Abstract
Mucosal surfaces are protected specifically by secretory immunoglobulin A (SIgA) and SIgM generated through external translocation of locally produced dimeric IgA and pentameric IgM. Their active transport is mediated by the epithelial polymeric Ig receptor (pIgR), also called the transmembrane secretory component. Paracellular passive external transfer of systemic and locally produced antibodies also provides mucosal protection, making the biological importance of secretory immunity difficult to assess. Here we report complete lack of active external IgA and IgM translocation in pIgR knockout mice, indicating no redundancy in epithelial transport mechanisms. The knockout mice were of normal size and fertility but had increased serum IgG levels, including antibodies to Escherichia coli, suggesting undue triggering of systemic immunity. Deterioration of their epithelial barrier function in the absence of SIgA (and SIgM) was further attested to by elevated levels of albumin in their saliva and feces, reflecting leakage of serum proteins. Thus, SIgA did not appear to be essential for health under the antigen exposure conditions of these experimental animals. Nevertheless, our results showed that SIgA contributes to maintenance of mucosal homeostasis. Production of SIgA might therefore be a variable in the initiation of human immunopathology such as inflammatory bowel disease or gluten-sensitive enteropathy.
Keywords: IgA, secretory; receptors, polymeric immunoglobulin; secretory component; immunity, mucosal; mice, knockout
The existence of an adaptive secretory immune system, based mainly on active external transport of locally produced dimeric IgA and operating fairly independently of systemic immunity, has been known for several decades 1 2. It is believed that secretory (S)IgA1 and, to a lesser extent, SIgM antibodies enhance the epithelial barrier function by a mechanism termed immune exclusion 3. These antibodies, which may act both within secretory epithelia and at mucosal surfaces 4 5 6, are generated by a unique cooperation between two distinct cell types 7 8: J chain–expressing plasma cells that produce polymeric (p)IgA (mainly dimers) or pentameric IgM, and secretory epithelial cells that express the pIgR, also known as the transmembrane secretory component (SC). This receptor mediates active transport of pIgA and pentameric IgM to exocrine secretions 9 10. Cleavage of unoccupied and ligand-complexed pIgR, respectively, releases free SC and SIgA or SIgM into the lumen.
Induction of a secretory immune response is often associated with elevation of corresponding serum IgG and IgA (in humans mainly monomeric) antibodies 6. These antibodies can reach external secretions by passive paracellular diffusion and may thus contribute to immune exclusion 3. As a consequence, it is often difficult if not impossible to distinguish between the role of secretory versus systemic immunity in local defense. Distinction between the effects of these types of protective mechanisms would be important for rational design of vaccines and understanding of immunopathology in important mucosal disorders, including inflammatory bowel disease (IBD). Therefore, to identify the effect of secretory immunity, we generated mice lacking functional pIgR and hence active external transport of pIgA and pentameric IgM.
Materials and Methods
Generation of pIgR Knockout Mice.
The polymeric Ig receptor locus (PIGR) was isolated from a genomic sv129 library in EMBL4 by probing with radiolabeled oligonucleotides based on the rat pIgR cDNA sequence. The targeting vector included (5′ to 3′): a 1.9-kb upstream arm; a neoR cassette inserted at the PmlI site in exon 3, disrupting the noncovalent pIg-binding site; a 7.5-kb downstream arm; and a herpes simplex virus thymidine kinase gene for negative selection of nonhomologous recombinants. E14.1 embryonic stem cells were electroporated with SalI linearized vector, and homozygous mutant mice were generated as described previously 11. 129/OLa × C57BL/6 black mixed males, 5–6 mo old, were kept in accordance with institutional guidelines and used for all analyses.
DNA and RNA Analysis.
Southern blots were performed with 10 μg of embryonic stem cell DNA or tail biopsy DNA digested with HindIII, separated by agarose gel electrophoresis, and probed with a 1.4-kb genomic NcoI fragment adjacent to the targeting construct. RNA was isolated from the small intestine with RNAesy kit (QIAGEN, Inc.), and 10 μg was separated on a formaldehyde agarose gel, blotted, and hybridized to radiolabeled murine pIgR cDNA 12 (gift from C.S. Kaetzel, University of Kentucky, Lexington, KY). For reverse transcription PCR, 500 ng of RNA was primed with oligo dT. PCR was performed with pigr-e2 for 5′-GCTCTACTTGTTCACGCTC versus pigr-e4.rev 5′-TTTCTGCCTATGTCCTTTG. The products were sequenced directly with a cycle sequencing kit (Amersham International PLC).
Immunohistochemistry.
Excised organs were washed briefly in ice cold PBS, fixed overnight in cold 70% ethanol, and paraffin embedded (56–57°C, 3–4 h) after graded dehydration. Primary rabbit antibody reagents against mouse IgA and mouse IgG were obtained commercially as fluorescein (Zymed Labs., Inc.) and Texas Red (Jackson ImmunoResearch Labs., Inc.) conjugates, respectively. Rabbit polyclonal antibody to murine SC (gift from B. Corthesy, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland) was used with a secondary rhodamine-labeled donkey IgG anti–rabbit conjugate (Jackson ImmunoResearch Labs., Inc.). Optimal working concentrations of all immune reagents were determined by performance testing on relevant tissue substrates.
Sampling and Analysis of Body Fluids.
Peripheral blood, whole saliva, extract of small intestinal wick-retrieved mucus, and extract of feces were sampled and processed as described 13. ELISA was used to determine IgA, IgG 13, and albumin (Bethyl Labs.) concentrations. ELISA was also used to measure serum IgG antibodies to formalin-inactivated murine Escherichia coli and Lactobacillus isolates (courtesy of T. Midtvedt, Karolinska Institutet, Stockholm, Sweden) and to wheat gluten (Sigma Chemical Co.). For Western blots, the indicated amount of sample was separated by nonreducing SDS-PAGE, transferred to nitrocellulose, and probed with polyclonal rabbit antiserum against murine IgA (DAKO Corp.) or murine SC. Secondary antibody was horseradish peroxidase–conjugated goat anti–rabbit IgG used at 1:3,000 followed by enhanced chemiluminescence revealing reaction (ECL; Amersham Corp.). All incubations were in PBS with 0.05% Tween.
Results and Discussion
Lack of Active Epithelial and Hepatic IgA Transport in pIgR Knockout Mice.
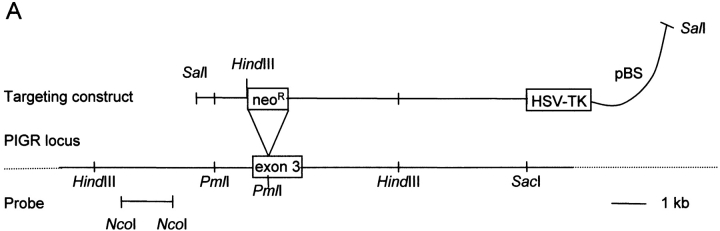
A targeting vector with a disruption in exon 3 that encodes the ligand-binding extracellular receptor domain 1 (D1) was used to knock out the pIgR gene (locus PIGR) in mice (Fig. 1 A). Wild-type and mutant chromosomes were distinguished by Southern blots (Fig. 1 B). To test expression of the mutant allele, we performed Northern blots with small intestinal RNA from wild-type and pIgR−/− mice (Fig. 1 C); the latter were expected to encode mRNA 1.7 kb larger than wild type, but mutant pIgR mRNA was in fact smaller and less abundant. Cloning and sequencing of pIgR cDNAs from pIgR−/− mice revealed two alternatively spliced mRNA forms (one in frame and one out of frame) that both deleted pIgR D1. Thus, there was a possibility that a truncated receptor lacking D1 might be produced, but this variant would not bind IgA.
Figure 1.
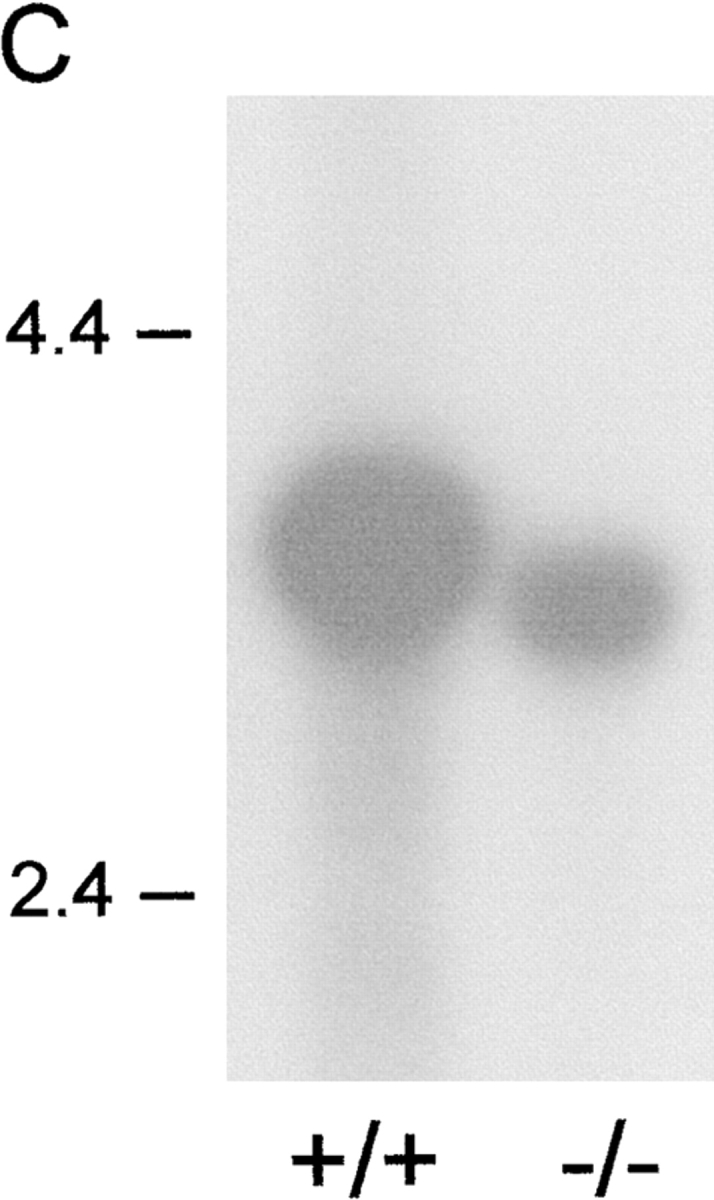
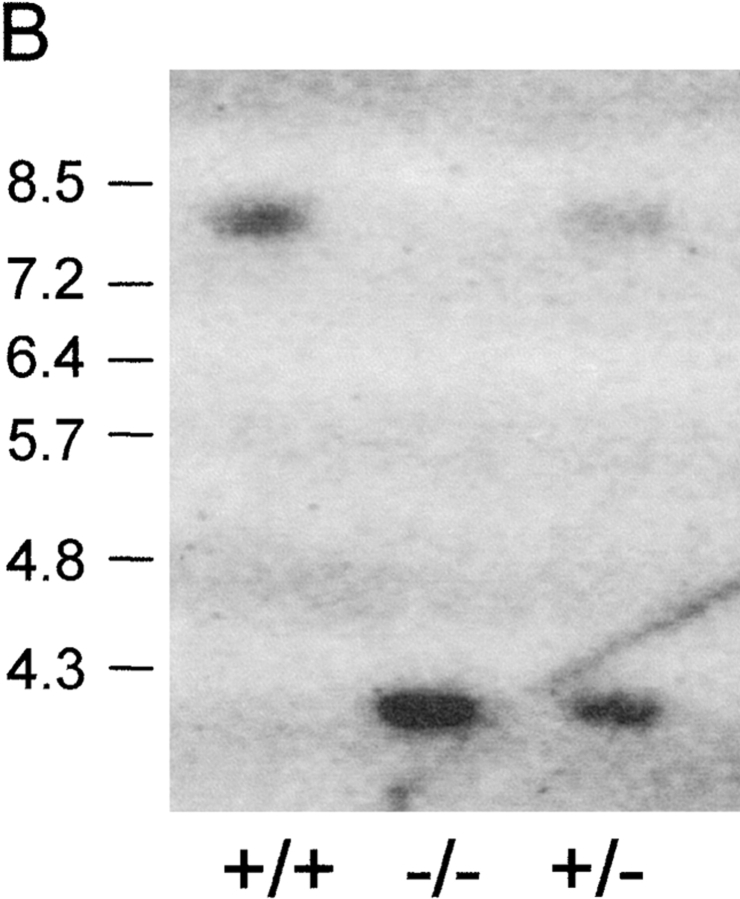
Generation of pIgR−/− mice. (A) The PIGR locus and gene targeting strategy. A neoR cassette was inserted in exon 3, disrupting the noncovalent pIg-binding site, and a herpes simplex virus thymidine kinase gene was inserted downstream for negative selection of nonhomologous recombinants. (B) Southern blot of tail DNA from wild-type, heterozygote, and pIgR−/− (+/+, +/−, and −/−, respectively) mice probed with the 1.4-kb NcoI fragment indicated in A. (C) Northern blot of RNA extracted from small intestines of +/+ and −/− mice probed with murine pIgR cDNA (gift from C. Kaetzel).
Sections of small intestinal mucosa from pIgR−/− and wild-type mice were immunostained for pIgR/SC, IgA, and IgG. The wild-type mice had relatively less interstitial IgA in their lamina propria than the pIgR−/− mice (Fig. 2, top panels). Conversely, the epithelium was IgA positive only in the wild-type mice; the staining was intensified at the apical face, indicating active external transport of pIgA. Thus, the pIgR−/− mice showed no evidence of intracellular IgA transport despite increased concentration of subepithelial IgA. Lack of epithelial transport was also evident for IgM (not shown). Likewise, immunostaining for SC demonstrated abundant cytoplasmic expression both in crypt and surface epithelium of wild-type mice, whereas only occasional faint crypt staining was seen in the pIgR−/− mice with a polyclonal reagent directed against D1–D5 (Fig. 2, bottom panels). This inconsistent expression might be spurious but could reflect expression of mutant pIgR lacking D1 and therefore not represent functional receptor protein. Immunofluorescence staining revealed a similar picture in colon mucosa and also confirmed that there was no vesicular IgA transport through hepatocytes in pIgR−/− mice in contrast to wild-type mice (not shown). Thus, the hepatic pIgR-mediated “IgA pump” was lacking in pIgR−/− mice. Similarly, the hepatic IgA transport was reported to be absent in J chain knockout mice because pIgA and pentameric IgM require this peptide for binding to pIgR 7 14.
Figure 2.
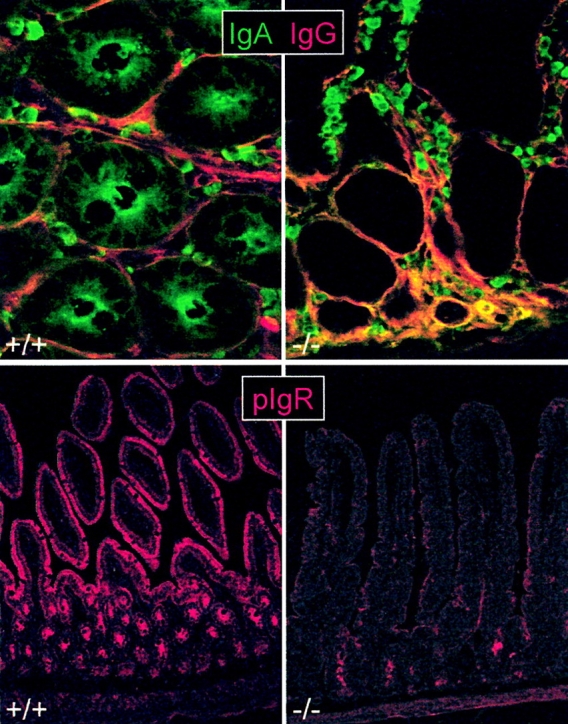
Lack of epithelial IgA transport in pIgR−/− mice. Immunofluorescence staining of IgA, IgG, and pIgR/SC in tissue sections from the small intestine of knockout (−/−; right panels) and wild-type (+/+; left panels) mice. Top panels: paired staining of IgA (fluorescein, green) and IgG (Texas Red) in cross section of crypt region. Note lack of IgA in epithelium and increased level of interstitial IgA in pIgR−/− mice (seen as yellow color due to mixing of green with red). Bottom panels: SC staining at low magnification showing abundant pIgR/SC expression in both crypt and villous epithelium of wild-type mice but only occasional faint staining deep in the crypts of pIgR−/− mice. Small intestinal mucosa is shown at full height (note obliquely cut villi in left panel due to tilted tissue orientation).
Epithelial Leakiness and Systemic IgG Antibody Production against E. coli in pIgR Knockout Mice.
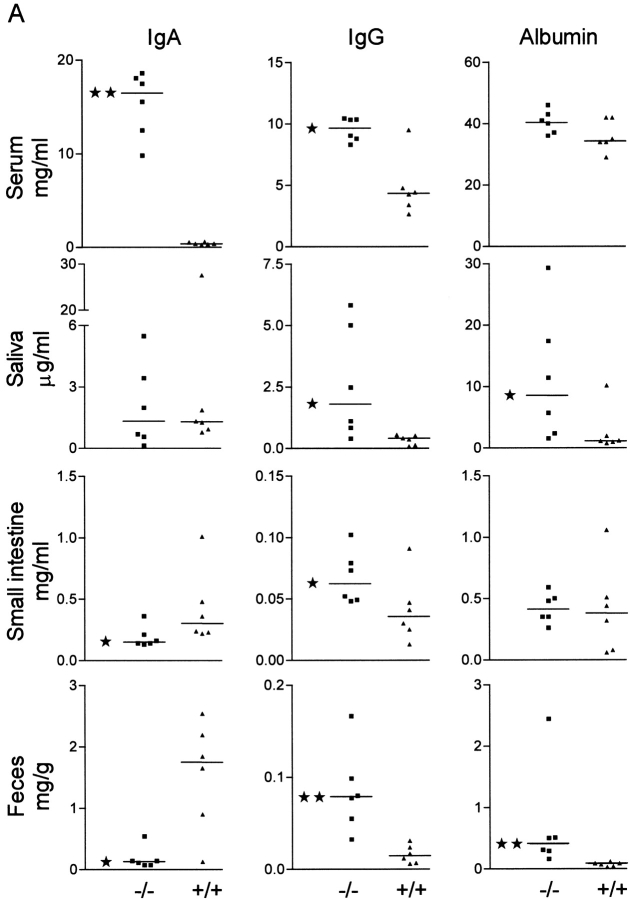
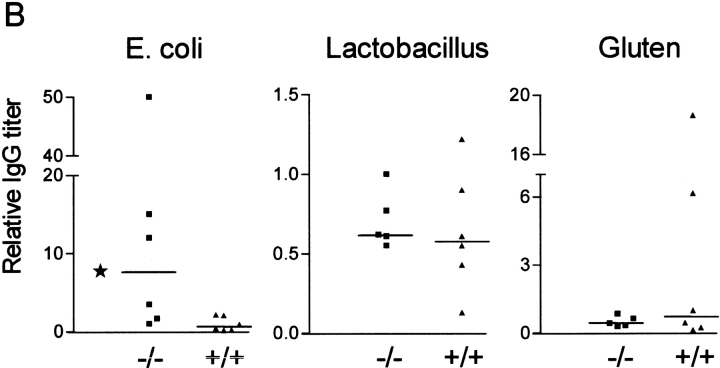
To measure the effect of pIgR mutation on total transfer of IgA to secretions compared with that of IgG and albumin (which are not actively transported), we tested serum, whole saliva, small intestinal secretions, and fecal extracts from pIgR−/− and wild-type mice by ELISA (Fig. 3 A). As expected, pIgR−/− animals had grossly elevated levels of IgA in serum due to lack of the hepatic IgA pump operating in rodents 15. Western blot analysis confirmed that this IgA was mostly polymeric (Fig. 4 A). More surprisingly, pIgR−/− animals also had increased levels of serum IgG. This elevation could be caused by less efficient IgG degradation due to catabolic competition with the elevated serum IgA. Alternatively, increased serum IgG in the knockout mice might reflect enhanced triggering of their systemic immune systems. We found that the serum IgG elevation was due in part to increased levels of antibodies to E. coli (Fig. 3 B), whereas there was no difference in the IgG antibody response to Lactobacillus or gluten (their main dietary protein). This finding provided strong support for a selective stimulation of systemic IgG response to E. coli in the absence of SIgA (and SIgM). The wide scatter of the IgG response among the knockout mice was not surprising in view of similar variability of intestinal immunopathology induced by gut bacteria in different cytokine-deficient and transgenic rodents 16 17.
Figure 3.
Altered Ig and albumin levels in serum and secretions of pIgR−/− mice. (A) Levels of IgA, IgG, and albumin in pIgR−/− (−/−, ▪) and wild-type (+/+, ▴) mice were measured by ELISA in serum, saliva, mucus extract from the small intestine, and extract of feces. Statistical analysis was performed with the Mann-Whitney test (★, P < 0.05 and ★★, P < 0.005). (B) Relative titers of serum IgG antibodies against E. coli, Lactobacillus, or gluten.
Figure 4.
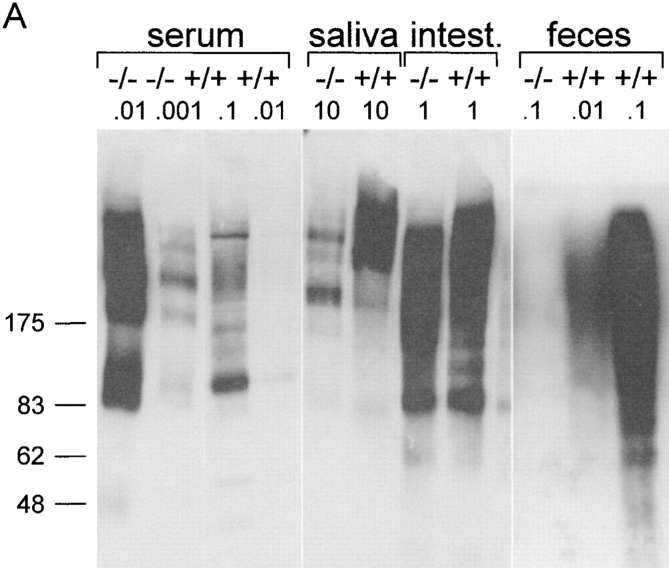
Increased pIgA serum level but lack of SIgA in pIgR−/− mice. Western blot of IgA in serum and secretions from pIgR−/− (−/−) and wild-type (+/+) mice. The indicated amounts of samples (in microliters for secretions and micrograms dry weight for feces) were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibody to α chain (A) or to SC as a marker of SIgA (B). The positions of migration of purified human SC and SIgA are indicated. Intest., intestine.
There was no statistical difference in the salivary IgA levels of wild-type and pIgR animals, whereas both IgG and albumin levels were elevated in saliva from the knockout mice, suggesting epithelial leakage of serum-derived proteins (Fig. 3 A). Each pIgR−/− mouse had higher levels of salivary IgG than IgA, whereas each wild-type mouse had more IgA than IgG. Furthermore, there was good correlation between albumin and both IgA and IgG levels in saliva of the knockout mice (r = 0.94, P = 0.02 for both), supporting the idea of increased external bulk diffusion of serum proteins. In wild-type mice, however, there was only a significant correlation between the much lower levels of albumin and IgG (r = 0.89, P = 0.03), presumably reflecting that their salivary IgA did not depend on leakage of serum IgA but rather on generation of SIgA.
At the mucosal surface in the small intestine, IgA concentrations were lower in pIgR−/− than in wild-type mice, despite elevated serum (and lamina propria) levels in the former. This contrasted with findings in J chain–deficient mice that showed no difference in IgA levels at this site compared with wild-type animals 18, although their fecal and biliary levels of IgA were reported to be significantly repressed 14. This disparity in the J chain knockout mice was proposed to be due to a putative alternative active epithelial transport mechanism for monomeric IgA and pIgA operating selectively at certain secretory sites 18. However, we found that small intestinal IgG levels were about twofold higher in the pIgR−/− than in the wild-type mice, clearly reflecting the difference in their serum IgG levels (Fig. 3 A). The high levels of albumin in the same samples also suggested a significant bulk leakage of serum proteins across the epithelium.
We believe that the harsh physical manipulation of the small intestine performed to collect secretions at the luminal surface, used in both this study and the J chain knockout study 18, considerably increased the leakage of proteins from the lamina propria. Thus, although the use of wicks to retrieve secretions may be appropriate for the determination of changes in specific production of antibodies along the intestinal tract, it may not be suitable to differentiate between luminal IgA derived via active or passive (in vivo or in vitro) external transfer. This is in good agreement with the documented liability of mucosal epithelia to allow profuse external bulk transfer of serum proteins after local irritation 19.
The IgA levels of fecal extracts were significantly lower in pIgR−/− than in wild-type mice, which probably was explained both by abrogated active transport and reduced stability of the paracellularly diffused IgA (compared with actively transported SIgA) in the knockout mice (see below). A similar disparity between small intestinal mucus and fecal extracts was reported in J chain knockout mice compared with wild-type mice 14 18. Importantly, the fecal levels of albumin were much higher in the pIgR−/− than in the wild-type mice, suggesting increased epithelial protein leakage even in the untouched large bowel, as was also noted for saliva (Fig. 3 A).
Nature of IgA in External Secretions of pIgR Knockout Mice.
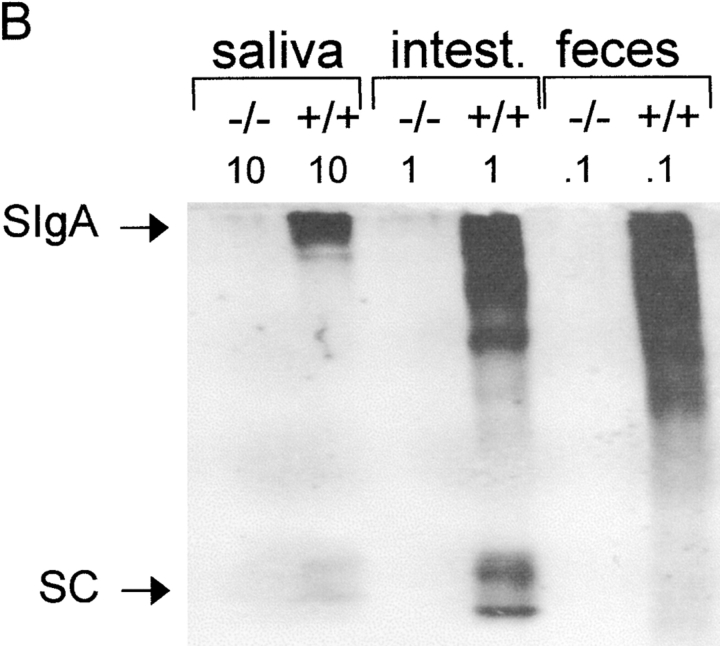
We performed Western blots to assess the molecular form of IgA collected from various compartments (Fig. 4a and Fig. b). Both monomeric and polymeric forms of IgA were present in serum from wild-type mice, whereas their salivary IgA was mainly polymeric (Fig. 4 A). By contrast, serum from pIgR−/− mice contained almost exclusively polymers of IgA, and their salivary IgA was of a similar molecular form. Fecal and small intestinal IgA from both wild-type and pIgR−/− mice migrated as a smear, suggesting some degradation, and the largest forms showed a size consistent with intact SIgA only in the wild-type mice. This was confirmed by blotting with an antibody to SC (Fig. 4 B). Together, these results demonstrated that only exocrine IgA from wild-type mice contained SIgA, whereas IgA in the secretions of pIgR−/− mice appeared to be derived from serum and interstitial fluid by passive external diffusion. As alluded to above, it is well known that such paracellular bulk transfer of proteins is greatly enhanced when mucous membranes are irritated 19, which was unavoidable in the small intestinal sampling procedure.
Possible Consequences of Absent Epithelial IgA Transport.
The functional basis of the now internationally accepted common model for receptor-mediated epithelial transport of pIgA and pentameric IgM into external secretions was proposed by this laboratory 25 years ago 8 20 21. It stated that J chain–expressing local plasma cells produce pIgA and pentameric IgM that, by virtue of their J chain incorporation, contain a noncovalent binding site for pIgR/SC. Mostov et al. first demonstrated in 1980 that free SC could be derived from a transmembrane precursor by endoproteolytic receptor cleavage 22. Subsequent cDNA cloning of transmembrane rabbit SC 23 and its functional expression in polarized Madin-Darby canine kidney cells 24 demonstrated that SC indeed operated as pIgR in being capable of external pIgA transport in vitro. Thus, SIgA and SIgM are generated by pIgR-mediated ligand transport across secretory epithelial cells and subsequent release into secretions after receptor cleavage. Secondary covalent bonding of SC to pIgA makes SIgA the most stable antibody operating in external secretions 6 25.
The relatively minor differences observed between pIgR−/− and wild-type mice in small intestinal mucus and salivary IgA levels showed that pIgR function is not essential for external IgA transfer. Thus, our results documented that pIgA, like IgG and monomeric IgA, can reach secretions by paracellular leakage and that this transfer is increased by epithelial irritation. External defense resulting from such passive luminal supply of antibodies probably explained that the intestinal mucosae of the knockout mice generally exhibited no histological signs of inflammation and that the animals were of normal size and fertility (data not shown). Nevertheless, their defective epithelial barrier function, as revealed by increased albumin levels at untouched mucosal surfaces (that is, in saliva and feces) and increased IgG anti–E. coli levels in serum, supported the notion that pIgR normally executes an important function in maintaining mucosal homeostasis.
Although not shown here, it is possible that increased bacterial colonization and related irritation of epithelial surfaces explained the “leaky phenotype” of our knockout mice. This possibility would harmonize with the striking role shown for SIgA from breast milk in inhibiting translocation of E. coli from the gut lumen to mesenteric lymph nodes in neonatal rabbits 26. Furthermore, the markedly reduced fecal IgA levels in the knockout mice confirmed a role of bound SC to stabilize pIgA in secretions 25. Also, apical recycling of pIgR–pIgA complexes has been reported 10, and the strong apical IgA staining of wild-type intestinal epithelium (Fig. 2) suggested that pIgA is normally associated with the epithelial cells for some time, in keeping with its ability to neutralize intracellular virus during pIgR-mediated transcytosis 4 27 28 29. Thus, in addition to mediating active external antibody transport, the function of pIgR/SC is most likely important at various other critical points of mucosal defense 5.
A reduced epithelial barrier function leading to abrogation of immunological tolerance against normally innocuous antigens, including dietary proteins and components of the indigenous bacterial flora, appears to be a significant immunopathogenic mechanism in several important mucosal disorders such as gluten-sensitive enteropathy (celiac disease) 30 and IBD 31 32. One marker of this untoward development is lack of mucosal integrity 33 and increased production of IgA and IgG antibodies to normally encountered luminal antigens, particularly against gluten in celiac disease 30 and E. coli in IBD 31 34. Complete lack of pIgR has not been convincingly identified in humans, perhaps reflecting evolutionary avoidance of such a deficiency 35. However, our results showed that this receptor is not essential for the health of mice in a conventional laboratory animal facility. This might reflect redundancy of SIgA in terms of local immunity under such conditions, but its presence might nevertheless protect against development of mucosal immunopathology over time. Thus, individuals with selective IgA deficiency show an increased frequency of mucosal disorders such as celiac disease 36 and IBD, particularly Crohn's disease (Hammarström, L., personal communication), despite generally eliciting compensatory SIgM responses 35. Also, increased mucosal leakiness is observed in patients with AIDS who have defective production of intestinal IgA antibodies against the infecting virus 37 38.
In conclusion, this study provides the first direct evidence that generation of SIgA and SIgM has a basic impact on the epithelial barrier function. It may therefore be an important factor for sustenance of mucosal homeostasis and thus a variable in local defense and immunopathology. Importantly, pIgR−/− mice present a unique opportunity to explore the relative contribution of secretory antibodies versus systemic immunity in protection against mucosal pathogens and maintenance of immune tolerance. Such studies may promote a rational development of efficient mucosal vaccines.
Acknowledgments
We thank Profs. B. Corthesy, C.S. Kaetzel, and T. Midtvedt for kind gifts of rabbit antiserum against murine SC, murine pIgR cDNA, and bacterial isolates, respectively. We are grateful to Marie K. Johannesen and Inger Lise Haugen for technical assistance.
This work was supported by grants from the Research Council of Norway, the Norwegian Cancer Society, and Anders Jahre's Foundation.
Footnotes
1used in this paper: IBD, inflammatory bowel disease; p, polymeric; S, secretory; SC, secretory component
References
- Brandtzaeg P. History of oral tolerance and mucosal immunity. Ann. NY Acad. Sci. 1996;778:1–27. doi: 10.1111/j.1749-6632.1996.tb21110.x. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Moro I., Underdown B. Mucosal immunoglobulins. In: Mestecky J., Bienstock J., McGhee J., Lamm M., Strober W., Ogra P., editors. Mucosal Immunology. Academic Press; San Diego, CA: 1999. p. 133. [Google Scholar]
- Stokes C.R., Soothill J.F., Turner M.W. Immune exclusion is a function of IgA. Nature. 1975;255:745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- Mazanec M.B., Kaetzel C.S., Lamm M.E., Fletcher D., Nedrud J.G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec M.B., Nedrud J.G., Kaetzel C.S., Lamm M.E. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today. 1993;14:430–435. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Farstad I.N., Johansen F.-E., Morton H.C., Norderhaug I.N., Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol. Rev. 1999;In press doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand. J. Immunol. 1985;22:111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Johansen F.-E., Krajci P., Natvig I. Human secretory component (the polymeric immunoglobulin receptor) In: Roitt D.P., editor. Encyclopedia of Immunology. 2nd Ed. Academic Press; London: 1998. pp. 2152–2158. [Google Scholar]
- Mostov K.E. Transepithelial transport of immunoglobulins. Annu. Rev. Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- Leveen P., Pekny M., Gebre M.S., Swolin B., Larsson E., Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Piskurich J.F., Blanchard M.H., Youngman K.R., France J.A., Kaetzel C.S. Molecular cloning of the mouse polymeric Ig receptor. Functional regions of the molecule are conserved among five mammalian species. J. Immunol. 1995;154:1735–1747. [PubMed] [Google Scholar]
- Haneberg B., Kendall D., Amerongen H.M., Apter F.M., Kraehenbuhl J.P., Neutra M.R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect. Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson B.A., Conner D.A., Ladd D.J., Kendall D., Casanova J.E., Corthesy B., Max E.E., Neutra M.R., Seidman C.E., Seidman J.G. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. J. Exp. Med. 1995;182:1905–1911. doi: 10.1084/jem.182.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D.L., Malburny G.N., Vaerman J.P. Hepatobiliary transport of plasma IgA in the mousecontribution to clearance of intravascular IgA. Eur. J. Immunol. 1985;15:893–899. doi: 10.1002/eji.1830150906. [DOI] [PubMed] [Google Scholar]
- Elson C.O., Sartor R.B., Tennyson G.S., Riddell R.H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Morales V.M., Snapper S.B., Blumberg R.S. Probing the gastrointestinal immune function using transgenic and knockout technology. Curr. Opin. Gastroenterology. 1996;12:577–583. [Google Scholar]
- Hendrickson B.A., Rindisbacher L., Corthesy B., Kendall D., Waltz D.A., Neutra M.R., Seidman J.G. Lack of association of secretory component with IgA in J chain-deficient mice. J. Immunol. 1996;157:750–754. [PubMed] [Google Scholar]
- Persson C.G., Erjefalt J.S., Greiff L., Erjefalt I., Korsgren M., Linden M., Sundler F., Andersson M., Svensson C. Contribution of plasma-derived molecules to mucosal immune defence, disease and repair in the airways. Scand. J. Immunol. 1998;47:302–313. doi: 10.1046/j.1365-3083.1998.00317.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Presence of J chain in human immunocytes containing various immunoglobulin classes. Nature. 1974;252:418–420. doi: 10.1038/252418a0. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin componentsdifferential localization of free and bound SC in secretory epithelial cells. J. Immunol. 1974;112:1553–1559. [PubMed] [Google Scholar]
- Mostov K.E., Kraehenbuhl J.P., Blobel G. Receptor-mediated transcellular transport of immunoglobulinsynthesis of secretory component as multiple and larger transmembrane forms. Proc. Natl. Acad. Sci. USA. 1980;77:7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K.E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984;308:37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Mostov K.E., Deitcher D.L. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Crottet P., Corthesy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab')2a possible implication for mucosal defense. J. Immunol. 1998;161:5445–5453. [PubMed] [Google Scholar]
- Dickinson E.C., Gorga J.C., Garrett M., Tuncer R., Boyle P., Watkins S.C., Alber S.M., Parizhskaya M., Trucco M., Rowe M.I. Immunoglobulin A supplementation abrogates bacterial translocation and preserves the architecture of the intestinal epithelium. Surgery. 1998;124:284–290. [PubMed] [Google Scholar]
- Bomsel M., Heyman M., Hocini H., Lagaye S., Belec L., Dupont C., Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- Burns J.W., Siadat-Pajouh M., Krishnaney A.A., Greenberg H.B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- Fujioka H., Emancipator S.N., Aikawa M., Huang D.S., Blatnik F., Karban T., DeFife K., Mazanec M.B. Immunocytochemical colocalization of specific immunoglobulin A with sendai virus protein in infected polarized epithelium. J. Exp. Med. 1998;188:1223–1229. doi: 10.1084/jem.188.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H., Nilsen E., Sollid L.M., Lundin K.E., Rugtveit J., Molberg O., Thorsby E., Brandtzaeg P. Immunopathology of gluten-sensitive enteropathy. Springer Semin. Immunopathol. 1997;18:535–553. doi: 10.1007/BF00824057. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Haraldsen G., Rugtveit J. Immunopathology of human inflammatory bowel disease. Springer Semin. Immunopathol. 1997;18:555–589. doi: 10.1007/BF00824058. [DOI] [PubMed] [Google Scholar]
- Duchmann R., Neurath M., Marker-Hermann E., Meyer Zum Buschenfelde K.H. Immune responses towards intestinal bacteria—current concepts and future perspectives. Z. Gastroenterol. 1997;35:337–346. [PubMed] [Google Scholar]
- Nejdfors P., Wang Q., Ekelund M., Westrom B.R., Jansson O., Lindstrom C.L., Karlsson B., Jeppsson B. Increased colonic permeability in patients with ulcerative colitisan in vitro study. Scand. J. Gastroenterol. 1998;33:749–753. doi: 10.1080/00365529850171701. [DOI] [PubMed] [Google Scholar]
- Macpherson A., Khoo U.Y., Forgacs I., Philpott H.J., Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Nilssen D.E., Rognum T.O., Thrane P.S. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol. Clin. North Am. 1991;20:397–439. [PubMed] [Google Scholar]
- Cataldo F., Marino V., Ventura A., Bottaro G., Corazza G.R. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac diseasean Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and “Club del Tenue” Working Groups on Coeliac Disease. Gut. 1998;42:362–365. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K., Lindner C., Frieling T., Niederau C., Reinauer H., Haussinger D. Intestinal protein leakage in the acquired immunodeficiency syndrome. J. Clin. Gastroenterol. 1997;25:426–428. doi: 10.1097/00004836-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Schneider T., Zippel T., Schmidt W., Pauli G., Heise W., Wahnschaffe U., Riecken E.O., Zeitz M., Ullrich R. Abnormal predominance of IgG in HIV-specific antibodies produced by short-term cultured duodenal biopsy specimens from HIV-infected patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997;16:333–339. doi: 10.1097/00042560-199712150-00004. [DOI] [PubMed] [Google Scholar]