Abstract
During lymphocyte homing, L-selectin mediates the tethering and rolling of lymphocytes on high endothelial venules (HEVs) in secondary lymphoid organs. The L-selectin ligands on HEV are a set of mucin-like glycoproteins, for which glycosylation-dependent cell adhesion molecule 1 (GlyCAM-1) is a candidate. Optimal binding in equilibrium measurements requires sulfation, sialylation, and fucosylation of ligands. Analysis of GlyCAM-1 has revealed two sulfation modifications (galactose [Gal]-6-sulfate and N-acetylglucosamine [GlcNAc]-6-sulfate) of sialyl Lewis x. Recently, three related sulfotransferases (keratan sulfate galactose-6-sulfotransferase [KSGal6ST], high endothelial cell N-acetylglucosamine-6-sulfotransferase [GlcNAc6ST], and human GlcNAc6ST) were cloned, which can generate Gal-6-sulfate and GlcNAc-6-sulfate in GlyCAM-1. Imparting these modifications to GlyCAM-1, together with appropriate fucosylation, yields enhanced rolling ligands for both peripheral blood lymphocytes and Jurkat cells in flow chamber assays as compared with those generated with exogenous fucosyltransferase. Either sulfation modification results in an increased number of tethered and rolling lymphocytes, a reduction in overall rolling velocity associated with more frequent pausing of the cells, and an enhanced resistance of rolling cells to detachment by shear. All of these effects are predicted to promote the overall efficiency of lymphocyte homing. In contrast, the rolling interactions of E-selectin transfectants with the same ligands are not affected by sulfation.
Keywords: L-selectin, high endothelial venule, sulfation, sulfotransferases, rolling
Lymphocytes home from the blood into most secondary lymphoid organs by interacting with high endothelial cells (HECs)1 lining the walls of venules called high endothelial venules (HEVs) 1 2. The interaction is initiated with tethering and rolling of lymphocytes along the endothelium, a process mediated by binding of L-selectin to a set of sulfated glycoprotein ligands expressed by HECs 3. Four discrete HEV-expressed glycoproteins have thus far been identified as potential L-selectin ligands in mice and humans: glycosylation-dependent cell adhesion molecule 1 (GlyCAM-1), CD34, mucosal addressin cell adhesion molecule 1 (MAdCAM-1), and podocalyxin 3. These glycoproteins all contain mucin-like domains. The O-glycans in these regions present sulfated, sialylated, and fucosylated determinants that are recognized by the C-type lectin domain of L-selectin 3.
Direct evidence for a critical role of sulfation in L-selectin ligand binding initially came from experiments with chlorate, a metabolic inhibitor of sulfation 4 5. Subsequent evidence derived from studies with the MECA-79, an mAb that stains HEV in a variety of lymphoid organs and inhibits L-selectin–mediated attachment of lymphocytes 1 2. This mAb cross-reacts with all of the L-selectin ligands mentioned above, suggesting that it recognizes a shared posttranslational modification. Although the exact structure of the MECA-79 epitope has not been elucidated, sulfation is required 5 6.
The analysis of oligosaccharides within GlyCAM-1 led to identification of two equal sulfation modifications (Gal-6-sulfate and N-acetylglucosamine [GlcNAc]-6-sulfate) within its O-linked chains 7. Two of the sulfated oligosaccharides identified, known respectively as sialyl 6′-sulfo Lewis x (Lex) and sialyl 6-sulfo Lex (Table ), occur as capping groups on O-linked chains 8 9. Recently, an mAb called G72 was described that reacts with sialyl 6-sulfo Lex in a sialic acid– and sulfate-dependent manner 10. This antibody stains lymph node HEVs and blocks the binding of an L-selectin/IgG chimera to these vessels, providing independent evidence for the importance of the sialyl 6-sulfo Lex structure.
Table 1.
Nomenclature and Structure of Oligosaccharides
| Name | Structure |
|---|---|
| sLex (sialyl Lewis x) | Siaα2→3Galβ1→4[Fucα1→3]GlcNAc |
| sialyl 6′- sulfo Lex | Siaα2→3[SO3→6]Galβ1→4[Fucα1→3]GlcNAc |
| sialyl 6- sulfo Lex | Siaα2→3Galβ1→4[Fucα1→3][SO3→6]GlcNAc |
The importance of sulfation to the structure and function of L-selectin ligands has focused attention on the identity of HEV sulfotransferases that can catalyze the appropriate modifications of these ligands. Recently, progress has been made toward this objective with the identification of a family of highly homologous carbohydrate sulfotransferases 11. This family includes two human GlcNAc-6-sulfotransferases (STs), i.e., HEC-GlcNAc6ST 11 and human (hu) GlcNAc6ST 12, and one human Gal-6-sulfotransferase, i.e., keratan sulfate galactose-6-sulfotransferase (KSGal6ST) 11 13. In contrast to KSGal6ST and huGlcNAc6ST, which show a broad tissue distribution, HEC-GlcNAc6ST is predominantly expressed in HECs. We have shown that KSGal6ST and HEC-GlcNAc6ST can catalyze the sulfation of GlyCAM-1 carbohydrates with the appropriate regiospecificity 11. Furthermore, expression of either of these sulfotransferases in Chinese hamster ovary (CHO) cells, along with CD34 and fucosyltransferase VII (FTVII), results in ligand activity, as detected by binding of an L-selectin/IgM chimera 11. Transfection of huGlcNAc6ST and FTVII cDNAs into ECV304 cells yields binding of L-selectin transfectants 6. These results concur with studies that examined the effect of sulfation of sLex-containing synthetic compounds on equilibrium binding to recombinant L-selectin 14 15, although the importance of sialyl 6′-sulfo Lex as a recognition epitope is controversial 16.
As noted above, L-selectin is specialized to mediate the tethering and rolling of lymphocytes under shear flow conditions in the microvasculature. In this study, we show that recombinant GlyCAM-1, when sulfated by KSGal6ST, HEC-GlcNAc6ST, or huGlcNAc6ST, supports increased L-selectin–dependent rolling of PBLs and Jurkat T cells in a parallel plate flow chamber. We report that sulfation of GlyCAM-1 on the C-6 position of either Gal or GlcNAc has a marked effect on the tethering efficiency, overall velocity, and binding strength of rolling interactions, all of which could significantly enhance the efficiency of in vivo homing.
Materials and Methods
Reagents.
The following individuals provided reagents: T.K. Kishimoto (DREG56 anti–L-selectin mAb), John Lowe (FTVII cDNA), Minoru Fukuda (core 2 β-1,6-N-acetylglucosminyltransferase [C2GnT] cDNA), G.S. Kansas (300.19 B cells transfected with E-selectin), and Arthur Weiss (Jurkat cells). Cells were maintained in RPMI 1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and 5% heat-inactivated FCS (Hyclone), with 100 μM 2-ME added to the 300.19 cells. Human PBLs were isolated by Ficoll-Hypaque sedimentation.
Generation of Recombinant Sulfated GlyCAM-1/IgG.
For generation of GlyCAM-1/IgG fusion proteins, COS-7 cells (80% confluent in 10-cm dishes) were transfected with plasmids encoding C2GnT (pCDNA1.1, 1 μg), FTVII (pCDM8, 1 μg), GlyCAM-1/IgG (pIG1, 2 μg), and KSGal6ST (pCDNA3.1/Myc-His, 0.5/1/2 μg), huGlcNAc6ST (pCDNA3.1, 0.5/1/2 μg), HEC-GlcNA c6ST (pCDNA1.1, 0.5/1/2 μg), or the empty vector (pCDNA3.1, 1 μg) using Lipofectamine (Life Technologies) and were cultured for several days. For radiolabeling, cells were grown for 24 h in Opti-MEM supplemented with 0.1 mCi of Na2 35SO4 (1,400 Ci/mmol; ICN Biomedicals, Inc.). The GlyCAM-1/IgG chimeras were purified on protein A–agarose and were exhaustively exchanged into PBS. In two independent experiments, the following specific activities (cpm/μg protein) were measured (mean ± range): no exogenous sulfotransferase (2,367 ± 142); KSGal6ST (19,262 ± 1,824); huGlcNAc6ST (9,624 ± 1,804); and HEC-GlcNAc6ST (10,068 ± 1,347).
Laminar Flow Assays.
To equalize coating densities of the GlyCAM-1/IgG chimeras, ELISAs were performed. Proteins were coated onto 96-well polystyrene plates (Costar Corp.) in Tris-buffered saline, pH 9, at 4°C. After blocking with BSA, the chimeras were detected with biotinylated anti–GlyCAM-1 peptide Ab 5 or biotinylated anti–human IgG (Fc specific) and streptavidin-conjugated alkaline phosphatase. For flow experiments, polystyrene dishes coated similarly with the chimeras as in the ELISA assays were incorporated as the lower wall of a parallel plate flow chamber 17 18. Cells were perfused through the flow chamber at 1–2 × 106 cells/ml. For inhibition studies, cells were pretreated with 5 μg/ml DREG56 or 10 μg/ml fucoidin (Sigma Chemical Co.; 10 min, 22°C) or resuspended in Ca2+, Mg2+-free HBSS with 5 mM EDTA. For sialidase experiments, coated substrates were incubated with 5 mU/ml Vibrio cholera sialidase (Oxford Glycosystems) for 30 min in 50 mM sodium acetate, 4 mM CaCl2, and 0.1% BSA, pH 5.5, or with buffer alone for the control.
For the initial comparison of rolling behavior on the different substrates, cells were perfused for 2 min through the flow chamber, after which equilibrium was reached and counts of rolling cells were taken. For analysis of tethering, cells were perfused through the chamber over a range of shear stresses (3–0.2 dyn/cm2). The fraction of cells that came into close proximity with the substrate and tethered stably (rolling for >1 s after initial attachment) was determined. For velocity and detachment determinations, cells were infused for 2 min at 1 dyn/cm2, after which shear stress was increased in 1.5–2-fold increments up to 35 dyn/cm2 at intervals of 5 s. Cell displacement was followed for 1–3 s to determine rolling velocities. For single-cell analysis, ≥4 randomly chosen cells were tracked at 1 dyn/cm2 over a period of 4 s in successive video frames (1/30 s) on each substrate, and duration of pauses and the velocity between pauses was determined. In detachment assays, the number of rolling cells was determined at each shear stress and calculated as the percentage of the peak value.
Results
PBLs and Jurkat Cells Roll on Fucosylated and Sulfated GlyCAM-1/IgG in Shear Flow.
Optimal equilibrium binding of L-selectin to its HEV ligands requires sialylation, fucosylation, and sulfation 3 4 19. To determine the contribution of sulfation to ligand activity under flow conditions, we examined the rolling of PBLs or Jurkat cells on recombinant GlyCAM-1/IgG that was immobilized on the bottom plate of a flow chamber. To produce the different forms of GlyCAM-1/IgG, COS cells were cotransfected with cDNAs for GlyCAM-1/IgG, FTVII 19, and C2GnT 20 and a cDNA encoding a sulfotransferase (KSGal6ST, huGlcNAc6ST, or HEC-GlcNAc6ST). The sialylation requirement for ligand activity was met by endogenous sialyltransferases within the COS cells. FTVII was included to satisfy the known fucosylation requirement 19, and C2GnT was added because it elaborates a core structure for O-linked glycans that allows the formation of sLex capping groups 9. The activity of the three sulfotransferases was verified by direct measurement of [35S]sulfate incorporation into the recombinant proteins. Transfection with the individual sulfotransferase cDNAs increased sulfate incorporation four- to eightfold over background (see Materials and Methods).
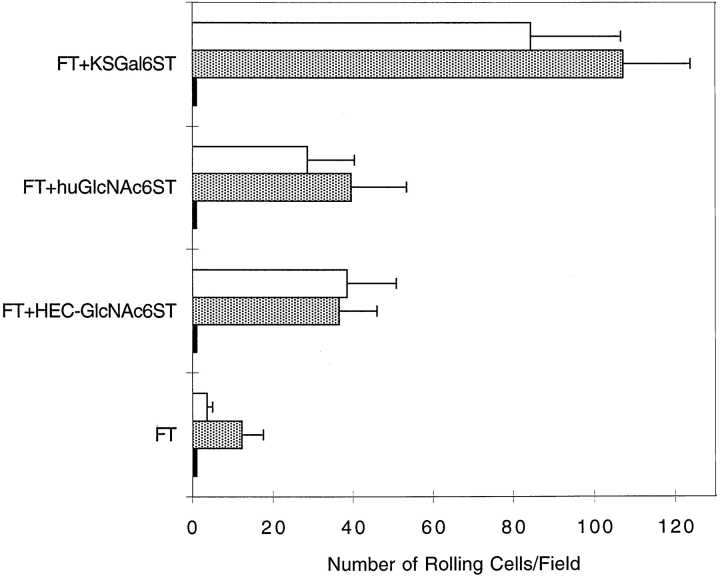
The recombinant proteins were purified and coated onto the flow chamber polystyrene plate at equal site densities, as determined by ELISA using similar conditions and polystyrene ELISA plates. Rolling of Jurkat cells and PBLs on GlyCAM-1/IgG required fucosylation, as there was no rolling on GlyCAM-1/IgG produced without FTVII transfection, with or without the inclusion of sulfotransferase cDNAs (Fig. 1). The addition of FTVII cDNA without a sulfotransferase cDNA resulted in a very low number of rolling PBLs and Jurkat cells (Fig. 1). The number of rolling PBLs and Jurkat cells was markedly increased on KSGal6ST-, HEC-GlcNAc6ST–, or huGlcNAc6ST-modified GlyCAM-1 when 1 μg of each sulfotransferase cDNA was used for transfection. The use of 0.5 or 2.0 μg sulfotransferase cDNA yielded smaller or comparable effects (not shown). An anti–L-selectin mAb (DREG56) abrogated the interaction of PBLs and Jurkat cells with the substrates (Fig. 1). EDTA or fucoidin also blocked rolling completely (Fig. 1), as expected for L-selectin–mediated binding 3. Treatment of the substrates with sialidase (Fig. 1) completely prevented tethering and rolling of Jurkat cells, consistent with previous observations made with native HEV ligands 3.
Figure 1.
Rolling of PBLs and Jurkat cells on various GlyCAM-1/IgG chimeras under flow conditions. Purified recombinant GlyCAM-1/IgG chimeras were coated at equal site densities. PBLs (white bars) and Jurkat cells (gray bars) at 2 × 106 cells/ml were perfused through the flow chamber at a wall shear stress of 1.25 or 1 dyn/cm2, respectively. At 2 min of flow, the number of rolling cells was determined. For inhibition studies (black bars), parallel samples of cells were preincubated with DREG56 mAb, fucoidin, or EDTA, or the immobilized GlyCAM-1/IgG was treated with sialidase. Stable tethering was completely absent (zero rolling cells) on nonfucosylated substrates that were sulfated by KSGal6ST, HEC-GlcNAc6ST. The values represent the mean ± SD of the number of rolling cells in at least two independent experiments, each performed in duplicate using two different fields of view. Statistical analysis using an unpaired two-tailed Student's t test showed that the enhanced binding of PBLs and Jurkat cells to sulfated GlyCAM-1/IgG was statistically significant in all cases (P < 0.0001).
Sulfation of Fucosylated GlyCAM-1/IgG Stabilizes L-Selectin–mediated Rolling Adhesion in Shear Flow.
We measured the ability of Jurkat cells to tether on the different GlyCAM-1/IgG substrates as a function of shear stress. We confirmed the shear threshold phenomenon that has been previously documented 21. Thus, stable tethering of Jurkat cells occurred at 0.4–0.6 dyn/cm2, below which little or none was observed (Fig. 2). Although a maximum tethering rate of 9% was found for the interaction of cells with fucosylated GlyCAM-1/IgG (FT), the frequency of tethered cells was increased threefold upon sulfation of C-6 on GlcNAc (FT plus huGlcNAc6ST; FT plus HEC-GlcNAc6ST) and increased sixfold upon sulfation on C-6 of Gal (FT plus KSGal6ST). The latter modification also resulted in a shift of the threshold toward lower shear stresses (Fig. 2).
Figure 2.
Jurkat cell tethering onto various GlyCAM-1/IgG chimeras in shear flow. Jurkat cells (106 cells/ml) were perfused into the chamber, and the fraction of cells that came into close proximity with the substrate and tethered stably onto different GlyCAM-1/IgG chimeras was determined. Data points represent the mean ± SD of the frequency of stable tethers in three independent experiments, each performed in duplicate using two different fields of view. Statistical analysis using an unpaired two-tailed Student's t test showed that the enhanced tethering frequency of Jurkat cells to sulfated GlyCAM-1/IgG as compared with nonsulfated GlyCAM-1 was statistically significant (for KSGal6ST, P < 0.004 in the range of 0.4–1.5 dyn/cm2; for huGlcNAc6ST, P < 0.0002 in the range of 0.6–1 dyn/cm2; and for HEC-GlcNAc6ST, P < 0.002 in the range of 0.6–1.25 dyn/cm2).
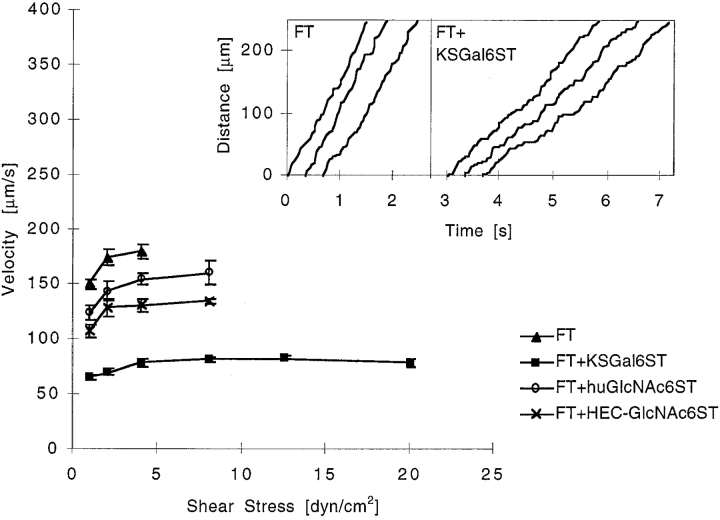
Sulfation on C-6 of GlcNAc or Gal significantly reduced the overall rolling velocity of Jurkat cells relative to that measured on fucosylated GlyCAM-1/IgG over a wide range of sheer stresses (Fig. 3). The same result was obtained with PBLs (not shown).
Figure 3.
Comparison of Jurkat cell rolling velocities on various GlyCAM-1/IgG chimeras. Jurkat cells (2 × 106 cells/ml) were tethered onto different GlyCAM-1/IgG chimeras at a wall shear stress of 1 dyn/cm2 for 2 min. Wall shear stress was then increased at intervals of 5 s to a maximum of 35 dyn/cm2, and rolling velocities over 1–3 s were measured. The data points represent the mean rolling velocity ± SE of the mean of three to four independent experiments, each performed in duplicate using two different fields of view. Statistical analysis using an unpaired two-tailed Student's t test showed that the reduced rolling velocity of Jurkat cells on sulfated GlyCAM-1/IgG as compared with nonsulfated GlyCAM-1 was statistically significant (for KSGal6ST, huGlcNAc6ST, and HEC-GlcNAc6ST, P < 0.0001 at ≥1 dyn/cm2). The inset shows the frame-by-frame (1/30 s) displacement of several randomly chosen cells as described in Materials and Methods.
To determine if these differences in tethering and rolling velocity were maintained at different site densities of coated ligand, we reduced the coating concentrations of GlyCAM-1/IgG in steps of threefold dilutions. As coating concentrations were decreased, tethering rates decreased (Fig. 4 A) and rolling velocities increased at 1 dyn/cm2 (Fig. 4a and Fig. b), consistent with a reduced coating density of immobilized ligand. GlyCAM-1/IgG modified by FT plus KSGal6ST yielded higher tethering rates (Fig. 4 A) and slower rolling velocities (Fig. 4 B) than GlyCAM-1/IgG modified by FT or FT plus HEC-GlcNAc6ST at all site densities tested. Notably, cells still tethered and rolled on FT plus KSGal6ST–modified GlyCAM-1/IgG at a very low site density (27-fold diluted), whereas no interactions were observed with the other substrates. For HEC-GlcNAc6ST plus FT–modified GlyCAM-1/IgG as compared with the FT-modified ligand, we observed a significant increase in the frequency of tethering and a decrease of rolling velocity at the two highest coating concentrations, whereas there were not significant differences at lower concentrations (Fig. 4a and Fig. b).
Figure 4.
Tethering and rolling of Jurkat cells on various GlyCAM-1/IgG chimeras over a range of site densities. Jurkat cells (2 × 106 cells/ml) were perfused into the chamber at 1 dyn/cm2, and the frequency of stable tethers (A) and the velocity of cells (B) was determined after 2 min of flow. Data points represent the mean ± SD (A) and the mean ± SEM (B) in three independent experiments, each performed at least in duplicate. Statistical analysis using an unpaired two-tailed Student's t test showed that the enhanced tethering frequencies and slower velocities of Jurkat cells on sulfated GlyCAM-1/IgG as compared with nonsulfated GlyCAM-1/IgG were statistically significant (for KSGal6ST, P < 0.0001 [A and B] at all site densities; for HEC-GlcNAc6ST at 33.3 and 100%, P < 0.008 [A] and P < 0.005 [B]).
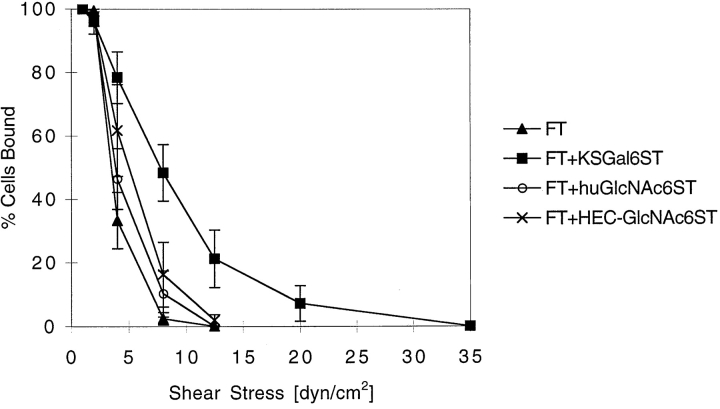
As others have observed 17 22, we found that L-selectin–mediated rolling was “jerky,” with periods of smooth movement interrupted by pauses. Analysis of the behavior of individual cells (Fig. 3, inset) revealed that sulfation did not affect the duration of pauses but that each modification significantly increased the frequency of pauses. Sulfation of C-6 on Gal significantly reduced the rolling velocity of cells between pauses, whereas sulfation on C-6 of GlcNAc caused a marginal reduction (Table ). These effects resulted in a net decrease in the rolling velocity measured over long distances (Fig. 3). Sulfation of GlyCAM-1/IgG also resulted in enhanced adhesive strength, as measured by an increase in the resistance of rolling Jurkat cells to shear-induced detachment (Fig. 5).
Table 2.
Single-Cell Analysis of Jurkat Cells Rolling on Sulfated GlyCAM-1/IgG Substrates
| Average duration of pauses | Frequency of pauses | Velocity between pauses | Overall velocity | |
|---|---|---|---|---|
| s | s−1 | μm/s | μm/s | |
| FT | 0.039 | 1.7 ± 0.7 | 183 ± 13 | 162 ± 9 |
| FT plus KSGal6ST | 0.047 | 6.7 ± 0.9 | 121 ± 4 | 73 ± 10 |
| FT plus huGlcNAc6ST | 0.042 | 4.7 ± 0.8 | 158 ± 8 | 115 ± 17 |
| FT plus HEC-GlcNAc6ST | 0.043 | 4.0 ± 0.8 | 160 ± 7 | 122 ± 14 |
Cell behavior was analyzed as described in Materials and Methods.
Figure 5.
Resistance of rolling Jurkat cells to shear-induced detachment on various GlyCAM-1/IgG chimeras. Jurkat cells (2 × 106 cells/ml) were allowed to tether onto different GlyCAM-1/IgG chimeras at a wall shear stress of 1 dyn/cm2 for 2 min. Wall shear stress was then increased at intervals of 5 s to a maximum of 35 dyn/cm2, and the number of rolling cells remaining bound at each shear stress was determined and expressed as the percentage of the maximum number of adherent cells. The data points represent the mean ± SD of three to four independent experiments, each performed in duplicate in two different fields of view. A statistical comparison of the detachment curves using a two-factor analysis of variance showed that the increased binding to sulfated GlyCAM-1/IgG as compared with nonsulfated GlyCAM-1 was statistically significant (for KSGal6ST, P < 0.0001; for huGlcNAc6ST, P < 0.02; and for HEC-GlcNAc6ST, P < 0.0003).
We recently demonstrated that coexpression of two sulfotransferases (KSGal6ST and HEC-GlcNAc6ST) in CHO/FTVII/CD34 cells resulted in much better binding of an L-selectin/IgM chimera than expression of the individual sulfotransferases 11. However, in several independent experiments, we were not able to demonstrate synergistic or even additive effects when we compared rolling on GlyCAM-1/IgG modified with individual sulfotransferases to that on GlyCAM-1/IgG modified with a combination of two sulfotransferases. For example, when GlyCAM-1/IgG was produced with FT plus 1 μg KSGal6ST cDNA, 69 ± 3% of cells tethered stably onto the substrate and rolled at a velocity of 71 ± 3 μm/s at 1 dyn/cm2. The combination of KSGal6ST and HEC-GlcNAc6ST (0.5 μg of each cDNA) plus FT resulted in a decrease of tethering frequency (43 ± 5% at 1 dyn/cm2) and an increase of velocity (103 ± 6 μm/s). Furthermore, we failed to observe synergistic effects of the sulfotransferases when a wide range of coating densities was tested (not shown).
Sulfation of Fucosylated GlyCAM-1/IgG Has No Effect on E-Selectin–mediated Rolling.
E-selectin is capable of binding to and supporting the rolling of transfectants on L-selectin ligands 22, although this interaction is of questionable functional significance. Because E-selectin has a C-type lectin domain highly homologous to that of L-selectin, we wanted to determine the effects of ligand sulfation on E-selectin–mediated rolling. To address this issue, we allowed 300.19 cells transfected with E-selectin to interact with the various forms of GlyCAM-1/IgG under flow conditions. Whereas there was no rolling of the parental 300.19 cells (not shown), the E-selectin transfectants rolled on fucosylated GlyCAM-1/IgG (Table ). Consistent with previous results 17 22, E-selectin–mediated rolling was significantly slower than L-selectin–mediated rolling. Also, the shear resistance of rolling adhesions was much greater for the E-selectin interactions (Table , Fig. 5). However, sulfation of the substrates produced no significant effects on tethering frequency, rolling velocity, or the strength of rolling adhesions (Table ). The comparable rolling behavior suggests similar degrees of fucosylation and sialylation in all of the substrates.
Table 3.
Rolling of E-Selectin/300.19 Cells on Various GlyCAM-1/IgG Chimeras
| Transfection | Rolling velocity (μm/s) | Shear stress for 50% detachment | Tethering frequency at 0.4 dyn/cm2 | ||
|---|---|---|---|---|---|
| 1 | 8 | 20 | |||
| dyn/cm2 | dyn/cm2 | % | |||
| FT | 3.7 ± 0.9 | 7.6 ± 1.7 | 13.5 ± 2.7 | 15.0 ± 3.4 | 61.4 ± 4.3 |
| FT plus KSGal6ST | 4.0 ± 0.8 | 7.3 ± 1.5 | 11.5 ± 2.0 | 15.2 ± 1.2 | 61.8 ± 3.8 |
| FT plus huGlcNAc6ST | 4.3 ± 0.9 | 8.8 ± 1.5 | 13.4 ± 2.6 | 11.4 ± 0.9 | 59.9 ± 6.4 |
| FT plus HEC-GlcNAc6ST | 4.4 ± 0.9 | 7.6 ± 1.8 | 11.9 ± 1.8 | 17.1 ± 2.6 | 63.7 ± 8.3 |
Statistical analysis (unpaired two-tailed Student's t test) comparing sulfated (FT plus ST) versus nonsulfated (FT) GlyCAM-1/IgG substrates showed no significant differences.
Discussion
Sulfation has been well established as a key modification of L-selectin ligands in HEVs of lymphoid organs and HEV-like vessels that are induced at sites of chronic inflammation 1. The molecular cloning of a novel family of sulfotransferases 11 12 13 allows the detailed study of the functional impact of Gal-6-sulfate and GlcNAc-6-sulfate modifications on the interactions between L-selectin and its physiological ligands. Previously, native GlyCAM-1 has been shown to be an excellent ligand for supporting L-selectin–dependent rolling in a parallel plate flow chamber 23. For our purposes, we generated a set of recombinant GlyCAM-1/IgG chimeras in which we could control fucosylation and sulfation by transfection of appropriate enzyme cDNAs. We evaluated the recombinant chimeras as adhesive substrates for lymphocytes under physiological flow conditions. As anticipated, L-selectin interactions with the sulfated ligands were calcium dependent and required both fucosylation and sialylation of the ligand. The sulfation modifications had a pronounced effect on L-selectin–dependent tethering and rolling and the strength of rolling adhesions. In agreement with previous data indicating that E-selectin interactions with its ligands are sulfation independent 24, we observed that E-selectin–mediated rolling on the chimeras was not affected by sulfation, further demonstrating a difference in the nature of the recognition determinants for these two selectins.
Cell tethering and rolling through L-selectin is a complex phenomenon that depends on the kinetic rate constants for the selectin–ligand bond 17 25 as well as various cellular factors 22 26 27. Our detailed tracking of individual cells indicated that the dominant contributing factor to decreased rolling velocities on the sulfated substrates was an increase in the frequency of pauses. There was no significant effect of sulfation on pause duration. These results are consistent with the possibility that sulfation increases the intrinsic on-rate of bond formation rather than decreasing the off-rate 17. The enhanced tethering rates and strengths of rolling adhesion that we observed with sulfated ligands in this study and the increased equilibrium binding observed previously 11 are also compatible with this explanation. However, because our rolling studies were performed on a relatively high ligand density, we cannot draw firm conclusions regarding the effects of sulfation on single bond properties. The application of biophysical techniques, such as surface plasmon resonance 28, will be required to address these questions.
In the assays described herein and in the CHO cell transfection experiments reported previously 11, sulfation on C-6 of Gal or GlcNAc within GlyCAM-1 and CD34 enhanced L-selectin interactions. At this point, we cannot judge the relative importance of each modification, because we were able to measure only overall sulfation levels without knowing how much of the incorporated sulfate actually participated in L-selectin binding. An interesting possibility is that the optimal recognition determinant for L-selectin involves both sulfation modifications. However, in experiments to date, we have not been able to demonstrate synergistic effects on the velocities or tethering rates of lymphocytes when GlyCAM-1/Ig was modified by both sulfotransferases. These results contrast with those of our previous study 11, in which we measured the binding of an L-selectin/IgM probe to CHO cells transfected with CD34, FTVII, C2GnT, and combinations of the sulfotransferases. Further experiments are needed to reconcile these equilibrium binding observations with measurements of dynamic parameters in the flow chamber reported herein.
Lymphocyte homing to lymph nodes and other secondary lymphoid organs is a multistep process in which lymphocytes must tether and roll on HEVs before firmly arresting via chemokine-induced integrin activation 1 2. The findings of this study indicate that sulfation of ligands could promote the overall process at several levels: (a) by increasing the extent of tethering, (b) by increasing the strength of rolling adhesions, and (c) by decreasing overall rolling velocity and thereby facilitating the effects of integrin-activating stimuli. The family of newly cloned sulfotransferases will allow further analysis of the functional contribution of the different sulfation modifications to these dynamic processes in lymphoid organs and sites of inflammation.
Acknowledgments
This work was supported by grants from the National Institutes of Health (merit award R37GM23547 and RO1GM5741), University of California Cancer Research Coordinating Committee, and Roche Bioscience (to S.D. Rosen).
Footnotes
1used in this paper: CHO, Chinese hamster ovary; FT, fucosyltransferase; GlyCAM-1, glycosylation-dependent cell adhesion molecule 1; HECs, high endothelial cells; HEVs, high endothelial venules; hu, human; STs, sulfotransferases
References
- Girard J.-P., Springer T.A. High endothelial venulesspecialized endothelium for lymphocyte migration. Immunol. Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Rosen S.D. Selectins. In: Vale R., Kreis T., editors. Guide to the Extracellular Matrix and Adhesion Proteins. 2nd Edition. Oxford University Press; Oxford, UK: 1999. pp. 290–297. [Google Scholar]
- Imai Y., Lasky L.A., Rosen S.D. Sulphation requirement for GlyCAM-1, an endothelial ligand for L-selectin. Nature. 1993;361:555–557. doi: 10.1038/361555a0. [DOI] [PubMed] [Google Scholar]
- Hemmerich S., Butcher E.C., Rosen S.D. Sulfation-dependent recognition of HEV-ligands by L-selectin and MECA 79, an adhesion-blocking mAb. J. Exp. Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N., Mitsuoka C., Kanamori A., Hiraiwa N., Uchimura K., Muramatsu T., Tamatani T., Kansas G.S., Kannagi R. Reconstitution of functional L-selectin ligands on a cultured human endothelial cell line by cotransfection of α1→3 fucosyltransferase VII and newly cloned GlcNAcβ:6-sulfotransferase cDNA. Proc. Natl. Acad. Sci. USA. 1999;96:4530–4535. doi: 10.1073/pnas.96.8.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich S., Bertozzi C.R., Leffler H., Rosen S.D. Identification of the sulfated monosaccharides of GlyCAM-1, an endothelial derived ligand for L-selectin. Biochemistry. 1994;33:4820–4829. doi: 10.1021/bi00182a010. [DOI] [PubMed] [Google Scholar]
- Hemmerich S., Rosen S.D. 6′-sulfated, sialyl Lewis X is a major capping group of GlyCAM-1. Biochemistry. 1994;33:4830–4835. doi: 10.1021/bi00182a011. [DOI] [PubMed] [Google Scholar]
- Hemmerich S., Leffler H., Rosen S.D. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J. Biol. Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- Mitsuoka C., Sawada-Kasugai M., Ando-Furui K., Izawa M., Nakanishi H., Nakamura S., Ishida H., Kiso M., Kannagi R. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis X. J. Biol. Chem. 1998;273:11225–11233. doi: 10.1074/jbc.273.18.11225. [DOI] [PubMed] [Google Scholar]
- Bistrup A., Bhakta S., Lee J.K., Belov Y.C., Gunn M.D., Zuo F.-R., Huang C.-C., Kannagi R., Rosen S.D., Hemmerich S. Sulfotransferases of two specificities function in the reconstitution of high-endothelial-cell ligands for L-selectin. J. Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura K., Muramatsu H., Kaname T., Ogawa H., Yamakawa T., Fan Q.W., Mitsuoka C., Kannagi R., Habuchi O., Yokoyama I. Human N-acetylglucosamine-6-O-sulfotransferase involved in the biosynthesis of 6-sulfo sialyl Lewis Xmolecular cloning, chromosomal mapping, and expression in various organs and tumor cells. J. Biochem. (Tokyo). 1998;124:670–678. doi: 10.1093/oxfordjournals.jbchem.a022164. [DOI] [PubMed] [Google Scholar]
- Fukuta M., Inazawa J., Torii T., Tsuzuki K., Shimada E., Habuchi O. Molecular cloning and characterization of human keratan sulfate Gal-6-sulfotransferase. J. Biol. Chem. 1997;272:32321–32328. doi: 10.1074/jbc.272.51.32321. [DOI] [PubMed] [Google Scholar]
- Scudder P.R., Shailubhai K., Duffin K.L., Streeter P.R., Jacob G.S. Enzymatic synthesis of a 6-sulfated sialyl-Lewisx which is an inhibitor of L-selectin binding to peripheral addressin. Glycobiology. 1994;4:929–933. doi: 10.1093/glycob/4.6.929. [DOI] [PubMed] [Google Scholar]
- Koenig A., Jain R., Vig R., Norgard-Sumnicht K.E., Matta K.L., Varki A. Selectin inhibitionsynthesis and evaluation of novel sialylated, sulfated and fucosylated oligosaccharides, including the major capping group of GlyCAM-1. Glycobiology. 1997;7:79–93. doi: 10.1093/glycob/7.1.79. [DOI] [PubMed] [Google Scholar]
- Galustian C., Lubineau A., le Narvor C., Kiso M., Brown G., Feizi T. L-selectin interactions with novel mono- and multisulfated Lewis x sequences in comparison with the potent ligand 3′-sulfated Lewis a. J. Biol. Chem. 1999;274:18213–18217. doi: 10.1074/jbc.274.26.18213. [DOI] [PubMed] [Google Scholar]
- Alon R., Chen S., Puri K.D., Finger E.B., Springer T.A. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J. Cell Biol. 1997;138:1169–1180. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangemann K., Giblin P., Gunn M.D., Rosen S.D. A high endothelial cell derived chemokine induces rapid, efficient, and subset-selective arrest of rolling T-lymphocytes on a reconstituted endothelial substrate. J. Immunol. 1998;161:6330–6337. [PubMed] [Google Scholar]
- Maly P., Thall A.D., Petryniak B., Rogers C.E., Mith P.L., Marks R.M., Kelly R.J., Gersten K.M., Cheng G., Saunders T.L. The Fuc-TVII α(1,3)fucosyltransferase Fuc-TVII controls lymphocyte homing, and blood leukocyte emigration through an essential role in L-,E- and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Bierhuizen M.F., Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal β 1-3-GalNAc-R (GlcNAc to GalNAc) β 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc. Natl. Acad. Sci. USA. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger E.B., Puri K.D., Alon R., Lawrence M.B., Von Andrian U.H., Springer T.A. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- Stein J.V., Cheng G., Stockton B.M., Fors B.P., Butcher E.C., von Andrian U.H. L-selectin–mediated leukocyte adhesion in vivomicrovillous distribution determines tethering efficiency, but not rolling velocity. J. Exp. Med. 1999;189:37–50. doi: 10.1084/jem.189.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwir O., Shimron F., Chen C., Singer M.S., Rosen S.D., Alon R. GlyCAM-1 supports leukocyte rolling in flowevidence for a greater dynamic stability of L-selectin rolling of lymphocytes than of neutrophils. Cell Adhes. Commun. 1998;6:349–370. doi: 10.3109/15419069809010793. [DOI] [PubMed] [Google Scholar]
- Pouyani T., Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- Puri K.D., Finger E.B., Springer T.A. The faster kinetics of L-selectin than of E-selectin and P-selectin rolling at comparable binding strength. J. Immunol. 1997;158:405–413. [PubMed] [Google Scholar]
- Hafezi-Moghadam A., Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J. Exp. Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Springer T.A. An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J. Cell Biol. 1999;144:185–200. doi: 10.1083/jcb.144.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe P.A., Barclay A.N. Transient intercellular adhesionthe importance of weak protein-protein interactions. Trends Biochem. Sci. 1994;19:354–358. doi: 10.1016/0968-0004(94)90109-0. [DOI] [PubMed] [Google Scholar]