Abstract
The function of natural killer T (NKT) cells in the immune system has yet to be determined. There is some evidence that their defect is associated with autoimmunity, but it is still unclear how they play a role in regulating the pathogenesis of T cell–mediated autoimmune diseases. It was originally proposed that NKT cells could control autoimmunity by shifting the cytokine profile of autoimmune T cells toward a protective T helper 2 cell (Th2) type. However, it is now clear that the major function of NKT cells in the immune system is not related to their interleukin (IL)-4 secretion. In fact, NKT cells mainly secrete interferon (IFN)-γ and, activated in the presence of IL-12, acquire a strong inflammatory phenotype and cytotoxic function.
Keywords: natural killer T cells, interferon γ, interleukin 4, autoimmunity, regulatory cells
In this study, we have focused our attention on peripheral, IFN-γ–secreting NKT cells of nonobese diabetic (NOD) mice and their ability to immunomodulate the pathogenesis of insulin-dependent diabetes mellitus (IDDM). We found that in NOD mice, the lack of immunoregulatory function of NKT cells in vivo correlated with a dramatic defect in proliferation and differentiation toward an IFN-γ–secreting phenotype upon T cell receptor engagement and IL-12 stimulation. Peripheral NKT cells may have a critical role balancing inflammatory immune responses and avoiding autoimmunity, such that the functional defect we found in the NOD mice may ultimately result in autoimmunity through inadequate counterregulation of the immune response.
Although the role of natural killer T (NKT) cells in the immune system is not yet fully understood 1 2, it is now clear that their dysfunction correlates with the pathogenesis of T cell–mediated autoimmune diseases. lpr/lpr mice carry a mutation in the Fas gene that is responsible for the development of a spontaneous autoimmune syndrome resembling human systemic lupus erythematosus. In these mice, NKT cells disappear from the periphery by the time the autoimmune disease develops. Moreover, the treatment of lpr/lpr mice with anti-Vα14 antibody, which selectively depletes NKT cells, results in early onset and exacerbation of autoimmune phenomena 3. NKT cell dysfunction also correlates with the pathogenesis of experimental allergic encephalomyelitis (EAE),1 a murine model of T cell–mediated autoimmune disease of the central nervous system. The high susceptibility of SJL/J mice to EAE is associated with a striking functional defect of NKT cells 4. Furthermore, the depletion of NK cells in mice not prone to autoimmune diseases, such as C57BL/6, renders them extremely susceptible to the induction of severe EAE 5. Nonobese diabetic (NOD) mice, a strain that develops spontaneous autoimmune diabetes (insulin-dependent diabetes mellitus [IDDM]), have fewer NKT cells compared with immunologically normal mice. The quantitative defect is associated with a reduced secretion of cytokines 6. NKT cell dysfunction and IDDM were unequivocally linked when the injection of thymic NKT cells from nonautoimmune (NOD × BALB/c)F1 mice into young NOD recipients completely protected the latter from the onset of IDDM 7 8. But, whether the increase in NKT cell number or the restoration of NKT cell function caused the protection was not determined. In fact, Vα14 TCR transgenic NOD mice carrying a large number of peripheral NKT cells were only partially protected against IDDM 9, suggesting that the functional, rather than quantitative, defect of NKT cells correlates with the pathogenesis of IDDM.
Although it is clear that NKT cell dysfunction is related to the onset of autoimmune diseases, so far there is little understanding of the mechanisms underlying NKT cell–mediated protection against autoimmunity. NKT cells can directly downmodulate T cells in antitumor immunity 10 11, graft-versus-host disease 12 13, and rejection of allogenic bone marrow stem cells 14. In some cases, the suppression seemed to be mediated by secretion of downmodulatory cytokines such as TGF-β 10. For this reason, it was originally proposed that NKT cells could modulate T cell–mediated autoimmunity by secreting downmodulatory cytokines 8 9. In fact, NKT cells transferred from thymi of nonautoimmune mice to NOD donors seemed to mediate protection through IL-4 and IL-10 secretion. However, it is still unclear if the cytokine pattern and functional features of thymic NKT cells are comparable to those of NKT cells in the periphery. Originally, because NKT cells are the only T cell subset able to secrete IL-4 primarily upon in vivo anti-CD3 stimulation, they have been suggested as the primary source of this cytokine and, therefore, required for the generation of Th2-type immune responses 15 16 17. Yet, since Th2 immune responses can be generated in β2-microglobulin– or CD1-deficient mice that completely lack NKT cells 18 19 20, a fundamental role for this cell subset in driving Th2 responses can be excluded 21. In fact, upon TCR-induced stimulation, NKT cells secrete much more IFN-γ than IL-4, suggesting that their function in the periphery may be related to their IFN-γ–secreting phenotype and cytotoxic properties rather than to IL-4 secretion 22 23 24. In the presence of IL-12, these cells acquire a strongly biased IFN-γ–secreting phenotype completely lacking IL-4 secretion 25 26. Moreover, activated NKT cells, which already share several phenotypic markers with NK cells, can express NK1 and acquire strong, MHC-unrestricted cytotoxicity against various tumoral NK targets 27 28 29 30, particularly in the presence of IL-12 31 32 33 34. Finally, NKT cell receptor (Vα14-Jα281 invariant chain paired with Vβ8.2, Vβ7, or Vβ2 chains) recognizes glycolipid antigens presented by CD1, a nonclassical MHC class I–like molecule highly conserved through mammalian evolution 35 36 37. All together, these lines of evidence suggest that peripheral NKT cells, carrying a strong inflammatory phenotype and cytotoxic properties, could be part of innate immunity and critical for early clearance of pathogens 38. It is now to be determined how the defect of peripheral, IFN-γ–secreting NKT cells relates to T cell–mediated autoimmunity.
To correlate the defect of peripheral NKT cells with the pathogenesis of IDDM, we purified NKT cells from spleens of NOD mice and analyzed their proliferation and IFN-γ secretion in vitro. Our results revealed a generalized dysfunction in NKT cells from NOD mice which encompasses their lack of TCR-mediated activation and differentiation toward an IFN-γ–secreting phenotype in response to IL-12 stimulation. In addition, we adoptively transferred peripheral NKT cells purified from adult NOD mice to 3-wk-old recipients to normalize their otherwise depleted NKT cell number. The transfer of NKT cells with a defective IFN-γ–secreting phenotype from NOD donors did not protect from IDDM, suggesting that the functional defect of NOD NKT cells renders them unable to prevent autoimmunity.
Materials and Methods
Mice.
C57BL/6, NOD/shi, and NOD/Scid mice were bred and maintained under specific pathogen-free housing conditions at the Scripps Institute Rodent Colony. Female 3-wk-old NOD mice were used as recipients in transfer experiments, and 8–10-wk-old female mice were used as donors and for in vitro experiments.
Antibodies.
Anti-CD3 mAb (clone 2C11, hamster Ig) for in vivo treatment was purified from tissue culture supernatants on a protein G column. Purified anti–TCR-β (clone H57-597), PE-conjugated hamster anti–mouse CD3, FITC-conjugated rat anti–mouse CD122 (IL-2 receptor β chain), and biotinylated rat anti–mouse Ly49A and streptavidin-cychrome to detect biotinylated antibodies were purchased from PharMingen. For ELISA assay, primary mAbs anti–IL-4 (clone 11B11) and anti–IFN-γ (clone R46A.2) and secondary biotinylated anti–IFN-γ (clone XMG1.2) were purified from ascites, and secondary biotinylated anti–IL-4 (clone BDV6-24G2) was purchased from PharMingen.
Anti-CD3 In Vivo Treatment.
To evaluate NKT cell homeostasis in vivo, a group of NOD and C57BL/6 mice were injected intravenously with purified anti-CD3∈ mAb (0.2 μg/mouse) in 200 μl of PBS. The mice were killed after 20 h, and their livers were collected for cell purification.
Cell Preparation.
Single cell suspension was prepared from liver, thymus, and spleen by passing them through nylon mesh. Splenocytes were treated with hypotonic solution to remove red blood cells. Total liver cells were resuspended in 40% Percoll solution (Sigma Chemical Co.) and underlaid with 80% isotonic Percoll solution. Mononuclear cells were then isolated at the 40/80% interface after centrifugation at 2,000 rpm for 20 min.
Flow Cytometric Analysis and Cell Sorting.
Single cell suspensions were washed twice with PBS containing 20 mM Hepes solution to stabilize the pH at 7.3, incubated with PE–anti-CD3, FITC–anti-CD122, and biotinylated anti-Ly49A primary antibodies at 4°C for 30 min, and after two washes, with the secondary reagent, streptavidin-cychrome, at 4°C for 20 min. The cells were washed again twice and then analyzed on a FACScan™ using the CELLQuest™ program. Triple-positive (CD3+, Ly49A+, and CD122+) T cells were sorted using a FACStarPLUS™ (Becton Dickinson). Simultaneously, CD3+, Ly49A−, and CD122− T cells were sorted. The sorted T cell populations were 99% pure and were used directly for cell culture or adoptive transfer experiments.
Adoptive Transfer Experiments.
3 × 105 NKT cells purified by FACS® sorting from total splenocytes of 8–10-wk-old NOD mice were injected in each 3-wk-old NOD recipient mouse. A control group was injected with the same number of CD3+NK− T cells. A different group of mice received NKT cells that were stimulated in vitro in the presence of IL-7 (103 U/ml) for 3 d after the sorting. In different experiments, total splenocytes were completely deleted from NKT cells by FACS® sorting and then injected in NOD/Scid mice either with or without 6 × 105 purified NKT cells. Blood glucose levels were measured weekly using Glucofilm blood glucose strips (Miles Diagnostics Division, Inc.). Mice were considered diabetic after two consecutive measurements of blood glucose level ≥250 mg/dl.
T Cell Culture and Cytokine Secretion In Vitro.
NKT cells freshly sorted from total thymocyte or splenocyte populations were stimulated in vitro with plate-bound anti–TCR-β and grown in complete RPMI 1640 medium (GIBCO BRL) supplemented with 10% FBS, 50 μM 2-ME, 2 mM l-glutamine, 100 U/ml penicillin, 200 μg/ml streptomycin, 1 mM sodium pyruvate, and 100 μM nonessential amino acids. The cells were cultured at a density of 5 × 104 cells/ml in the presence of IL-2 (10 U/ml) alone, or with IL-7 (103 U/ml) or IL-12 (10ng/ml). IL-2 was added to the cultures every 3–4 d, and every week they were restimulated with plate-bound anti–TCR-β. 48 h after each restimulation, 100 μl of supernatants was collected from NKT cell cultures for cytokine measurement. The number of NKT cells/well was the same for NOD and C57BL/6 samples. We did not observe differences in NKT cell viability between the two groups after cytokine stimulation.
NKT Cell Proliferation Assay.
96-well plates were coated with anti–TCR-β mAb at 10 μg/ml for 2 h at 37°C and washed three times with cold PBS. Freshly sorted NKT cells were added to the wells at 104 cells/well in 200 μl of complete RPMI in duplicates in the presence of IL-2 alone or with IL-7 or IL-12. After a 2-d incubation at 37°C, 5% CO2, cells were pulsed with 1 μCi of [3H]thymidine for 16 h, and radioactive thymidine incorporation was measured by a scintillation β-counter (Wallac).
Cytokine Determination by ELISA Assay.
Supernatants of anti–TCR-β stimulated NKT cell cultures were analyzed for the presence of IL-4 or IFN-γ using an ELISA assay. In brief, ELISA 96-well plates (Nunc) were coated with primary mAb anti–IL-4 at 4 μg/ml or anti–IFN-γ at 2 μg/ml diluted in PBS overnight at 4°C. After washes with PBS containing 0.05% Tween and incubation with blocking solution (PBS/Tween containing 1% BSA and 10% FBS), the supernatant samples were added to the wells at serial fivefold dilutions and incubated overnight at 4°C. Recombinant murine IFN-γ and IL-4 (PharMingen) were used as standards. Biotinylated secondary antibody anti–IFN-γ or anti–IL-4 at 1 μg/ml and streptavidin-peroxidase plus H2O2–based developing system were used to detect the amount of cytokines. The concentrations of IFN-γ and IL-4 were interpolated using the Softmax program against the linear range on the standard curves (40–2,500 pg/ml).
Statistical Analysis.
Differences in proliferation assay, cytokine secretion, and diabetes incidence were analyzed using the Student's t test.
Results
NKT Cells of NOD Mice Have a Defect in TCR-mediated Activation.
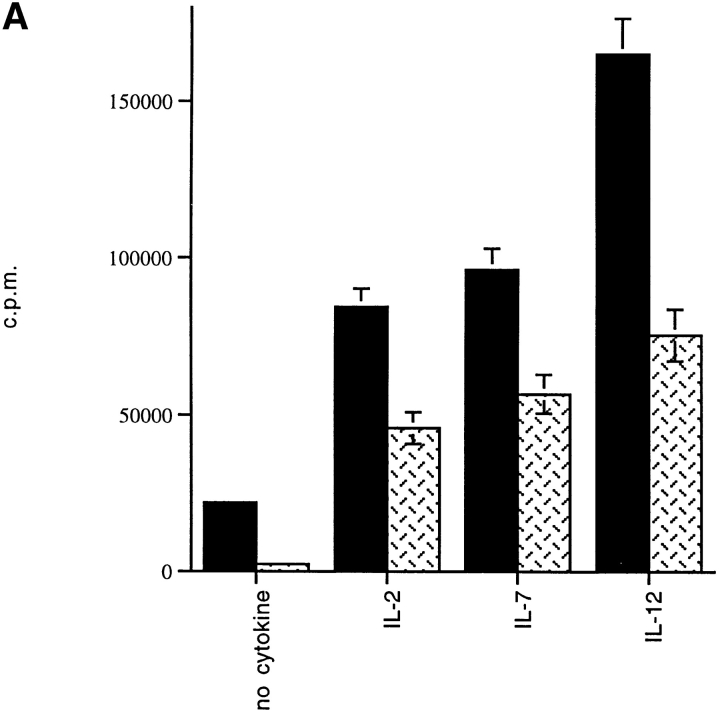
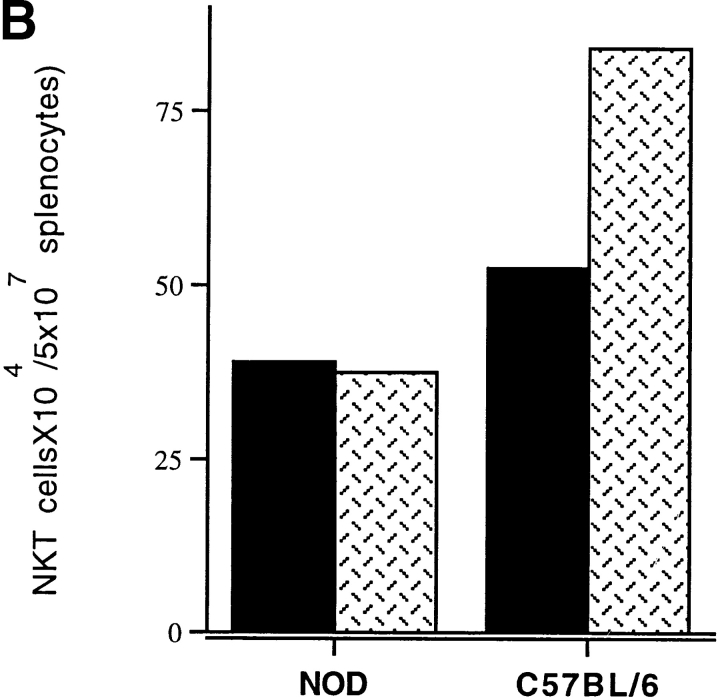
It has been reported that primary cytokine secretion upon anti-CD3 stimulation of thymic NKT cells is defective in NOD mice, but it is still unclear where the functional defect of peripheral NKT cells lies. We analyzed the ability of NKT cells from spleens of NOD mice to respond to anti-TCR–mediated activation. NKT cells were isolated by three-color staining with antibodies against markers of T cells (anti-CD3–PE) and NK cells (anti-Ly49A–cychrome and anti–IL-2 receptor β chain–FITC) followed by FACS® sorting of triple-positive lymphocytes. Freshly sorted NKT cells of NOD mice were activated in vitro with plate-bound anti–TCR-β antibody. NKT cells from C57BL/6 mice were used as controls. Several cytokines known to act as growth factors for NKT cells were then added to the NKT cell cultures to determine their effect on the anti-TCR–mediated proliferation. As Fig. 1 A illustrates, the proliferative response of NKT cells from NOD mice either without cytokines or in the presence of IL-2 was significantly reduced compared with the same T cell population of C57BL/6 mice (P < 0.05). Next, IL-7 was also added because it has been shown to induce in vitro maturation and growth of thymic NKT cells from NOD mice 39. However, in our experiment IL-7 did not modify the defect in TCR-mediated stimulation in peripheral NKT cells of NOD mice. This defect became even more dramatic in the presence of IL-12, a critical cytokine for NKT cell activation and differentiation toward the IFN-γ–secreting phenotype. As expected, IL-12 added to like cultures from C57BL/6 mice enhanced TCR-mediated proliferation of NKT cells. Strikingly, IL-12 had no effect on NKT cells of NOD mice. In addition, we found that the expansion of the NKT cell population in response to anti-TCR plus IL-12 stimulation was also effected in NOD mice. In fact, after 3 d of culture in the presence of IL-12, anti-TCR–stimulated NKT cells of C57BL/6 mice showed a numerical increase of 50%. In contrast, there was no increase and even a slight decrease (5%) in the number of NKT cells from NOD mice, most likely related to cell death (Fig. 1 B).
Figure 1.
The proliferative response to TCR plus IL-12–mediated stimulation is affected in peripheral NKT cells of NOD mice. (A) The NKT cells were stained with the cocktail of three antibodies against markers of T cells (anti-CD3–PE) and NK cells (anti-Ly49A–cychrome and anti–IL-2 receptor β chain–FITC) and purified by FACS® sorting. Freshly sorted NKT cells from splenocytes of NOD and C57BL/6 mice were in vitro stimulated in TCR-β–bound 96-well plates. IL-2 alone or with IL-7 or IL-12 was added to the cultures to measure their effect on TCR-mediated stimulation of NKT cells. The degree of proliferation was measured by [3H]thymidine incorporation and expressed as cpm ± SD. NKT cells of NOD (hatched bars) mice showed a reduced proliferation with or without the addition of cytokines to the cultures compared with the same T cell population isolated from spleens of age-matched C57BL/6 mice (black bars). The impaired proliferation was even more striking in the NOD NKT cell cultures stimulated with IL-12. Data are from one representative experiment out of three and represent the geometric mean of duplicate determinations. (B) NKT cells sorted from NOD or C57BL/6 splenocytes were cultured in anti–TCR-β bound 24-wells plate in the presence of IL-12. The number of peripheral NKT cells found in spleens of NOD mice was lower than that of C57BL/6 mice (black bars). The NKT cell number difference became more evident after TCR plus IL-12–mediated stimulation. While NKT cells of C57BL/6 expanded significantly after 4 d of culture (50% increase), NKT cells of NOD mice did not amplify in culture (hatched bars).
IL-12–induced Differentiation toward an IFN-γ–secreting Phenotype Is Defective in Peripheral NKT Cells of NOD Mice.
It has been proposed that NKT cells may regulate autoimmunity by secreting cytokines such as IL-4 and IL-10 that are downmodulatory for the Th1 cytokine pathway. In addition, it has been reported that patients affected by IDDM have a defect selectively on IL-4 secretion by NKT cells 40, although it recently emerged that the functional defect of NKT cells of IDDM patients does not involve only IL-4 secretion (Wilson, B., personal communication). Moreover, it is now clear that the function of peripheral NKT cells is related to their IFN-γ–secreting phenotype and inflammatory features 22 23 24 27 28 29 30. Therefore, we investigated the ability of NKT cells from NOD mice to differentiate toward an IL-4– or IFN-γ–secreting phenotype to determine if they carry a defect in differentiation toward a specific cytokine pathway.
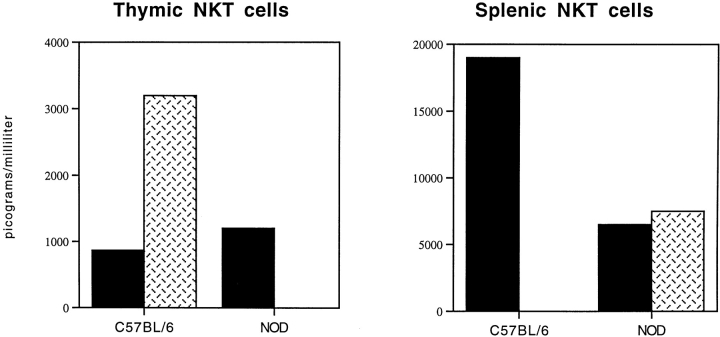
To establish cytokine phenotype, NKT cells isolated from the thymi and spleens of NOD and C57BL/6 mice were examined by IL-4 and IFN-γ ELISA assays on supernatants of anti-TCR–stimulated NKT cells. We found that normal NKT cells from thymocytes of C57BL/6 mice had a predominant IL-4–secreting phenotype in accordance with the observation that NKT cells from the thymi of normal (BALB/c × NOD)F1 mice protected NOD mice through secretion of IL-4 8. NKT cells isolated from thymi of NOD mice not only secreted lower amounts of both cytokines but, in particular, were unable to secrete IL-4. This defect can be related to the lack of maturation of NOD NKT cells in the thymus. Several studies have now shown that NKT cells, once they reach secondary lymphoid tissue, acquire a strong IFN-γ–secreting phenotype. In fact, when we measured cytokine secretion of NKT cells isolated from spleens of C57BL/6, we observed a complete shift toward an IFN-γ–secreting phenotype (Fig. 2, and Table ). Strikingly, peripheral NKT cells from spleens of NOD mice showed no increase in cytokine secretion and did not differentiate toward the IFN-γ cytokine phenotype as did normal C57BL/6 NKT cells.
Figure 2.
NKT cells of NOD mice failed to differentiate toward an IFN-γ–secreting phenotype in the periphery. The cytokine profile of NKT cells in the thymi and spleens of normal C57BL/6 and NOD mice was evaluated by IFN-γ and IL-4 ELISA assays. Freshly sorted NKT cells were briefly stimulated in vitro by plate-bound anti-TCR antibody, and supernatants were analyzed for IFN-γ (black bars) and IL-4 secretion (hatched bars). Normal NKT cells of C57BL/6 mice in the thymus secrete a larger amount of IL-4 than IFN-γ. Once in the periphery, their cytokine phenotype changed, and they differentiated toward a strongly biased IFN-γ–secreting phenotype with almost undetectable IL-4 secretion. Peripheral NKT cells of NOD mice fail to acquire the IFN-γ-secreting phenotype and they do not increase their IFN-γ secretion; however, at the same time, they retain the ability to secrete IL-4.
Table 1.
Cytokine-secreting Phenotype of NKT Cells
| Thymus | Spleen | |
|---|---|---|
| C57BL/6 mice | IL-4 > IFN-γ | IFN-γ >> IL-4 |
| NOD mice | IFN-γ > IL-4 | IFN-γ = IL-4 |
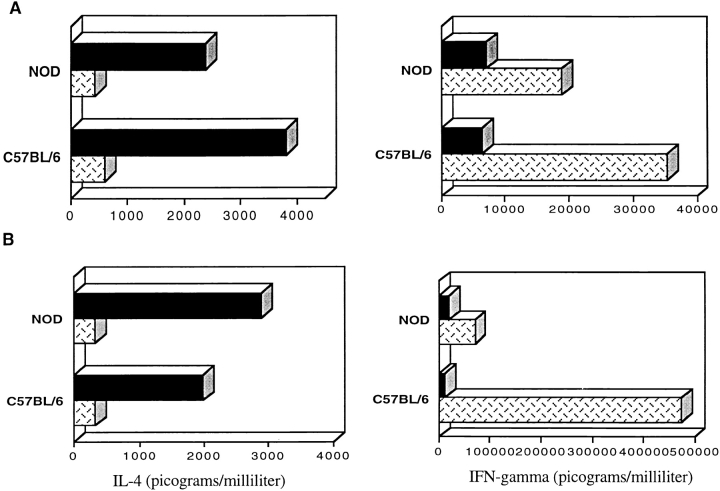
Since IL-12 is considered a critical cytokine in maturation and differentiation of NKT cells 25 26 31 32 33 34, we investigated the ability of IL-12 to drive peripheral NKT cells from NOD mice toward a strong IFN-γ–secreting phenotype. NKT cells purified from spleens of NOD and C57BL/6 mice were activated in vitro by anti-TCR stimulation in the presence of IL-7 or IL-12. Secretion of IL-4 and IFN-γ was then measured both during primary stimulation and after continuous stimulation in the presence of cytokines. As expected, peripheral NKT cells from C57BL/6 mice secreted higher amounts of IFN-γ than IL-4. In addition, IL-12 abolished IL-4 secretion while dramatically increasing IFN-γ secretion in the NKT cell cultures (Fig. 3 A). On the other hand, in the NOD mice the secretion of IFN-γ in response to IL-12 stimulation was significantly lower (P < 0.05; Fig. 3 B). The lack of differentiation toward an IFN-γ phenotype was even more striking in NOD NKT cell cultures repetitively stimulated in the presence of IL-12 (Fig. 3 B).
Figure 3.
IL-12 did not induce IFN-γ secretion in NKT cells of NOD mice. We analyzed the ability of IL-12 to induce IFN-γ secretion in peripheral NKT cells of NOD mice. NKT cells from total splenocytes of C57BL/6 and NOD mice were isolated by FACS® sorting and cultured with repeated anti-TCR stimulations in the presence of IL-2 with IL-7 (black bars) or IL-12 (hatched bars). The supernatants of the NKT cell cultures were collected 48 h after each restimulation and analyzed by IFN-γ and IL-4 ELISA assays. (A) Already at the first stimulation, NKT cells of C57BL/6 mice secreted IFN-γ in larger amounts than IL-4, particularly when IL-12 was added to the cultures. Noticeably, IL-12 was able to inhibit IL-4 secretion by NKT cells. NKT cells of NOD mice responded less intensively to IL-12 and secreted lower amounts of IFN-γ. (B) The defect of IL-12–induced IFN-γ secretion in NKT cells of NOD mice became more evident after continuous in vitro stimulations. At the third restimulation, while NKT cells from normal mice secreted ∼500 ng of IFN-γ per milliliter of supernatant, the same cells from NOD mice did not increase their IFN-γ secretion at all. These data are from one representative experiment out of three.
Lack of Rapid Turnover of NKT Cells in the NOD Mice.
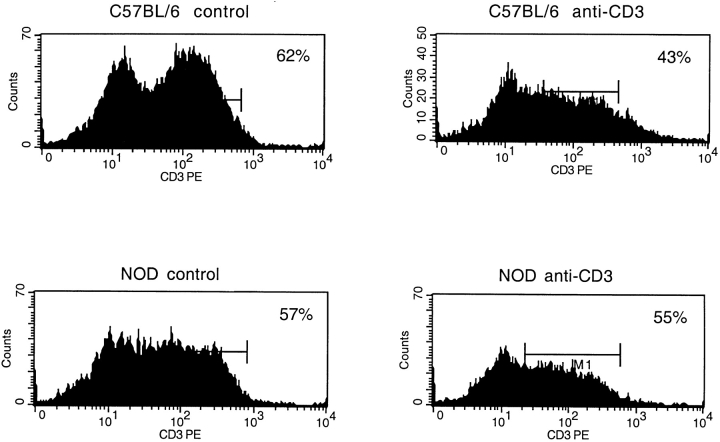
It has been shown recently that IL-12– or anti-CD3–mediated activation in vivo induces pronounced cell death in peripheral NKT cells in <24 h, after which their number is rapidly restored by regeneration from bone marrow stem cells 41. This uniquely rapid turnover seems necessary to prevent NKT cells from interfering with acquired immune responses. In other words, activated NKT cells with a biased IFN-γ–secreting phenotype must be rapidly removed once they clear pathogens, so as to halt inflammatory immune responses that are no longer required. To evaluate homeostasis of peripheral NKT cells in NOD mice, we analyzed the degree of CD3+ cell depletion in the liver, where ∼50% of the T cells are NKT cells. NOD and C57BL/6 mice were injected with anti-CD3 antibody, and the total number of CD3+ lymphocytes in the liver was measured by FACS® analysis. Fig. 4 shows that CD3+ lymphocytes of C57BL/6 mice decrease by 50% at 20 h after anti-CD3 antibody injection. This CD3+ cell depletion could reflect the death of NKT cells, since previous studies have shown that other T cell populations in the liver are not affected by anti-CD3 administration. We confirmed that the depleted CD3+ T cell population in C57BL/6 mice carried markers of NKT cells such as NK1.1 (data not shown). Strikingly, in NOD mice the same CD3+ population was not at all affected by anti-CD3 stimulation. Our results suggest that peripheral NKT cells of NOD mice, once activated in vivo, did not undergo a rapid deletion, a phenomenon that can be ascribed to their defective TCR-mediated activation.
Figure 4.
Lack of rapid turnover in peripheral NKT cells of NOD mice. The homeostasis of peripheral NKT cells was evaluated by measuring CD3+ lymphocytes in the liver of C57BL/6 and age-matched NOD mice injected intravenously with anti-CD3 mAb. Control mice were injected with PBS. After 20 h, the lymphocyte population of the liver was isolated and analyzed by FACS® sorting. In C57BL/6 mice, anti-CD3 treatment reduced the CD3+ cells in the liver ∼50% (from 62.3 to 31.7% lymphocytes), a reduction that can be integrally related to NKT cell depletion. On the contrary, the same T cell population in the liver of NOD mice showed only a slight decrease (from 52.6 to 48%). The data are from one representative experiment out of two.
Peripheral NKT Cells Isolated from NOD Mice Do Not Mediate Protection against IDDM.
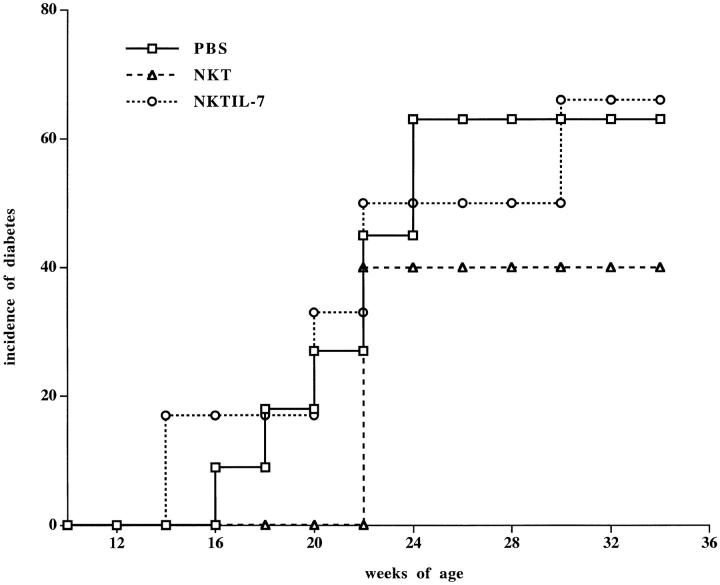
Because NOD mice have fewer circulating NKT cells than nonautoimmune strains of mice, we questioned whether this quantitative defect is the only defining factor, or whether the functional defect we found in peripheral NKT cells of NOD mice could be crucial for the pathogenesis of IDDM. First, we transferred 3 × 105 purified NKT cells from adult NOD mice to markedly enlarge the NKT cell subset in young syngeneic recipients. NKT cells from splenocytes of 8–10-wk-old donors were stained with markers of T cells (anti-CD3) and NK cells (anti-Ly49A and anti–IL-2 receptor β chain). The triple-positive cells were isolated by FACS® sorting and injected into 3-wk-old NOD mice. A control group received only phosphate buffer solution. The NOD transfer recipients, despite carrying an NKT cell repertoire double that of untreated NOD mice, were only partially protected against IDDM (Fig. 5). However, the difference in diabetes incidence between the two groups of mice was not statistically significant (P > 0.05) at any point in the age-related curve.
Figure 5.
Peripheral NKT cells of NOD mice can only mediate incomplete protection against IDDM. The NKT cell repertoire of 3-wk-old NOD mice was significantly enlarged by intravenous injection of 3 × 105 NKT cells (CD3+, CD122+, and Ly49A+ cells) purified by FACS® sorting from spleens of 8–10-wk-old NOD donors. Some of the freshly isolated NKT cells were cultured for 3 d in the presence of IL-7 to restore a normal IL-4 secretion. A control group received phosphate buffer solution. Blood glucose levels were measured weekly starting at 10 wk of age. Mice with blood glucose values ≥250 mg/dl were considered diabetic. Starting at 16 wk of age, the PBS-injected controls (□; n = 11) as well as the mice that received IL-7–stimulated NKT cells (○; n = 6) developed diabetes with a similar time course. Mice that received a large number of peripheral NKT cells started developing diabetes later and with a lower incidence (▵; n = 7). However, at 22 wk of age, 42% of the NKT-injected mice had developed diabetes, and the difference in diabetes incidence compared with the control group was not statistically significant (P > 0.05) at any time point on the age-related curve. The data are cumulative from three different experiments.
Normal NKT cells in the periphery predominantly carry an IFN-γ–secreting phenotype, so the lack of protection from IDDM by a large number of peripheral NKT cells could be integrally ascribed to their functional defect in IFN-γ secretion. To further support this hypothesis, we isolated NKT cells from the spleens of NOD donors and stimulated them in vitro with IL-7, after which they were transferred into 3-wk-old recipients. This cytokine has been reported to be capable of stimulating IL-4 secretion by NOD NKT cells 39. In fact, after 3 d in vitro stimulation with IL-7, NKT cells of NOD mice showed a normal IL-4 secretion but were weak in IFN-γ secretion (as shown in Fig. 3A and Fig. B). Although their IL-4 secretion was amplified, IL-7–stimulated NKT cells were still unable to protect against IDDM (Fig. 5). Furthermore, the group of mice that received IL-7–stimulated NKT cells did not show the slight decrease in IDDM incidence that we found in NOD mice treated with freshly sorted, IFN-γ–secreting NKT cells. This demonstrates that the administration of Th2-differentiated NKT cells does not counterregulate, but rather worsens, IDDM pathogenesis. We found that the dramatic defect in proliferation and IFN-γ secretion in response to IL-12 rendered us unable us to induce NOD NKT cells toward the Th1 cytokine–secreting phenotype.
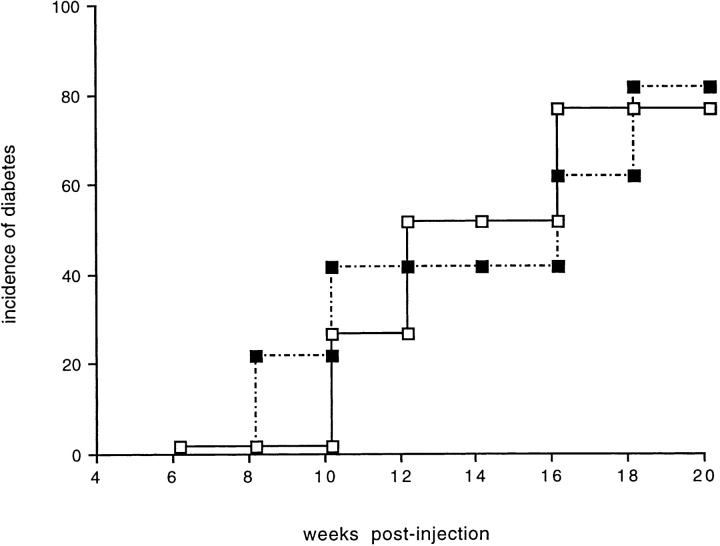
We also tested the ability of NOD NKT cells to immunoregulate the effector phase of IDDM and downmodulate diabetogenic T cells. For that purpose, 107 total splenocytes were isolated from prediabetic NOD mice (14-wk-old), depleted of NKT cells by FACS® sorting, and injected in NOD/Scid recipients. Another group of NOD/Scid mice received the same number of NKT-depleted splenocytes plus 6 × 105 purified NKT cells. The onset and incidence of IDDM in the two groups of mice were identical (Fig. 6), indicating that a functional rather than numerical defect in peripheral NKT cells renders them unable to mediate immunoprotection against IDDM.
Figure 6.
NKT cells of NOD mice had no immunomodulatory effect on the effector phase of IDDM. To test if NKT cells from NOD mice could have any immunoregulatory role on diabetogenic effector T cells, we transferred diabetogenic splenocytes from NOD mice (14-wk-old) to NOD/Scid recipients with or without NKT cells. In brief, total splenocytes were stained for the three NKT cell markers (CD3, CD122, and Ly49A) and FACS® sorted. The NKT cell–negative splenocytes were then injected in NOD/Scid recipients with or without 6 × 105 triple-positive NKT cells. The NOD/Scid mice that received NKT cells together with the diabetogenic splenocytes (▪; n = 4) showed the same clinical course and incidence of diabetes as the mice that were injected with NKT cell–depleted splenocytes (□; n = 4). The data are cumulative from two different experiments.
Discussion
The function of NKT cells in the immune system appears strongly related to their IFN-γ secretion and cytotoxic properties 22 23 24 27 28 29 30. Our results clearly showed that peripheral NKT cells of NOD mice carry a pronounced defect in their TCR-mediated– and IL-12–induced activation and IFN-γ secretion. The lack of these inflammatory features associated with the inability to offset IDDM, which we found in NKT cells of NOD mice, suggests that the defect of their IFN-γ–secreting phenotype may be involved in T cell–mediated autoimmunity.
The involvement of the NKT cells in modulation of autoimmunity is, in fact, proved by the observation that a defect of the NKT cell population is invariably associated with T cell–mediated autoimmune diseases. NOD mice which develop spontaneous IDDM, as well as other autoimmune-prone strains of mice, have a paucity of NKT cells 4 6. In young NOD mice, the restoration of a normal NKT cell repertoire by transfer of NKT cells from non–autoimmune-prone mice conferred protection from IDDM 7 8. However, we showed here that the restoration of a functional NKT cell population rather than simply an increase in cell number may be critical to offset IDDM. In fact, the transfer of NKT cells from adult, prediabetic NOD mice to young NOD recipients did not significantly affect the disease. Yet, transfer of diabetogenic T cells into NOD/Scid mice induced IDDM whether NKT cells were absent or coinjected in large numbers. These findings agree with a recent report showing that Vα14 TCR transgenic mice, despite an extremely large NKT cell population, developed IDDM, albeit at a reduced frequency 9. Therefore, it is now clear that a functional rather than quantitative defect of NKT cell correlates with the pathogenesis of IDDM. However, the nature of this defect in NOD mice is still unclear. Previous studies in NOD mice and individuals affected by IDDM have shown that NKT cells are defective specifically in IL-4 secretion 6 40. This finding, together with the observation that IL-4 is required for (BALB/c × NOD)F1 NKT cells to mediate protection against IDDM 8, had supported the hypothesis that the secretion of IL-4 is associated with NKT cell–mediated regulation of autoimmunity. However, this hypothesis is in sharp contrast to many studies showing that normal NKT cells in the periphery have a strong IFN-γ–secreting phenotype and do not secrete large amounts of IL-4. Therefore, we analyzed peripheral, IFN-γ–secreting NKT cells in NOD mice and found that their defect was more generalized, involved their IFN-γ secretion, and could relate to the pathogenesis of IDDM in distinct ways.
The marked lack of proliferation and expansion of NKT cells in response to TCR-mediated activation that we reported could account for the typically limited number of these cells in NOD mice 6. Moreover, the defect in TCR-mediated activation might dramatically compromise the ability of NKT cells to respond to their specific ligand, the CD1 molecule 42. CD1 recognition in the thymus, and probably also in the periphery, is a critical signal for maturation of NKT cells 43 44 45 46. In fact, CD1 knockout mice carry an NKT cell repertoire that is defective in cytokine secretion 19, a defect similar to that which we found in NOD mice.
The defect in NKT cell activation and response to IL-12 may also affect their homeostasis, i.e., turnover rate, in NOD mice. Like many cells belonging to the innate immune system, activated NKT cells, differentiated toward a strong IFN-γ–secreting phenotype, are required in the early stages of an immune response against pathogens, and then they need to be rapidly removed from the site of inflammation so as not to interfere with the secondary T cell responses. Both IL-12 and anti-CD3 activation induce a dramatic cell death among the NKT cell subset 40. NKT cells may be refractory to activation through the IL-12 receptor or TCR–CD3 complex. The lack of rapid homeostasis of peripheral, IFN-γ–secreting NKT cells that we found in NOD mice could be the result of this defect, which predisposes them to sustain unnecessary Th1 inflammatory responses, including autoreactive responses, and ultimately poses the risk of T cell–mediated autoimmunity.
An alternative hypothesis holds that peripheral NKT cells with a strong IFN-γ–secreting phenotype could be critical to directly dampen the destructive potential of autoreactive T cells. Contrary to the belief that NKT cells could prevent the onset of autoimmune diseases by secreting downmodulatory cytokines such as IL-4, we found that IL-4 secretion by peripheral NKT cells may be not so critical to offset IDDM. In fact, the release of IL-4 by peripheral NKT cells is minimal compared with IFN-γ secretion. In other words, normal NKT cells in the thymus secreted large amounts of IL-4 in our experiments, perhaps the route of IL-4–mediated protection from IDDM that NKT cells from thymocytes of nonautoimmune mice induced in NOD mice. However, our results showed that once normal NKT cells of C57BL/6 mice reach the periphery, they acquire a strongly biased IFN-γ–secreting phenotype. On the other hand, peripheral NKT cells of NOD mice failed to respond to activation and underwent IFN-γ phenotype differentiation induced by IL-12, thus suggesting that the defect in the IFN-γ–secreting phenotype of peripheral NKT cells may correlate with the pathogenesis of IDDM in the NOD mice. This hypothesis has been further supported by our finding that restoring a normal IL-4–secreting phenotype in NOD NKT cells before their transfer in young syngeneic recipients did not enhance their ability to offset the pathogenesis of IDDM. Several lines of evidence indicate that cytokines belonging to the Th1 pathway are ultimately needed to prevent pathogenic autoimmunity. Interestingly, the expression of IFN-γ in the pancreatic islets protects NOD mice from IDDM, and removal of Th1 cytokines such as IFN-γ exacerbated T cell–mediated autoimmune diseases 47 48 49. IFN-γ has been proposed to play a direct role in downmodulation of T cell immunity 50 51 52. In fact, IFN-γ is able to induce cell death on effector T cells that are activated by TCR cross-linking in the absence of costimulatory signals, a mechanism that seems critical for maintaining immune tolerance to self-antigens 53. It has been previously shown that regulatory T cells such as TCR-specific CD4+ T cells protect from T cell–mediated autoimmunity through secretion of IFN-γ 54. We believe that peripheral NKT cells with a strong IFN-γ–secreting phenotype could directly downmodulate Th1-type autoimmunity by releasing large amounts of IFN-γ in the microenvironment. Alternatively, they could directly eliminate Th1-autoreactive cells by Fas-mediated cytotoxicity 28 30. NKT cells with a strongly biased IFN-γ–secreting phenotype and cytotoxic properties could participate in T cell–mediated immunity. At the same time, the burst of IFN-γ secretion induced by NKT cells in the microenvironment could be critical to turn off unnecessary T cell immune responses, including autoreactive responses. Our viewpoint is paradoxical with the earlier finding that administration of IL-12 leads to accelerated diabetes in the NOD mouse 55. Indeed, widespread polarized Th1 responses are highly diabetogenic; however, the amplification of such responses may be thwarted by the NKT cell counterregulatory functions described here. We believe that the secretion of IL-4 by peripheral NKT cells is less critical for downregulation of T cell–mediated autoimmunity. In fact, the amount of IL-4 released by NKT cells in the periphery is minimal compared with the secretion of IFN-γ. Moreover, we found that the restoration of normal IL-4 secretion by NOD NKT cells before their transfer in young syngeneic recipients did not improve, but rather worsened, their ability to downregulate autoimmunity.
There is now extensive evidence that a defect of the NKT cell subset is invariantly associated with T cell–mediated autoimmunity in mice as well as in patients affected by IDDM. We believe that the association between a lack of IFN-γ–secreting phenotype of NKT cells and IDDM in NOD mice suggests a critical homeostatic role for IFN-γ in regulation of T cell immunity. IFN-γ–secreting T cell subsets such as NKT cells that actively participate in innate immune responses against pathogens may also be critical for counterregulation of autoimmunity so that their functional defect leads, by default, to disease.
Acknowledgments
We thank Joanna Davies for providing antibodies for ELISA assays, Phyllis Minick for editing the manuscript, and Massimo Degano, Mitchell Kronenberg, and members of the laboratory for stimulating discussion.
Marika Falcone was supported by a postdoctoral fellowship from the Myasthenia Gravis Foundation and from Juvenile Diabetes Foundation International. This work was supported by the National Institutes of Health, and by a Diabetes Interdisciplinary Research Program from Juvenile Diabetes Foundation International.
Footnotes
1used in this paper: EAE, experimental autoimmune encephalomyelitis; IDDM, insulin-dependent diabetes mellitus; NOD, nonobese diabetic
References
- MacDonald H.R. NK1.1+ T cell receptor-α/β1 cellsnew clues to their origin, specificity, and function. J. Exp. Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari A.P., Zlotnik A. Mouse NK1.1+ T cellsa new family of T cells. Immunol. Today. 1996;17:71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- Mieza M.A., Itoh T., Cui J.Q., Makino Y., Kawano T., Tsuchida K., Koike T., Shirai T., Yagita H., Matsuzawa A. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J. Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Hu-Li J., Paul W. Defective IgE production by SJL/J mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc. Natl. Acad. Sci. USA. 1995;92:11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Yamamura T., Kondo T., Fujiwara M., Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J. Exp. Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombert J.M., Herbelin A., Tancrede-Bohin E., Dy M., Carnaud C., Bach J.F. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur. J. Immunol. 1996;26:2989–2998. doi: 10.1002/eji.1830261226. [DOI] [PubMed] [Google Scholar]
- Baxter A.G., Kinder S.J., Hammond K.J.L., Scollay R., Godfrey D.I. Association between α/β TCR+CD4− CD8− T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 1997;46:572–582. doi: 10.2337/diab.46.4.572. [DOI] [PubMed] [Google Scholar]
- Hammond K.J.L., Poulton L.D., Palmisano L., Silveira P., Godfrey D.I., Baxter A.G. α/β-T cell receptor (TCR)+ CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in non-obese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J. Exp. Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehuen A., Lantz O., Beaudoin L., Laloux V., Carnaud C., Bendelac A., Bach J.F., Monteiro R.C. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J. Exp. Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada K., Harada M., Ito O., Takenoyama M., Mori T., Matsuzaki G., Nomoto K. The emergence of non-cytolytic NK1.1+ T cells in the long-term culture of murine tumour-infiltrating lymphocytesa possible role of transforming growth factor-β. Immunology. 1996;89:627–635. doi: 10.1046/j.1365-2567.1996.d01-771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada K., Harada M., Abe K., Li T., Tada H., Onoe Y., Nomoto K. Immunosuppressive activity of cloned natural killer (NK1.1+) T cells established from murine tumor-infiltrating lymphocytes. J. Immunol. 1997;158:4846–4854. [PubMed] [Google Scholar]
- Weerasinghe A., Kawamura T., Moroda T., Seki S., Watanabe H., Abo T. Intermediate TCR cells can induce graft-versus-host disease after allogeneic bone marrow transplantation. Cell. Immunol. 1998;185:14–29. doi: 10.1006/cimm.1998.1263. [DOI] [PubMed] [Google Scholar]
- Onoe Y., Harada M., Tamada K., Abe K., Li T., Tada H., Nomoto K. Involvement of both donor cytotoxic T lymphocytes and host NK1.1+ T cells in the thymic atrophy of mice suffering from acute graft-versus-host disease. Immunology. 1998;95:248–256. doi: 10.1046/j.1365-2567.1998.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara A., Kawamura H., Iiai T., Moroda T., Suzuki S., Tada T., Minagawa M., Musha N., Hatakeyama K., Abo T. Participation of NK1.1+ T cells in the rejection of lpr αβT cells when bone marrow cells of lpr mice are transplanted into B6 mice. Microbiol. Immunol. 1998;42:447–456. doi: 10.1111/j.1348-0421.1998.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Paul W.E. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Watson C., Hu-Li J., Paul W.E. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Hunziker R.D., Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J. Exp. Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Rogers K.H., Lewis D.B. β2-microglobulin–dependent T cells are dispensable for allergen-induced T helper 2 responses. J. Exp. Med. 1996;184:1507–1512. doi: 10.1084/jem.184.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.H., Chiu N.M., Mandal M., Wang N., Wang C.R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Mendiratta S.K., Martin W.D., Hong S., Boesteanu A., Joyce S., Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- Foucras G., Coureau C., Beijleveld L., Druet P., Saoudi A., Guery J.C. β2-microglobulin-dependent T cells are not necessary for alloantigen-induced Th2 responses after neonatal induction of lymphoid chimerism in mice. J. Immunol. 1998;161:1751–1757. [PubMed] [Google Scholar]
- Arase H., Arase N., Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Paul W.E. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J. Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- Badovinac V., Boggiano C., Trajkovic V., Frey A.B., Vujanovic N.L., Gold D.P., Mostarica-Stojkovic M., Vukmanovic S. Rat NKR-P1+ CD3+ T cellsselective proliferation in interleukin-2, diverse T-cell-receptor-Vβ repertoire and polarized interferon-γ expression. Immunology. 1998;95:117–125. doi: 10.1046/j.1365-2567.1998.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-de-Moraes M.C., Moreau G., Arnould A., Machavoine F., Garcia C., Papiernik M., Dy M. IL-4-producing NK-T cells are biased towards IFN-γ production by IL-12. Influence of the microenvironment on the functional capacities of NK T cells. Eur. J. Immunol. 1998;28:1507–1515. doi: 10.1002/(SICI)1521-4141(199805)28:05<1507::AID-IMMU1507>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Takeda K., Mendiratta S.K., Kawamura H., Van Kaer L., Yagita H., Abo T., Okumura K. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J. Immunol. 1998;160:16–19. [PubMed] [Google Scholar]
- Arase H., Arase-Fukushi N., Good R.A., Onoe K. Lymphokine-activated killer cell activity of CD4− CD8− TCRαβ+ thymocytes. J. Immunol. 1993;151:546–555. [PubMed] [Google Scholar]
- Arase H., Arase N., Kobayashi Y., Nishimura Y., Yonehara S., Onoe K. Cytotoxicity of fresh NK1.1+ T cell receptor α/β+ thymocytes against a CD4+8+ thymocyte population associated with intact Fas antigen expression on the target. J. Exp. Med. 1994;180:423–432. doi: 10.1084/jem.180.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto M., Emoto Y., Kaufmann S.H. TCR-mediated target cell lysis by CD4+NK1+ liver T lymphocytes. Int. Immunol. 1997;9:563–571. doi: 10.1093/intimm/9.4.563. [DOI] [PubMed] [Google Scholar]
- Moroda T., Iiai T., Suzuki S., Tsukahara A., Tada T., Nose M., Hatakeyama K., Seki S., Takeda K., Watanabe H., Abo T. Autologous killing by a population of intermediate T-cell receptor cells and its NK1.1+ and NK1.1− subsets, using Fas ligand/Fas molecules. Immunology. 1997;91:219–226. doi: 10.1046/j.1365-2567.1997.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto W., Takeda K., Anzai R., Ogasawara K., Sakihara H., Sugiura K., Seki S., Kumagai K. Cytotoxic NK1.1Ag+ α/β T cells with intermediate TCR induced in the liver of mice by IL-12. J. Immunol. 1995;154:4333–4340. [PubMed] [Google Scholar]
- Anzai R., Seki S., Ogasawara K., Hashimoto W., Sugiura K., Sato M., Kumagai K., Takeda K. Interleukin-12 induces cytotoxic NK1+ αβ T cells in the lungs of euthymic and athymic mice. Immunology. 1996;88:82–89. doi: 10.1046/j.1365-2567.1996.d01-638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Ogasawara K., Takeda K., Hashimoto W., Sakihara H., Kumagai K., Anzai R., Satoh M., Seki S. LPS induces NK1.1+ αβ T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J. Immunol. 1996;156:2436–2442. [PubMed] [Google Scholar]
- Cui J., Shin T., Kawano T., Sato H., Kondo E., Toura I., Kaneko Y., Koseki H., Kanno M., Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Spada F.M., Koezuka Y., Porcelli S.A. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S.A., Segelke B.W., Sugita M., Wilson I.A., Brenner M.B. The CD1 family of lipid antigen-presenting molecules. Immunol. Today. 1998;19:362–368. doi: 10.1016/s0167-5699(98)01289-4. [DOI] [PubMed] [Google Scholar]
- Brossay L., Chioda M., Burdin N., Koezuka Y., Casorati G., Dellabona P., Kronenberg M. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers E.Y., Scharton-Kersten T., Barbieri S., Caspar P., Sher A. A role for CD4+ NK1.1+ T lymphocytes as major histocompatibility complex class II–independent helper cells in the generation of CD8+ effector function against intracellular infection. J. Exp. Med. 1996;184:131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombert J.M., Tancrede-Bohin E., Hameg A., Leite-de-Moraes M.C., Vicari A., Bach J.F., Herbelin A. IL-7 reverses NK1+ T cell-defective IL-4 production in the non-obese diabetic mouse. Int. Immunol. 1996;8:1751–1758. doi: 10.1093/intimm/8.11.1751. [DOI] [PubMed] [Google Scholar]
- Wilson S.B., Kent S.C., Patton K.T., Orban T., Jackson R.A., Exley M., Porcelli S.A., Schatz D.A., Atkinson M.A., Balk S.P. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- Eberl G., MacDonald H.R. Rapid death and regeneration of NKT cells in anti-CD3∈- or IL-12-treated micea major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Lantz O., Quimby M.E., Yewdell J.W., Bennick J.R., Brutkiewicz R.R. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Coles M.C., Raulet D.H. Class I dependence of the development of CD4+ CD8− NK1.1+ thymocytes. J. Exp. Med. 1994;180:395–399. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adahi Y., Koseki H., Zijlstra M., Taniguchi M. Positive selection of invariant Vα14+ T cells by non-major histocompatibility complex-encoded class I-like molecules expressed on bone-marrow-derived cells. Proc. Natl. Acad. Sci. USA. 1995;92:1200–1204. doi: 10.1073/pnas.92.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R.-J., Parkes A., Mizoguchi E., Bhan A.K., Koyasu S. Development of CD4−CD8−αβTCR+ NK1.1+ T lymphocytesthymic selection by self antigen. J. Immunol. 1996;157:4379–4389. [PubMed] [Google Scholar]
- Ferber I.A., Brocke S., Taylor-Edwards C., Ridgway W., Dinisco C., Steinman L., Dalton D., Fathman C.G. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Krakowski M., Owens T. Interferon-γ confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- Heremans H., Dillen C., Groenen M., Martens E., Billiau A. Chronic relapsing experimental autoimmune encephalomyelitis (CREAE) in miceenhancement by monoclonal antibodies against interferon-γ. Eur. J. Immunol. 1996;26:2393–2398. doi: 10.1002/eji.1830261019. [DOI] [PubMed] [Google Scholar]
- Holda J.H., Maier T., Claman H.N. Natural suppressor activity in graft-vs-host spleen and normal bone marrow is augmented by IL 2 and interferon-γ. J. Immunol. 1986;137:3538–3543. [PubMed] [Google Scholar]
- Holda J.H., Maier T., Claman H.N. Evidence that IFN-γ is responsible for natural suppressor activity in GVHD spleen and normal bone marrow. Transplantation. 1988;45:772–777. doi: 10.1097/00007890-198804000-00021. [DOI] [PubMed] [Google Scholar]
- Huchet R., Bruley-Rosset M., Mathiot C., Grandjon D., Halle-Pannenko O. Involvement of IFN-γ and transforming growth factor-β in graft-vs-host reaction-associated immunosuppression. J. Immunol. 1993;150:2517–2524. [PubMed] [Google Scholar]
- Liu Y., Janeway C.A., Jr. Interferon γ plays a critical role in induced cell death of effector T cella possible third mechanism of self-tolerance. J. Exp. Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Sercarz E. Induction or protection from experimental autoimmune encephalomyelitis depends on the cytokine secretion profile of TCR peptide-specific regulatory CD4 T cells. J. Immunol. 1998;161:6585–6591. [PubMed] [Google Scholar]
- Trembleau S., Penna G., Bose E., Mortara A., Gately M.K., Adorini L. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J. Exp. Med. 1995;181:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]