Abstract
T1/ST2 is an orphan receptor of unknown function that is expressed on the surface of murine T helper cell type 2 (Th2), but not Th1 effector cells. In vitro blockade of T1/ST2 signaling with an immunoglobulin (Ig) fusion protein suppresses both differentiation to and activation of Th2, but not Th1 effector populations. In a nascent Th2-dominated response, anti-T1/ST2 monoclonal antibody (mAb) inhibited eosinophil infiltration, interleukin 5 secretion, and IgE production. To determine if these effects were mediated by a direct effect on Th2 cells, we next used a murine adoptive transfer model of Th1- and Th2-mediated lung mucosal immune responses. Administration of either T1/ST2 mAb or T1/ST2-Ig abrogated Th2 cytokine production in vivo and the induction of an eosinophilic inflammatory response, but failed to modify Th1-mediated inflammation. Taken together, our data demonstrate an important role of T1/ST2 in Th2-mediated inflammatory responses and suggest that T1/ST2 may prove to be a novel target for the selective suppression of Th2 immune responses.
Keywords: inflammation, eosinophil, asthma, cytokines, immunoglobulin superfamily
While the molecular basis underlying Th1 and Th2 differentiation still remains to be fully elucidated, recent work has demonstrated that during the commitment of naive cells to either pathway, distinct molecular events occur that result in differential gene expression. In this respect, the transcription factors c-maf 1 and GATA-3 2 3 have recently been shown to be induced in Th2 cells and demonstrated to play an important role in Th2 cytokine secretion. Moreover, GATA-3 not only appears to directly regulate Th2 phenotype differentiation, but also functions to inhibit commitment to the Th1 phenotype by inhibiting IFN-γ secretion and the acquisition of the β2 subunit of the IL-12 receptor 4. However, overexpression of GATA-3 in differentiated effector populations has minimal effects on IL-4 or IFN-γ secretion 4, suggesting that other as yet unidentified factors regulate Th2 cytokine production from effector cells.
More recently, several transmembrane receptors have been shown to be differentially expressed on Th subsets. The CC chemokine receptors (CCR)1 CCR3 and CCR4 are expressed on Th2 cells, whereas CCR1 and CCR5 are expressed on Th1 effector populations 5 6, providing an attractive mechanism by which Th subsets are preferentially recruited to distinct inflammatory sites. In addition to chemokine receptors, two members of the IL-1 receptor superfamily, IL-1Rrp (7; subsequently identified as the IL-18 receptor 8) and T1/ST2 9, have also been shown to be differentially expressed on Th cells. The IL-18 receptor is expressed on activated Th1 cells and regulates IFN-γ secretion, IL-12R β2 expression, and Th1-mediated inflammation in vivo 10. T1/ST2, originally identified as a gene induced by serum stimulation of fibroblasts 9, has more recently been demonstrated to be overexpressed on Th2 effector cells 11 12 although its function still remains unclear.
In this report, we provide evidence to suggest that T1/ST2 is more than a stable marker on the surface of Th2 cells, and demonstrate that T1/ST2 is a crucial cell surface receptor that is required for Th2 effector responses. These data suggest that T1/ST2, like other members of the IL-1 receptor superfamily including human Toll (hToll, Toll-like receptor [TLR]-4), TLR-2, and the IL-18 receptor, are critical regulators of both innate and adaptive immunity 10 13 14 15.
Materials and Methods
Generation of T1/ST2-Ig and T1/ST2 mAbs.
A DNA sequence containing the extracellular domain of T1/ST2 was PCR amplified and cloned into an expression vector containing the CD5 signal sequence and the hIgG1 constant region. COS cells were transiently transfected with T1/ST2-Ig cDNA, and the recombinant proteins were purified via affinity chromatography (protein A). The purity of T1/ST2-Ig was subsequently assessed by Coomassie-stained SDS-PAGE and determined to be >90%. The identify of the T1/ST2-Ig was further confirmed by mass spectrometry by comparing the trypsin peptides generated from the extracted gel band with a theoretical trypsin digest (peptide mass fingerprinting by matrix-associated laser desorption ionization time-of-flight [MALDI-TOF] analysis). We also PCR amplified a DNA sequence containing the extracellular domain of a novel Ig superfamily member identified in a murine brain cDNA library unique to a Millennium Proprietary Database. This gene, termed H1, was cloned into the identical vector as T1/ST2 containing the CD5 signal sequence. H1-Ig failed to bind to either T, B, or dendritic cells and unlike T1/ST2, was not detectable by PCR analysis in resting or activated Th1 or Th2 cells (data not shown); therefore, we used H1-Ig as an irrelevant control reagent in some experiments. The rat anti-T1/ST2 mAb (clone 3E10) was generated and characterized as described elsewhere 12.
Surface Expression of T1/ST2 on Th1 and Th2 Effector Cells.
Mice expressing the transgene for the DO11.10 α/β-TCR, which recognizes residues 323–339 of chicken OVA in association with I-Ad, were provided by Dr. D. Loh (Washington University, St. Louis, MO 16). Naive TCR transgenic CD4+ T cells were isolated as described 12 and cultured in complete RPMI 1640 with OVA323–339 (10 μg/ml) and mitomycin C–treated splenocytes in a 1:5 ratio. For Th1 phenotype development, recombinant murine IL-12 (10 ng/ml) and neutralizing anti–IL-4 mAb (11B11, 40 μg/ml; R&D Systems) were added, and for Th2 development, recombinant murine IL-4 (10 ng/ml) and neutralizing polyclonal anti–murine IL-12 (TOSH-2, 3 μg/ml; Endogen) were used. After 5–7 d, cells were washed and restimulated up to three times under identical polarizing conditions. Cells were stained after 5–7 d with digoxigenin-labeled 3E10, and the number of T1/ST2-positive cells was detected by antidigoxigenin Fab fragments (Boehringer Mannheim) conjugated to PE. Expression of T1/ST2 was analyzed on a FACSCalibur™ (Becton Dickinson). To determine the cytokine profile at each time point, cells were washed and a viable CD4 population was isolated over a ficoll gradient and activated (2 × 105/well) in a 96-well plate for 24 h using plate-bound CD3 (2C11, 10 μg/ml; PharMingen). IL-4 and IFN-γ levels were measured in the supernatant by ELISA (Endogen).
In Vitro Differentiation of Effector Cells in an Accessory Cell–dependent System.
CD4+ T cells from DO11.10 α/β-TCR mice were activated as described above in the absence of exogenous cytokines (termed neutral conditions) or in the presence of IL-12 or IL-4, together with T1/ST2-Ig (100 μg/ml) or hIg as the appropriate isotype control. Cells were washed and replated in 96-well plates (5 × 104/well) together with 105 splenocytes/well and restimulated with OVA peptide, and cytokines were measured 48 h later. To determine the effect of T1/ST2-Ig in effector cells, Th1 and Th2 cells were reactivated with OVA peptide in the presence of either hIg or T1/ST2-Ig. In some experiments, H1-Ig was used as a second control reagent for the specificity of T1/ST2-Ig.
In Vivo Measurement of Th1- or Th2-mediated Immune Responses.
Recipient normal BALB/c mice were injected intravenously with 2 × 106 Th1 or Th2 effector cells. 24 h later, mice were exposed to an aerosol of OVA (50 mg/ml) for 20 min on two consecutive days. 1 h before allergen exposure, mice were injected intravenously with either 20 or 100 μg of mAb against T1/ST2 or 100 μg of rat IgG1. 24 h later, the trachea was cannulated, a bronchoalveolar lavage (BAL) was performed as described 17, and cytokine levels in the lavage fluid were measured by ELISA. A second series of experiments was also performed using T1/ST2-Ig (100 μg i.v.) or hIg as the appropriate isotype control. Cytospin preparations were prepared (Shandon), stained with Giemsa reagent, and a total of 200 cells were counted differentially. Lungs were then removed, inflated with 10% neutral buffered formalin, and paraffin embedded. 4-μm sections were stained for cyanide-resistant peroxidase and counterstained with hematoxylin using standard techniques. Airway inflammation was determined by semiquantitative scoring using an arbitrary system where a score of +1 represents one small focus of cells and +5 indicates widespread infiltrates. All scoring was performed by an investigator (C. Lloyd) unaware of the treatment.
Measurement of Airway Hyperresponsiveness.
Airway responsiveness was measured in Th2 recipient mice 24 h after the last aerosol challenge by recording respiratory pressure curves by whole body plethysmography (Buxco; EMKA Technologies) in response to inhaled methacholine (Sigma Chemical Co.) at concentrations of 2.5–20 mg/ml for 1 min. Airway responsiveness was expressed in enhanced pause (P enh), a calculated value, which correlates with measurement of airway resistance, impedance, and intrapleural pressure in the same mouse: P enh = (t e/t r1) × Pef/Pif (t e = expiration time, t r = relaxation time, Pef = peak expiratory flow, Pif = peak inspiratory flow) 18.
Active Immunization Protocol and IgE Measurement.
Male BALB/c mice (15–20 g) were immunized intraperitoneally with 7.5 μg of OVA and 1.5 mg AI(OH)3 in saline on days 0 and 7. On days 14 and 21, the mice were challenged with aerosolized OVA (10 mg/ml) for 1 h. Control mice were challenged with PBS instead of OVA. 1 h before antigen sensitization and challenge, the mice were injected with 100 μg of mAb against T1/ST2 or 100 μg of rat IgG1. 24 h after the second challenge, a BAL was performed and IL-5 levels in the BAL fluid were measured. Serum OVA-specific IgE was determined by specific ELISA.
Results and Discussion
T1/ST2 Is Expressed on Th2, but Not Th1 Cells.
The percentage of T1/ST2-positive cells increased under Th2 polarizing conditions from 5.2% after primary restimulation to 41% after tertiary restimulation (Fig. 1), and correlated with an enhanced capacity of cells to secrete IL-4 upon restimulation (data not shown). Naive cells and Th1 effector cells fail to express T1/ST2. These results extend our previous observations that the majority of IL-4– and IL-5–producing cells either under bulk culture conditions 12 or ex vivo from Th2-dominated immune responses 19 are contained within the T1/ST2-positive cell populations. Taken together, these data suggest that T1/ST2 is a useful surface marker for identifying IL-4– and IL-5–producing cells in vitro and in vivo.
Figure 1.
T1/ST2 is expressed on the surface of Th2 cells. T1/ST2 expression was determined by flow cytometry on (A) splenocytes, (B) purified naive CD4+ (CD4+/CD62L+), or (C) Th2 and (D) Th1 effector populations after primary, secondary, or tertiary restimulation under the indicated polarizing conditions.
T1/ST2 Signaling Is Important for Differentiation to Th2 Effector Cells.
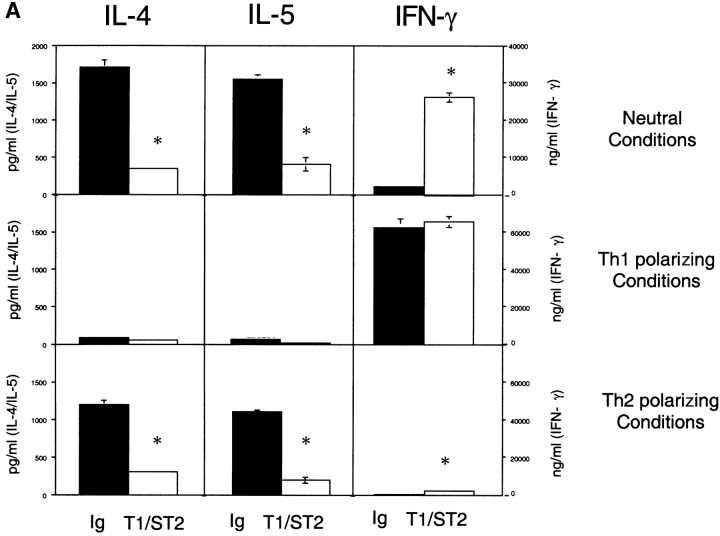
To determine whether T1/ST2 plays a critical role as a signaling molecule required for Th2 function, experiments were performed using a T1/ST2-Ig fusion protein. Under neutral conditions, cells acquired the capacity to secrete high levels of IL-4 (≈1,700 ng/ml), IL-5 (≈1,600 ng/ml), and IFN-γ (≈2,500 pg/ml) upon restimulation. T1/ST2-Ig treatment inhibited IL-4 and IL-5 secretion by >70% and resulted in a 10-fold augmentation in IFN-γ production. Under Th2 polarizing conditions, cells produced equivalent amounts of IL-4 and IL-5 as in neutral conditions, but produced only ≈300 pg/ml of IFN-γ. In the presence of T1/ST2-Ig, Th2 cytokine production was also reduced, and a modest but reproducible increase in IFN-γ secretion (≈1,500 pg/ml) was observed. In contrast to these observations, when cells were cultured in the presence of IL-12, inhibition of T1/ST2 failed to modify IFN-γ production. These results are in some respects similar to recent data generated using either CTLA-4–Ig fusion protein to inhibit CD28/B7 interactions 20 or B7-deficient APCs 21. However, in contrast to CD28-mediated costimulation, which is required for optimal secretion of both IL-4 and IFN-γ when cells were cultured under neutral conditions 21, inhibition of T1/ST2 resulted in skewing of the immune response from a Th2 to a Th1 phenotype. Moreover, while the absence of CD28 costimulation results in an attenuation of IFN-γ and IL-4 secretion when cells are cultured under either Th1 or Th2 polarizing conditions, respectively, inhibition of T1/ST2 signaling selectively inhibited cytokine secretion from Th2 cells without modifying IFN-γ secretion from Th1 cells (Fig. 2 A). These data suggest that T1/ST2 delivers an important signal instructing naive cells to switch to Th2 cytokine production.
Figure 2.
T1/ST2 signaling is critical for differentiation to and activation of Th2 effector cells. (A) Cytokine production from antigen-restimulated CD4+ T cells differentiated with OVA peptide alone (Neutral Conditions), IL-12 plus anti–IL-4 mAb (Th1 polarizing Conditions), or IL-4 and anti–IL-12 (Th2 polarizing Conditions) for 5 d in the presence of hIg (black bars) or T1/ST2-Ig (white bars). Data are shown as the mean ± SEM of triplicate wells and are representative of three different experiments. Statistical significance (*P < 0.01) was determined by Student's t test. (B) Th1 and Th2 effector populations were generated by two rounds of stimulation under the appropriate conditions. Effector populations were then activated with peptide and mitomycin C–treated splenocytes in the presence of either hIg (100 μg/ml; □) or T1/ST2-Ig (1–100 μg/ml) as indicated. In some experiments, a second irrelevant hIg fusion protein was included (H1-Ig; ▪). Data are shown as the mean ± SEM of triplicate wells and are representative of four different experiments.
T1/ST2 Signaling Is Important for Activation of Th2, but Not Th1 Effector Cells.
To determine the requirement of T1/ST2 signaling for activation of effector cells, Thp cells were differentiated for two rounds of polarization to Th1 or Th2 effector populations. Effector populations were then activated with peptide and APCs in the presence of different concentrations of T1/ST2-Ig. Under these circumstances, blockade of T1/ST2 signaling reduced cytokine production from Th2, but not Th1 effector cells in a dose-dependent manner (Fig. 2 B). The specificity of the T1/ST2-Ig protein is further supported by experiments with the control H1-Ig protein. These studies are in marked contrast to recent data generated using B7-deficient APCs demonstrating that cytokine production from Th1 and Th2 effector cells, respectively, is largely independent of CD28/B7-mediated costimulation 21. Taken together, our data suggest that signaling through T1/ST2 can account, at least in part, for CD28/B7-independent activation of Th2 but not Th1 effector cells.
Contribution of T1/ST2 to Cellular and Humoral Response Induced by Active Immunization.
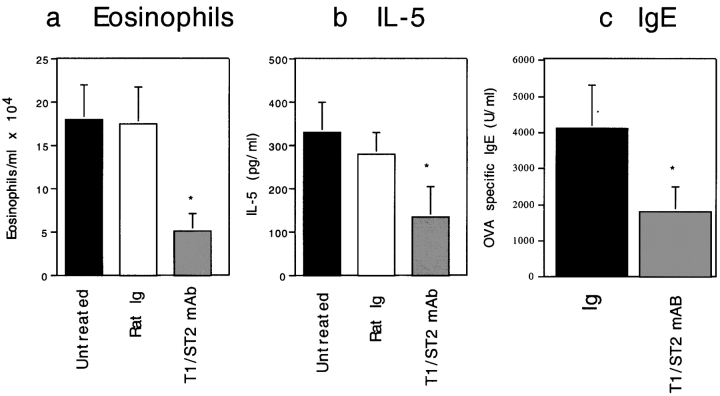
We next determined whether T1/ST2 contributes to a nascent Th2-dominated immune response in vivo. Mice were immunized systemically with antigen in adjuvant before allergen provocation, and the cellular and humoral responses were evaluated. Anti-T1/ST2 mAb was effective in inhibiting allergen-induced lung eosinophilic inflammation, IL-5 production, and the induction of OVA-specific IgE (Fig. 3, a–c). Taken together, our data demonstrate that T1/ST2 is a critical regulatory molecule for both cellular and humoral allergic inflammation in mice in vivo.
Figure 3.
Inhibition of cellular and humoral responses in an active immunization model by anti-T1/ST2 mAb. 1 h before each allergen administration, mice were injected with 100 μg of either rat IgG1 (white bars) or anti-T1/ST2 mAb (gray bars). Untreated allergen-exposed mice (black bars) are shown for comparison. Data are shown as the mean ± SEM of n = 4–9 animals. Statistical significance (*P < 0.05) was determined by Student's t test.
Central Role of T1/ST2 in a Th2-, but Not Th1-driven Mucosal Immune Response.
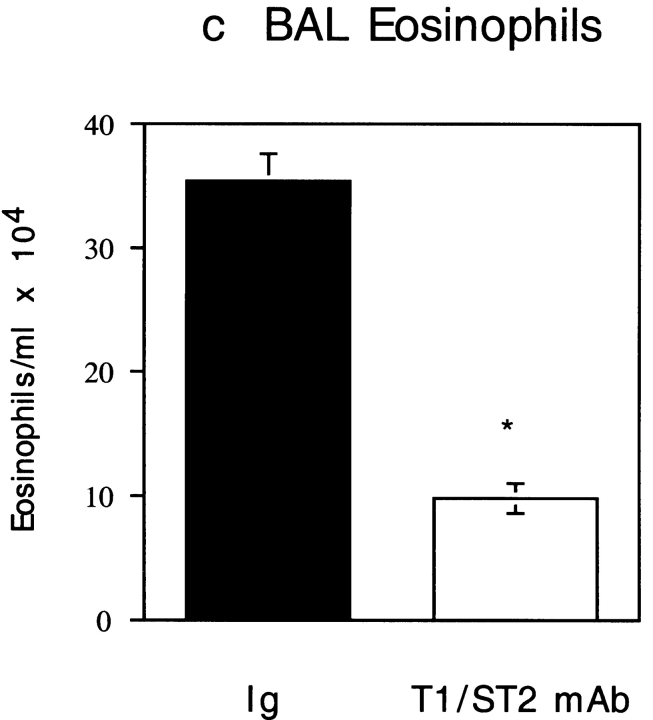
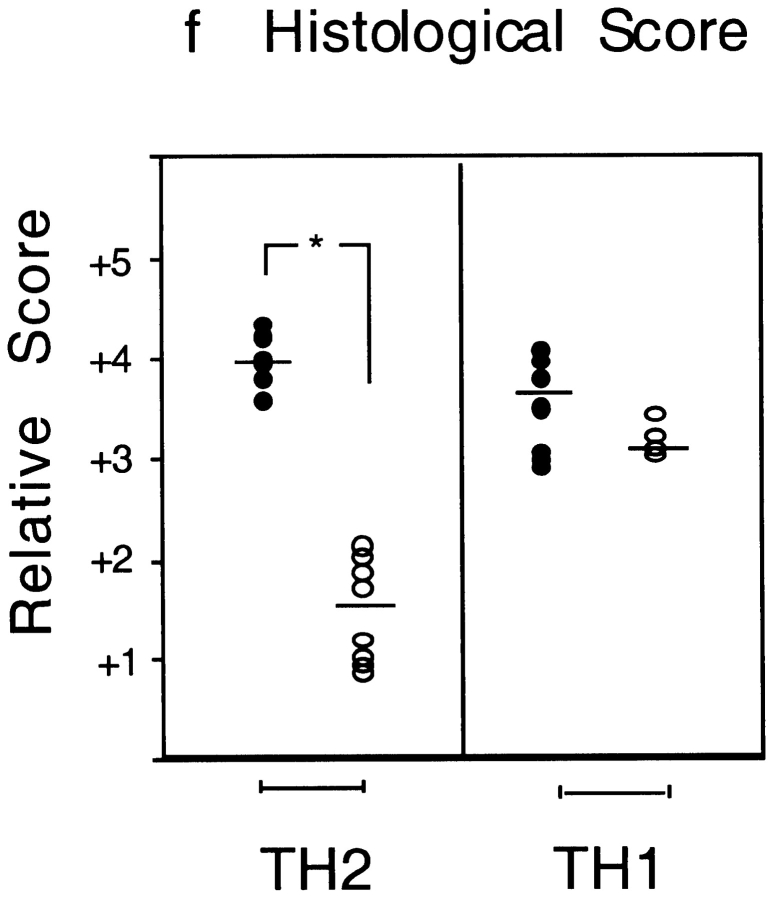
While the above data demonstrate an important role for T1/ST2 in a Th2-dominated response induced by antigen and adjuvant, it is possible that the observed effects on airway inflammation are not mediated via suppression of Th2 cells, as other cell types, including mast cells, also express T1/ST2 22. To address this issue, we next used a model of Th1 and Th2 cell adoptive transfer 23. Aeroallergen provocation of Th1 or Th2 effector cell recipient mice resulted in either a neutrophilic or eosinophilic lung mucosal inflammatory response, respectively 23. Inhibition of T1/ST2 in OVA-exposed Th2 recipient mice with either anti-T1/ST2 mAb (Fig. 4) or T1/ST2-Ig (Table ) inhibited the secretion of IL-4, IL-5, IL-6, and IL-13 in the BAL fluid by >90%. Intriguingly, IL-10 secretion was independent of T1/ST2, suggesting either that the majority of IL-10–producing cells are from a population distinct from cells that produce other Th2 cytokines or that the mechanisms of IL-10 secretion are regulated differently from other Th2 cytokines. Administration of anti-T1/ST2 mAb or T1/ST2-Ig also markedly suppressed eosinophilic inflammation of the airways as assessed both histologically (Fig. 5, a and b) and by analysis of the number of eosinophils in the BAL fluid (Fig. 5 c, and Table ). In contrast to the effects of anti-T1/ST2 mAb in Th2-mediated inflammation, inhibition of T1/ST2 did not modify Th1 effector responses as revealed either by IFN-γ secretion (Fig. 4) or Th1-mediated neutrophilic lung inflammation (Fig. 5d Fig. f). Likewise, using whole body plethysmography 18, both anti-T1/ST2 mAb and T1/ST2-Ig treatment suppressed the development of airway hyperresponsiveness induced by OVA challenge in Th2 recipient mice (Fig. 5 g, and Table ) or in the active immunization model (data not shown). However, whether the ability of T1/ST2 to suppress airway hyperresponsiveness is secondary to attenuated eosinophilic inflammation or is via the suppression of other key effector molecules such as IL-13 24 25 remains to be determined.
Figure 4.
Anti-T1/ST2 mAb administration inhibits IL-4, IL-5, IL-6, and IL-13 secretion in the BAL fluid. Allergen exposure of Th2 recipient mice resulted in marked elevations in IL-4, IL-5, IL-6, IL-10, and IL-13 in the BAL fluid (black bars). Cytokine levels were below the level of detection (<10 pg/ml) in Th2 recipient mice that were exposed to PBS (data not shown). Mice were treated with either 20 or 100 μg of anti-T1/ST2 mAb (white bars). OVA challenge of Th1 recipient mice (inset) resulted in high levels of IFN-γ in the BAL fluid (black bars) that were not inhibited by T1/ST2 mAb (white bars). Data are shown as the mean ± SEM of n = 5–6 animals. Statistical significance (*P < 0.01) was determined by Student's t test.
Table 1.
Suppression of Eosinophilic Lung Inflammation, IL-5, and Airway Hyperresponsiveness by Inhibition of T1/ST2
| Treatment (aerosol + i.v.) | BAL eosinophils | IL-5 | PD400 |
|---|---|---|---|
| cells/ml × 104 | pg/ml | mg/ml | |
| PBS + rat Ig | <1× 104 | <10 | 14.1 ± 2.1 |
| OVA + rat Ig | 36.2 ± 2.1 | 142 ± 32 | 5.0 ± 0.6 |
| OVA + anti-T1/ST2 | 10.2 ± 1.9* | 23 ± 6* | 10.7 ± 1.2* |
| PBS + hIg | <1 × 104 | <10 | 17.9 ± 4.2 |
| OVA + hIg | 43.3 ± 5.7 | 119 ± 32 | 5.8 ± 1.7 |
| OVA + T1/ST2-Ig | 16.1 ± 3.5* | 32.3 ± 11.0* | 14.3 ± 3.0* |
The effect of anti-T1/ST2 mAb or T1/ST2-Ig on the number of eosinophils and IL-5 in the BAL after Th2 transfer in mice that were exposed to either PBS or OVA. Control mice were treated with either hIg or rat Ig as the appropriate isotype controls. Airway hyperresponsiveness is shown as provocative dose 400 (PD400), i.e., the dose of methacholine (mg/ml) required to induce an increase of 400% from baseline P enh values. Data are shown as the mean ± SEM and represent n = 4–9 mice. Statistical significance was determined by Student's t test, and a value of *P > 0.05 was considered significant.
Figure 5.

Anti-T1/ST2 mAb inhibits Th2-mediated allergic lung inflammation and airway hyperresponsiveness. Representative lung histology for Th2 recipient, OVA-exposed (a) control isotype-treated or (b) anti-T1/ST2 mAb–treated mice. Panel c shows eosinophil number in the BAL fluid (cells/ml × 104) in rat Ig–treated (black bars) or anti-T1/ST2 mAb–treated Th2 recipient mice (white bars), and data are shown as mean ± SEM of n = 5–6 animals. Similar data were generated using T1/ST2-Ig fusion protein (data not shown). Statistical significance (*P < 0.01) was determined by Student's t test. In contrast to the effects of anti-T1/ST2 mAb on Th2-mediated pathology, there was no effect of T1/ST2 mAb on Th1-mediated lung pathology (e) compared with Th1 recipient mice treated with control rat Ig (d), summarized in panel f where the circles represent individual mice treated with either rat Ig (•) or anti-T1/ST2 mAb (○). Statistical significance (*P < 0.01) was determined by Student's t test. (g) OVA exposure in control Ig–treated, Th2 recipient mice resulted in airway hyperresponsiveness (⋄) compared with recipient mice that were exposed to PBS (and treated with anti-T1/ST2 mAb; □). Pretreatment with anti-T1/ST2 mAb inhibited OVA-induced bronchial hyperresponsiveness (○). The results are shown as the mean ± SEM of n = 5–10 mice.
In conclusion, our data suggest that T1/ST2 is more than a useful marker for detecting Th2 cells, but plays a crucial role in the differentiation to and activation of Th2, but not Th1 cells. These in vitro observations are supported by in vivo data that inhibition of T1/ST2 signaling attenuates Th2-mediated inflammatory responses without affecting Th1-mediated inflammation. Our data add to the increasing appreciation of IL-1 receptor superfamily members as central regulators of a number of key events in both innate and adaptive immunity 10 13 14 15.
Acknowledgments
We would like to thank Chris Groves and Max Löhning for advice on the FACS® analysis, Mike Gosselin for fusion protein and antibody production, and Marci Melzer for editorial assistance.
The respiratory inflammation group at Millennium Pharmaceuticals is funded by Astra Draco. The Deutsches Rheumaforschungs Zentrum is funded by the Senatsverwaltung für Wissenschaft und Kultur, Berlin, Germany.
Footnotes
1used in this paper: BAL, bronchoalveolar lavage; CCR, CC chemokine receptor; TLR, Toll-like receptor
References
- Ho I.C., Hodge M.R., Rooney J.W., Glimcher L.H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Zheng W., Flavell R. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Zhang D.-H., Cohn L., Ray P., Bottomly K., Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J. Biol. Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- Ouyang W., Ranganath S.H., Weindel K., Bhattacharya D., Murphy T.L., Sha W.C., Murphy K.M. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Mackay C.R., Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- Bonecchi R., Bianchi G., Bordignon P.P., D'Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A., Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. 1998. J. Exp. Med. 1998;5:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnet P., Garka K.E., Bonnert T.P., Dower S.K., Sims J.E. IL-1Rrp is a novel receptor-like molecule similar to the type I interleukin-1 receptor and its homologues T1/ST2 and IL-1R AcP. J. Biol. Chem. 1996;271:3967–3970. doi: 10.1074/jbc.271.8.3967. [DOI] [PubMed] [Google Scholar]
- Torigoe K., Ushio S., Okura T., Kobayashi S., Taniai M., Kunikata T., Murakami T., Sanou O., Kojima H., Fujii M. Purification and characterization of the human interleukin-18 receptor. J. Biol. Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Hoffman S., Werenskiold A.K. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA. 1989;86:5708–5712. doi: 10.1073/pnas.86.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Chan W.L., Leung B.P., Hunter D., Schulz K., Carter R.W., McInnes I.B., Robinson J.H., Liew F.Y. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J. Exp. Med. 1988;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Chan W.L., Leung B.P., Huang B.P., Wheeler R., Piedrafita D., Robinson J.H., Liew F.Y. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhning M., Stroehmann A., Coyle A.J., Grogan J.L., Lin S., Gutierrez-Ramos J.C., Levinson D., Radbruch A., Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock F.L., Hardiman G., Timans J.C., Kastelein R.A., Bazan J.F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Preston-Hurlburt P., Janeway C.A., Jr. A human homologue of Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Kirschning C.J., Wesche H., Merrill Ayres T., Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.M., Heimberger A.B., Loh D.Y. Induction by antigen of intrathymic apoptosis of CD4+ CD8+TCRlo thymocytes in vivo. Science. 1990;21:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Coyle A.J., Le Gros G., Bertrand C., Tsuyuki S., Heusser C.H., Kopf M., Anderson G.P. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am. J. Respir. Cell Mol. Biol. 1995;13:54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G.L., Irvin C.G., Gelfand E.W. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 1997;156:766–771. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- Löhning M., Grogan J.L., Coyle A.J., Yazdanbakhsh M., Meisel C., Gutierrez-Ramos J.C., Radbruch A., Kamradt T. T1/ST2 expression is enhanced on CD4+ T cells from schistosome egg-induced granulomas. Analysis of T helper cell cytokine coexpression ex vivo. J. Immunol. 1999;162:3882–3889. [PubMed] [Google Scholar]
- Seder R.A., Germain R.N., Linsley P.S., Paul W.E. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon γ production. J. Exp. Med. 1994;179:299–304. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer A.N., Sharpe A.H. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 1998;161:2762–2771. [PubMed] [Google Scholar]
- Gachter T., Werenskiold A.K., Klemenz R. Transcription of the interleukin-1 receptor-related T1 gene is initiated at different promoters in mast cells and fibroblasts. J. Biol. Chem. 1996;271:124–129. doi: 10.1074/jbc.271.1.124. [DOI] [PubMed] [Google Scholar]
- Cohn L., Homer R.J., Marinov A., Rankin J., Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cellsa critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M., Luyimbazi J., Xu X., Schofield B., Neben T.Y., Karp C.L., Donaldson D.D. Interleukin-13central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- Grunig G., Warnock M., Wakil A.E., Venkayya R., Brombacher F., Rennick D.M., Sheppard D., Mohrs M., Donaldson D.D., Locksley R.M., Corry D.B. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]