Abstract
Inhibitory receptors expressed on natural killer (NK) cells abrogate positive signals upon binding corresponding major histocompatibility complex (MHC) class I molecules on various target cells. By directly micromanipulating the effector–target cell encounter using an optical tweezers system which allowed temporal and spatial control, we demonstrate that Ly49–MHC class I interactions prevent characteristic cellular responses in NK cells upon binding to target cells. Furthermore, using this system, we directly demonstrate that an NK cell already bound to a resistant target cell may simultaneously bind and kill a susceptible target cell. Thus, although Ly49-mediated inhibitory signals can prevent many types of effector responses, they do not globally inhibit cellular function, but rather the inhibitory signal is spatially restricted towards resistant targets.
Keywords: natural killer cell, major histocompatibility complex class I, optical tweezers, Ly49, video microscopy
Natural killer (NK) cells are large granular lymphocytes that can kill tumor and virally infected target cells without a requirement for MHC restriction 1. The ability of NK cells to kill target cells is believed to be controlled by both positive (activating) and negative (inhibitory) signals 2 3 4. Several putative activation receptors, such as CD2, NKR-P1, and FcRγIII, have all been implicated in target cell recognition by NK cells 5 6 7.
Extensive families of inhibitory receptors have been found on mouse, rat, and human NK cells 8 9 10. Inhibitory receptors belonging to the murine Ly49 receptor family abrogate NK cell effector functions upon engagement of MHC class I ligands on target cells 11 12 13. These receptors contain inhibitory cytoplasmic immune tyrosine-based inhibitory motifs (ITIMs), and additional ITIM-bearing receptor families have been identified on a variety of cell types, including macrophages, dendritic cells, T cells, B cells, and mast cells 14 15 16 17. Thus, ITIM-bearing receptors may mediate inhibitory effects on numerous functions in many different cell types.
Considerable efforts have been made to study the effects of inhibitory receptors on NK cell effector functions. In vitro NK cytotoxicity assays have been important in dissecting the mechanisms of inhibitory signaling in NK cells. Bone marrow allograft rejection and tumor resistance are representative examples of in vivo responses that are regulated by inhibitory signals. Such in vivo and in vitro experimental systems have provided an overall view of signals mediated by bulk populations of NK cells, but these systems have provided little information on the function of individual NK cells during target engagement. An overall activating response may mask subtle responses by individual cells, which may provide important information on the mechanisms by which inhibitory and activating signals are integrated to control cellular function.
To study the effect of inhibitory signaling on NK cell responses at the microscopic level, we have used a clonal rat NK cell leukemia line, RNK-16, transfected with the murine Ly49A inhibitory receptor. As target cells, we have used a susceptible rat myeloma B cell line (YB) and YB cells transfected to express H-2Dd MHC class I molecules (YB-Dd) 18 19. Ly49A binds specifically to H-2Dd and H-2Dk MHC class I molecules on target cells and inhibits lysis of cells expressing these MHC class I ligands 11 20 21. Using an optical tweezers system 22 that allows the precise positioning of target cells, we have found that NK cell morphologic and cytotoxic responses are altered in the presence of protective H-2Dd class I molecules on targets. Furthermore, we have directly demonstrated that NK cells can bind to susceptible and H-2Dd–protected target cells simultaneously, selectively killing the susceptible target cell but not killing the protected target cell.
Materials and Methods
Cell Lines and Immunofluorescence.
The following cells were used in these experiments: the RNK-16 49A.9 rat NK leukemia (RNK.Ly-49A) and the YB2/0 (YB) and YB2/0-Dd (YB-Dd) rat B cell lines have been described previously 19. The cells were cultured in RPMI 1640 glutamax (GIBCO BRL) supplemented with 50 μM 2-ME, 10 U/ml penicillin, 10 μg/ml streptomycin, and 10% FCS in 5% CO2 at 37°C. The transfectants were maintained in 1 mg/ml G418 (GIBCO BRL) for selection purposes. All cells were mycoplasma free. To distinguish target cells from RNK.Ly-49A cells, 5 × 105 target cells were first labeled with biotin (300 μg/ml) in 100 μl PBS for 25 min at room temperature, extensively washed, and labeled with StreptaLite™ Cascade Blue®, streptavidin-FITC, or streptavidin-TRITC (Molecular Probes) for 30 min at 4°C. Cells were then washed and used as target cells in subsequent cell–cell conjugation experiments. This labeling procedure has been demonstrated not to alter NK cell cytotoxicity 23.
Cold Target Competition Assays.
Chromium-release assays were performed as described previously 24. YB target cells were labeled with 100 μCi of Na2 51CrO4 (Nycomed Amersham plc) for 1 h and washed before their use as targets in subsequent cytotoxicity assays. Varying amounts of unlabeled target cells were then added to the effector cells in triplicate wells of U-bottomed 96-well microtiter plates followed by the addition of “hot” YB targets in a final volume of 200 μl. E/T ratio was 40:1. After 4 h of incubation, 100 μl of the supernatant was harvested and its radioactive content was assayed in a gamma-irradiation counter. The mean percent specific lysis of triplicate wells was calculated using the following formula: % specific lysis = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Optical Tweezers and Video Microscopy.
The main components of the optical tweezers system were an ion laser–pumped titanium-sapphire laser (models 2060-10SAH and 3900, respectively; Spectra Physics) with a tuning range of 675–980 nm and an average peak power of 2.3 W, and an inverted microscope (model IX 70; Olympus). The microscope was rebuilt to work as an optical tweezers system by replacing the holder for the bottom mirror with a custom-built holder for a dichroic mirror (Plane plate, BK7, dichroitic coating FLP-5 Lam0 = 590 nm; Spindler & Hoyer). The dichroic mirror directed the infrared laser light into the microscope and onto the specimen plane while reflecting the visible observation light. An extra protective short-pass edge filter, 640 nm cut off (35-5420; Coherent), was mounted in the detection path to prevent scattered laser light from reaching the eyes or the camera. The trapping beam at 810 nm and 200 mW laser output power was focused to a diffraction-limited spot by a high numerical aperture objective (UPlanApo100×OI/1.35NA; Olympus). The cells were moved in the objective plane relative to the laser focus by a motor-driven scanning stage, Scan IM 100×100 (Märzhäuser). The microscope was combined with an Argus-20 image processor and a C2400-75i camera (Hamamatsu) for video microscopy.
Single and Dual Target Cell Analysis.
NK cells were first allowed to settle and adhere onto a small area at the bottom of a PetriPerm™ dish (Heraeus Instruments) placed on a heated stage. The temperature was continuously monitored with a temperature probe near the area where the cells were observed, and temperature was maintained at 35–37°C. Fluorescently labeled tumor target cells were then carefully added to these NK cells. The target cells were then captured in the optical trap, their identity confirmed by fluorescence microscopy, and placed at the leading edge of a migrating NK cell. Effector–target interactions were then analyzed using bright field microscopy and recorded onto videotapes for detailed analysis.
For the dual target experiments, an H-2Dd–expressing target (YB-Dd) was trapped by the optical tweezer, placed in front of a migrating NK cell, and allowed to interact for ∼2 min. Thereafter, a second target (YB) was placed next to the NK cell and allowed to bind. The identity of the cells was confirmed by fluorescence before selecting the target cells. Cellular events were then recorded as described in the single target experiments.
Online Supplemental Material.
We have included three videoclips from representative experiments for Fig. 2, Fig. 3, and Fig. 6. Video 1, Fig. 2: 7× speed, 0.5× original magnification; second part of clip is shown at 1× original magnification for a better view of cell–cell interactions. Video 2, Fig. 3: 7× speed, 1× original magnification. Video 3, Fig. 6: 7× and 21× speed, 1× original magnification. Still images at various time points are presented in the videos, also indicated by the timer.
Figure 2.
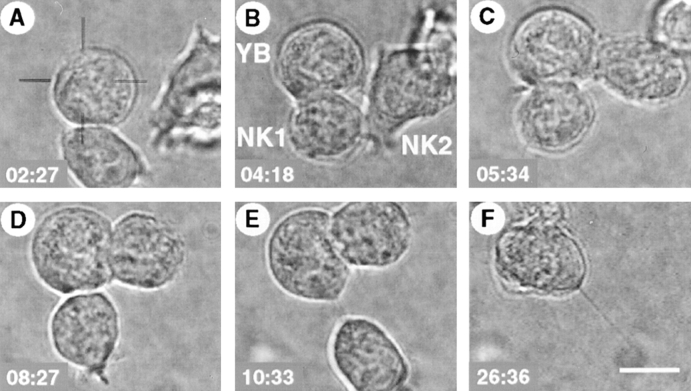
Videomicroscopic analysis of NK cell interactions with single target cells (type II interaction). Bright field microscopy images taken at different time points after contact of an RNK.Ly-49A cell with a susceptible (YB) tumor target cell (00 min:00 s). (A) An NK cell (below) has bound a YB tumor target cell (middle), and a second NK cell (right) approached the first NK cell (02:27). (B) The second NK cell contacted the first NK cell and scanned its surface (04:18). (C) The second NK cell contacted the YB target cell, and extensive membrane interaction could be seen to form between effector and target (05:34). (D) The second NK cell was rounded, and the first NK cell formed lamellipodia on the opposite side of the target contact point (08:27). (E) The first NK cell pulled away from the target (10:33). (F) The target cell had undergone cellular disruption, and the second NK cell had started to leave the target (26:36). Scale bar, 10 μm. Video available at http://www.jem.org/cgi/content/full/190/7/1005/F2/DC1.
Figure 3.
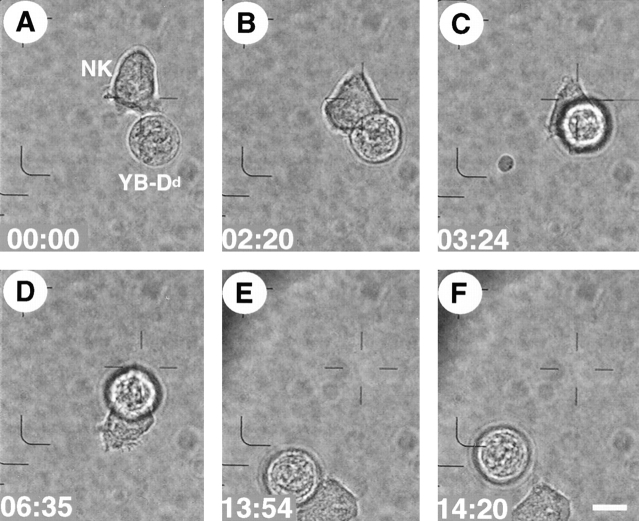
Videomicroscopic analysis of NK cell interactions with single target cells (type IV interaction). Bright field microscopy images taken at different time points after contact of an RNK.Ly-49A cell with a resistant (YB-Dd) tumor target cell. (A) The NK cell contacted the YB-Dd target cell (00:00). (B) The target cell was lifted up from the surface of the dish (02:20). (C) The effector cell continued to crawl under the target (03:24). (D) The target attached at the back of the NK cell as the NK cell moved forward (06:35). (E and F) The YB-Dd target cell was carried along and finally released into the surrounding medium (13:54–14:20). Scale bar, 10 μm. Video available at http://www.jem.org/cgi/content/full/190/7/1005/F3/DC1.
Figure 6.
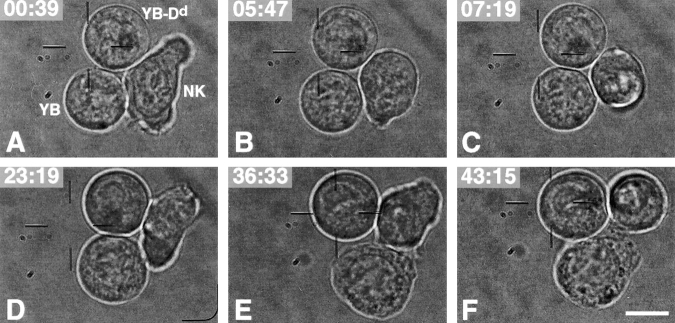
Directional inhibition of NK cell cytotoxicity. Images of NK cell–dual target cell interactions at various time points after contact with a resistant tumor target cell (NK/YB-Dd; −02:22) and a susceptible target cell (NK/YB; 00:00). (A) The NK cell was elongated just after first contact with both YB and YB-Dd (00:39). (B) The NK cell started to round up (05:47). (C) The effector cell was almost completely rounded (07:19). (D) The NK cell was elongated, and the YB target started to swell (23:19). (E and F) The YB target underwent loss of cell integrity (36:33–43:15). Scale bar, 10 μm. Video available at http://www.jem.org/cgi/content/full/190/7/1005/F6/DC1.
Results
Videomicroscopic Analysis of NK Cell–Target Interactions.
To determine how inhibitory MHC class I receptors modulate the cellular responses of individual NK cells, we used an optical tweezers system 25 combined with videomicroscopy to analyze RNK.Ly-49A cell interactions with susceptible (YB) or resistant (YB-Dd) target cells. In these assays, individual NK cells were allowed to settle and adhere to the surface of a dish filled with warm medium on a heated microscope stage. With the temperature confirmed at 35–37°C, a migrating NK cell was selected as an “active” effector cell. Tumor target cells were then carefully added above the NK cell, and a healthy target cell was trapped using laser optical tweezers before it could adhere to the surface of the dish. The identity of the target cell was confirmed by fluorescence, and the cell was then directly placed near the leading edge of the designated NK cell. Thereafter, the effector–target cell interaction was observed and recorded in real time. During trapping, positive identification, and positioning, the total time the target cell spent in the optical trap was between 20 and 60 s.
Changes in YB Target Cell Morphology during Interaction with NK Cells.
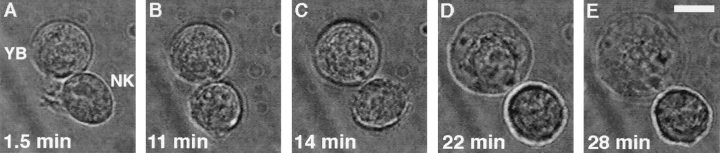
The data in Fig. 1 illustrate typical morphological changes induced in a susceptible YB tumor target cell during an interaction with an NK cell. Early in the interaction, the NK cell began to round up, and small moving lamellipodia were still seen (Fig. 1 A, 1.5 min). Next, the NK cell completely rounded up (Fig. 1 B, 11 min), and the YB target cell began to swell (Fig. 1 C, 14 min). 8 min later, the target further increased its volume and lost intracellular integrity (Fig. 1 D, 22 min). In the final panel, the target cell had completely lost its original appearance and had acquired the typical morphology of a dead target cell (Fig. 1 E, 28 min). To rule out that laser light was harmful to the cells, target cells were held in the trap for >15 min, and no obvious morphological changes were induced (data not shown).
Figure 1.
Changes in target cell morphology during NK cell cytotoxicity. Light microscopy images of NK cell–YB target cell interaction at various time points after target cell binding (0 min). (A) The NK cell had some moving filaments and began to round up (1.5 min). (B) The NK cell had rounded (11 min). (C) The target began to swell (14 min). (D) The target cell continued to increase in volume (22 min). (E) The YB target had completely lost membrane integrity (28 min). Scale bar, 10 μm.
Ly49A–H-2Dd Interactions Alter NK Cell Responses towards Susceptible Target Cells.
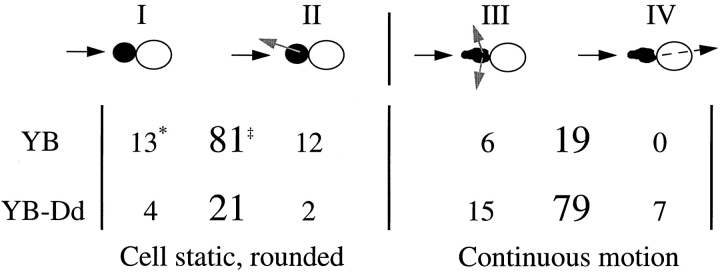
We analyzed NK cell interactions with susceptible (YB) and resistant (YB-Dd) target cells (summarized in Table ). Upon first contact with a susceptible target cell (YB), the NK cell typically stopped migrating, rounded up within minutes, and acquired a dark rim along its membrane surface. We categorized the responses of NK cells after target cell encounters into two general groups: the NK cell became static and rounded after binding the target cell, or the NK cell maintained continuous motion when engaging the target cell. Effector cell rounding was observed in 81% of the NK–YB cell conjugates analyzed (31 interactions total), whereas only 21% of the NK–YB-Dd cell interactions resulted in this type of effector response (28 interactions total). In the group where NK cells became static and rounded, cell death of susceptible YB cells was often noted (88%), whereas death of the YB target cells was rarely seen in the group where the NK cells maintained their motion (17%). There was no evidence for death of the YB-Dd target cells in these experiments. These two major categories were further divided into two subcategories each, giving a total of four types of NK cell responses. In type I responses, the NK cell stopped migrating, rounded up, and stayed attached to the target cell for the remainder of the experiment (17–55 min). In type II, the NK cell responded to the target by cell rounding, stayed attached to the target for several minutes, and then moved away. In type III, the NK cell bound the target cell, but maintained its motion during the entire encounter, although the NK cell changed its direction of movement. The NK cell remained attached to the target for some minutes, but no NK cell rounding was seen. Eventually the NK cell left the target. Finally, in type IV responses, the NK cell continued to move after target cell contact and migrated under the target without significantly altering its direction of movement. In some cases, the NK cell attached to the target cell and carried it along for a period of time as it moved forward.
Table 1.
Categorization of NK/Target Cell Interactions
*No. of total observations for each interaction type. Small filled circles, NK cells; large open circles, target cells.
‡Percentage of interactions that were of types (I + II) or (III + IV).
A typical example of an interaction included in the first group, where the NK cell became static and rounded, is presented in Fig. 2 (type II interaction; Video 1 available at http://www.jem.org/cgi/content/full/190/7/1005/F2/DC1). In this example, an NK cell started to interact with a YB target cell (Fig. 2 A, 02:27). A second NK cell approached the first NK cell, and out of focus, a third NK cell was also seen attaching to the second NK cell (Fig. 2 A). The first NK cell rounded up, whereas the second NK cell made contact with the first NK cell (Fig. 2 B, 04:18). Then, the second NK cell also started to interact with the YB target cell, and an extensive membrane interaction could be seen (Fig. 2 C, 05:34). 8.5 min after contact (Fig. 2 D, 08:27), the first NK cell formed lamellipodia on the opposite side from the YB cell, and pulled itself away from the YB target cell. 2 min later (Fig. 2 E, 10:33), the first NK cell separated from the YB cell, pulling two or three membrane filaments with it, while the YB cell began to swell, undergoing obvious morphological changes. By the last panel (Fig. 2 F, 26:36), the YB cell had lost its membrane integrity, and was eventually lysed. In the upper part of the panel, the second NK cell had started to separate from the YB target cell.
The data in Fig. 3 show an example from the group where the NK cell maintained continuous motion (type IV interaction; Video 2 available at http://www.jem.org/cgi/content/full/190/7/1005/F3/DC1). The NK cell made contact with a YB-Dd target cell (Fig. 3 A, 00:00) and failed to stop or round up (Fig. 3 B, 02:20). Instead, the NK cell continued to move under the YB-Dd cell, carried it along (Fig. 3C–E, Fig. 03:24–13:54), and then released the target cell unharmed into the surrounding medium (Fig. 3 F, 14:20).
NK Cell Interaction Times between YB-Dd and YB Target Cells.
We next compared effector–target contact times between RNK.Ly-49A cells and YB tumor target cells that did (YB-Dd, 21 interactions total) or did not (YB, 19 interactions total) express the protective MHC class I molecule for Ly49A (Fig. 4). These experiments showed that the NK–target cell interaction times varied for each target cell type. However, there was a small difference in the duration of interaction between the two targets. NK–YB-Dd cell conjugates preferentially lasted for shorter times, and a higher percentage (73%) of the NK–YB-Dd cell conjugates separated earlier (<10 min) compared with NK–YB cell pairs (38%). Occasionally, longer interaction times (>25 min) were observed with both target cell types (YB, six interactions; YB-Dd, six interactions). In 74% of the NK–YB cell interactions, the encounter resulted in target cell death, whereas none of the NK–YB-Dd cell conjugates resulted in obvious morphologic changes in the target cell. Taken together, these data suggest that NK cells interact for somewhat shorter times with targets expressing an inhibitory MHC class I ligand, compared with target cells that do not.
Figure 4.
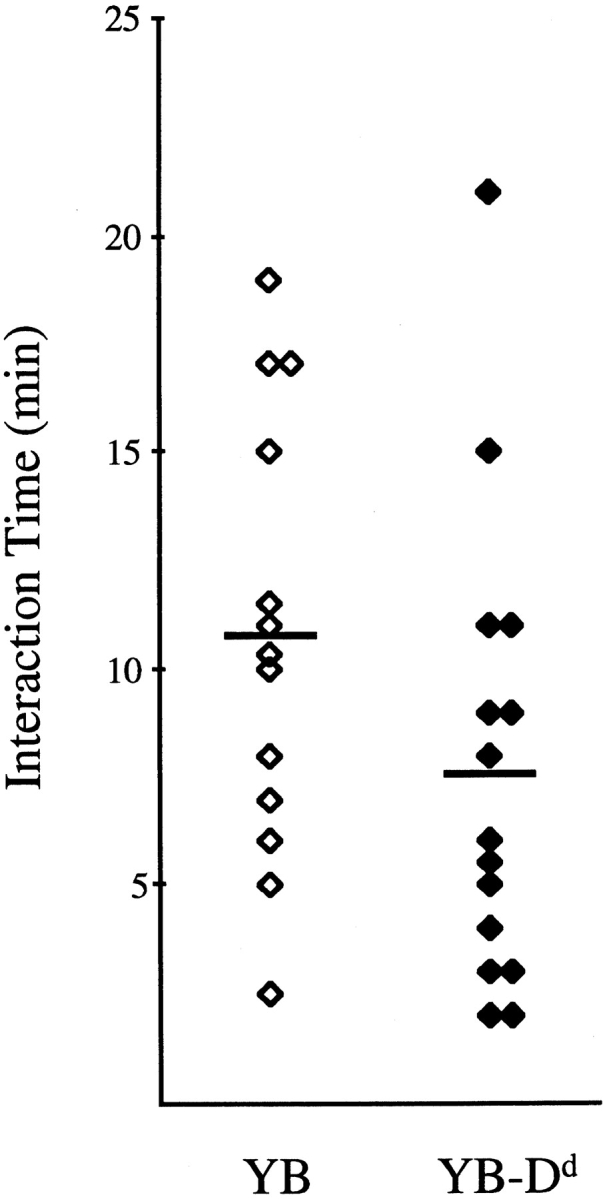
Duration of NK cell–target cell binding. Scatter plot representing interaction times between NK cells and susceptible (YB) and resistant (YB-Dd) tumor target cells. Conjugations that lasted for 25 min or less are shown. For each target, six conjugations lasted >25 min (not shown). The total number of effector–target observations that included YB or YB-Dd were 19 and 21, respectively. Horizontal bars indicate mean values of interaction times (YB: 10.7 ± 5.1 [mean ± SD], n = 13; YB-Dd: 7.6 ± 5.3, n = 15).
Bystander YB-Dd Target Cells Do Not Inhibit Killing of Susceptible YB Target Cells by Ly49A+ NK Cells.
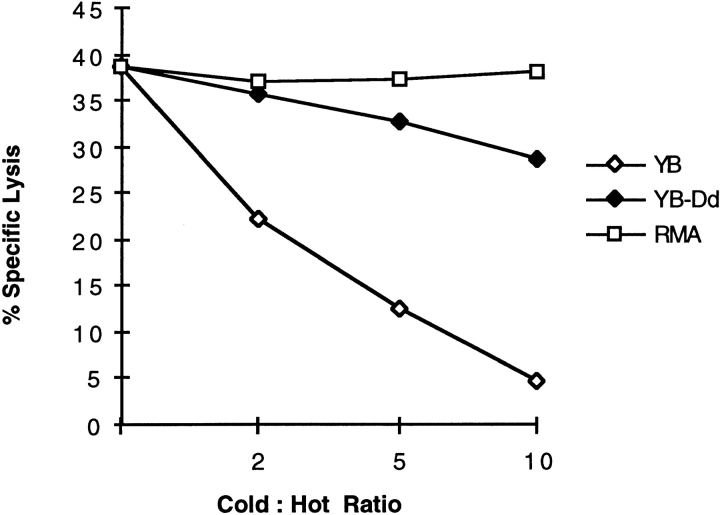
To investigate bystander effects of protected YB-Dd cells on the killing of susceptible target cells, we performed cold target competition experiments using susceptible YB cells as “hot” targets. Increasing amounts of unlabeled YB-Dd target cells did not compete for YB target killing by NK cells, yet unlabeled “cold” YB cells competed very efficiently with their hot labeled YB counterparts (Fig. 5). These data indicated that killing of susceptible target cells (YB) by NK cells was not significantly affected by the presence of increasing numbers of resistant neighboring cells, regardless of the expression of H-2Dd inhibitory ligands by bystander YB-Dd targets. These data are similar to those reported with other target cell combinations, and they suggest that H-2Dd positive target cells fail to deliver global inhibitory signals to Ly49A+ NK cells 18.
Figure 5.
YB-Dd cells do not compete for YB killing. Lysis of 51Cr-labeled YB cells by RNK.Ly-49A NK cells in the presence of increasing amounts of unlabeled YB, YB-Dd, or RMA target cells. E/T ratio was 40:1. The results are representative of three independent experiments.
Directional Inhibition of RNK.Ly-49A towards YB-Dd Target Cells.
There are several possible explanations for the lack of competition between YB-Dd and YB cells, including differences in binding of YB and YB-Dd cells, shorter release times for YB-Dd cells (temporally restricted inhibition), or compartmentalization of Ly49A signaling (spatially restricted inhibition). As such, only YB-Dd was protected from lysis, but not other targets. We wondered whether Ly49A+ NK cells could bind to both YB-Dd and YB cells simultaneously, and if so, were NK cells able to lyse YB, even though the NK effectors were bound to YB-Dd targets? To test this idea directly, we first allowed the NK cell to establish contact with a YB-Dd cell for ∼2 min before the addition of a susceptible target (YB). The videomicroscopic laser tweezers system allowed us to freely move the target cells and to monitor the resulting interactions. Fig. 6 (Video 3 available at http://www.jem.org/cgi/content/full/190/7/1005/F6/DC1) shows a sequence of images from an interaction between an RNK.Ly-49A cell bound to a resistant YB-Dd and a susceptible YB target. We found that in most cases (73%, 11 total interactions), the susceptible YB target cell underwent morphological changes associated with cell death, whereas the resistant YB-Dd cell did not (Fig. 6). The percentage of YB cells killed (73%) was similar to that observed when the NK cells encountered YB targets alone (74%). These data indicate that NK cells can bind to both susceptible and resistant target cells simultaneously and still retain the ability to selectively kill the susceptible target cell. Thus, inhibitory signals mediated via Ly49 receptors do not completely inhibit cellular effector functions. Rather, Ly49A directionally inhibits NK cell activation against protected, but not unprotected target cells.
Discussion
NK cells express inhibitory receptors which prevent the lysis of cells that express sufficient quantities of self MHC class I molecules. This system likely allows NK cells to distinguish between normal self cells and abnormal, or nonself, cells due to differences in MHC class I expression and polymorphism. There are significant variations in the expression of MHC class I molecules between different cell types and tissues, and it has been proposed that some cell types are unable to activate NK cells under normal circumstances. These cell types are likely to be ignored by NK cells. Data have accumulated from a variety of laboratories indicating that inhibitory receptors of the Ly49, KIR, and CD94 families are able to mediate inhibition of all NK cell effector functions 8 10 26. Such “global” inhibition is believed to act as a fail-safe mechanism, protecting normal tissue from deleterious NK cell responses. In vivo, cells with reduced or absent expression of MHC class I molecules due to malignant transformation or viral infection are likely to be outnumbered by normal cells with high MHC class I expression. Thus, if NK cells continuously encounter normal cells able to engage their inhibitory receptors, how are NK cells able to mediate effector functions within the in vivo environment?
There are many possible scenarios that could explain inhibitory NK cell functions in vivo. It is clear from in vitro and in vivo analyses that NK cells can readily distinguish target cells expressing the proper MHC class I from those that do not 27. The final experimental readout from such bulk assays is the result of numerous individual cell–cell encounters, each making a small contribution to the overall response. To understand how NK cells function, we examined how inhibitory receptors affect individual NK cells when they encounter various target cell types. In these studies, we have directly tested the effects of inhibitory signals on individual NK cells using an optical tweezers system. This system has allowed us to temporally and spatially direct the entire NK cell encounter with divergent target cell types. We have used this system to understand how the response of individual NK cells accounts for the overall response of an NK cell population.
Using videomicroscopic analysis of NK–target cell interactions, we found typical changes in NK cell movement upon contact with a susceptible target cell (NK cell rounding). These experiments are consistent with previously published studies using a T cell hybridoma, where a similar behavior was observed upon T helper cell interactions with B cells 28. In addition, similar observations were seen in studies of a human T cell clone interacting with MHC class II–transfected L cells 29. Our studies have uniquely enabled us to examine receptor-mediated NK cell inhibition during effector–target cell interactions in real time. Recognition of H-2Dd MHC class I molecules on targets by Ly49A inhibitory receptors on NK cells considerably altered the behavior of the rat NK cell line (RNK.Ly-49A) towards potentially susceptible tumor target cells (Table ). In the majority of cases, Ly49A ligation by target H-2Dd prevented NK cell migratory arrest and the associated rounding seen when susceptible target cells were engaged. There was continuous NK cell movement even though NK–YB-Dd contact had been made. Thus, inhibitory receptors prevent NK cell effector functions, and they prevent target-induced migratory arrest. These data also suggest that inhibitory receptors fail to globally inhibit the biochemical pathways required for cell movement.
The NK–target interaction times varied for both protected and unprotected target cells (from 2 to 55 min). However, there was a trend toward shorter NK–YB-Dd interactions compared with NK–YB interactions, although the observed differences were not statistically significant. It is possible that under physiological conditions in vivo, NK cells interacting with, or binding to, normal cells are likely to continue migrating in the presence of nearby cells and tissues, which may shorten the time spent with a resistant target cell compared with in vitro conditions in a petri dish. With respect to susceptible target cells, we believe that the absence of such mechanical forces imposed upon the isolated effector–target cell couple may increase the duration of the interaction we have observed in vitro, and account for some of the variation in Fig. 4. Interestingly, the separation of an NK cell from a susceptible target during type II interactions was in many cases associated with the formation of one or more very flexible, thin membrane filaments between the cells (Fig. 2E and Fig. F). The significance of these filaments is unclear, but they may induce additional physical damage to susceptible targets during NK cell–mediated cytotoxicity.
The experiments analyzing single NK–target cell interactions demonstrated that inhibitory receptors did not prevent all cell functions. In vivo, NK cells are surrounded by normal cells, and are thus likely to encounter normal and resistant cells simultaneously. Therefore, we have addressed the question of whether an NK cell surrounded and in contact with normal cells would recognize and kill an aberrant target. Using a cold target competition assay, our data demonstrated that expression of protective MHC class I molecules on surrounding targets does not significantly affect lysis of susceptible targets (Fig. 5). The slight decrease in killing of YB cells in the presence of YB-Dd cells may be due to the fact that NK cells bind to the YB-Dd cells, and although they can continue to move, the NK cells will be slightly delayed due to membrane interactions with abundant YB-Dd cells. Previous in vitro data with RNK.Ly-49A cells support these data 18. Murine RMA cells did not influence YB cell lysis (Fig. 5), and this could be correlated with the fact that these cells were not themselves killed and showed little or no binding to NK cells on videomicroscopic analysis (data not shown). These data indicate that RMA cells are “ignored” by the RNK.Ly-49A cells, perhaps due to lack of the proper adhesion and/or triggering molecules.
Previous cold target competition studies have shown that NK cells are able to kill unprotected cells in the presence of MHC-protected targets 18 27 30. One interpretation of these data is that NK cells might not kill susceptible targets when coengaged with MHC-protected targets, but susceptible cells might be killed after NK cell disengagement from protected cells (temporally restricted inhibition). Alternatively, the inhibitory effects of target MHC class I might be localized to the effector–target interface, allowing for the lysis of susceptible targets while simultaneously coupled to an MHC-protected target (spatially restricted inhibition). Our videomicroscopic demonstration that an NK cell coupled to an MHC-protected target can kill a susceptible target while leaving the protected cell undamaged (Fig. 6) proves that NK cell inhibitory receptors such as Ly49 do not deliver global inhibitory signals per se, but rather interrupt activation signals in a spatially restricted manner at the effector–target interface. The NK cell response to the YB target cell was similar to the behavior of an NK cell interacting with a susceptible target cell alone (Fig. 2). Our data analyzing the individual cell response support the conclusions from molecular studies that demonstrated tyrosine phosphorylation in human NK cells upon conjugation with either sensitive or resistant target cells, which suggested that there was not complete effector cell inhibition 31 32. However, the data presented here go much further since they demonstrate that an NK cell can mediate effector functions while bound to a resistant cell. In summary, our data show that Ly49 inhibition occurs in a spatially restricted manner and help explain how the inhibitory receptor system allows NK cells to distinguish normal cells from abnormal cells in an efficient manner.
Acknowledgments
This work was supported by grants from the Stiftelsen för Strategisk Forskning and the Swedish Medical Research Council. M. Eriksson was partly supported by a grant from the Kempe Foundation. O. Axner, E. Fällman, and G. Leitz acknowledge support from the Swedish Council for Planning and Coordination of Research (FRN; grant 950057:3), the Swedish Research Council for Engineering Science (TFR; grant 95-110/287), the Swedish Natural Science Research Council (NFR; grant I-AA/LS 09354-338/344/347) and Magnus Bergvalls Stiftelse. M.C. Nakamura is supported by a Veterans Administration Career Development Award, the Arthritis Foundation, and the American Cancer Society. J.C. Ryan is supported by the Veterans Administration and is the recipient of National Institutes of Health grant R01 AI44126.
Footnotes
The online version of this article contains supplemental material.
Abbreviation used in this paper: ITIM, immune tyrosine-based inhibitory motif.
References
- Trinchieri G. Biology of natural killer cells. Adv. Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumperz J.E., Parham P. The enigma of the natural killer cell. Nature. 1995;378:245–248. doi: 10.1038/378245a0. [DOI] [PubMed] [Google Scholar]
- Lanier L.L. Natural killer cell receptors and MHC class I interactions. Curr. Opin. Immunol. 1997;9:126–131. doi: 10.1016/s0952-7915(97)80169-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama W.M. Natural killer cell receptors. Curr. Opin. Immunol. 1998;10:298–305. doi: 10.1016/s0952-7915(98)80168-4. [DOI] [PubMed] [Google Scholar]
- Seaman W.E., Eriksson E., Dobrow R., Imboden J.B. Inositol trisphosphate is generated by a rat natural killer cell tumor in response to target cells or to crosslinked monoclonal antibody OX-34possible signaling role for the OX-34 determinant during activation by target cells. Proc. Natl. Acad. Sci. USA. 1987;84:4239–4243. doi: 10.1073/pnas.84.12.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J.C., Niemi E.C., Nakamura M.C., Seaman W.E. NKR-P1A is a target-specific receptor that activates natural killer cell cytotoxicity. J. Exp. Med. 1995;181:1911–1915. doi: 10.1084/jem.181.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone J.C., Lu Y., Trevillyan J.M., Björndahl J.M., Phillips C.A. Association of the p56lck tyrosine kinase with the FcγIIIA/CD16 complex in human natural killer cells. Eur. J. Immunol. 1993;23:2488–2497. doi: 10.1002/eji.1830231017. [DOI] [PubMed] [Google Scholar]
- Yokoyama W.M. Natural killer cell receptors. Curr. Opin. Immunol. 1995;7:110–120. doi: 10.1016/0952-7915(95)80036-0. [DOI] [PubMed] [Google Scholar]
- Takei F., Brennan J., Mager D.L. The Ly-49 familygenes, proteins and recognition of class I MHC. Immunol. Rev. 1997;155:67–77. doi: 10.1111/j.1600-065x.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Moretta A., Moretta L. HLA class I specific inhibitory receptors. Curr. Opin. Immunol. 1997;9:694–701. doi: 10.1016/s0952-7915(97)80051-9. [DOI] [PubMed] [Google Scholar]
- Karlhofer F.M., Ribaudo R.K., Yokoyama W.M. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- Yu Y.Y., George T., Dorfman J.R., Roland J., Kumar V., Bennett M. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 1996;4:67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- Mason L.H., Ortaldo J.R., Young H.A., Kumar V., Bennett M., Anderson S.K. Cloning and functional characteristics of murine large granular lymphocyte-1a member of the Ly-49 gene family (Ly-49G2) J. Exp. Med. 1995;182:293–303. doi: 10.1084/jem.182.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M., Navarro F., Bellón T., Llano M., García P., Samaridis J., Angman L., Cella M., López-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A.M., Lanier L.L., Weiss A. Phosphotyrosines in the killer cell inhibitory receptor motif of NKB1 are required for negative signaling and for association with protein tyrosine phosphatase 1C. J. Exp. Med. 1996;184:295–300. doi: 10.1084/jem.184.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko G.W., Tridandapani S., Damen J.E., Liu L., Krystal G., Coggeshall K.M. Negative signaling in B lymphocytes induces tyrosine phosphorylation of the 145-kDa inositol polyphosphate 5-phosphatase, SHIP. J. Immunol. 1996;157:2234–2238. [PubMed] [Google Scholar]
- Ono M., Bolland S., Tempst P., Ravetch J.V. Role of the inositol phosphatase SHIP in negative regulation of the immune receptor by the receptor FcγRIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- Nakamura M.C., Niemi E.C., Fisher M.J., Shultz L.D., Seaman W.E., Ryan J.C. Mouse Ly-49A interrupts early signaling events in natural killer cell cytotoxicity and functionally associates with the SHP-1 tyrosine phosphatase. J. Exp. Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundbäck J., Nakamura M.C., Waldenström M., Niemi E.C., Seaman W.E., Ryan J.C., Kärre K. The α2 domain of H-2Dd restricts the allelic specificity of the murine NK cell inhibitory receptor Ly-49A. J. Immunol. 1998;160:5971–5978. [PubMed] [Google Scholar]
- Kane K.P. Ly-49 mediates EL4 lymphoma adhesion to isolated class I major histocompatibility complex molecules. J. Exp. Med. 1994;179:1011–1015. doi: 10.1084/jem.179.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B.F., Karlhofer F.M., Seaman W.E., Yokoyama W.M. A natural killer cell receptor specific for a major histocompatibility complex class I molecule. J. Exp. Med. 1994;180:687–692. doi: 10.1084/jem.180.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkin A. Optical trapping and manipulation of neutral particles using lasers. Proc. Natl. Acad. Sci. USA. 1997;94:4853–4860. doi: 10.1073/pnas.94.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M., Ryan J.C., Nakamura M.C., Sentman C.L. Ly49A inhibitory receptors redistribute on natural killer cells during target cell interaction. Immunology. 1999;97:341–347. doi: 10.1046/j.1365-2567.1999.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman C.L., Olsson M.Y., Salcedo M., Höglund P., Lendahl U., Kärre K. H-2 allele-specific protection from NK cell lysis in vitro for lymphoblasts but not tumor targetsprotection mediated by α1/α2 domains. J. Immunol. 1994;153:5482–5490. [PubMed] [Google Scholar]
- Fällman E., Axner O. Design for fully steerable dual-trap optical tweezers. Applied Optics. 1997;36:2107–2113. doi: 10.1364/ao.36.002107. [DOI] [PubMed] [Google Scholar]
- Lanier L.L. NK cell receptors. Annu. Rev. Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- Ljunggren H.G., Öhlén C., Höglund P., Yamasaki T., Klein G., Kärre K. Afferent and efferent cellular interactions in natural resistance directed against MHC class I deficient tumor grafts. J. Immunol. 1988;140:671–678. [PubMed] [Google Scholar]
- Negulescu P.A., Krasieva T.B., Khan A., Kerschbaum H.H., Cahalan M.D. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- Donnadieu E., Bismuth G., Trautmann A. Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium. Curr. Biol. 1994;4:584–595. doi: 10.1016/s0960-9822(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Colonna M., Borsellino G., Falco M., Ferrara G.B., Strominger J.L. HLA-C is the inhibitory ligand that determines dominant resistance to lysis by NK1- and NK2-specific natural killer cells. Proc. Natl. Acad. Sci. USA. 1993;90:12000–12004. doi: 10.1073/pnas.90.24.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D.S., Schoon R.A., Robertson M.J., Leibson P.J. Inhibition of selective signaling events in natural killer cells recognizing major histocompatibility complex class I. Proc. Natl. Acad. Sci. USA. 1995;92:6484–6488. doi: 10.1073/pnas.92.14.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.S., Dessing M., López-Botet M., Cella M., Colonna M. Tyrosine phosphorylation of a human killer inhibitory receptor recruits protein tyrosine phosphatase 1C. J. Exp. Med. 1996;184:93–100. doi: 10.1084/jem.184.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]