Abstract
Cytotoxic T lymphocyte antigen 4 (CTLA-4) is a T cell costimulation receptor that delivers inhibitory signals upon activation. Although the tyrosine-based motif (165YVKM) within its cytoplasmic tail has been shown to associate in vitro with Src homology 2 domain–containing tyrosine phosphatase (SHP-2) and phosphatidylinositol 3 kinase upon phosphorylation, the mechanism of negative signaling remains unclear. Here, we report a new mechanism of negative signaling based on the analysis of murine T cell clones transfected with various mutants of CTLA-4. Upon T cell activation by cross-linking with anti-CD3 and anti-CD28 antibodies, CTLA-4 engagement inhibited both proliferation and interleukin 2 production in tyrosine mutants as well as in wild-type CTLA-4 transfectants. Furthermore, the mutant CTLA-4 lacking most of the cytoplasmic region strongly suppressed interleukin 2 production as well. These data suggest that negative signals by CTLA-4 could be mediated through the membrane-proximal region of CTLA-4 but not through the YVKM motif and that the association of CTLA-4 with SHP-2 is not required for CTLA-4–mediated suppression of T cell activation.
Keywords: CTLA-4, costimulation, negative signal, tyrosine motif
CTLA-4 (CTL antigen 4)1 is a T cell costimulation receptor and critical negative regulator of T cell activation 1 2 3. CTLA-4 is homologous to CD28 and shares common ligands, CD80 and CD86, on APCs. Whereas CD28 is constitutively expressed at high levels on the surfaces of both resting and activated T cells and delivers positive costimulation signals, the regulation of the cell surface expression of CTLA-4 is more complex. CTLA-4 cannot be detected on resting T cells, but after T cell activation, CTLA-4 mRNA is rapidly induced and the protein becomes detectable on the cell surface, with a peak expression 48–72 h after stimulation. However, even under conditions of optimal stimulation, CTLA-4 is localized predominantly within intracellular compartments and its expression level on the cell surface is only a small fraction of that of CD28 4 5.
Functional in vitro analysis using Abs to murine CTLA-4 demonstrated that the addition of soluble Abs augmented T cell responses 6. In contrast, cross-linking of CTLA-4 by immobilized Ab or secondary Abs resulted in inhibition of T cell activation upon TCR/CD3 and CD28 stimulation 7 8. These data suggested that CTLA-4 functions as a negative regulator of T cell activation.
Strong evidence to support the inhibitory role of CTLA-4 was provided by the analysis of mice deficient in CTLA-4 9 10. CTLA-4 null mutant mice exhibited a massive lymphoproliferative disorder and died between 3 and 4 wk of age. The majority of peripheral T cells in these mice were in an activated state and exhibited spontaneous production of cytokines. When CTLA-4−/− mice were crossed with TCR-transgenic mice, the progeny did not develop the lymphoproliferative disorder, demonstrating that T cells from CTLA-4−/− mice are autoreactive 11.
Recent studies have demonstrated that the cytoplasmic tail of CTLA-4 controls its expression on the cell surface. This cell surface expression is limited by the mechanism in which CTLA-4 is rapidly internalized by clathrin-mediated endocytosis and accumulates within the endosomes of activated T cells. Endocytosis of CTLA-4 is induced by the association of its cytoplasmic tail with the medium chain (μ2) of the clathrin-associated adaptor protein complex 2 (AP-2), and the tyrosine-based motif containing 165YVKM within the cytoplasmic tail is responsible for the binding to μ2 12 13 14 15. Within the tyrosine motif, Y-165 is critical for the association with μ2. Furthermore, phosphorylation of this tyrosine prevents the association with AP-2 complex, resulting in the inhibition of endocytosis and the accumulation of CTLA-4 on the cell surface 14.
It has been shown that the same tyrosine-based motif, 165YVKM, associates with a phosphatase, Src homology (SH)2 domain–containing tyrosine phosphatase (SHP-2) 15 16, and phosphatidylinositol 3 (PI3) kinase 17 through their SH2 domains upon phosphorylation of the tyrosine motif of CTLA-4. Using a cotransfection system of various kinases with CTLA-4 into Cos or 293T cells, we identified Fyn and Lck src kinases as the tyrosine kinases responsible for phosphorylating both Y-165 and Y-182 in the cytoplasmic tail of CTLA-4 through their direct association with CTLA-4 12 18. However, in spite of the identification of these kinases and phosphatases as CTLA-4–associated molecules, the mechanism of negative signaling of CTLA-4 remains unclear.
Here, we have identified a new mechanism of negative signal transduction by CTLA-4. We analyzed murine normal T cell clones transfected with various forms of mutant CTLA-4. Upon stimulation through TCR in the presence of CD28-mediated costimulation, cross-linking with anti–CTLA-4 mAb induced the suppression of both proliferation and IL-2 production in T cells expressing tyrosine-substituted or cytoplasmic tail–deleted mutants of CTLA-4 as well as wild-type (WT) CTLA-4. These data demonstrate that negative signals through CTLA-4 can be mediated through the membrane-proximal region of CTLA-4 rather than the 165YVKM motif, probably through the association with unidentified molecules. We also suggest here a novel regulation of the cell surface expression of CTLA-4.
Materials and Methods
Cells and Abs.
A KLH-specific and I-Ak–restricted murine Th1-type T cell clone, 23-1-8 19 20, was maintained in RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-ME, and 5 ng/ml murine IL-2. These T cells were stimulated with KLH (100 μg/ml; Calbiochem Corp.) and irradiated C3H/HeJ spleen cells every 3–4 wk. Retrovirus packaging cell line BOSC23 was provided by Dr. T. Kitamura (The University of Tokyo, Tokyo, Japan) and cultured in DMEM supplemented with 10% FCS, 100 μg/ml l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 250 μg/ml xanthine, 15 μg/ml hypoxanthine, 6 μg/ml mycophenolic acid, and 50 μM 2-ME. Anti–CTLA-4 mAb (UC10.4F10.11) and anti-CD3∈ mAb (145-2C11) were provided by Dr. J. Bluestone (University of Chicago, Chicago, IL). Anti-CD3ζ mAb (H146-968) was provided by Dr. R. Kubo (Cytel Corp., San Diego, CA). Stimulatory anti-CD28 mAb (PV-1) was previously described 21.
Retrovirus-mediated Gene Transfer into T Cell Clones.
Retrovirus vector pMXneo 22 was provided by Dr. T. Kitamura and used for stable transfection of WT CTLA-4 and its mutants into T cell clone 23-1-8. To construct the retrovirus vector carrying cDNA for WT CTLA-4, the full length cDNA (a gift from Dr. P. Golstein, INSERM-CNRS, Marseille, France) was subcloned into the EcoRI site of pMXneo. DNA fragments carrying point mutations and deletions of the cytoplasmic region of CTLA-4, Y165G (165Tyr-Gly), Y182G (182Tyr-Gly), Y165/182G (165Tyr-Gly and 182Tyr-Gly), and ΔCP7 (deletion of the cytoplasmic region except for the membrane-proximal 7 amino acids) were generated by PCR and also introduced into the same vector. These retroviral constructs were transiently transfected into the packaging cell line BOSC23 by lipofection using LipofectAMINE (GIBCO BRL). 24 h later, the T cells prestimulated with KLH and irradiated C3H/HeJ spleen cells were added to the culture and cocultivated for 24 h with the virus-producing BOSC23 cells. Thereafter, T cells were harvested and subjected to G418 (Geneticin; GIBCO BRL) selection at 1 mg/ml.
Flow Cytometric Analysis of CTLA-4 and Cell Sorting.
T cells were incubated with biotinylated anti–CTLA-4 mAb for 30 min at 4°C, followed by PE–streptavidin. Flow cytometry was performed using a FACScan™ flow cytometer (Becton Dickinson), and 104 cells were analyzed using the CELLQuest™ program (Becton Dickinson).
Metabolic Labeling, Immunoprecipitation, and Deglycosylation.
T cells (107) were precultured in a methionine-free medium for 30 min, metabolically labeled with 18.5 mBq/ml [35S]methionine (Tran 35S-Label™, ICN Pharmaceuticals, Inc.) for 3 h, harvested, washed three times in PBS, and then lysed in 1% NP-40 lysis buffer (1% NP-40, 50 mM Tris, 150 mM NaCl, 5 mM EDTA, 10 μg/ml aprotinin, 12.5 μg/ml antipain, 12.5 μg/ml chymostatin, 50 μg/ml leupeptin, 25 μg/ml pepstatin A, 1 mM PMSF, and 2 mM Na3VO4). The precleared lysates were immunoprecipitated with either anti–CTLA-4 or anti-CD3∈ mAb as control. Where indicated, immunoprecipitates were treated with N-glycosidase F to remove N-linked oligosaccharide chains. Immunoprecipitated samples were resuspended with 20 μl of 0.1 M 2-ME/0.5% SDS, denatured by boiling, resuspended in 60 μl of a buffer containing 1.5 mU N-glycosidase F (Calbiochem Corp.), 167 mM Tris-Cl, pH 8.0, 13 mM 1,10-phenanthrorine, and 1.3% NP-40, incubated overnight at 37°C, and analyzed on 13% SDS-PAGE. The dried gels were analyzed with a BAS-2000 image analyzer (Fuji Photo Film Co.).
Cell Surface Biotinylation.
Cell surface biotinylation was performed as previously described 23. Cells were solubilized in 0.5% NP-40 lysis buffer. Immunoprecipitates were treated with N-glycosidase F as described above and then analyzed on 14% SDS-PAGE.
Proliferation Assay of T Cells.
For antigen stimulation, 105 23-1-8 T cells were cultured with 5 × 105 irradiated C3H/HeJ spleen cells and 10 μg/ml KLH in a 96-well flat-bottom plate for 48 h at 37°C in 5% CO2. For stimulation by FcR-dependent cross-linking with anti-CD3∈ and anti-CD28 mAbs, 2 × 105 T cells were cultured with 105 irradiated C3H/HeJ spleen cells in the presence of 0.1 μg/ml anti-CD3∈ alone or in combination with 1 μg/ml anti-CD28 mAb. In both stimulations, anti–CTLA-4 mAb (or control anti-CD3ζ mAb) was added at 50 μg/ml. For the last 8 h of incubation, 37 kBq [3H]TdR (Amersham Life Sciences) was pulsed and the uptake was measured with a liquid scintillation counter (MicroBeta™; Pharmacia).
Analysis of IL-2 Production upon T Cell Activation.
Flat-bottom 96-well plates were sequentially coated with 0.1 μg/ml of anti-CD3∈ mAb and then with anti–CTLA-4 mAb and/or control anti-CD3ζ mAb to keep a constant sum concentration of mAbs of 10 μg/ml. T cells were added at 105/well in 200 μl of complete RPMI 1640 in the presence or absence of 1 μg/ml anti-CD28 mAb. Supernatants from triplicate cultures were collected, and the titer of IL-2 was determined by ELISA with rIL-2 as a standard as described previously 24. The Ab to detect IL-2 was purchased from PharMingen.
Reverse Transcriptase–PCR Analysis.
Total RNA was extracted from stimulated or unstimulated T cells by the AGPC (acid guanidium-phenol-chloroform) method. Reverse transcriptase (RT) reaction was carried out using random hexamer primers (Takara Biomedicals) and Superscript II (GIBCO BRL). Twofold dilutions of the RT product were amplified with the specific primers for IL-2, IFN-γ, Bcl-XL, and β-actin to ensure that the PCR reactions were performed in a linear range.
Immune Complex Kinase Assay for Extracellular Signal-regulated Kinase 2 Activity.
T cells were stimulated with 0.5 μg/ml immobilized anti-CD3∈ mAb and 10 μg/ml soluble anti-CD28 mAb and lysed for 30 min at 4°C with a lysis buffer (20 mM Hepes, pH 7.4, 300 mM NaCl, 2 mM EGTA, 1% Triton X-100, 1 mM dithiothreitol, 20 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, 10 μg/ml aprotinin, 12.5 μg/ml antipain, 12.5 μg/ml chymostatin, 50 μg/ml leupeptin, and 25 μg/ml pepstatin A). Protein concentration in the lysate was determined using the Bradford method (Bio-Rad Labs.), and 50 μg of total protein was subjected to immunoprecipitation with polyclonal rabbit anti–extracellular signal-regulated kinase 2 (ERK2) Ab (Santa Cruz Biotechnology, Inc.). After the immunoprecipitates were washed with a kinase buffer (50 mM Tris, pH 7.4, 10 mM MgCl2, and 1 mM dithiothreitol), kinase reactions were carried out at 30°C in 30 μl of the kinase buffer containing 20 μM ATP, 5 μCi of γ-[32P]ATP, and 3 μg of myelin basic protein (MBP; Sigma Chemical Co.) as a substrate and stopped after 20 min. Samples were resolved on 15% SDS-PAGE, and phosphorylation of MBP was visualized and the radioactivity of each band was quantified using a BAS-2000 image analyzer (Fuji Photo Film Co.).
ERK2 immunoblots were performed as previously described 25. 40 μg of whole cell extracts was analyzed on 10% SDS-PAGE and transferred onto a polyvinyl difluoride membrane (Immobilon-P; Millipore Corp.). The membranes were blotted with murine monoclonal anti-ERK2 (Santa Cruz Biotechnology, Inc.), followed by a peroxidase-conjugated anti–murine IgG. Blots were developed using the enhanced chemiluminescence system (ECL kit; Amersham Life Sciences).
Results
Surface Expression of CTLA-4 Mutants on Resting T Cell Clones.
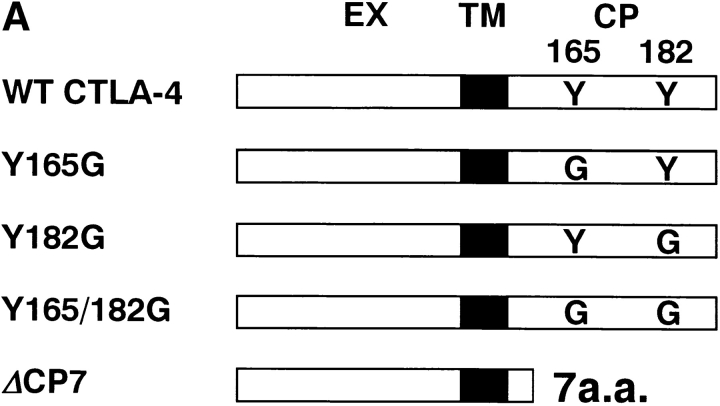
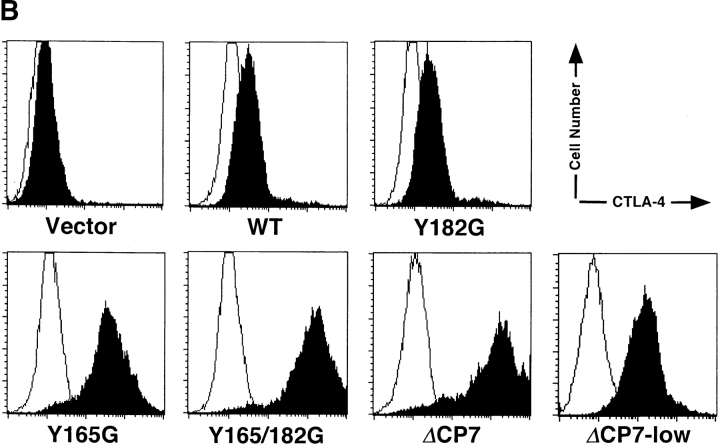
Although it has been shown that Y-165 of CTLA-4 is crucial for association with AP-2 complex and endocytosis from the cell surface in a model system using nonlymphoid cells 12 13 14 15, the regulation of the cell surface expression of CTLA-4 in normal T cells has not been analyzed. Therefore, we examined which of the two tyrosines in the cytoplasmic tail of murine CTLA-4 (Y-165, Y-182, or both) are important for the regulation of the cell surface expression and inhibitory signals of CTLA-4 in normal T cells. For this purpose, we generated various forms of mutant CTLA-4 with a single tyrosine→glycine mutation (Y165G and Y182G), double mutations (Y165/182G), and a deletion of most of the cytoplasmic tail except for the membrane-proximal 7 amino acids (ΔCP7) (Fig. 1 A). These constructs were transfected into a murine T cell clone, 23-1-8, by retrovirus-mediated gene transfer, and the G418-resistant bulk population of transfected T cells was analyzed for the surface expression of CTLA-4 (Fig. 1 B). CTLA-4 was hardly detected on the surfaces of resting T cells transfected with the vector alone (CT T cells). T cells transfected with the WT CTLA-4 (WT T cells) showed only a dull expression of CTLA-4 on the cell surface, as did T cells transfected with the Y182G mutant CTLA-4 (Y182G T cells). In contrast, the mutation of 165Tyr→Gly (Y165G), which blocks the association with AP-2, induced constitutively high expression of CTLA-4 on the surfaces of resting T cells. Interestingly, Y165/182G mutants exhibited a higher expression of surface CTLA-4 than Y165G mutants, and ΔCP7 mutants showed even greater expressions than Y165/182G mutants.
Figure 1.
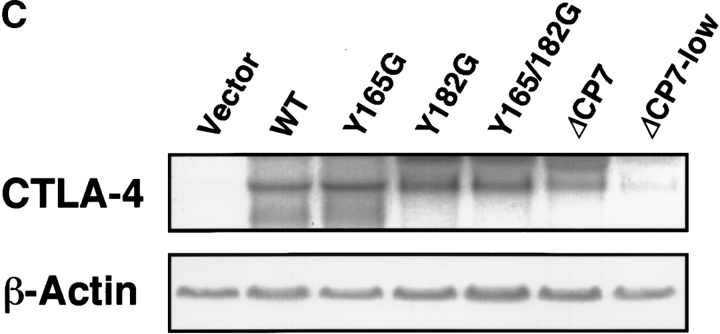
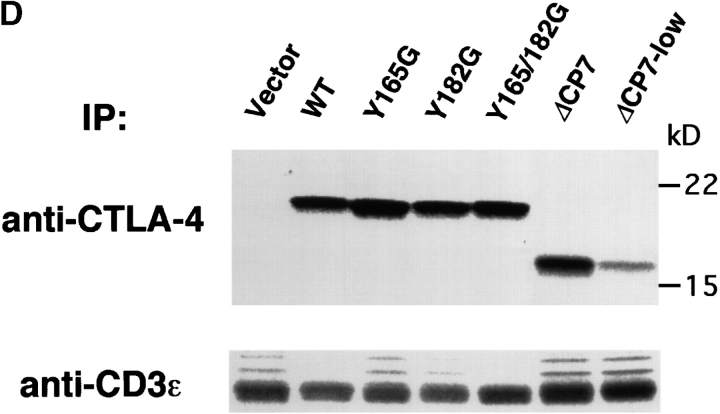
Surface expression of transfected CTLA-4 on resting T cell clones. (A) Schematic structure of WT and mutant forms of CTLA-4. These constructs were subcloned into the retrovirus vector pMXneo and transfected into murine Th1 cell clone 23-1-8. Black boxes represent the transmembrane region. EX, extracellular; TM, transmembrane; CP, cytoplasmic domains; a.a., amino acid. (B) Surface expression of CTLA-4 on resting T cell clones transfected with control vector, WT, or CTLA-4 mutants. ΔCP7-low T cells were isolated from ΔCP7 T cells as T cells expressing low levels of the cell surface CTLA-4 by cell sorting. T cells were stained with biotinylated anti–CTLA-4 mAb and PE–streptavidin and analyzed by FACS™. (C) Northern blot analysis of WT and various mutant CTLA-4–transfected T cells. 10 μg of total RNA was analyzed using a full length CTLA-4 cDNA as a probe. Quantitation of each band showed the following relative values when normalized by the vector-transfectant cells as 1: vector, 1.0; WT, 33.4; Y165G, 55.3; Y182G, 45.8; Y165/182G, 33.8; ΔCP7, 26.5; and ΔCP7-low, 6.6. (D) Total protein levels of CTLA-4 in WT and various mutant CTLA-4–transfected T cells. T cells expressing control vector, WT CTLA-4, mutant CTLA-4 (Y165G, Y182G, Y165/182G, ΔCP7), and ΔCP7-low T cells were metabolically labeled with [35S]methionine and lysed in 1.0% NP-40 lysis buffer. The lysates were immunoprecipitated with anti–CTLA-4 mAb or anti-CD3∈ mAb as control. Immunoprecipitates (IP) by anti–CTLA-4 mAb were treated with N-glycosidase F and then resolved on 13% SDS-PAGE. Molecular size is indicated, right (kD).
To test whether the significant differences in the surface expression of CTLA-4 can be attributed to its transcriptional level in these T cells, we performed Northern blot analysis using a CTLA-4 cDNA probe. The expression level of CTLA-4 mRNA of these WT and mutants of CTLA-4 T cells at resting stage varied within a twofold difference (Fig. 1 C). We also examined the protein level of CTLA-4 in T cells expressing these mutants and WT CTLA-4. T cells were metabolically labeled with [35S]methionine, and the cell lysates were immunoprecipitated with either anti–CTLA-4 mAb or anti-CD3∈ mAb as control. As CTLA-4 is heavily glycosylated, simple immunoprecipitation of the labeled CTLA-4 showed very broad bands. Therefore, all precipitates were treated with N-glycosidase F to remove all N-linked oligosaccharide chains and then the core proteins were analyzed. As shown in Fig. 1 D, CTLA-4 protein was not precipitated in control cells. WT and all mutant CTLA-4 cells (Y165G, Y182G, Y165/182G, and ΔCP7) showed fairly similar amounts of CTLA-4 proteins. These data demonstrate that the difference in the surface expression of CTLA-4 was not due to the different expression level of mRNA nor protein of CTLA-4 but rather due to translocation of CTLA-4 protein from the cytoplasm to plasma membrane by the mutation or deletion of the tyrosine motif in the cytoplasmic tail. Preliminary analysis with confocal microscopy disclosed that CTLA-4 protein was located predominantly in the cytoplasmic granules in resting WT T cells, whereas the major portion was localized on the cell surface in Y165G or ΔCP7 T cells (Iida, T., C. Nakaseko, H. Ohno, and T. Saito, manuscript in preparation).
The finding that Y165/182G T cells and ΔCP7 T cells exhibited higher expressions of surface CTLA-4 than Y165G T cells indicates that Y-165 is basically the tyrosine required for regulation of the surface expression of CTLA-4 through endocytosis mediated by the clathrin–AP-2 complex, but Y-182 or other regions of the cytoplasmic tail of CTLA-4 are also involved in the regulation of the surface expression of the molecule.
Suppression of Ag-specific Proliferation and Restoration by Anti–CTLA-4 mAb in Mutant CTLA-4–expressing T Cells.
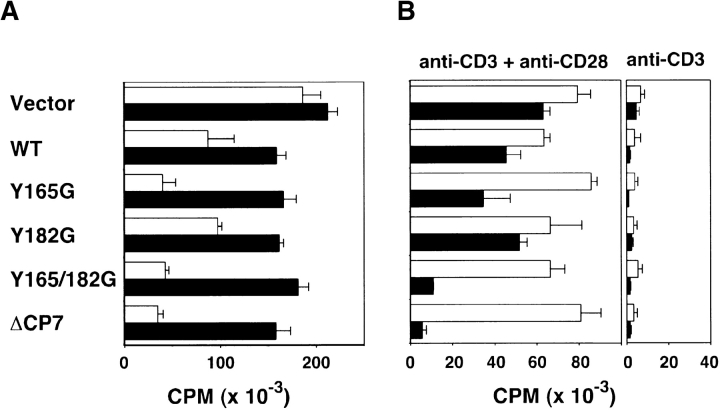
Previous reports demonstrated that both intact and monovalent fragments of CTLA-4 augmented T cell proliferation in allogeneic MLR, whereas the same Ab inhibited proliferation under the condition where FcR cross-linking was provided 6 7. To investigate the region in the cytoplasmic tail of CTLA-4 responsible for delivering negative signals, we performed functional analyses using these T cells transfected with various mutant forms of CTLA-4. First, we examined the effect of CTLA-4 mutants in terms of Ag-specific proliferative response. Because 23-1-8 is a KLH-specific T cell clone, transfectants expressing various CTLA-4 mutants were stimulated with KLH and APCs in the presence or absence of anti–CTLA-4 mAb. As shown in Fig. 2 A, the proliferative response of CT T cells was not altered by the addition of anti–CTLA-4 mAb. However, proliferation of WT T cells was suppressed in comparison with CT T cells. The addition of anti–CTLA-4 mAb restored the response to a level almost equal to that in control cells. In T cells with high expression of surface CTLA-4, such as Y165G, Y165/182G, and ΔCP7 T cells, the suppression was more significant than in WT T cells, in accordance with their surface expression. For these cells, the addition of anti–CTLA-4 mAb induced dramatic restoration of the Ag-specific response. These results suggest that mutant forms of CTLA-4, with either the tyrosine mutation or deletion of the cytoplasmic region, could deliver a negative signal for Ag-specific proliferation. Alternatively, these molecules might inhibit the proliferation by merely competing for ligand binding with CD28.
Figure 2.
Inhibition of proliferative response in mutant CTLA-4–transfected T cells. (A) Suppression of Ag-specific response in various forms of CTLA-4–transfected T cells and restoration by anti–CTLA-4 mAb. 105 T cells transfected with control vector and WT or mutant forms of CTLA-4 were stimulated with KLH (10 μg/ml) and 5 × 105 irradiated spleen cells in the presence of anti–CTLA-4 mAb (▪) or anti-CD3ζ mAb as control (□) at 50 μg/ml. Proliferation was measured by [3H]TdR uptake. Data are shown as mean ± SD of triplicates. (B) Suppression of T cell proliferation by CTLA-4 cross-linking in T cells expressing various forms of CTLA-4 upon TCR/CD28-mediated and FcR-dependent stimulation. 2 × 105 T cells were stimulated with 0.1 μg/ml anti-CD3∈ mAb alone (right) or in combination with 1 μg/ml soluble anti-CD28 mAb (left) in the presence of irradiated spleen cells as FcR+ cells. Anti–CTLA-4 mAb (▪) or anti-CD3ζ mAb as control (□) was added at 50 μg/ml. The data represent the mean ± SD of triplicates.
Inhibition of Costimulation-dependent Proliferation by Engagement of CTLA-4 Lacking Tyrosine Motifs.
To examine the effect of cross-linking various forms of CTLA-4 with anti–CTLA-4 mAb, T cells were stimulated with a suboptimal dose of anti-CD3∈ mAb and optimal costimulation with anti-CD28 mAb by cross-linking on FcR-bearing syngeneic APCs. In this system, suboptimal stimulation with anti-CD3∈ mAb resulted in a minimum costimulation effect by CD80/CD86 on APCs, and the addition of stimulatory anti-CD28 mAb greatly augmented T cell proliferation (Fig. 2 B). Under these conditions, the cross-linking of CTLA-4 mutants with Y165G, Y165/182G, and ΔCP7 by anti–CTLA-4 mAb resulted in significant inhibition of CD3/CD28-mediated proliferation. Together with the results in Fig. 2 A, these data strongly suggest that these mutant forms of CTLA-4 as well as WT CTLA-4 could transduce negative signals for proliferative response upon engagement of TCR and CD28.
CTLA-4 Without Tyrosine Motifs Delivers a Negative Signal for IL-2 Production.
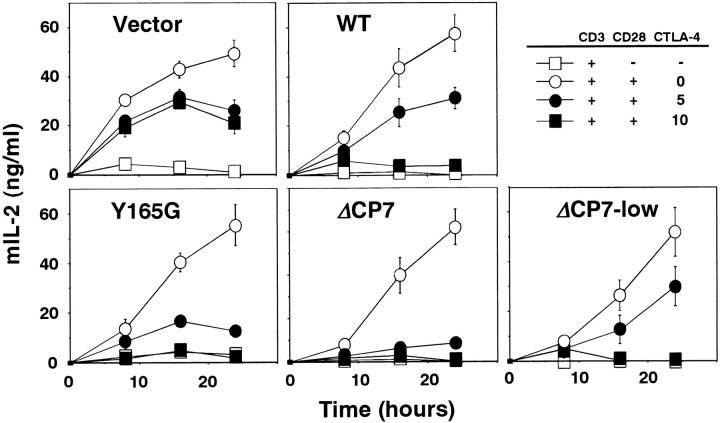
For detailed analysis of the suppressive mechanism via CTLA-4, we used another system in which T cells were stimulated by cross-linking of CD3, CD28, and CTLA-4 separately with the respective specific Abs in the absence of APCs and examined the early response by measuring IL-2 secretion. This system made it possible to avoid the effect of interaction between CTLA-4 and/or CD28 and CD80 and/or CD86 on APCs and to analyze the molecular events of CTLA-4 cross-linking both in the early and late phases after T cell activation. To this end, T cells expressing WT CTLA-4, Y165G, ΔCP7, and control T cells were stimulated by cross-linking with a suboptimal dose of immobilized anti-CD3∈ mAb in the presence or absence of soluble stimulatory anti-CD28 mAb, and the negative effect via CTLA-4 in IL-2 production was examined by cross-linking with immobilized anti–CTLA-4 mAb. Under this condition, the costimulatory effect of anti-CD28 mAb was optimal for IL-2 production (Fig. 3). In CT T cells, IL-2 secretion was inhibited by endogenous CTLA-4 by at most 60–70% of the response upon CTLA-4 cross-linking (Fig. 3). In WT T cells, CTLA-4 cross-linking resulted in significant inhibition of IL-2 production in a dose-dependent manner (Fig. 3). In ΔCP7 as well as Y165G T cells, consistent with the results of the proliferative response shown in Fig. 2, cross-linking of these CTLA-4 mutants induced stronger inhibition of IL-2 production than was seen in WT T cells (Fig. 3). As ΔCP7 T cells express extremely high levels of mutant CTLA-4 on their cell surfaces (severalfold higher than those expressed by Y165G T cells), we intended to analyze the function of ΔCP7 T cells with relatively low expression of the mutant CTLA-4 on the cell surface. For this purpose, we isolated low-expressing cells (ΔCP7-low) by cell sorting. The cell surface expression of ΔCP7 on these cells was much lower than that of Y165G T cells, though it was still higher than that of WT T cells. However, when mRNA and protein of the mutant CTLA-4 in ΔCP7-low T cells was analyzed, the expression of both mRNA and protein was found to be extremely low (Fig. 1b and Fig. c). Analysis of the function of such ΔCP7-low T cells revealed that these cells with much lower expression level of ΔCP7 strongly inhibited IL-2 production upon CTLA-4 cross-linking (Fig. 3). These results demonstrated that the engagement of not only tyrosine mutants but also the cytoplasmic tail deletion mutant of CTLA-4 could inhibit IL-2 secretion as an early T cell response as well as proliferation as a late response.
Figure 3.
Inhibition of IL-2 production by CTLA-4 lacking tyrosine motif upon T cell activation. 105 T cells expressing control vector, WT, Y165G, ΔCP7 CTLA-4, and ΔCP7-low T cells were stimulated with 0.1 μg/ml immobilized anti-CD3∈ mAb alone (□) or in combination with 1 μg/ml soluble anti-CD28 mAb (○, •, ▪). Anti–CTLA-4 was cocoated at 0 (○), 5 (•), and 10 (▪) μg/ml. Culture supernatants of triplicates were collected at indicated times and murine (m)IL-2 concentration was determined by ELISA.
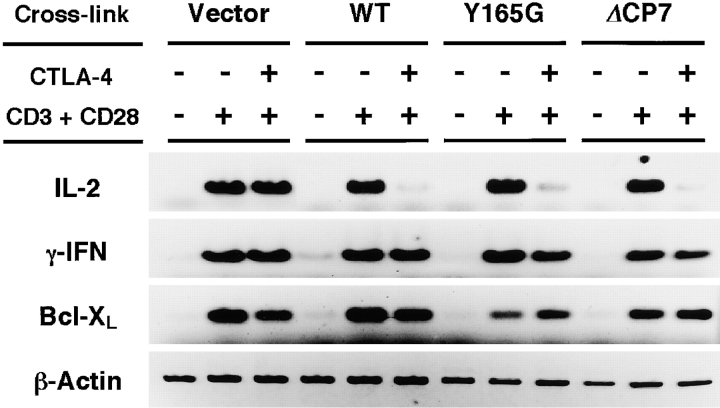
Specific suppression of IL-2 secretion was demonstrated by analyzing mRNA expression using a semiquantitative RT-PCR analysis (Fig. 4). mRNAs were isolated from various T cells after stimulation by cross-linking of CD3 and CD28 for 4 h. Similar to IL-2 secretion, ligation with anti–CTLA-4 mAb induced strong inhibition of IL-2 mRNA expression in WT, Y165G, and ΔCP7 T cells but not in CT T cells. This inhibitory effect was not observed for the expression of IFN-γ or Bcl-XL. The expression of the latter is known to be induced upon TCR stimulation in the presence of CD28 costimulation 26 27 28. These data support the notion that the high expression of cell surface CTLA-4 did not physically block TCR and CD28 stimulation and that the engagement of these mutant CTLA-4, which induced high expression on the cell surface, actively inhibited IL-2 mRNA production as early as 4 h after T cell activation. These results also demonstrate that CTLA-4 does not merely inhibit the signal through CD28, because the induction of CD28-dependent molecules such as Bcl-XL were not affected by CTLA-4 cross-linking.
Figure 4.
RT-PCR analysis of cytokines and Bcl-XL. T cells transfected with the control vector, WT, and various mutant forms of CTLA-4 were unstimulated or stimulated with immobilized anti-CD3∈ (0.1 μg/ml) and soluble anti-CD28 (1 μg/ml) mAbs. Anti–CTLA-4 mAb or anti-CD3ζ mAb as the control was cocoated at 10 μg/ml. After 4-h stimulation, total RNA was extracted and RT-PCR was performed with specific primers for IL-2, IFN-γ, Bcl-XL, and β-actin.
Suppression of ERK2 Activity through Tailless CTLA-4.
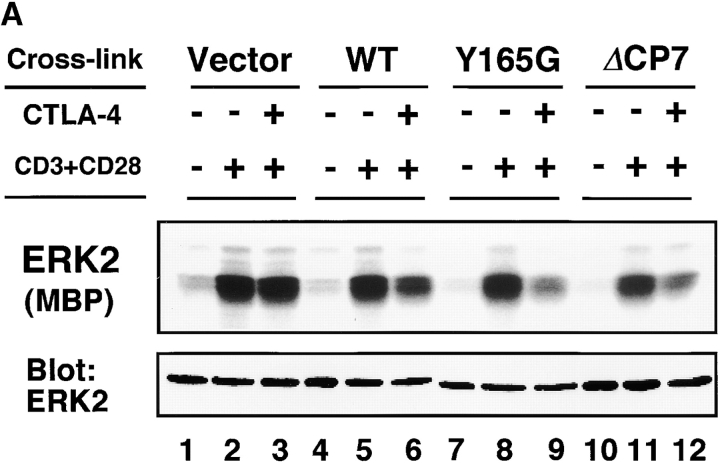
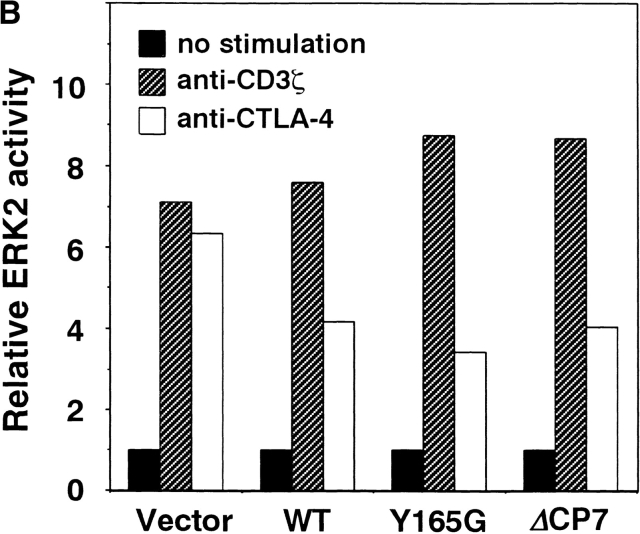
As ERKs are involved in the induction of IL-2 gene transcription and it has been shown that CTLA-4 engagement downregulates ERK activity in preactivated T cells 25, we investigated whether mutant CTLA-4 such as ΔCP7 CTLA-4 has an inhibitory effect on ERK2 upon activation of resting T cells by immune complex kinase assay. Under the stimulation condition with suboptimal anti-CD3 and optimal anti-CD28 mAbs as described above, the ERK2 activity was so weakly upregulated that the effect of CTLA-4 cross-linking was hardly observed. Therefore, T cells were stimulated by optimal CD3/CD28 stimulation, and the effect of CTLA-4 cross-linking was examined. As shown in Fig. 5 A, ERK2 activity was significantly upregulated by the optimal CD3/CD28 costimulation within 20 min. The cross-linking of CTLA-4 significantly inhibited ERK2 activity to about half the level of the control in WT, Y165G, and ΔCP7 T cells but not in CT T cells. These data demonstrated that WT and tailless CTLA-4 might inhibit ERK2 activity during early activation of resting T cells when expressed on the cell surface.
Figure 5.
Reduced ERK2 activity by cross-linking of mutant CTLA-4. (A) Kinase activities of ERK2. T cells were stimulated with immobilized anti-CD3∈ (0.5 μg/ml) and soluble anti-CD28 (10 μg/ml) mAbs. Anti–CTLA-4 and anti-CD3ζ mAbs as the control were cocoated at 10 μg/ml. Cell lysates (50 μg/lane) were immunoprecipitated with anti-ERK2 Ab. Immune complex kinase assay was performed using MBP as a substrate and resolved on 15% SDS-PAGE (top). The same lysates were immunoblotted with anti-ERK2 Ab for protein abundance (bottom). (B) Quantitation of ERK2 activities. ERK2 activity in A was quantified using an image analyzer and is represented as relative values compared with those from unstimulated cells (with unstimulated equaling 1).
Responsible CTLA-4 Molecule for Suppression in CTLA-4 Mutant–expressing Cells.
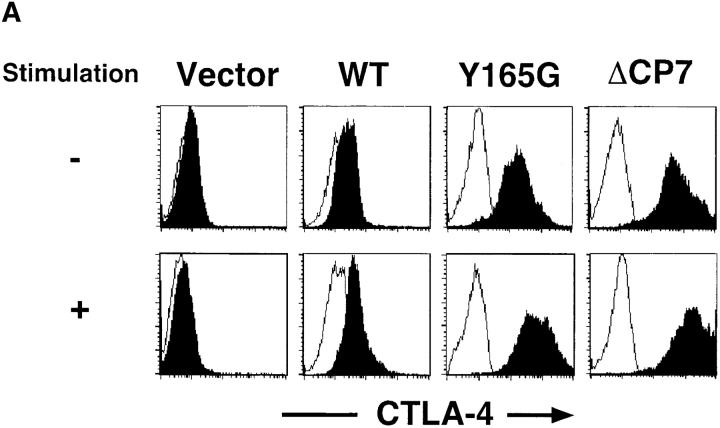
The inhibition of IL-2 production in the transfectants expressing mutant CTLA-4, such as Y165G or ΔCP7, is likely to be mediated through the expressed mutant CTLA-4. However, the possibility still remains that this suppression could be mediated through the endogenous CTLA-4 accumulated on the cell surface by forming heterodimers with the mutant CTLA-4. To examine this possibility, T cells were activated with immobilized anti-CD3 and anti-CD28 mAbs for 48 h, surface-biotinylated, and immunoprecipitated with anti–CTLA-4 mAb. We analyzed the surface expression of CTLA-4 on CT, WT, Y165G, and ΔCP7 T cells before and after T cell activation (Fig. 6 A). Whereas CT T cells did not induce the surface expression of CTLA-4, WT T cells slightly enhanced the expression upon activation. The enhancement of the surface expression of CTLA-4 upon stimulation was also observed in Y165G and ΔCP7 mutant CTLA-4 transfectants. Because CTLA-4 is heavily glycosylated (Fig. 1 D), it migrates broadly on SDS-PAGE, and we can hardly distinguish WT from mutant CTLA-4. Therefore, we treated the immunoprecipitates similarly to those in Fig. 1 D. In CT T cells, whereas CTLA-4 on the cell surface was hardly detected without N-glycosidase F treatment, a small amount of endogenous CTLA-4 of 18 kD was observed by immunoprecipitation with anti–CTLA-4 mAb after the treatment (Fig. 6 B, lane 4). In WT T cells, WT CTLA-4 was clearly detected by the treatment, and the amount of WT CTLA-4 was almost 10-fold greater than that on CT T cells (Fig. 6 B, lane 5). In ΔCP7 T cells, although the mutant CTLA-4 on the cell surface was detected as a strong band of 15 kD, the endogenous CTLA-4 of 18 kD was hardly detected (Fig. 6 B, lane 6). These data demonstrate that the enhancement of the surface expression of CTLA-4 on ΔCP7 T cells was not caused by accumulation of the endogenous CTLA-4 but rather by the upregulation of the ΔCP7 mutant upon activation. Considering that proliferation and IL-2 production were strongly inhibited in ΔCP7 but not in WT T cells, these data demonstrate that CTLA-4 with tyrosine mutation or tailless mutant did transduce negative signals through the mutant CTLA-4 molecules themselves rather than by forming heterodimers with endogenous CTLA-4.
Figure 6.
Biochemical analysis of endogenous and transfected CTLA-4 on the cell surface of activated ΔCP7 CTLA-4 transfectants. (A) Cell surface expression of CTLA-4 on various CTLA-4–expressing T cells. T cells transfected with control vector, WT, Y165G, and ΔCP7 mutant CTLA-4 were unstimulated (top) or stimulated (bottom) with immobilized anti-CD3∈ (1.0 μg/ml) and anti-CD28 (1.0 μg/ml) mAbs for 48 h. Surface expression of CTLA-4 was analyzed by FACS™ after staining with anti–CTLA-4 mAb. (B) Biochemical analysis of the cell surface CTLA-4 on various CTLA-4–expressing T cells. Control vector (lanes 1 and 4), WT (lanes 2 and 5), and ΔCP7 mutant–transfected T cells (lanes 3 and 6) were stimulated as in A, surface biotinylated, and lysed in 0.5% NP-40 lysis buffer. The same amount of protein per sample was immunoprecipitated with anti–CTLA-4 (lanes 4–6) or a control hamster IgG (lanes 1–3). The immunoprecipitated samples were treated with N-glycosidase F and resolved on 14% SDS-PAGE. Closed and open arrows indicate the band of endogenous and ΔCP7 mutant CTLA-4, respectively. Biotinylated proteins were visualized with streptavidin–peroxidase and enhanced chemiluminescence system.
Discussion
Recently, we and other groups demonstrated that endocytosis of CTLA-4 is induced by association of its cytoplasmic tail with the μ2 subunit of clathrin-associated adaptor complex AP-2 12 13 14 15. The tyrosine-based motif containing 165YVKM within the cytoplasmic tail of CTLA-4 is responsible for the binding to μ2. However, there has been no data so far that demonstrated the same regulation in normal T cells. In this study, we demonstrated that the surface expression of CTLA-4 on WT CTLA-4–transfected T cells was dull at the resting stage and that CTLA-4 was predominantly localized in the cytoplasm rather than on the cell surface. Whereas the expression of CTLA-4 on T cells transfected with the Y182G mutant was similar to WT CTLA-4 T cells, Y165G mutants exhibited a constitutively high expression of the cell surface CTLA-4, suggesting that Y-165 is critical for the regulation of surface expression of CTLA-4 in normal T cells.
In addition, we found that the surface expression of Y165/182G and ΔCP7 mutant CTLA-4 at the resting stage was higher than that of the Y165G mutant. Furthermore, the surface expression of these mutants as well as WT CTLA-4 was enhanced upon activation. These data strongly suggest that Y-182 and/or other regions of the cytoplasmic tail are also involved in the regulation of the surface expression of CTLA-4. The mechanism of transport of CTLA-4 from intracellular endosomal pool to the plasma membrane might contribute to this regulation along with clathrin-associated endocytic machinery.
Although functional analyses of CTLA-4 engagement have been extensively performed in vivo and in vitro, very little is known about the signaling events resulting from ligation of CTLA-4. PI3 kinase has been shown to associate with the cytoplasmic tail of CTLA-4 upon receptor ligation 17. The same kinase also binds to the cytoplasmic tail of CD28 29 30, and then the suppressive effect of CTLA-4 can only be explained by sequestering PI3 kinase by the high expression of CTLA-4. However, the fact that the expression level of CD28 is much higher than that of CTLA-4 suggests that this possibility is unlikely. It has also been suggested that the role of PI3 kinase binding to CTLA-4 may regulate the transport of molecules from intracellular vesicles to the cell surface 4 5 29. Other investigators have reported that phosphatase SHP-2 associates with the same tyrosine of CTLA-4 upon activation and can be coimmunoprecipitated with phosphorylated CTLA-4 peptide 15 16. Such CTLA-4 immunoprecipitates could dephosphorylate p52Shc in vitro. Furthermore, it has been shown that the kinases Fyn, Lck, and ZAP-70 were activated and other proteins, including CD3ζ and p52Shc, were constitutively hyperphosphorylated in T cells from CTLA-4–deficient mice. However, these findings might be attributed to the hyperactive status of T cells in CTLA-4–deficient mice rather than being a direct consequence of the lack of CTLA-4 9 10. Frearson et al. reported that SHP-2 plays a critical role in connecting TCR to the Ras/MAPK (mitogen-activated protein kinase) pathway in TCR stimulation of Jurkat T cells 31, in which the expression of mutant SHP-2 significantly inhibited TCR-induced activation of ERK2 but had no effect on CD3ζ tyrosine phosphorylation or TCR-elicited Ca2+ mobilization. Taken together with the observation in signaling through several receptor tyrosine kinases that SHP-2 has been demonstrated to stimulate rather than inhibit growth factor–induced Ras/MAPK activation 32 33, these reports suggest that SHP-2 plays a positive role in TCR stimulation rather than serving as a negative regulator of CTLA-4 engagement.
In this study, we identified a new mechanism of negative signal transduction by CTLA-4. We analyzed CTLA-4–transfected T cells by cross-linking CD3, CD28, and CTLA-4 separately with specific Abs to avoid the complexity derived from the T cell–APC interaction. One of the mechanisms of suppression by CTLA-4 has been thought to be the competition of the ligand CD80/86 with CD28 34 35. In our system, we proved that ligand-independent cross-linking of CTLA-4 induces suppression. Engagement of mutant forms of CTLA-4 with Y-165 substitution or complete deletion resulted in strong inhibition of IL-2 production upon CD3/CD28 costimulation. Under these conditions, IL-2 mRNA expression was significantly suppressed by CTLA-4 cross-linking just 4 h after stimulation. If these mutants were functionally inactive and served as dominant negative mutants of CTLA-4, their engagement would augment proliferation or IL-2 secretion. However, these mutants lacking the tyrosine motif did not serve as dominant negative forms for endogenous CTLA-4. Another argument for the strong inhibition of T cell activation by CTLA-4 mutants might be explained if the cross-linking of highly expressed CTLA-4 could physically interfere with the CD3/CD28 stimulation on the cell surface. However, the observation that the induction of IFN-γ or Bcl-XL mRNA expression was not inhibited by CTLA-4 cross-linking in the same T cells ruled out this possibility. Thus, we demonstrated that CTLA-4 cross-linking inhibits IL-2 production and proliferation induced by CD3/CD28 stimulation even in the absence of its tyrosine motif within the cytoplasmic tail. Therefore, in contrast to the current model for an inhibitory mechanism through SHP-2 activation, our results clearly show that the association of CTLA-4 with PI3 kinase and SHP-2 is not required for CTLA-4–mediated suppression of T cell activation, although it is still possible that the tyrosine motif may also partly contribute to CTLA-4–induced inhibition.
Recently, CTLA-4 was found to associate with the CD3ζ chain in primary T cells 36. It has been suggested that the interaction of CTLA-4 and CD3ζ recruits SHP-2 into the complex that induces CD3ζ dephosphorylation. However, these analyses showed only the effect on ζ phosphorylation; the functional consequence has not yet been determined. Considering that this analysis demonstrated that the association depends on the cytoplasmic tail of CTLA-4 and the tailless CTLA-4 did not associate with CD3ζ 36, the inhibition through ΔCP7 CTLA-4 in our study could be induced by a different mechanism than CTLA-4–CD3ζ interaction.
Upon CD3/CD28 stimulation, the surface CTLA-4 expression on Y165G or ΔCP7 mutant T cells was significantly increased. Analysis of CTLA-4 species on the surfaces of ΔCP7 T cells demonstrated that the mutant CTLA-4 did not form heterodimers with endogenous CTLA-4 on the cell surface, ruling out the possibility that endogenous CTLA-4 accumulated on the cell surface upon activation and delivered a negative signal. Inhibition of both proliferation and IL-2 production in WT T cells was much less than that in ΔCP7 mutant T cells. These results prove that cross-linking of the ΔCP7 mutant did deliver an inhibitory signal. Heterodimers between endogenous CTLA-4 and the mutant were not detected; if there are any, they are probably endocytosed by internalization machinery.
The target molecule of inhibition by CTLA-4 has not yet been identified. As CTLA-4 inhibits CD28-dependent TCR activation, the target molecules may be related to CD28 signals. Both ERKs and Jun NH2-terminal kinases (JNKs) have been reported to be involved in the induction of IL-2 gene transcription, and it has been reported that CTLA-4 engagement downregulates these MAPKs 25. We investigated whether Y165G or ΔCP7 mutant CTLA-4 has an inhibitory effect on ERK2 upon activation of resting T cells. However, under the condition of suboptimal CD3/optimal CD28 stimulation, where IL-2 secretion was maximally inhibited by CTLA-4, the ERK2 activity was only weakly upregulated and the effect of CTLA-4 cross-linking was not clearly observed. When we examined ERK2 activity upon optimal CD3/CD28 stimulation, it was upregulated in T cells and was reduced to an approximately half level by CTLA-4 cross-linking, even in Y165G or ΔCP7 mutant CTLA-4 as well as WT CTLA-4–transfected cells. In addition, the suppression of JNK was not clearly observed in our system (data not shown). Taken together with the results of Frearson et al. 31, the inhibition of ERK2 upon CTLA-4 engagement is not mediated by SHP-2. Although CTLA-4 may suppress more efficiently upon stimulation with suboptimal CD3/optimal CD28 stimulation under physiological conditions, it is difficult to conclude from these results that the suppression of the early event of ERK2 upregulation upon TCR/CD3 stimulation is the primary cause of CTLA-4–mediated inhibition of IL-2 production. Further analysis will be required.
Collectively, these data suggest that negative signals by CTLA-4 for IL-2 production and proliferation could be mediated through the membrane-proximal region of CTLA-4 rather than the 165YVKM motif. The suppression is probably mediated through the association with as yet unidentified transmembrane or intracellular molecules, or, alternatively, is due to the proper positioning of the extracellular region upon T cell activation. The recent finding that CD28 mediates reorganization of TCR signaling machinery to rafts 37 may suggest that CTLA-4 inhibits such CD28 function. The tyrosine motif plays a role in regulating the surface expression of CTLA-4 through clathrin-mediated endocytosis and, in addition, might have other unknown signaling functions.
Acknowledgments
We thank Dr. T. Kitamura for retroviral vectors, D.J. Bluestone and Dr. R. Kubo for mAbs, Dr. P. Golstein for cDNA, Dr. H. Arase for helpful discussion, Ms. M. Sakuma and Ms. R. Shiina for technical help, and Ms. H. Yamaguchi for secretarial assistance.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education of Japan and in part by The Mochida Memorial Foundation For Medical and Pharmaceutical Research.
Footnotes
1used in this paper: AP-2, adaptor protein complex 2; CTLA-4, CTL antigen 4; ERK2, extracellular signal-regulated kinase 2; MAPK, mitogen-activated protein kinase; MBP, myelin basic protein; PI3, phosphatidylinositol 3; RT, reverse transcriptase; SH2, Src homology 2; SHP-2, SH2 domain–containing tyrosine phosphatase; WT, wild-type
H. Ohno's present address is the Division of Molecular Membrane Biology, Cancer Research Institute, Kanazawa University, Kanazawa 920-0934, Japan.
References
- Chambers C.A., Allison J.P. Co-stimulation in T cell responses. Curr. Opin. Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- Saito T. Negative regulation of T cell activation. Curr. Opin. Immunol. 1998;10:313–321. doi: 10.1016/s0952-7915(98)80170-2. [DOI] [PubMed] [Google Scholar]
- Thompson C.B., Allison J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Leung H.T., Bradshaw J., Cleaveland J.S., Linsley P.S. Cytotoxic T lymphocyte-associated molecule-4, a high-avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplasmic tail. J. Biol. Chem. 1995;270:25107–25114. doi: 10.1074/jbc.270.42.25107. [DOI] [PubMed] [Google Scholar]
- Linsley P.S., Bradshaw J., Greene J., Peach R., Bennett K.L., Mittler R.S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas T.L., Bakker C.Y., Bluestone J.A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., Mak T.W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Bachmann M.F., Penninger J.M., Ohashi P.S., Mak T.W. Normal thymic selection, normal viability and decreased lymphoproliferation in T cell receptor-transgenic CTLA-4-deficient mice. Eur. J. Immunol. 1997;27:1887–1892. doi: 10.1002/eji.1830270811. [DOI] [PubMed] [Google Scholar]
- Bradshaw J.D., Lu P., Leytze G., Rodgers J., Schieven G.L., Bennett K.L., Linsley P.S., Kurtz S.E. Interaction of the cytoplasmic tail of CTLA-4 (CD152) with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation. Biochemistry. 1997;36:15975–15982. doi: 10.1021/bi971762i. [DOI] [PubMed] [Google Scholar]
- Chuang E., Alegre M.L., Duckett C.S., Noel P.J., Vander Heiden M.G., Thompson C.B. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J. Immunol. 1997;159:144–151. [PubMed] [Google Scholar]
- Shiratori T., Miyatake S., Ohno H., Nakaseko C., Isono K., Bonifacino J.S., Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6:583–589. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Allison J.P. Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein. Proc. Natl. Acad. Sci. USA. 1997;94:9273–9278. doi: 10.1073/pnas.94.17.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengere L.E., Waterhouse P., Duncan G.S., Mittrucker H.W., Feng G.S., Mak T.W. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- Schneider H., Prasad K.V., Shoelson S.E., Rudd C.E. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J. Exp. Med. 1995;181:351–355. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Nakaseko C., Umemori H., Yamamoto T., Saito T. Src family tyrosine kinases associate with and phosphorylate CTLA-4 (CD152) Biochem. Biophys. Res. Commun. 1998;249:444–448. doi: 10.1006/bbrc.1998.9191. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Kubo R.T., Kubo M., Fujisawa I., Kishimoto H., Asano Y., Tada T., Asano Y. Epitopes associated with major histocompatibility complex (MHC) restriction site of T cells. IV. I-J epitopes on MHC-restricted cloned T cells. Eur. J. Immunol. 1988;18:761–765. doi: 10.1002/eji.1830180516. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Sakuma M., Saito T. Induction of interleukin-2 unresponsiveness and down-regulation of the JAK-STAT system upon activation through the T cell receptor. Eur. J. Immunol. 1997;27:1816–1823. doi: 10.1002/eji.1830270733. [DOI] [PubMed] [Google Scholar]
- Abe R., Vandenberghe P., Craighead N., Smoot D.S., Lee K.P., June C.H. Distinct signal transduction in mouse CD4+ and CD8+ splenic T cells after CD28 receptor ligation. J. Immunol. 1995;154:985–997. [PubMed] [Google Scholar]
- Onishi M., Kinoshita S., Morikawa Y., Shibuya A., Phillips J., Lanier L.L., Gorman D.M., Nolan G.P., Miyajima A., Kitamura T. Applications of retrovirus-mediated expression cloning. Exp. Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- Takase K., Wakizaka K., von Boehmer H., Wada I., Moriya H., Saito T. A new 12-kilodalton dimer associated with pre-TCR complex and clonotype-independent CD3 complex on immature thymocytes. J. Immunol. 1997;159:741–747. [PubMed] [Google Scholar]
- Arase N., Arase H., Park S.Y., Ohno H., Ra C., Saito T. Association with FcRγ is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1+ T cells. J. Exp. Med. 1997;186:1957–1963. doi: 10.1084/jem.186.12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo C.R., Amsen D., Kruisbeek A.M. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and Jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor ζ and ZAP70. J. Exp. Med. 1997;186:1645–1653. doi: 10.1084/jem.186.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling A.I., Auger J.A., Ehst B.D., Rulifson I.C., Thompson C.B., Bluestone J.A. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J. Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- Boise L.H., Minn A.J., Noel P.J., June C.H., Accavitti M.A., Lindsten T., Thompson C.B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL . Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Blair P.J., Riley J.L., Levine B.L., Lee K.P., Craighead N., Francomano T., Perfetto S.J., Gray G.S., Carreno B.M., June C.H. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-XL induction. J. Immunol. 1998;160:12–15. [PubMed] [Google Scholar]
- Hutchcroft J.E., Bierer B.E. Signaling through CD28/CTLA-4 family receptorspuzzling participation of phosphatidylinositol-3 kinase. J. Immunol. 1996;156:4071–4074. [PubMed] [Google Scholar]
- Rudd C.E. Upstream-downstreamCD28 cosignaling pathways and T cell function. Immunity. 1996;4:527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- Frearson J.A., Alexander D.R. The phosphotyrosine phosphatase SHP-2 participates in a multimeric signaling complex and regulates T cell receptor (TCR) coupling to the Ras/mitogen-activated protein kinase (MAPK) pathway in Jurkat T cells. J. Exp. Med. 1998;187:1417–1426. doi: 10.1084/jem.187.9.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche S., McGlade J., Jones M., Gish G.D., Pawson T., Courtneidge S.A. Requirement of phospholipase Cγ, the tyrosine phosphatase Syp and the adaptor proteins Shc and Nck for PDGF-induced DNA synthesisevidence for the existence of Ras-dependent and Ras-independent pathways. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:4940–4948. [PMC free article] [PubMed] [Google Scholar]
- Tang T.L., Freeman R.M., Jr., O'Reilly A.M., Neel B.G., Sokol S.Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- Linsley P.S., Brady W., Urnes M., Grosmaire L.S., Damle N.K., Ledbetter J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe P.A., Bodian D.L., Daenke S., Linsley P., Davis S.J. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J. Exp. Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Chuang E., Griffin M., Khattri R., Hong D.K., Zhang W., Straus D., Samelson L.E., Thompson C.B., Bluestone J.A. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]