Abstract
Development of effectors from naive CD4 cells occurs in two stages. The early stage involves activation and limited proliferation in response to T cell receptor (TCR) stimulation by antigen and costimulatory antigen presenting cells, whereas the later stage involves proliferation and differentiation in response to growth factors. Using a TCR-transgenic (Tg+) model, we have examined the effect of aging on effector generation and studied the ability of γc signaling cytokines to reverse this effect. Our results indicate that responding naive CD4 cells from aged mice, compared with cells from young mice, make less interleukin (IL)-2, expand poorly between days 3 to 5, and give rise to fewer effectors with a less activated phenotype and reduced ability to produce cytokines. When exogenous IL-2 or other γc signaling cytokines are added during effector generation, the Tg+ cells from both young and aged mice proliferate vigorously. However, IL-4, IL-7, and IL-15 all fail to restore efficient effector production. Only effectors from aged mice generated in the presence of IL-2 are able to produce IL-2 in amounts equivalent to those produced by effectors generated from young mice, suggesting that the effect of aging on IL-2 production is reversible only in the presence of exogenous IL-2.
Keywords: T cell receptor transgenic, CD4 cells, aging, IL-2, γc-binding cytokines
The in vitro generation of CD4 T cell effectors from naive resting T cells has been extensively described, and many of the factors involved in this response have been defined 1. Naive CD4 cell activation requires TCR stimulation with peptide Ag bound to MHC class II on an APC as well as ligation of T cell coreceptors by costimulatory ligands on the APC 2 3 4 5 6 7. In vitro, this stimulation induces IL-2 production, which then drives proliferation of activated T cells and the differentiation of these cells into effectors. It has been proposed that in vitro effector development involves two steps 8 9, the first step being a TCR-dependent response to Ag and the second step, IL-2 driven expansion and differentiation to effectors. In vitro, effectors are defined as highly differentiated, activated cells able to produce high levels of cytokines rapidly upon restimulation, even in the absence of costimulation. This confers upon effectors the ability to produce cytokines in response to nonprofessional APCs. The key characteristic that distinguishes fully differentiated effectors from activated CD4 T cells that have not yet reached this stage is the production of high levels of cytokines other than IL-2.
Adequate IL-2 secretion by naive T cells is essential, as effector development requires IL-2 at several crucial stages. When isolated CD4 cells are cultured in vitro, the initial amount of IL-2 produced upon T cell stimulation may determine the extent of clonal expansion, because IL-2 is the only cytokine produced in these cultures that can drive CD4 T cell cycle progression. Therefore, the size of the effector population is closely linked to the levels of IL-2 present, as indicated by results from our laboratory 9. IL-2 is also required for the efficient differentiation of responding CD4 T cells into effectors secreting high titers of other cytokines 9. Once effectors are generated, IL-2 promotes their expansion and is required for their survival as long as they remain activated 1 10 11. In the absence of IL-2, effectors rapidly undergo apoptosis. As IL-2 is critical for the generation, expansion, and survival of effector CD4 cells, the reduction in IL-2 that has been reported 12 13 14 15 in the aged could lead to inadequate effector cell development. Perhaps paradoxically, IL-2 is also implicated in driving effector CD4 cells to apoptosis 16, a result we interpret as part of the differentiative effect of IL-2 11.
In aged individuals, two of the most significant defects in CD4 T cell function are a decline in the frequency of CD4+ T cells producing IL-2 and a decreased expression of IL-2Rs 17 18. There is also evidence of a decrease in the early events of signal transduction and decreased cellular proliferation in response to TCR stimulation in both the absence and presence of costimulation 19 20 21 22, but this may be a defect confined to the Ag-experienced T cell population 19. Using a TCR-transgenic (Tg)1 mouse model, we have previously shown a defect in IL-2 production by naive CD4 cells from aged AND TCR-Tg mice (>13 mo old) responding to both specific Ag (pigeon cytochrome c peptide fragment 88–104 [PCCF]) and anti-CD3/anti-CD28 stimulation 23 24. In this report, we show that one effect of decreased IL-2 production by aged naive CD4 T cells is indeed greatly diminished late phase effector expansion and differentiation to effectors. We find, however, that this defect can be corrected by the addition of exogenous IL-2 but that other γc signaling cytokines only partially reverse the defects. This indicates that the aged cells are capable of responding normally to IL-2 and that even the loss of IL-2 production is reversible. It also indicates that only IL-2, and not other γc-binding cytokines, can correct the defect found in naive CD4 cells in aged animals. Thus, defects in naive CD4 T cells in aged animals might be overcome by strategies inducing greater IL-2 availability, leading to vigorous primary responses.
Materials and Methods
Animals.
AND H-2k/k TCR-Tg mice were bred from a C57BL/6 × SJL founder, provided by Dr. S. Hedrick (University of California, San Diego, CA), by successive backcrosses (12N) onto the B10.Br background. These mice express a Vβ3/Vα11 TCR transgene that recognizes PCCF in the context of I-Ek. The mice were housed at either the animal facilities at the Trudeau Institute or the Scripps Research Institute (La Jolla, CA) until their use at 2–4 (young) and 13–16 mo old (aged). Mice with evidence of gross pathology were excluded from the study.
Cell Isolations.
Each experiment was performed at least two times with pooled spleens and peripheral lymph nodes from two young and two or three aged mice. The isolation of spleen cells enriched for CD4 cells has been described previously 23. In brief, the cells were passed through a nylon wool column or a mouse CD4 enrichment column (R & D Systems, Inc.), and the nonadherent cells were stained with Cy-Chrome–anti-CD4, FITC–anti-Vα11, and PE–anti-Vβ3 or APC–anti-CD4, PE–anti-Vα11, biotin–anti-Vβ3, and streptavidin red613. The CD4+Vβ3+Vα11+ cells were sorted using either FACStarPLUS™ or FACSVantage™ (Becton Dickinson). Alternatively, small resting CD4 cells were prepared as described 25 by passage over nylon wool and antibody and complement depletion of CD8+ and class II+ cells followed by Percoll gradient separation. Both methods of CD4 enrichment yielded similar results in all experiments.
Immunofluorescent Staining.
All staining was done at 4°C in PBS with 1% BSA and 0.1% NaN3. The following antibodies and fluorescent reagents were used: Cy-Chrome– and APC–anti-CD4 (clone RM4-5; PharMingen), FITC–anti-CD44 (clone IM7), FITC–anti-CD62L (clone Mel 14), FITC– and PE–anti-Vα11 (clone RR8-1; PharMingen), PE– and biotin–anti-Vβ3 (clone KJ25; PharMingen), FITC–anti-CD25 (IL-2R α chain; clone PC61), FITC–anti-CD122 (IL-2R β chain; clone 5H4), biotin–anti-CD132 (common γ [γc] chain; clone 4G3) streptavidin red613 (GIBCO BRL), streptavidin Per-CP (Becton Dickinson), streptavidin–PE, and streptavidin–FITC (both from Southern Biotechnology). All isotype control antibodies were purchased from PharMingen: FITC–hamster Ig, FITC–rat IgG1, FITC–rat IgG2a, and FITC–rat IgG2b. Flow cytometry was carried out using FACScan™, FACStarPLUS™, or FACSCalibur™ flow cytometers, and the data were analyzed with CELLQuest™ software (all from Becton Dickinson).
Carboxy-Fluorescein Succinimidyl Ester Labeling.
Naive cell populations were stained with the dye CFSE (carboxy-fluorescein succinimidyl ester; Molecular Probes, Inc.) as previously described 26. In brief, cells were resuspended in PBS at 5 × 107 cells/ml. CFSE (1 mM stock) was added to the cell suspension at 1:250 and incubated in a 37°C water bath for 13 min. Cells were then washed two times, recounted, and cultured as described below.
Cell Culture and Effector Generation.
Cells were cultured in RPMI 1640 (GIBCO BRL) supplemented with penicillin (200 μg/ml), streptomycin (200 μg/ml), glutamine (4 mM), 2-ME (50 μM), Hepes (10 mM), and 8% fetal bovine serum (Intergen). DCEK-ICAM, a fibroblast cell line that expresses B7.1 constitutively and is stably transfected with intercellular adhesion molecule (ICAM)-1 and class II MHC (I-Ek) molecules, was used as APC at 2:1 T cell/APC. These cells do not express other costimulatory molecules such as lymphocyte-associated function antigen (LFA) type 1, CD48, or heat-stable Ag.
Recombinant murine cytokines IL-2, IL-4, IFN-γ, and IL-5 were obtained from culture supernatant of X63.Ag8-653 cells transfected with cDNA for the respective cytokines 27. Recombinant murine IL-12 was a gift of Dr. Stanley Wolf (Genetics Institute, Cambridge, MA). For experiments involving the addition of cytokines to T cell proliferation assays, IL-2 (35 U/ml), IL-4 (25 ng/ml), IL-7 (950 U/ml; a gift from Dr. Albert Zlotnik, DNAX, Palo Alto, CA), and human IL-15 (60 ng/ml; R & D Systems, Inc.) were added at the initiation of the cultures.
CD4 effectors were generated by culturing Tg+ CD4 cells (2 × 105 cells/ml) with 5 μM PCCF and mitomycin c–treated (100 μg/ml for 30 min at 37°C) DCEK-ICAM cells (2:1 T cell/APC) in the presence of polarizing cytokines. Th1 effectors were generated with IL-2 (80 U/ml), IL-12 (2 ng/ml), and anti-IL4 (11B11; 10 μg/ml). Th2 effectors were generated with IL-2, IL-4 (200 U/ml), and anti–IFN-γ (XMG1.2; 10 μg/ml). IL-2 effectors were generated with IL-2 (80 U/ml). “No cytokine” effectors were generated in the presence of PCCF/DCEK-ICAM alone. Day 4 effectors were used in all experiments and were restimulated in 1-ml cultures with PCCF/DCEK-ICAM; 24-h supernatants were collected and assayed for cytokine secretion.
DNA Synthesis.
[3H]thymidine incorporation assays to detect DNA synthesis were performed in 96-well plates. Varying numbers of Tg+ CD4 cells (104–5 × 105 cells/ml; 0.2-ml cultures) were incubated along with DCEK-ICAM cells and 5 μM PCCF for 3 d. The cultures were pulsed for the last 16 h with 0.4 μCi [3H]TdR (6.7 Ci/mmol; NEN Research Products), harvested, and counted on a Wallac 1205 Betaplate counter.
Cytokine Detection.
Culture supernatants collected after 24 h of culture or as indicated and were assayed for the presence of IL-2 in a bioassay with NK-3 cells and for IL-4, IL-5, and IFN-γ by ELISA as previously described 23. IL-4 and IFN-γ concentrations are expressed in ng/ml; IL-2 and IL-5 concentrations are expressed in U/ml. 1 U of IL-2 is equal to 1.2 ng.
Statistical Analysis.
Differences in IL-2 production between young and aged effector populations were analyzed by paired Student's t test. Values of P < 0.05 were considered significant.
Intracellular Cytokine Staining.
Cytokine production by effectors was detected by intracellular cytokine staining as previously described 28 29. Effectors (5 × 105 per milliliter) were restimulated overnight with DCEK-ICAM cells (2:1 T cell/APC) with or without 5 μM PCCF. Brefeldin A (Epicentre Technologies; 10 μg/ml final concentration) was added 2 h after culture initiation. 16 h later, cells were collected and surface stained for CD4 and Tg+ expression as described above. The cells were then divided into two tubes, washed, and fixed in 75 μl 4% paraformaldehyde plus 25 μl PBS containing 10 μg/ml Brefeldin A and incubated for 20 min at room temperature. The tubes were washed once with PBS, resuspended in 50 μl saponin buffer (PBS containing 1% fetal bovine serum, 0.1% NaN3, and 0.1% saponin, pH 7.4–7.6) containing anti–mouse IL-2–FITC (clone S4B6; PharMingen) or isotype control (FITC–IgG2a; PharMingen) and incubated for 30 min at room temperature. All samples were then washed with PBS and analyzed on a FACScan™ or FACSCalibur™ cytometer.
Results
To analyze the impact of the low IL-2 levels produced by naive CD4 cells from aged mice and to determine whether the defects could be reversed simply by providing IL-2, we used the AND TCR-Tg model to follow effector generation and the function and phenotype of the effectors generated in detail. As almost all Tg+ cells are naive and because we used peptide Ag plus exogenous fibroblast APCs to stimulate effector generation, we can assess the intrinsic defect(s) due to age on naive T cell responses without the complications of age effects on other T cells or APCs.
Effect of Decreased IL-2 Production on Late Phase Proliferation of Tg+ CD4 Cells from Aged Mice.
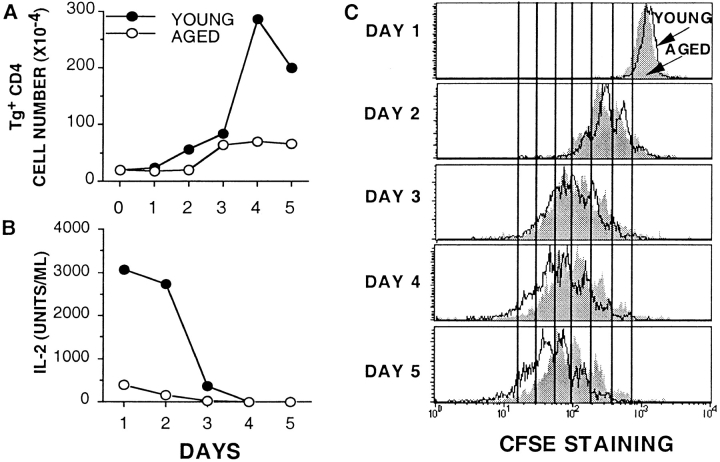
We have previously shown that when naive young and aged Tg+ CD4 cells were stimulated with PCCF and DCEK-ICAM, the young cells synthesized DNA at a higher rate in a 3-d [3H]TdR incorporation assay 23. To pinpoint when the response of aged naive CD4 T cells becomes defective, similar numbers (2 × 105) of young and aged naive Tg+ CD4 cells were stimulated with PCCF/DCEK-ICAM, and the number of cells on each day of a 5-d culture was determined (Fig. 1 A). Both young and aged Tg+ CD4 populations expanded at a similar rate up to day 3 (early phase proliferation). After day 3, the young cells showed a large burst of expansion that was not seen in the aged cultures (late phase proliferation). This burst of expansion resulted in a greatly increased number of cells by days 4 and 5 in the young cultures when compared with the aged cultures.
Figure 1.
Kinetics of decreased expansion and IL-2 production by aged Tg+ CD4 cells. Tg+ CD4 cells from young (•) and aged (○) mice (2 × 105 cells/well) were stimulated with DCEK-ICAM APCs at 2:1 T cell/APC and 5 μM PCCF in a 24-well plate. At each time point, cell counts were performed (A) and supernatants were collected and assayed for IL-2 in a bioassay (B). Data is representative of five experiments. (C) Tg+ CD4 cells (2 × 105 cells/ml) from young (open histograms) and aged (shaded histograms) mice were labeled with the dye CFSE and then stimulated with DCEK-ICAM APCs at 2:1 T cell/APC and 5 μM PCCF in a 24-well plate. At each time point, cells were collected and assayed for CFSE expression on a flow cytometer. Each histogram is gated on Vβ3+CD4+ propidium iodide–negative cells. Data is representative of three experiments.
We suspected that this lack of late phase proliferation of the aged cultures could potentially be attributed to decreased IL-2 production in these cultures. Fig. 1 B shows the IL-2 recovered in supernatants of young and aged cultures on each day. The young cultures contain large quantities (∼3,000 U/ml) of IL-2 on days 1 and 2 that is then consumed by day 3, just as the cells begin to undergo rapid expansion. In contrast, the supernatants of cultures of aged CD4 T cells contain only small quantities of IL-2 (<500 U/ml), and expansion does not continue beyond day 3, supporting the concept that availability of IL-2 may be the critical factor in driving later expansion.
The decreased late phase proliferation of aged cells can also be visualized by labeling with the dye CFSE. CFSE is distributed equally between cells upon each cell division, resulting in the sequential halving of fluorescence intensity with each round of division. This sequential loss of fluorescence is visualized as distinct peaks when analyzed by flow cytometry 26. Fig. 1 C shows a kinetic analysis of young and aged CFSE-labeled Tg+ CD4 cells stimulated with Ag plus APC. On days 1–3, responding CD4 cells from both young and aged populations have undergone similar numbers of cell divisions, as shown by similar CFSE fluorescence profiles. On day 1, no divisions have yet occurred; on day 2, both young and aged populations show three peaks of division; and on day 3, both show four peaks of division. Importantly, these profiles also show that all, not just a subset, of both the young and aged Tg+ CD4 cells undergo several rounds of division in response to stimulation. If no Ag is present, neither young nor aged Tg+ CD4 cells divide (i.e., profile remains a single peak with high levels of CFSE staining; data not shown). This data suggests that all of the aged naive Tg+ CD4 cells are capable of being activated to respond and divide. On days 4 and 5, the recovered cells from cultures of young naive CD4 cells continue to divide (up to six peaks of division), whereas the aged cells seem to stop dividing after day 3 (day 4 and 5 profiles look very similar to those of day 3). These two additional cell divisions in the young population translate theoretically into a fourfold greater expansion, as is seen in Fig. 1 A. These results further confirm the observation that both young and aged Tg+ CD4 cells can initially proliferate (days 1–3) in response to Ag stimulation, but the aged cells are unable to sustain late phase proliferation.
Addition of IL-2 Restores Late Phase Expansion of Tg+ CD4 Cells from Aged Mice.
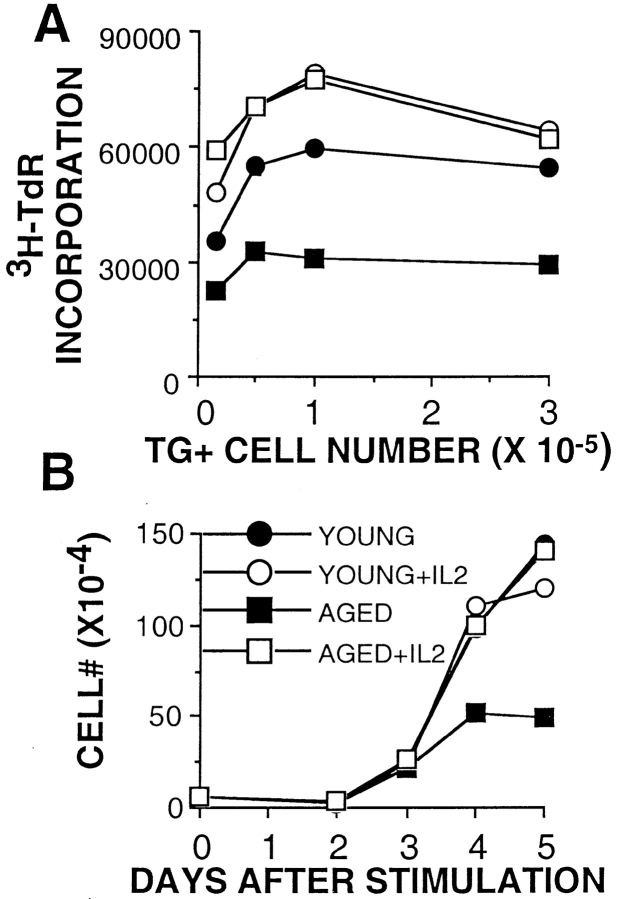
As IL-2 levels were significantly decreased in the cultures of aged Tg+ CD4 cells, we examined the ability of aged Tg+ CD4 cells to expand in the presence of exogenous IL-2. As shown in our previous experiments, young CD4 cells synthesized DNA at a twofold higher rate on day 3 than aged cells (as measured by [3H]TdR incorporation) when no exogenous IL-2 was added (Fig. 2 A, filled symbols). When IL-2 was added on day 0 to the Ag-stimulated cultures of naive young and aged CD4 cells, both effector populations incorporated radiolabel to the same extent (Fig. 2 A, open symbols). Fig. 2 B shows a kinetic analysis of cell counts from young and aged Tg+ CD4 cell cultures with and without exogenous IL-2 through day 5. As in the experiment shown in Fig. 1 A, all cultures expanded similarly through day 3. On days 4 and 5, both young and aged cells cultured with IL-2 expanded to the same extent, as did young cells cultured with Ag/APC alone. Only the aged Tg+ CD4 cells cultured without added IL-2 showed greatly reduced expansion. These results indicate that the aged Tg+ CD4 cells are capable of proliferating in response to Ag in a manner comparable to young cells if they are provided with adequate amounts of exogenous IL-2. This suggests that there is no defect in signaling through the IL-2R in these aged naive T cells.
Figure 2.
Addition of IL-2 restores the proliferative capacity of aged Tg+ CD4 cells. (A) 3-d [3H]TdR incorporation assay of young (circles) and aged (squares) Tg+ CD4 cells stimulated with DCEK-ICAM APCs and 5 μM PCCF with (open symbols) and without (filled symbols) exogenous IL-2. [3H]TdR was added for the last 16 h of culture. Data is representative of three experiments. (B) Tg+ CD4 cells from young (circles) and aged (squares) mice were stimulated with DCEK-ICAM APCs at 2:1 T cell/APC and 5 μM PCCF with (open symbols) or without (filled symbols) exogenous IL-2 in a 24-well plate. At each time point, cell counts were performed. Data is representative of three experiments.
Effect of Other γc Signaling Cytokines.
As IL-2 can induce naive aged Tg+ cells to proliferate and differentiate like young cells, we examined the effect of other common receptor γ chain signaling cytokines on proliferation of young and aged cells to determine if the important components of IL-2 signaling were related only to γc or unique to the IL-2R chains. IL-2, IL-4, IL-7, and IL-15 were titrated over a broad range to determine the concentration for optimal proliferation in our assay (data not shown). The optimal doses were then added to cultures of young and aged naive CD4 T cells, and [3H]TdR incorporation was determined at day 4. Fig. 3 A, like the experiment shown in Fig. 2, shows that aged cells are dividing less but that addition of exogenous IL-2 can induce aged Tg+ cells to synthesize DNA to the same extent as young cells. IL-15 and IL-4 can induce similarly high levels (greater than threefold) of DNA synthesis of aged cells as are observed with IL-2. IL-7 can also somewhat enhance proliferation (about twofold) of aged cells but has little effect on young cells. The expansion and IL-2 production of young and aged effectors generated in the presence of these cytokines was also examined. Fig. 3 B shows the fold expansion of young and aged cells from day 0 to 4 of culture. Young cells expanded two to three times as much as aged cells when no cytokines were added, whereas expansion of both young and aged cells was equivalent when IL-2 was present. IL-4, IL-7, and IL-15 also augment the expansion of aged cells to some extent. IL-4, however, also enhances young CD4 T cell expansion so that young cell expansion remained greater than that of aged CD4 T cells. The effect of IL-15 on expansion was equivalent to that of IL-2.
Figure 3.
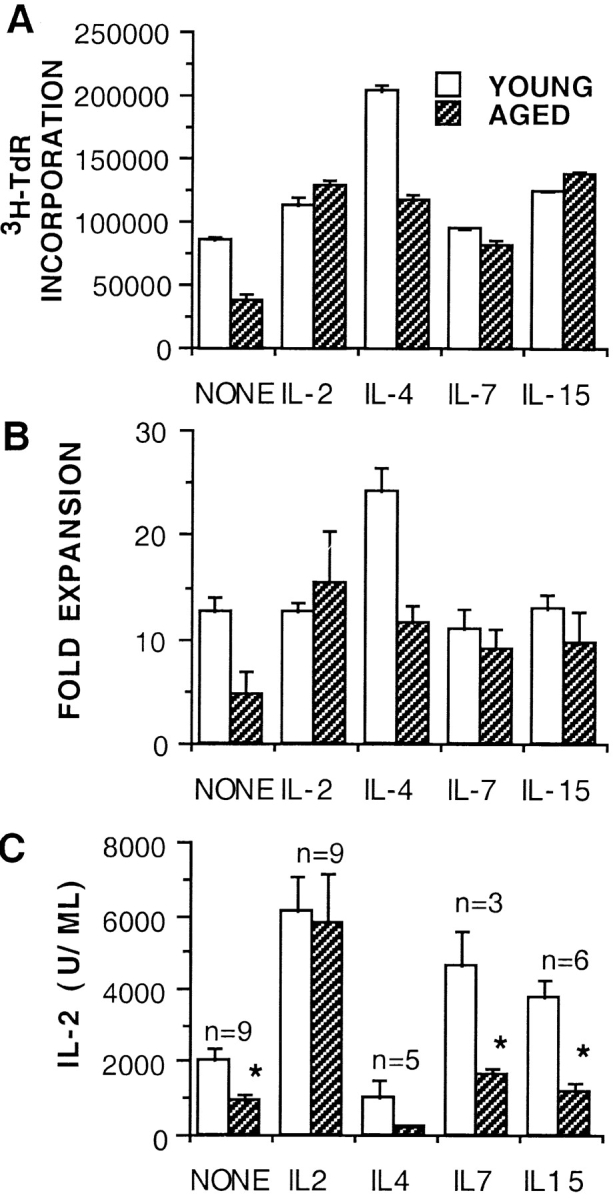
Enhancement of proliferation of aged naive Tg+ CD4 cells by γc signaling cytokines. (A) Optimum concentrations of IL-2 (35 U/ml), IL-4 (25 ng/ml), IL-7 (950 U/ml), and IL-15 (60 ng/ml) were added at the initiation of cultures of young and aged naive Tg+ cells (2 × 104/well) with DCEK-ICAM APCs at 2:1 T cell/APC and 5 μM PCCF in 96-well plates. Cultures were pulsed with [3H]TdR on day 3 and harvested 18 h later. Data presented is mean of triplicates ± SE and is representative of two experiments. (B) Fold expansion of young and aged day 4 Tg+ CD4 effectors generated with DCEK-ICAM APCs (2:1 T cell/APC) and PCCF without added cytokines (NONE) or optimal concentrations of IL-2, IL-4, IL-7, or IL-15. Data presented is mean of three separate experiments ± SE. (C) IL-2 production by young and aged Tg+ CD4 effectors from B. On day 4, 2.5 × 105 effectors were restimulated for 24 h with DCEK-ICAM and PCCF. Supernatants were assayed for IL-2 content (expressed in units per milliliter); Data presented is the mean ± SE of the number of experiments (n). *Statistically significant difference between the young and aged populations (P < 0.05).
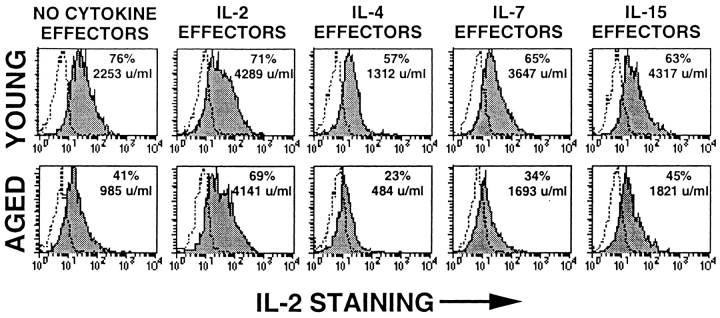
Because the most important outcome of the primary CD4 T cell response is the generation of effectors, we also examined the function of recovered effectors by assaying for IL-2 production upon restimulation with Ag/APC. Fig. 3 C shows mean ± SE of IL-2 secretion from all experiments performed, and Fig. 4 shows a representative experiment with both intracellular staining and cytokine secretion. IL-2 is the only cytokine that can cause an increase in IL-2 production by aged effectors to the levels seen for young cells. IL-4, IL-7, and IL-15 have little or no enhancing effect on IL-2 production by aged effectors. As IL-4 causes differentiation to a Th2 cytokine pattern, the effect of IL-4 on IL-2 production is negative in young as well as aged CD4 T cells. Despite the ability of IL-7 and IL-15 to restore proliferation, neither cytokine restores levels of IL-2 production by the aged cells to those achieved by the young CD4 T cells. These results suggest that IL-2 causes generation of effectors both by inducing proliferation and, via a separate mechanism, upregulation of IL-2R expression, whereas IL-4, IL-7, and IL-15 can support proliferation but not all aspects of further differentiation.
Figure 4.
Intracellular cytokine staining of young and aged effectors. Effectors were generated from young (top) and aged (bottom) Tg+ CD4 cells with DCEK-ICAM APCs (2:1 T cell/APC) and PCCF alone (NO CYTOKINE EFFECTORS) or with optimum concentrations of added exogenous IL-2, IL-4, IL-7, or IL-15. On day 4, 5 × 105 effectors were restimulated for 16 h with DCEK-ICAM and PCCF. Brefeldin A was added 2 h after culture initiation for intracellular staining; parallel cultures generated supernatants that were assayed for IL-2 content (expressed in U/ml). Shaded histograms represent anti–IL-2 FITC staining for Tg+ CD4 gated effectors; dotted lines represent isotype controls for the anticytokine antibodies. Data is representative of three experiments.
Phenotype of Young and Aged Tg+ CD4 Cells.
We also examined the cell surface phenotype of effectors generated from Tg+ CD4 cells of young and aged mice in the absence of exogenous cytokines (no cytokine effectors) and presence of exogenous IL-2, IL-4, IL-7, or IL-15 in addition to Ag/APC. Previously, we reported 23 that the Tg+ CD4 cells obtained from both aged and young mice express a naive phenotype (Fig. 5 A, bottom), i.e., they express low levels of CD44 and high levels CD62L (L-selectin) and do not express CD25 (IL-2Rα). As shown in Fig. 5 A, the aged no cytokine effectors (Fig. 5, shaded histograms) express a partially activated phenotype (CD44hiCD62LhiCD25lo) by day 4 after stimulation, whereas the young no cytokine effectors (Fig. 5, open histograms) express a more differentiated phenotype (CD44hiCD62Llower CD25hi). The addition of IL-2 at the initiation of the day 4 cultures produced IL-2 effectors from aged and young mice that expressed a similar and fully differentiated activated phenotype (CD44hiCD62LlowerCD25hi). This again supports the role of IL-2 in the full differentiation to an effector stage 9. Both young and aged IL-4 effectors showed a more pronounced downregulation of CD62L compared with the other effectors, also consistent with a more differentiated state. But IL-4, IL-7, and IL-15 cannot induce upregulation of CD25 on the aged effectors. Therefore, effectors that were generated under conditions where the level of IL-2 was limiting (aged no cytokine effectors and IL-4, IL-7, and IL-15 effectors) expressed lower levels of CD25, whereas effectors that were generated under conditions where IL-2 was not limiting, either due to the adequate production of IL-2 by the effectors (all groups of young effectors) or the addition of exogenous IL-2 to the cultures (aged IL-2 effectors), expressed high levels of CD25 with no apparent age-related differences.
Figure 5.
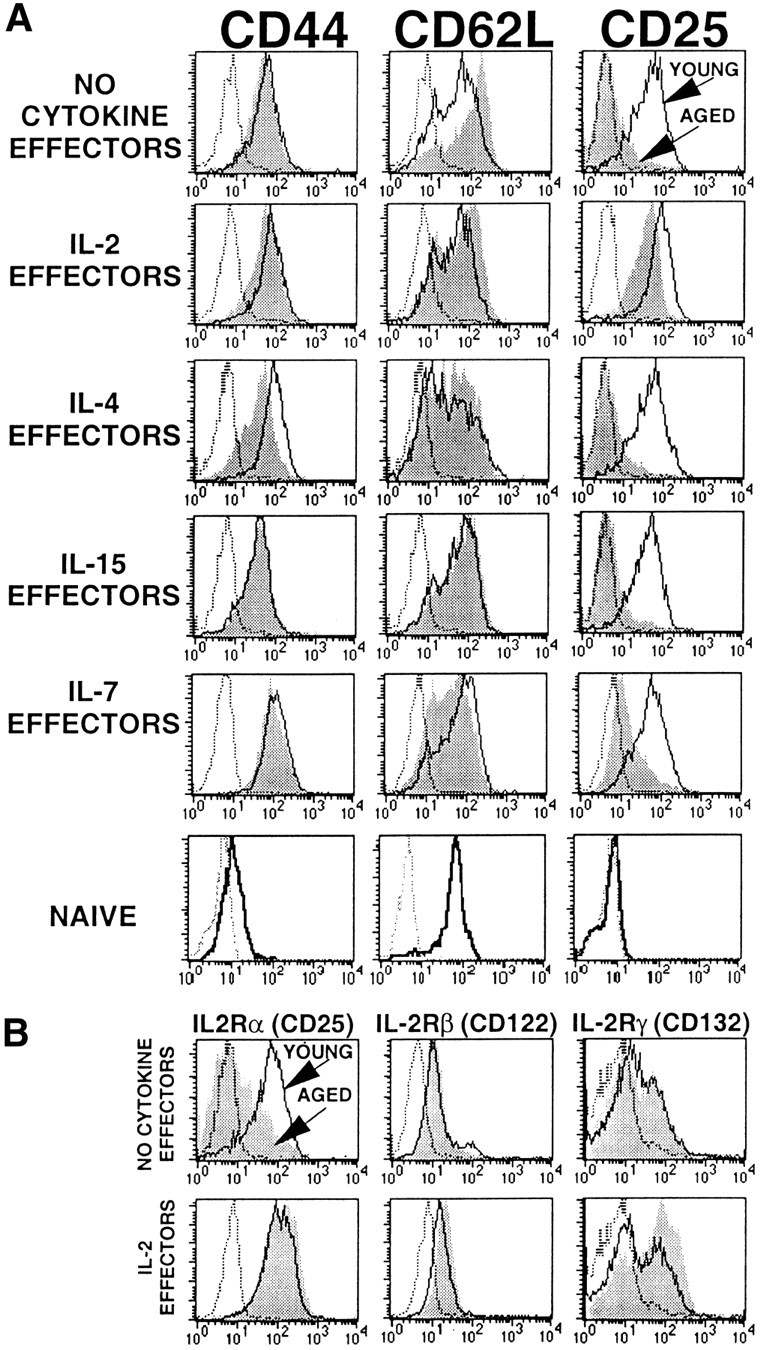
Phenotype of young and aged Tg+ CD4 effectors. Effectors were generated from young and aged Tg+ CD4 cells with DCEK-ICAM APCs (2:1 T cell/APC) and PCCF alone (NO CYTOKINE EFFECTORS) or with added optimum concentrations of exogenous IL-2, IL-4, IL-7, or IL-15. (A) Day 4 young (open histograms) and aged (shaded histograms) effectors were stained for CD4 and TCR transgene expression as well as CD44, CD62L, and CD25. Naive Tg+ CD4 cells (bottom row) and isotype controls (dashed gray lines) are also shown. (B) Day 4 young (open histograms) and aged (shaded histograms) no cytokine and IL-2 effectors were stained for expression of IL-2R α, β, and γ chains. All histograms are gated on Tg+ CD4 cells. Data is representative of five experiments.
To further investigate IL-2R expression, we examined the expression of all three chains of the receptor. Fig. 5 B shows IL-2Rα, -β, and -γc chain expression on young and aged no cytokine and IL-2 effectors. As shown in Fig. 5 A, α chain (CD25) expression on aged no cytokine effectors is lower than on young no cytokine effectors and is upregulated on the aged effectors when they are provided with exogenous IL-2. There are no age-related differences in β (CD122) or γ chain (CD132) expression on either no cytokine or IL-2 effectors.
Cytokine Production by Young and Aged Tg+ Th1 and Th2 CD4 Effectors.
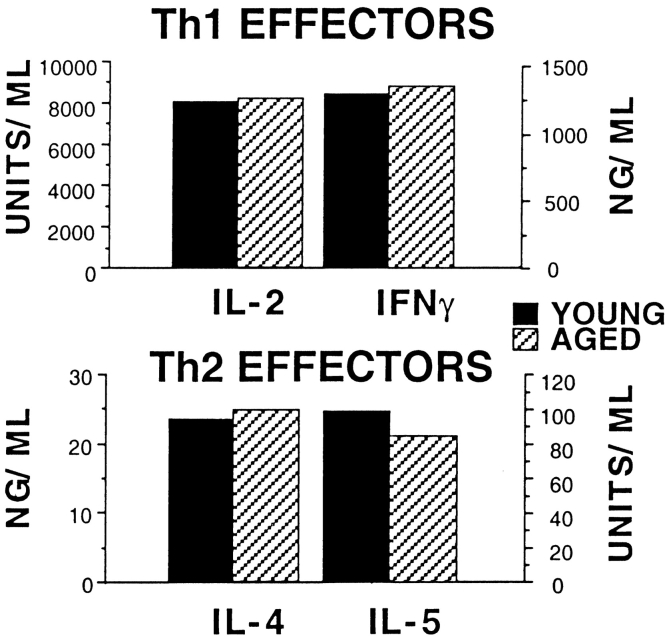
To further evaluate the functional impact of aging, we examined whether naive CD4 T cells from aged mice could become polarized effectors in the presence of polarizing cytokines and blocking antibodies. Polarized Th1 and Th2 effector populations can be generated from naive Tg+ CD4 cells in vitro by the addition of cytokines and anticytokine blocking antibodies in addition to Ag and APC. For optimum polarization, Th1 effectors require IL-2, IL-12, and anti-IL-4, whereas Th2 effectors require IL-2, IL-4, and anti–IFN-γ. Although not shown here, the expansion by young versus aged naive Tg+ CD4 cells cultured with Th1 or Th2 polarizing conditions were comparable. When Tg+ Th1 and Th2 effectors are restimulated, they secrete high levels of specific cytokines. Th1 effectors secrete IL-2 and IFN-γ, whereas Th2 effectors secrete IL-4, IL-5, and a small amount of IL-2. To determine whether clear polarization could occur with aged CD4 T cells, we generated effectors from both young and aged CD4 T cells in the presence of cytokines (including IL-2) and blocking antibodies. When we restimulated Tg+ Th1 and Th2 effectors after 4 d of culture, effectors generated from both young and aged populations secreted similar levels of the appropriate cytokines. Fig. 6 shows that Tg+ Th1 effectors produced IL-2 and IFN-γ and Tg+ Th2 effectors produced IL-4 and IL-5 with no age-related deficiencies. These results demonstrate that aged naive Tg+ CD4 cells are just as capable of differentiating into highly polarized Th1 and Th2 effectors as young Tg+ cells, given the appropriate polarizing conditions and adequate amounts of IL-2.
Figure 6.
Cytokine production by young and aged Th1 and Th2 effectors. Effectors were generated from young and aged Tg+ CD4 cells with DCEK-ICAM APCs (2:1 T cell/APC), PCCF, and Th1 or Th2 polarizing cytokines and anticytokine antibodies (see Materials and Methods). All supernatants (24 h) were collected from 3 × 105 day 4 effectors restimulated with DCEK-ICAM and PCCF and assayed for IL-2, IL-4, IL-5, and IFN-γ. Data is representative of three experiments.
Rescue of Polarized Effector Generation with IL-15.
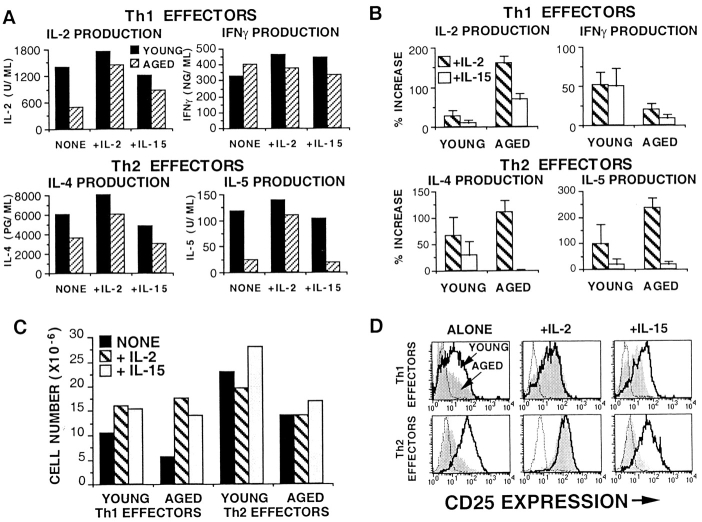
IL-15 acts by binding to a three-chain receptor composed of the IL-2Rβ and IL-2Rγ chains and a unique α chain. To see if there was any difference in the ability of IL-2 and IL-15 to support effector generation of aged CD4 T cells, we tested the ability of IL-15 to substitute for IL-2 in the differentiation of young and aged Th1 and Th2 effectors. Polarized effectors were generated in the presence of polarizing cytokines and antibodies (IL-12 and anti–IL-4 for Th1 and IL-4 and anti–IFN-γ for Th2) alone, with exogenous IL-2, or with exogenous IL-15. Fig. 7 A shows cytokine production data from a representative experiment, whereas Fig. 7 B shows the mean percent increase ± SE in cytokine production in cultures generated with added IL-2 or IL-15 over that seen with polarizing cytokines alone for three separate experiments. IL-2 and IFN-γ production is from Th1 effectors, and IL-4 and IL-5 production is from Th2 effectors after restimulation with Ag and APC. Th1 effectors from CD4 T cells of young mice make high levels of IL-2 and IFN-γ regardless of whether IL-2 or IL-15 is added. Aged Th1 effectors make enhanced levels of IL-2 (a 150% increase) when exogenous IL-2 is added, compared with a 70% increase when IL-15 is added; IFN-γ production by Th1 cells from aged mice is for the most part unaffected by exogenous IL-2 or IL-15. Th2 effectors from CD4 T cells of young mice produce IL-4 and IL-5, which can be enhanced up to 100% when IL-2 is added. Importantly, the production of high levels of IL-4 and IL-5 by aged Th2 effectors is uniquely IL-2 dependent (a 110% increase for IL-4 and a 235% increase for IL-5) and cannot be induced by the presence of IL-15 (a 0.7% increase for IL-4 and a 20% increase for IL-5).
Figure 7.
IL-15 cannot substitute for IL-2 in promoting efficient Th1/Th2 differentiation of aged effectors. Young and aged Th1 and Th2 effectors were generated without exogenous IL-2, with IL-2 (+IL-2; hatched bars), or with IL-15 (+IL-15; shaded bars) in addition to polarizing cytokines and blocking antibodies. (A) Cytokine production by young (solid bars) and aged (hatched bars) cultures generated with no exogenous IL-2 (NONE), with IL-2 (+IL-2), or with IL-15 (+IL-15). IL-2 and IFN-γ production graphs are data from Th1 effectors; IL-4 and IL-5 production graphs are data from Th2 effectors. All supernatants (24 h) were collected from 3 × 105 day 4 effectors restimulated with DCEK-ICAM and PCCF and assayed for IL-2, IL-4, IL-5, and IFN-γ. Data is representative of three experiments. (B) Percent increase in cytokine production by cultures with IL-2 or IL-15 above the cultures with polarizing conditions alone. IL-2 and IFN-γ production graphs are data from Th1 effectors; IL-4 and IL-5 production graphs are data from Th2 effectors. Data are the means ± SE of the three experiments represented in A. (C) Tg+ CD4 effector recovery from effectors generated in A. 106 naive CD4 T cells were cultured for 4 d with DCECK-ICAM and PCCF plus polarizing cytokines alone (solid bars) or with IL-2 (hatched bars) or IL-15 (shaded bars). (D) CD25 expression by day 4 young (open histograms) and aged (shaded histograms) Th1 and Th2 effectors generated in A. Histograms are gated on Tg+ CD4 T cells. Data is representative of three experiments.
The cell recoveries of the effectors generated in Fig. 7 A are shown in Fig. 7 C. Both IL-2 and IL-15 can enhance the expansion of young and aged Th1 effectors but have little effect on Th2 cells, because the Th2 effectors expand due to the presence of IL-4. These results indicate that even though both IL-4 and IL-15 can induce high levels of proliferation of aged Tg+ CD4 cells, other additional factors are necessary to induce differentiation into highly polarized Th1 or Th2 effectors in vitro. Fig. 7 D shows CD25 expression by the young and aged effectors from Fig. 7 A. As expected, only the addition of IL-2 can increase CD25 expression by aged effectors (both Th1 and Th2) to the levels seen on young effectors. Aged Th1 and Th2 effectors generated with polarizing cytokines alone or in the presence of IL-15 express lower levels of CD25 compared with young effectors.
Discussion
During a primary response, generation of large numbers of CD4 effectors able to secrete high titers of cytokines is critical in order to quickly clear the immunogen. Using a TCR-Tg mouse model, we have found several profound alterations in CD4 effector cell generation that are associated with aging, and our results suggest that the low production of IL-2 by aged naive cells is largely (or totally) responsible for these deficiencies. Aged CD4 T cell defects included lower production of IL-2, loss of late phase expansion, and failure to differentiate into effectors that express phenotypic markers associated with that stage and that secrete high levels of cytokines. Importantly, provision of exogenous IL-2 both supports the expansion and differentiation of aged naive CD4 cells and leads to generation of effectors that themselves are capable of optimum IL-2 production. Furthermore, we show here that whereas both IL-2 and IL-15 as well as IL-4 and IL-7 can enhance the expansion of aged CD4 T cells, only IL-2 can support the efficient generation of polarized Th2 effectors, indicating a unique role for IL-2 signaling through the high affinity IL-2R in effector development.
Aged Tg+ CD4 cells expand less than young cells during the 5-d period of in vitro effector generation (Fig. 1A–C). The early stage of expansion (days 1–3) is quite similar in both the young and aged cultures, whereas later expansion (days 4 and 5) is three to five times greater in the cultures of young cells. This is most dramatically observed by flow cytometric analysis of CFSE-labeled young and aged Tg+ CD4 cells (Fig. 1 C), in which we can actually visualize that the aged cells do not divide after day 3, whereas the young cells continue dividing. This effect is mirrored in the expansion of cells, which continues after day 3 in young but not aged populations. Moreover, the aged CD4 effectors expressed decreased levels of CD25 (IL-2Rα). We suggest that the early stage of proliferation is more dependent upon Ag-induced TCR stimulation, and the latter stage is more dependent upon IL-2, which is present in greater amounts in the young cultures. The phenotype of the day 4 effectors generated from aged naive cells was between that of a naive cell and a day 4 effector from young mice, i.e., the aged effector cells had a less differentiated phenotype that had high levels of CD44 but did not substantially downregulate CD62L and did not upregulate IL-2R (CD25) expression. This suggests that cytokines (IL-2 and perhaps others) acting midway (days 2–3) in the primary response (when IL-2 disappears from cultures of aged cells) are required for full differentiation into effectors. Effectors generated from these Tg+ CD4 cells from aged mice produced less IL-2, and the frequency of effector cells secreting IL-2, as detected by intracellular staining, was also significantly reduced in the aged, supporting dependence on the continued presence of IL-2 for efficient effector generation.
These results thus support a large impact of aging on CD4 effector development but also suggest that the spectrum of deficiencies found in effector generation of aged CD4 T cells may be attributable largely or totally to the decreased secretion of IL-2 upon initial TCR stimulation. In vitro, IL-2 supports the progression of activated T cells through the cell cycle, the regulation of the differentiation of T cell effectors, and the differentiation of CD4 T cells so they become susceptible to Fas/FasL-induced activation-induced cell death 30 31 32. Although other cytokines that signal through the γc chain may substitute for IL-2, IL-2 is the only γc chain–binding cytokine produced when naive CD4 T cells are stimulated in vitro and is thus solely responsible under these circumstances where no other T cells are present and other cell populations are limited. Therefore, the finding that IL-2 secretion by CD4 Tg+ cells upon TCR stimulation is markedly reduced in the aged is predicted to have profound effects on the generation of effector cells and the ensuing effector response. Moreover, as IL-2 regulates its own receptor expression 33 34 35, the effects of any reduction in IL-2 production in the early stages of an immune response may be amplified enormously. It then becomes important to know whether these deficiencies may be overcome simply by the addition of IL-2 or other cytokines or if other intrinsic defects in the effector cells' ability to respond to IL-2 are present.
When aged CD4+ Tg+ effectors are generated in the presence of IL-2 (IL-2 effectors, Th1 and Th2 effectors), they become phenotypically (Fig. 4) and functionally (Fig. 5 Fig. 6 Fig. 7) similar to effectors derived from younger mice. In addition to upregulating CD44, they upregulate CD25 expression and downregulate CD62L. They also expand at the same rate and to the same extent as seen in cultures of young Tg+ cells (Fig. 2). When aged effectors generated in the presence of IL-2 are restimulated with Ag/APC, they secrete levels of cytokines that are the same as young effectors (Fig. 5 Fig. 6 Fig. 7). Additionally, similar proportions of IL-2 effectors in both the young and aged populations are actively secreting IL-2 (Fig. 4), and similar amounts of cytokines are secreted on a per-cell basis. These findings suggest that the major defect in effector cell generation in the aged is the inability to secrete sufficient IL-2 levels to sustain cell expansion and induce further differentiation to fully functional effector cells. However, the addition of sufficient levels of IL-2 during effector cell generation abrogates these age-related differences. Once adequately differentiated effectors are generated, it is not surprising that the effector progeny are capable of high levels of cytokine production, based on recent publications reporting the heritability of differential gene methylation patterns (epigenetic remodeling) that occurs during effector generation 36 37 38. This model proposes that activation and effector generation of T cells involves demethylation and increased accessibility of the cytokine gene locus (IL-2 in this case) to transcription factors as a consequence of signaling from the appropriate cytokine receptor. This remodeling is permanent and heritable, thus explaining our finding that aged IL-2 effectors generated in the presence of high levels of IL-2 can produce high levels of IL-2 upon restimulation. Experiments to test this hypothesis on young and aged effectors are planned.
When IL-2 remains low during effector generation in cultures of aged CD4 T cells, only a fraction of the potential effector generation is realized. It is not clear if this partially activated population can later be recruited into the responding population, as a lack of IL-2 during effector cell generation has been reported to lead to anergy in some models 39 40. The presence of a significant population of nonresponding or poorly responding T cells in aged individuals is well documented 12 19 41 42 43 44, and these cells have been shown to display a phenotype similar to the aged no cytokine effectors generated in our model. Furthermore, in IL-2−/−, IL-2Rα−/−, and IL-2β−/− mice 45 46 47, many CD4 T cells express an Ag-experienced phenotype but appear to be nonresponsive to TCR stimulation. There is also impaired in vivo peripheral deletion of these Ag-experienced cells in both models. This has lead to the hypothesis that in the absence of IL-2 induced signals, stimulation of T cells fails to activate the death pathway, which leads to the accumulation of Ag-experienced anergic cells in the periphery, and we suggest that this is what may be occurring in aged individuals.
During an in vivo response, other cytokines that signal through the γc chain may be present and could possibly replace IL-2 and restore function of aged naive cells. The experiments shown in Fig. 3 demonstrate that other cytokines that signal through the γc chain (IL-15, IL-4, and IL-7) can indeed increase the proliferative capacity of naive aged Tg+ CD4 cells. It seems likely that these effects are via an IL-2–independent mechanism that does not involve increased IL-2 production or upregulation of IL-2Rα expression. Most interesting, from a therapeutic viewpoint, is the effect of IL-15. IL-15 was able to support not only enhanced expansion of aged CD4 T cells without IL-2Rα expression but also development of Th1 polarized effectors. However, although IL-2 addition allows aged CD4 T cells to develop into polarized effectors under the influence of polarizing cytokines, IL-15 is unable to support optimum development of Th2 effectors. These data support the hypothesis that IL-2, acting through the high affinity IL-2R, plays a unique role in Th2 effector generation and that lower IL-2 production by aged CD4 T cells results in a defect in both expansion of responding naive cells and efficient generation of Th2-polarized effectors.
Recent reports have shown that adjuvants such as LPS, poly I:C, and nonvertebrate DNA can induce type 1 IFN, which then induces APCs to secrete IL-15 48 49. If these adjuvants can induce adequate IL-15 levels in vivo, they may be able to stimulate enhanced proliferation of activated T cells in aged individuals, which could lead to the development of more efficacious vaccines. Even though aged effectors generated in the presence of IL-15 in vitro are not as differentiated as those generated in the presence of IL-2, the increased expansion of these aged “IL-15 effectors” may boost the in vivo aged immune response sufficiently to gain an advantage over an invading pathogen. However, based on our results, one might expect defects in generation of Th2 polarized effectors and in response to pathogens cleared by Th2-dependent mechanisms. Experiments are currently underway in our laboratory to examine the in vivo potential of this approach.
In conclusion, it is clear that the decline in IL-2 production by naive CD4 T cells that occurs with aging has a dramatic negative impact in the generation of T cell effectors in vitro. Assuming that such a decrease also occurs in vivo, this would lead to decreased and ineffective responses, thus helping to explain the increased incidence of mortality and infection with aging. In fact, preliminary studies suggest that aged naive CD4 T cells also respond poorly in vivo (Haynes, L., and S.L. Swain, unpublished data). Moreover, it is conceivable that any cell that partially undergoes a limited response and receives subthreshold signals from IL-2 may become anergic, and these anergic cells (which do not undergo apoptosis) could potentially accumulate with aging and fill the peripheral immune system with nonresponsive lymphocytes. However, the ability of exogenous IL-2 and other γc-binding cytokines to reverse the proliferative defect of aged CD4 cells in vitro raises the possibility that adjuvants that work to induce inflammatory cytokines (IL-15 or other γc reacting cytokines) might boost the primary response and restore effector generation in the elderly.
Acknowledgments
This work was supported by National Institutes of Health grant AG01743.
Footnotes
1used in this paper: ICAM, intercellular adhesion molecule; PCCF, pigeon cytochrome c peptide fragment 88–104; Tg, transgenic
References
- Swain S.L., Croft M., Dubey C., Haynes L., Rogers P., Zhang X., Bradley L.M. From naive to memory T cells. Immunol. Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- DeBenedette M.A., Chu N.R., Pollok K.E., Hurtado J., Wade W.F., Kwon B.S., Watts T.H. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphomas by cAMP. J. Exp. Med. 1995;181:985–992. doi: 10.1084/jem.181.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata T., Agematsu K., Kameoka J., Schlossman S.F., Morimoto C. CD27 is a signal-transducing molecule involved in CD45RA+ naive T cell costimulation. J. Immunol. 1994;153:5422–5432. [PubMed] [Google Scholar]
- Liu Y., Jones B., Aruffo A., Sullivan K.M., Linsley P.S., Janeway C.A., Jr. Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J. Exp. Med. 1992;175:437–445. doi: 10.1084/jem.175.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Koyanagi M., Okada H., Takanshi T., Wong Y.W., Williams A.F., Okumura K., Yagita H. CD48 is a counterreceptor for mouse CD2 and is involved in T cell activation. J. Exp. Med. 1992;176:1241–1249. doi: 10.1084/jem.176.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N.K., Klussman K., Linsley P.S., Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J. Immunol. 1992;148:1985–1992. [PubMed] [Google Scholar]
- Dubey C., Croft M., Swain S.L. Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J. Immunol. 1995;155:45–57. [PubMed] [Google Scholar]
- Vella A.T., Mitchell T., Groth B., Linsley P.S., Green J.M., Thompson C.B., Kappler J.W., Marrack P. CD28 engagement and proinflammatory cytokines contribute to T cell expansion and long-term survival in vivo. J. Immunol. 1997;158:4714–4720. [PubMed] [Google Scholar]
- Rogers P.R., Huston G., Swain S.L. High antigen density and IL-2 are required for generation of CD4 effectors secreting Th1 rather than Th0 cytokines. J. Immunol. 1998;161:3844–3852. [PubMed] [Google Scholar]
- Zhang X., Giangreco L., Broome H.E., Dargan C.M., Swain S.L. Control of CD4 effector fatetransforming growth factor β1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J. Exp. Med. 1995;182:699–709. doi: 10.1084/jem.182.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Brunner T., Carter L., Dutton R.W., Rogers P., Bradley L., Sato T., Reed J.C., Green D., Swain S.L. Unequal death in T helper cell (Th)1 and Th2 effectorsTh1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerken L., Hertogh-Huijbregts A., Dobber R., Drager A. Age-related changes in lymphokine production related to a decreased number of CD45Bhi CD4+ T cells. Eur. J. Immunol. 1991;21:273–281. doi: 10.1002/eji.1830210206. [DOI] [PubMed] [Google Scholar]
- Kirman I., Zhao K., Tschepen I., Szabo P., Richter G., Nguyen H., Weksler M.E. Treatment of old mice with IL-2 corrects dysregulated IL-2 and IL-4 production. Int. Immunol. 1996;8:1009–1015. doi: 10.1093/intimm/8.7.1009. [DOI] [PubMed] [Google Scholar]
- Gilman S.C., Rosenberg J.S., Feldman J.D. T lymphocytes of young and aged rats. II. Functional defects and the role of interleukin-2. J. Immunol. 1982;128:644–650. [PubMed] [Google Scholar]
- Thoman M.L., Weigle W.O. Lymphokines and aginginterleukin-2 production and activity in aged animals. J. Immunol. 1981;127:2102–2106. [PubMed] [Google Scholar]
- Lenardo M.J. Interleukin-2 programs mouse α/β T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Nordin A.A., Collins G.D. Limiting dilution analysis of alloreactive cytotoxic precursor cells in aging mice. J. Immunol. 1983;131:2215–2218. [PubMed] [Google Scholar]
- Negoro S., Hara S., Miyata S., Saiki O., Tanaka T., Yoshizaki K., Igarashi T., Kishimoto S. Mechanisms of age-related decline in antigen-specific T cell proliferative responseIL-2 receptor expression and recombinant IL-2 induced proliferative response of purified TAC-positive T cells. Mech. Ageing Dev. 1986;36:223–241. doi: 10.1016/0047-6374(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Flurkey K., Stadecker M., Miller R.A. Memory T lymphocyte hyporesponsiveness to non-cognate stimulia key factor in age-related immunodeficiency. Eur. J. Immunol. 1992;22:931–935. doi: 10.1002/eji.1830220408. [DOI] [PubMed] [Google Scholar]
- Shi J., Miller R.A. Differential tyrosine-specific protein phosphorylation in mouse T lymphocyte subsetseffect of age. J. Immunol. 1993;151:730–739. [PubMed] [Google Scholar]
- Whisler R.L., Grants L.S. Age-related alterations in the activation and expression of phosphotyrosine kinase and protein kinase C (PKC) among human B cells. Mech. Ageing Dev. 1993;71:31–46. doi: 10.1016/0047-6374(93)90033-n. [DOI] [PubMed] [Google Scholar]
- Utsuyama M., Varga Z., Fukami K., Homma Y., Takenawa T., Hirokawa K. Influence of age on the signal transduction of T cells in mice. Int. Immunol. 1993;5:1177–1182. doi: 10.1093/intimm/5.9.1177. [DOI] [PubMed] [Google Scholar]
- Linton P.-J., Haynes L., Klinman N.R., Swain S.L. Antigen-independent changes in CD4 T cells with aging. J. Exp. Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L., Linton P.-J., Swain S.L. Age-related changes in CD4 T cells of T cell receptor transgenic mice. Mech. Ageing Dev. 1997;93:95–105. doi: 10.1016/s0047-6374(96)01826-x. [DOI] [PubMed] [Google Scholar]
- Jaiswal A.I., Croft M. CD40 ligand induction on T cell subsets by peptide-presenting B cells. Implications for development of the primary T and B cell response. J. Immunol. 1997;159:2282–2291. [PubMed] [Google Scholar]
- Wells A.D., Gudmundsdottir H., Turka L.A. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Melchars F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4, 5 using modified cDNA vectors. Eur. J. Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Assenmacher M., Schmitz J., Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytesexpression of interleukin-10 in interferon-γ and in interleukin-4-expressing cells. Eur. J. Immunol. 1994;24:1097–1101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- Openshaw P., Murphy E.E., Hosken N.A., Maino V., Davis K., Murphy K., O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J. Exp. Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Trageser C.L., Willerford D.M., Lenardo M.J. T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J. Immunol. 1998;160:763–769. [PubMed] [Google Scholar]
- Van Parijs L., Biuckians A., Ibragimov A., Alt F.W., Willerford D.M., Abbas A.K. Functional responses and apoptosis of CD25 (IL-2R α)-deficient T cells expressing a transgenic antigen receptor. J. Immunol. 1997;158:3738–3745. [PubMed] [Google Scholar]
- Lenardo M.J., Boehme S., Chen L., Combadiere B., Fisher G., Freedman M., McFarland H., Pelfrey C., Zheng L. Autocrine feedback death and the regulation of mature T lymphocyte antigen responses. Int. Rev. Immunol. 1995;13:115–134. doi: 10.3109/08830189509061742. [DOI] [PubMed] [Google Scholar]
- Smith K.A., Cantrell D.A. Interleukin 2 regulates its own receptors. Proc. Natl. Acad. Sci. USA. 1985;82:864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh K., Twardzik T., Kneitz B., Heyer J., Schimpl A., Serfling E. The interleukin 2 receptor α chain/CD25 promoter is a target for nuclear factor of activated T cells. J. Exp. Med. 1998;188:1369–1373. doi: 10.1084/jem.188.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depper J.M., Leonard W.J., Drogula C., Kronke M., Waldman T.A., Greene W.C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc. Natl. Acad. Sci. USA. 1985;82:4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D.R., Shirley K.M., McDonald L.E., Bielefeldt-Ohmann H., Kay G.F., Kelso A. Distinct methylation of the interferon γ (IFN-γ) and interleukin 3 (IL-3) genes in newly activated primary CD8+ T lymphocytesregional IFN-γ promoter demethylation and mRNA expression are heritable in CD44high CD8+ T cells. J. Exp. Med. 1998;188:103–117. doi: 10.1084/jem.188.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J.J., Brown D.R., Mullen A.C., Moskowitz N.H., Mahowald M.A., Sider J.R., Gajewski T.F., Wang C.-R., Reiner S.L. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- Agarwal S., Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- Schwartz R.H. T cell clonal anergy. Curr. Opin. Immunol. 1997;9:351–357. doi: 10.1016/s0952-7915(97)80081-7. [DOI] [PubMed] [Google Scholar]
- Jenkins M.K., Pardoll D.M., Mizuguchi J., Chused T.M., Schwartz R.H. Molecular events in the induction of a nonresponsive state in interleukin-2 producing helper T-lymphocyte clones. Proc. Natl. Acad. Sci. USA. 1987;84:5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst D.N., Hobbs M.V., Torbett B.E., Glasebrook A.L., Rehse M.A., Bottomly K., Hayakawa K., Hardy R.R., Weigle W.O. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J. Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- Lerner A., Yamada T., Miller R.A. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur. J. Immunol. 1989;19:977–982. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- Ernst D.N., Weigle W.O., Noonan D.J., McQuitty D.N., Hobbs M.V. The age-associated increase in IFN-γ synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J. Immunol. 1993;151:575–587. [PubMed] [Google Scholar]
- Utsuyama M., Hirokawa C., Kurashima C., Fukayama M., Inamatsu T., Suzuki K., Hashimoto W., Sato K. Differential age-change in the numbers of CD4+CA45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech. Ageing Dev. 1992;63:57–68. doi: 10.1016/0047-6374(92)90016-7. [DOI] [PubMed] [Google Scholar]
- Schorle H., Holtschke T., Hunig T., Schimpl A., Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- Kneitz B., Herrmann T., Yonehara S., Schimpl A. Normal clonal expansion but impaired Fas-mediated cell death and anergy induction in interleukin-2-deficient mice. Eur. J. Immunol. 1995;25:2572–2577. doi: 10.1002/eji.1830250925. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A.C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Zhang X., Sun S., Hwang I., Tough D., Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Tough D.F., Sun S., Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J. Exp. Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]