Abstract
The antiapoptotic protein cellular FLICE (Fas-associated death domain–like IL-1β–converting enzyme) inhibitory protein (cFLIP) protects cells from CD95(APO-1/Fas)-induced apoptosis in vitro and was found to be overexpressed in human melanomas. However, cytotoxic T cell–induced apoptosis, which is critically involved in tumor control in vivo, is not inhibited by cFLIP in vitro, as only CD95- and not perforin-dependent lysis is affected. This calls into question whether cFLIP is sufficient to allow escape from T cell–dependent immunity. Using two murine tumors, we directly demonstrate that cFLIP does result in escape from T cell immunity in vivo. Moreover, tumor cells are selected in vivo for elevated cFLIP expression. Therefore, our data indicate that CD95-dependent apoptosis constitutes a more prominent mechanism for tumor clearance than has so far been anticipated and that blockade of this pathway can result in tumor escape even when the perforin pathway is operational.
Keywords: CD95, apoptosis, CTL, perforin, cytotoxicity
Apoptosis is a carefully controlled suicide program that serves to dispose of superfluous or unwanted cells 1. It can be induced by the triggering of specific death receptors, such as CD95(APO-1/Fas) 2. Activation of CD95 can lead to recruitment and activation of caspase-8 3 4 5 6, which then engages the cell death machinery 7 8. Several mechanisms have been proposed over the last few years that can interfere with death receptor–induced apoptosis 7 9. Of these, cFLIP (also called Casper/iFLICE/FLAME-1/CASH/CLARP/MRIT/usurpin) 9 is probably the most receptor proximal. Interestingly, cFLIP expression is increased in human melanomas (references 10 and 11; Medema, J.P., and J. de Jong, unpublished observations), suggesting that these tumor cells become refractory to CD95-induced apoptosis.
CTLs play a critical role in the control of tumor growth 12. They lyse their targets through CD95- as well as perforin-dependent pathways 13 14 15. Although both pathways can converge at an early step in the signaling cascade that leads to target cell apoptosis 16, cFLIP was shown to only inhibit CD95-dependent and not perforin-dependent apoptosis 17. In effect, cFLIP overexpression fails to protect cells from lysis by an effector population consisting of polyclonal alloreactive CTLs in vitro 17. We obtained comparable results with tumor-specific CTL clones that have a defined peptide specificity and Raji, a human Burkitt B cell lymphoma, loaded with the relevant peptide epitope as target cell (Medema, J.P., and J. de Jong, unpublished observations). Clearly, cFLIP overexpression efficiently blocks the CD95 pathway but does not inhibit CTL-mediated lysis in vitro.
Here, we set out to analyze whether cFLIP overexpression can affect the sensitivity of tumor cells to T cell immunity in vivo and permit tumorigenesis. We employed two murine tumors whose eradication critically depends on the CTL response 18 19. We demonstrate that overexpression of cFLIP results in their escape from T cell immunity. Moreover, we show that tumor cells are selected in vivo for elevated cFLIP expression.
Materials and Methods
Mice.
C57BL/6 perforin−/− (PKO, H-2b; reference 20; a gift from Dr. M. van den Broek) and C57BL/6 Kh (B6, H-2b) mice were bred at TNO-PG (Leiden, The Netherlands). C57BL/6nu/nu (B6 nude, H-2b) mice were obtained from Bomholtgard (Ry, Denmark).
Cells.
Adenovirus type 5 E1A plus mutant EJ-ras–transfected (AR6; reference 19) and MBL2-Fas (MF; reference 20) (a gift from Dr. M. van den Broek, Zürich, Switzerland) were transfected by electroporation (240 V, 960 μF) with a plasmid encoding murine FLAG-tagged cFLIP under the control of a CMV promoter (cDNA provided by Dr. J. Tschopp, Lausanne, Switzerland) or a control plasmid. Sam-Db is a mouse embryo fibroblast transfected with a plasmid encoding the E1A epitope coupled to a signal sequence and murine H-2Db and therefore presents high levels of the Ad5E1A epitope in the context of H-2Db. CD95L-expressing C57BL/6lpr/lpr mouse embryo cells (MECs) were obtained by Ca3(PO4)2 transfection with a plasmid encoding murine CD95L. Clone 1 was obtained by immunizing mice with RMA and is specific for an epitope encoded by the FMR-MuLV gagLeader sequence (CCLCLTVFL). The E1A (line 5)– and gagLeader (clone 1)–specific CTLs were cultured as previously reported 16 19.
Cytotoxicity/Apoptosis Assays.
Detection of anti-CD95–triggered apoptosis in MF was performed using the Nicoletti assay as described 21. Cell-mediated cytotoxicity was determined either by incubating Na51CrO4- or [3H]thymidine-labeled target cells (1 h, 100 μCi and 4 h, 5 μCi, respectively) with CTL clones or CD95L-expressing MECs at different E/T ratios. Release of 51Cr was determined by collecting the medium after 6 h. Maximum release was determined by incubation of the labeled cells with 1 M HCl. [3H]thymidine retention was determined by harvesting the cells and counting in the presence of scintillation fluid. To degrade perforin, CTLs were preincubated for 2 h with 75 nM concanamycin A (CMA; Sigma Chemical Co.), which was present throughout the assay 22. E1A-specific CTL response in vivo was determined by isolation of splenocytes and subsequent restimulation with Sam-Db. After 6 d, CTL activity toward Sam-Db was analyzed with 51Cr release.
Immunoprecipitation/Western Blot.
Cell lysates and immunoprecipitates were generated as described 6 21. For immunoprecipitations, the polyclonal rabbit anti-FLAG antibody (Zymed Labs., Inc.) was used. SDS-PAGE and Western blot analysis were performed using a standard protocol 6 16 21 with the anti–FLAG-M2 antibody (Eastman Kodak Co.). Flow cytometry was performed as previously described 23 using Jo2–FITC (PharMingen) and anti–mouse mAb against H-2Db and H-2Kb.
Tumor Challenge.
AR6 tumors were injected subcutaneously into 6-wk-old male mice at 2 × 107 cells per mouse. Mice were killed when tumors reached a size >1,000 mm3. MF tumors were injected intraperitoneally into 6-wk-old female mice and were followed by weighing the animals. Mice were killed at >15% weight gain and/or at clear signs of intraperitoneal tumor growth.
Results and Discussion
The inability of cFLIP to prevent CTL killing in vitro makes it questionable whether increased cFLIP expression, as observed in human melanomas (references 10 and 11; Medema, J.P., and J. de Jong, unpublished observations), can protect tumor cells from CTL-mediated cytotoxicity in vivo and thereby provide a mechanism of tumor escape. To analyze this directly, we used the murine tumor line MF. MF is a CD95 transfectant of MBL2 20, a Moloney murine leukemia virus–induced lymphoma that can be controlled in vivo by virus-specific CTLs 18. Due to the high sensitivity of MF to CD95-induced apoptosis, it is well suited to testing the potential of cFLIP to modulate tumorigenicity.
Transfectants of MF were generated either expressing high levels (MF-FLIPhigh) or, as a control, very low levels (MF-FLIPlow) of FLAG-tagged cFLIP (Fig. 1 A, inset). Both lines express identical amounts of MHC class I and CD95 (data not shown), but only MF-FLIPlow is sensitive to apoptosis induced by the murine CD95–specific antibody Jo2 in vitro (Fig. 1 A). Overexpression of cFLIP does not affect tumor-specific CTL–induced cytotoxicity (Fig. 1 B). However, when the perforin pathway of this CTL is blocked by preincubation with CMA, a substance that results in specific degradation of perforin 22, the sensitivity of MF-FLIPhigh is completely lost, whereas MF-FLIPlow killing is only slightly reduced (Fig. 1 B). These data also indicate that for the MF tumor, cFLIP expression only affects CD95- and not perforin-dependent killing and that both pathways need to be inhibited for MF to escape from CTL-induced apoptosis in vitro.
Figure 1.
Increased tumorigenicity of MF cells by expression of cFLIP. (A) MF cells 20 were tested for their sensitivity to CD95-induced apoptosis with the murine CD95–specific antibody Jo2 at increasing concentrations. After 16 h, apoptotic nuclei were determined using the Nicoletti assay 21. Inset shows expression of FLAG-tagged cFLIP, which was immunoprecipitated using a rabbit polyclonal anti-FLAG mAb and subsequently detected on Western blot with an mAb against FLAG. (B) CTL-induced DNA fragmentation of MF-FLIPlow (circles) or MF-FLIPhigh (squares) in a 6-h DNA fragmentation assay using the Moloney virus gagLeader-specific CTL clone 1, either left untreated (open symbols) or preincubated for 2 h with CMA (filled symbols). Experiments shown are representative of at least three performed with similar results. (C) MF cells were injected intraperitoneally into PKO (filled symbols) or wild-type mice (open symbols) at 102 and 106 cells, respectively. (D) 103 (filled symbols) or 106 (open symbols) MF cells were injected into nude mice. In both C and D, squares represent MF-FLIPhigh and circles represent MF-FLIPlow.
We subsequently set out to analyze the role of perforin- and CD95-dependent cytotoxicity in vivo. Injection of 102 MF-FLIPlow cells into syngeneic PKO mice results in clearance of the tumor cells in the majority of these mice, whereas injection of the same dose of MF-FLIPhigh cells results in progressive tumor growth (Fig. 1 C). To our knowledge, this outcome represents the first direct proof that overexpression of cFLIP enables tumor cells to escape CD95-dependent cytotoxicity not only in vitro but also in vivo.
Next we tested whether cFLIP expression also allowed these tumor cells to escape destruction by the immune response in wild-type mice, in which not only the CD95- but also the perforin-dependent pathway is functional. Wild-type mice are far more resistant to MF-FLIPlow. Injection of up to 106 of these cells does not efficiently induce tumors (Fig. 1 C), whereas such doses give rise to progressively growing tumors in PKO mice (data not shown). This indicates that perforin-dependent killing is an essential aspect of the immune defense against this tumor, an observation that is in line with previous observations 20 and our in vitro data (Fig. 1 B). Nevertheless, we found that injection of 106 MF-FLIPhigh cells leads to much higher tumor-take in wild-type mice than injection of the same number of MF-FLIPlow cells (Fig. 1 C). Importantly, MF-FLIPlow and MF-FLIPhigh are equally efficient in inducing tumor growth in T cell–deficient nude mice (Fig. 1 D). This indicates that overexpression of cFLIP enables MF to escape from T cell–dependent immunity in immunocompetent mice. Even in the presence of perforin-dependent cytotoxicity, which increases the resistance of mice to these tumors and is sufficient to obtain complete lysis in vitro, the T cell–dependent immune response in vivo is not adequately equipped to eradicate MF-FLIPhigh tumors. These data underscore the crucial role of CD95-induced apoptosis in tumor clearance by a physiological T cell response and indicate that blockade of the CD95 pathway can tip the balance in favor of the tumor cells.
Due to the high levels of CD95 on MF, CTL activity against this line may be skewed toward this pathway. Therefore, we analyzed the effect of cFLIP on a tumor that expresses only modest levels of endogenous CD95. Tumor line AR6 has been generated by transfection of B6 MECs with the adenovirus type 5 E1A and mutant EJ-ras oncogenes. Subcutaneous injection of AR6 results in initial tumor growth, after which the tumor regresses due to an efficient CTL response directed against an epitope encoded by E1A 19. AR6 expresses low but detectable levels of CD95 (Fig. 2 A). We transfected AR6 with FLAG-tagged cFLIP (AR6–FLIP) or as control with vector alone (AR6–vector) (Fig. 2 B, inset). Two subclones were selected on the basis of comparable MHC class I expression, growth kinetics, and morphological features in vitro. AR6–vector is resistant to Jo2 (anti–murine CD95)–induced apoptosis, probably due to the low cytotoxic potential of this antibody (Medema, J.P., and J. de Jong, unpublished observation). However, in cocultures with CD95L-expressing MECs, apoptosis of AR6–vector is induced but AR6–FLIP is fully resistant (Fig. 2 B). Even though CD95-induced apoptosis is completely blocked, we find that cFLIP does not affect lysis of AR6–FLIP by E1A-specific CTLs (Fig. 2 C). Inhibition of the CD95 pathway during CTL-induced apoptosis by cFLIP is, however, suggested by the partial cleavage of FLAG-tagged cFLIP in AR6–FLIP (Fig. 2 C, inset), which has been shown to correlate with resistance 10 24.
Figure 2.
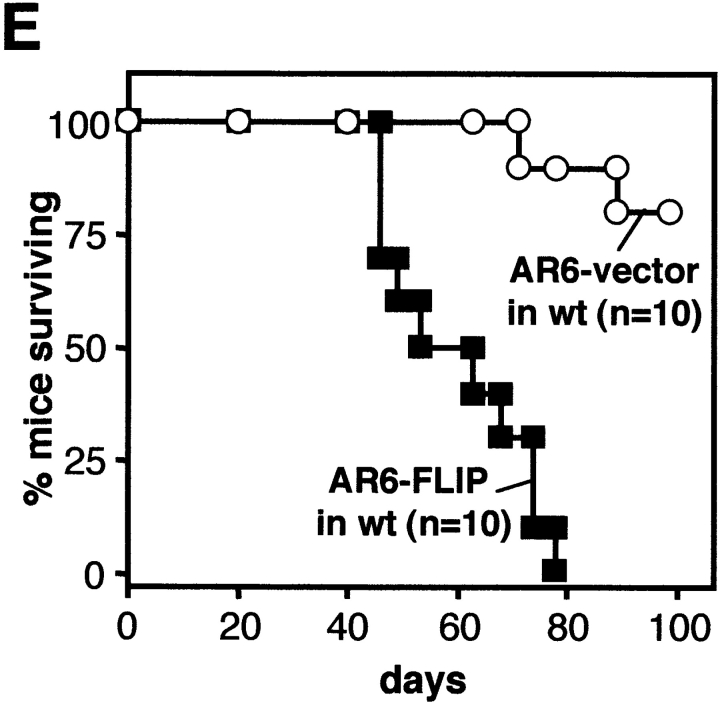
cFLIP renders AR6 cells resistant to CD95-induced apoptosis. (A) The parental AR6 line was tested for surface CD95 expression using Jo2–FITC in comparison with MF. (B) Both AR6 transfectants were analyzed for their sensitivity to CD95L-expressing MECs in a 16-h DNA fragmentation assay at different E/T ratios. Inset shows expression of tagged cFLIP in both AR6 lines as detected with the anti-FLAG antibody, M2. (C) AR6 lines were incubated with E1A-specific CTLs (line 5) in a 6-h 51Cr-release assay. Experiments shown are representative of at least three performed with similar results. Inset shows full length and cleaved cFLIP (anti-FLAG M2) in AR6–FLIP before and after incubation with CTL for 2 h. (D and E) AR6–vector (○) and AR6–FLIP (▪) were injected subcutaneously (2 × 107) into PKO (D; n = 5) or wild-type (wt) mice (E; n = 10). Survival of the mice is shown at different times after injection. Identical results were obtained with a separate pair of AR6 transfectants (data not shown).
When injected into nude mice, the AR6 lines display equal and uncontrolled tumor growth (data not shown). In contrast, both PKO and wild-type mice can control the AR6–vector tumor (Fig. 2D and Fig. e). However, comparable to our findings with MF-FLIPhigh, neither of these mouse strains is capable of rejecting the cFLIP-overexpressing tumor AR6–FLIP (Fig. 2D and Fig. e). Thus, escape from T cell–mediated immunity in vivo by cFLIP overexpression is not limited to tumors with very high CD95 surface expression.
It is important to note that the AR6–FLIP tumor grows almost as efficiently in PKO and wild-type mice (Fig. 2D and Fig. e), which suggests that eradication of this tumor depends almost exclusively on CD95. This observation is not without precedent, as other murine tumor lines, such as the T cell lymphoma RMA and the melanoma B16, were shown to be equally tumorigenic in PKO and wild-type mice 20. Apparently, clearance of certain tumors does not critically depend on perforin-based cytotoxicity but can be achieved by other mechanisms, which obviously include CD95-induced apoptosis. Importantly, this dependance is not determined by a high sensitivity to CD95-induced apoptosis, as is clear from the two tumor lines tested here. Further evaluation is required to determine the relative importance of perforin-dependent cytotoxicity among different tumor systems, but these data clearly indicate that CD95-mediated apoptosis constitutes a highly important mechanism for tumor clearance even in situations where the perforin pathway is operational.
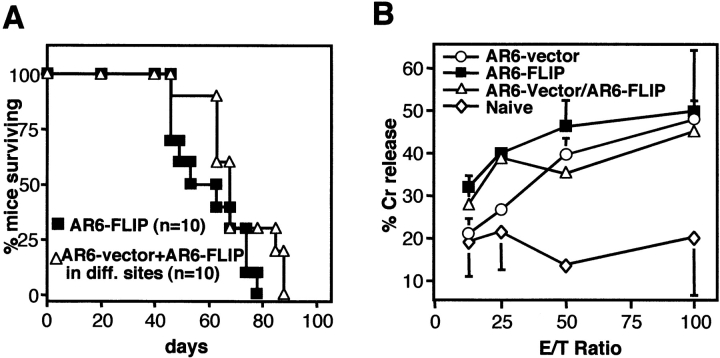
Recent findings have suggested that apoptosis of tumor cells may be required for efficient uptake and cross-presentation of tumor antigens by dendritic cells and therefore may be essential for priming of effective T cell immunity 25. To test whether immune escape by AR6–FLIP may involve prevention of T cell priming rather than escape from the T cell response, we injected B6 mice simultaneously with AR6–FLIP and AR6–vector in either flank. As expected, most mice were again capable of rejecting the AR6–vector cells, pointing to the induction of an efficient immune response. Despite this response, AR6–FLIP tumors in the other flanks of these mice developed progressively, indicating that these cFLIP-expressing tumors are capable of growing out in the face of an effective antitumor response (Fig. 3 A). To directly examine whether anti-tumor CTL immunity is induced in mice challenged with AR6–vector, AR6–FLIP, or both, we analyzed the CTL response against the E1A epitope in splenocytes from these mice. Importantly, comparable E1A-specific CTL immunity was detected in all mice (Fig. 3 B).
Figure 3.
cFLIP-induced immune evasion is due to CTL resistance. (A) Tumor growth was analyzed in wild-type mice injected simultaneously with AR6–vector (2 × 107) into the left flanks and AR6–FLIP (2 × 107) into the right flanks of mice (▵; n = 10). For comparison, mice injected in the right flanks with AR6–FLIP (2 × 107) alone is added (▪; n = 10). Percentage survival of the mice is shown. Death of these mice is in all cases due to growth of the AR6–FLIP tumor. (B) E1A-specific CTL response was determined by isolating splenocytes from naive or from tumor-injected mice 2 wk after tumor challenge (two mice per setting). Restimulated splenocytes were tested for their capacity to lyse E1A-expressing MECs. Error bars represent SEM. (C) Growing MF-FLIPlow tumors were isolated (ex vivo) from PKO or wild-type (WT) and were analyzed for their sensitivity to Jo2-induced apoptosis and for expression of tagged cFLIP on a Western blot using the anti-FLAG M2 antibody. This is in comparison with the MF cells that were used for injection (pre-inj.). CD95 sensitivity is indicated as percentage increase of apoptotic nuclei after incubation for 16 h with 1 μg/ml Jo2.
Also in the MF model, we obtained evidence that cFLIP overexpression enables escape from antitumor immunity rather than preventing the induction of this response. As shown in Fig. 1 C, a minority of the PKO and wild-type mice challenged with MF-FLIPlow developed progressively growing tumors. Intriguingly, isolation of these tumors and analysis in vitro showed that the cells had acquired resistance to CD95-mediated apoptosis, which correlates well with the increased expression of cFLIP in these cells (Fig. 3 C). Moreover, reinjection of such tumor cells revealed that they are as tumorigenic as MF-FLIPhigh (data not shown). As challenge of nude mice with MF-FLIPlow does not result in tumors with elevated cFLIP expression (data not shown), this process requires the selective pressure of the T cell immune system.
In conclusion, our data show that cFLIP-overexpressing tumors escape from T cell immunity in vivo despite the fact that they are efficiently killed in vitro. This apparent discrepancy is most likely due to limitations of in vitro assays, which do not accurately reflect the microenvironment in the tumor. For instance, in vitro CTL assays are generally performed at aphysiological E/T ratios and under conditions that allow prolonged CTL–target interactions that may give rise to a different killing potential of the CTL. Alternatively, the experimental generation of CTL clones often favors clones with high affinity, which does not necessarily reflect the response against a tumor in vivo. In addition, in in vitro assays target cells are often pretreated with IFN-γ to increase their MHC class I expression and thereby the avidity of the CTL–target interaction. Together, this may change the specificity of the response. Indeed, it has been suggested that decreasing the affinity/avidity of the CTL–target interaction shifts the balance of CTL-mediated cytotoxicity toward the CD95-dependent pathway 26 27 28. Although the exact reason for this disparity remains to be determined, our data do provide direct evidence that tumor clearance in vivo critically depends on the CD95 pathway. In view of this finding, it is interesting to note that a plethora of mechanisms has been reported by which tumors seem to block CD95-induced apoptosis. For instance, mutation 29 or simply downmodulation of CD95 30 31 32 33 is found in several tumors. Alternatively, secretion of CD95 decoy receptors 34 could provide a separate route to escape from CD95-dependent cytotoxicity. Our findings demonstrate that blockade of the CD95 pathway, for instance through overexpression of cFLIP as was found in human melanomas 10 11, can indeed serve as an efficient mechanism of immune escape by tumors.
Acknowledgments
We are grateful to Marcus Peter and Peter Krammer for providing the anti–APO-1 antibody, Jürg Tschopp for the cDNA encoding murine cFLIP and the RAJI cell lines, and Maries van den Broek and Hans Hengartner for providing the MBL2-Fas cell line and perforin-deficient mice. Furthermore, we would like to thank René Toes and Ferry Ossendorp for fruitful discussion from the beginning of this project and Andrea van den Elsas and Ton Schumacher for careful reading of the manuscript.
This work was supported by the Dutch Cancer Society.
References
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Krammer P.H. CD95(APO-1/Fas)-mediated apoptosislive and let die. Adv. Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- Kischkel F.C., Hellbardt S., Behrmann I., Germer M., Pawlita M., Krammer P.H., Peter M.E. Cytotoxicity-dependent APO-1(Fas/CD95)-associated proteins form a death-inducing signalling complex (DISC) with the receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldin M.P., Goncharov T.M., Goltsev Y.V., Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–816. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Muzio M., Chinnaiyan A.M., Kischkel F.C., O'Rourke K., Shevchenko A., Scaffidi C., Zhang M., Ni J., Gentz R., Mann M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex (DISC) Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Medema J.P., Scaffidi C., Kischkel F.C., Shevchenko A., Mann M., Krammer P.H., Peter M.E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M.E., Scaffidi C., Medema J.P., Kischkel F., Krammer P.H. The death receptors. Results Probl. Cell Differ. 1999;23:25–63. doi: 10.1007/978-3-540-69184-6_3. [DOI] [PubMed] [Google Scholar]
- Medema J.P., Borst J. T cell signalinga decision of life and death. Hum. Immunol. 1999;60:403–411. doi: 10.1016/s0198-8859(99)00008-7. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Irmler M., Thome M. Inhibition of fas death signals by FLIPs. Curr. Opin. Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- Irmler M., Thome M., Hahne M., Schneider P., Hofmann K., Steiner V., Bodmer J.L., Schroter M., Burns K., Mattmann C. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Griffith T.S., Chin W.A., Jackson G.C., Lynch D.H., Kubin M.Z. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J. Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- Melief C.J. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv. Cancer Res. 1992;58:143–175. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depreatere V., Nagata S., Hengartner H., Golstein P. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- Medema J.P., Toes R.E., Scaffidi C., Zheng T.S., Flavell R.A., Melief C.J., Peter M.E., Offringa R., Krammer P.H. Cleavage of FLICE (caspase-8) by granzyme B during cytotoxic T lymphocyte-induced apoptosis. Eur. J. Immunol. 1997;27:3492–3498. doi: 10.1002/eji.1830271250. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Schroter M., Hahne M., Schneider P., Irmler M., Thome M., Froelich C.J., Tschopp J. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J. Immunol. 1998;161:3936–3942. [PubMed] [Google Scholar]
- Jiang D., Flyer D.C. Immune response to Moloney murine leukemia virus nonviral, tumor-associated antigens fails to provide in vivo tumor protection. J. Immunol. 1992;148:974–980. [PubMed] [Google Scholar]
- Toes R.E., Offringa R., Blom R.J., Melief C.J., Kast W.M. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc. Natl. Acad. Sci. USA. 1996;93:7855–7860. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M.E., Kagi D., Ossendorp F., Toes R.E., Vamvakas S., Lutz W.K., Melief C.J., Zinkernagel R.M., Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema J.P., Scaffidi C., Krammer P.H., Peter M.E. Bcl-xL acts downstream of caspase-8 activation by the CD95 death-inducing signaling complex. J. Biol. Chem. 1998;273:3388–3393. doi: 10.1074/jbc.273.6.3388. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Shinohara N., Takayama H., Takaku K., Kondo S., Yonehara S., Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- Peter M.E., Kischkel F.C., Scheuerpflug C.G., Medema J.P., Debatin K.M., Krammer P.H. FLICE is involved in regulation of activation-induced cell death (AICD) of peripheral T cells. Eur. J. Immunol. 1997;27:1207–1212. doi: 10.1002/eji.1830270523. [DOI] [PubMed] [Google Scholar]
- Scaffidi C., Schmitz I., Krammer P.H., Peter M.E. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Cao W., Tykodi S.S., Esser M.T., Braciale V.L., Braciale T.J. Partial activation of CD8+ T cells by a self-derived peptide. Nature. 1995;378:295–298. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- Brossart P., Bevan M.J. Selective activation of Fas/Fas ligand–mediated cytotoxicity by a self peptide. J. Exp. Med. 1996;183:2449–2458. doi: 10.1084/jem.183.6.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, B., D. Hudrisier, M. Schroeter, J. Tschopp, J.C. Cerottini, and I.F. Luescher. Peptide modification or blocking of CD8, resulting in weak TCR signaling, can activate CTL for Fas- but not perforin-dependent cytotoxicity or cytokine production. 1998. J. Immunol. 161:6939–6946. [PubMed]
- Gronbaek K., Straten P.T., Ralfkiaer E., Ahrenkiel V., Andersen M.K., Hansen N.E., Zeuthen J., Hou-Jensen K., Guldberg P. Somatic Fas mutations in non-Hodgkin's lymphomaassociation with extranodal disease and autoimmunity. Blood. 1998;92:3018–3024. [PubMed] [Google Scholar]
- Hahne M., Rimoldi D., Schroter M., Romero P., Schreier M., French L.E., Schneider P., Bornand T., Fontana A., Lienard D. Melanoma cell expression of Fas(Apo-1/CD95) ligandimplications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- Strand S., Hofmann W.J., Hug H., Muller M., Otto G., Strand D., Mariani S.M., Stremmel W., Krammer P.H., Galle P.R. Lymphocyte apoptosis induced by CD95 (APO-1/Fas)ligand-expressing tumor cells—a mechanism of immune evasion? Nat. Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- Fenton R.G., Hixon J.A., Wright P.W., Brooks A.D., Sayers T.J. Inhibition of Fas (CD95) expression and Fas-mediated apoptosis by oncogenic Ras. Cancer Res. 1998;58:3391–3400. [PubMed] [Google Scholar]
- Peli J., Schroter M., Rudaz C., Hahne M., Meyer C., Reichmann E., Tschopp J. Oncogenic Ras inhibits Fas ligand-mediated apoptosis by downregulating the expression of Fas. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1824–1831. doi: 10.1093/emboj/18.7.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitti R.M., Marsters S.A., Lawrence D.A., Roy M., Kischkel F.C., Dowd P., Huang A., Donahue C.J., Sherwood S.W., Baldwin D.T. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]