immune systemidentification of two distinct natural killer T cell intraepithelial lineages. Eur. J. Immunol. 1996;26:2246–2258. doi: 10.1002/eji.1830260942. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Disanto J.P., Henchoz P., Malassis-Seris M., Vassalli P. Small bowel enteropathyrole of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. Eur. J. Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sydora B.C., Mixter P.F., Holcombe H.R., Eghtesady P., Williams K., Amaral M.C., Nel A., Kronenberg M. Intestinal intraepithelial lymphocytes are activated and cytolytic but do not proliferate as well as other T cells in response to mitogenic signals. J. Immunol. 1993;150:2179–2191. [PubMed] [Google Scholar]
- Van Kerckhove C., Russell G.J., Deusch K., Reich K., Bhan A.K., Dersimonian H., Brenner M.B. Oligoclonality of human intestinal intraepithelial T cells. J. Exp. Med. 1992;175:57–63. doi: 10.1084/jem.175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault A., Cumano A., Vassalli P., Guy-Grand D., Kourilsky P. Oligoclonal repertoire of the CD8αα and the CD8αβ TCR-α/β murine intestinal intraepithelial lymphocytesevidence for the random emergence of T cells. J. Exp. Med. 1994;180:1345–1358. doi: 10.1084/jem.180.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiura Y., Kawaguchi M., Kondo Y., Obana S., Yamamoto H., Nanno M., Ishikawa H. Development of CD8αα+ intestinal intraepithelial T cells in β2-microglobulin- and/or TAP1-deficient mice. J. Immunol. 1996;156:2710–2715. [PubMed] [Google Scholar]
- Sydora B.C., Brossay L., Hagenbaugh A., Kronenberg M., Cheroutre H. TAP-independent selection of CD8+ intestinal intraepithelial lymphocytes. J. Immunol. 1996;156:4209–4216. [PubMed] [Google Scholar]
- Blumberg R.S., Gerdes D., Chott A., Porcelli S.A., Balk S.P. Structure and function of the CD1 family of MHC-like cell surface proteins. Immunol. Rev. 1995;147:5–29. doi: 10.1111/j.1600-065x.1995.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Tourne S., van Santen H.M., van Roon M., Berns A., Benoist C., Mathis D., Ploegh H. Biosynthesis of major histocompatibility complex molecules and generation of T cells in Ii TAP1 double-mutant mice. Proc. Natl. Acad. Sci. USA. 1996;93:1464–1469. doi: 10.1073/pnas.93.4.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C., Lemonnier F.A., Perarnau B., Freitas A.A., Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Chiu N.M., Mandal M., Wang N., Wang C.R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N., Loring J., Raulet D., Jaenisch R. β2-microglobulin deficient mice lack CD4−8+ cytolytic cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- Calabi F., Jarvis J.M., Martin L., Milstein C. Two classes of CD1 genes. Eur. J. Immunol. 1989;19:285–292. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- Balk S.P., Bleicher P.A., Terhorst C. Isolation and expression of cDNA encoding the murine homologues of CD1. J. Immunol. 1991;146:768–774. [PubMed] [Google Scholar]
- Park S.H., Roark J.H., Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J. Immunol. 1998;160:3128–3134. [PubMed] [Google Scholar]
- Bendelac A., Rivera M.N., Park S.H., Roark J.H. Mouse CD1-specific NK1 T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Rocha B., Von Boehmer H., Guy-Grand D. Selection of intraepithelial lymphocytes with CD8α/α co-receptors by self-antigen in the murine gut. Proc. Natl. Acad. Sci. USA. 1992;89:5336–5340. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussier P., Teh H.S., Julius M. Thymus-independent positive and negative selection of T cells expressing a major histocompatibility complex class I restricted transgenic T cell receptor α/β in the intestinal epithelium. J. Exp. Med. 1993;178:1947–1957. doi: 10.1084/jem.178.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt C.N., de Jong Y.P., Mizoguchi E., O'Farrelly C., Bhan A.K., Tonegawa S., Terhorst C., Simpson S.J. High- and low-affinity single-peptide/MHC ligands have distinct effects on the development of mucosal CD8alpha/alpha and CD8alpha/beta T lymphocytes. Proc. Natl. Acad. Sci. USA. 1999;96:5628–5633. doi: 10.1073/pnas.96.10.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitell M., Cheroutre H., Panwala C., Holcombe H., Eghtesady P., Kronenberg M. Structure and function of H-2 T (Tla) region class I MHC molecules. Crit. Rev. Immunol. 1994;14:1–27. [PubMed] [Google Scholar]
- Tompkins S.M., Kraft J.R., Dao C.T., Soloski M.J., Jensen P.E. Transporters associated with antigen processing (TAP)-independent presentation of soluble insulin to α/β T cells by the class Ib gene product, Qa-1(b) J. Exp. Med. 1998;188:961–971. doi: 10.1084/jem.188.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich C.J., DeCloux A., Woods A.S., Cotter R.J., Soloski M.J., Forman J. Identification of a Tap-dependent leader peptide recognized by alloreactive T cells specific for a class Ib antigen. Cell. 1994;79:649–658. doi: 10.1016/0092-8674(94)90550-9. [DOI] [PubMed] [Google Scholar]