Abstract
Insulin-dependent diabetes mellitus (IDDM) is an autoimmune disease resulting from apoptotic destruction of β cells in the islets of Langerhans. Low expression of antioxidants and a predilection to produce nitric oxide (NO) have been shown to underscore β cell apoptosis. With this perspective in mind, we questioned whether β cells could mount an induced protective response to inflammation. Here we show that human and rat islets can be induced to rapidly express the antiapoptotic gene A20 after interleukin (IL)-1β activation. Overexpression of A20 by means of adenovirus-mediated gene transfer protects islets from IL-1β and interferon γ–induced apoptosis. The cytoprotective effect of A20 against apoptosis correlates with and is dependent on the abrogation of cytokine-induced NO production. The inhibitory effect of A20 on cytokine-stimulated NO production is due to transcriptional blockade of inducible NO synthase (iNOS) induction; A20 inhibits the activation of the transcription factor nuclear factor κB at a level upstream of IκBα degradation. These data demonstrate a dual antiapoptotic and antiinflammatory function for A20 in β cells. This qualifies A20 as part of the physiological cytoprotective response of islets. We propose that A20 may have therapeutic potential as a gene therapy candidate to achieve successful islet transplantation and the cure of IDDM.
Keywords: A20, β cells, nuclear factor κB, nitric oxide, apoptosis
Type I insulin-dependent diabetes mellitus (IDDM)1 is an autoimmune disease resulting from specific destruction of the insulin-producing β cell within the islet of Langerhans 1 2. Many studies have focused on the initiator phase of the disease, exploring the factors that permit or provoke the autoimmune attack 2 3 4. More recently, greater attention has been devoted to understanding the mechanisms of β cell susceptibility to death. Although multiple mechanisms are involved in the destruction of β cells, the common unifying theme remains that most of these trigger the apoptotic machinery of the β cell 5 6.
β cell apoptosis can be induced by either specific T lymphocyte–mediated killing or proinflammatory cytokines. T cell–mediated β cell damage occurs through direct cognate interactions using the granzyme/perforin or Fas/Fas ligand (FasL) systems 7 8. Cytokine-mediated β cell apoptosis requires the active participation of the β cells. The intraislet release of IL-1β, TNF-α, and IFN-γ by activated mononuclear cells activates β cells to upregulate inducible nitric oxide synthase (iNOS) 9 10. Generation of iNOS results in the production of high levels of nitric oxide (NO) and, to a lesser extent, superoxide 11 12. NO and its reactive oxygen species derivatives, including peroxynitrite (OONO−), are cytotoxic to β cells 13 14. NO-mediated toxicity is the predominant mechanism responsible for β cell dysfunction and apoptosis induced by soluble mediators. In addition to its direct toxic potential, NO induces Fas expression on β cells, priming them to T lymphocyte–mediated killing 15. The central role played by NO in the pathophysiology of β cell loss during IDDM is directly demonstrated by the acceleration of IDDM in nonobese diabetic (NOD) mice (a well-studied experimental model of autoimmune diabetes) carrying the inos transgene under the control of the insulin promoter 16.
Since the early work of Reckard et al. 17 and Ballinger 18 showing that islet transplantation could cure diabetes in rodents, islet transplantation for humans has been regarded as a potential cure for diabetes 17 18 19 20. However, several obstacles still need to be overcome before successful islet transplantation becomes a reality, namely, (a) primary nonfunction in the immediate posttransplantation period, (b) recurrence of autoimmune disease, and (c) allograft rejection 21 22 23. Whether related to hypoxia, loss of nutrients, induction of nonspecific inflammatory reactions, or immune effectors implicated in the development of autoimmune disease or allograft rejection, the final outcome of these processes is destruction of the transplanted islets by apoptosis.
One way to achieve successful islet transplantation for the treatment of IDDM would be to genetically engineer β cells to express antiapoptotic and antiinflammatory proteins 24. The zinc finger protein A20 represents one such candidate for genetic engineering of β cells. A20 was originally described as an antiapoptotic TNF-α–induced gene in endothelial cells 25 26. Besides protection from apoptosis, we have demonstrated previously that A20 also inhibits proinflammatory responses in endothelial cells 27 28. In this paper, we evaluate the efficacy of A20 to protect islets from apoptosis. We demonstrate that recombinant adenovirus (rAd)-mediated gene expression of A20 in rodent islets protects against cytokine-induced apoptosis and inhibits cytokine-induced NO generation. A20 suppresses cytokine-induced NO generation at the level of iNOS transcription through blockade of the transcription factor, nuclear factor κB (NF-κB). Furthermore, we report for the first time that A20 mRNA is rapidly induced in human and rat islets after cytokine stimulation. These later data indicate that A20 is part of the physiological protective response of islets, further supporting its consideration for human gene therapy.
Materials and Methods
Islets.
Rats (male Sprague-Dawley) were purchased from The Jackson Laboratory, and islets were isolated as described previously 23. Human islets were a gift from Dr. C. Ricordi (Diabetes Research Institute, University of Miami School of Medicine, Miami, FL). Both rodent and human islets were cultured in RPMI 1640, 10% FCS with 2 mM l-glutamine, 5 mM d-glucose, and 50 U/ml of penicillin and streptomycin, at 37°C with 5% CO2.
Analysis of A20 mRNA Expression in Islets.
Total mRNA was isolated from human and rodent islets (RNeasy Mini Protocol; Qiagen), and cDNA was synthesized using random hexamers (Superscript Preamplification System for First Strand cDNA Synthesis; GIBCO BRL). PCR reactions were performed with the following primers: rodent β-actin: sense, 5′-CCTGACCGAGCGTGGCTACAGC-3′, and antisense, 5′-AGCCTCAGGGCATCGGAAC-3′; A20: sense, 5′-TTTGAGCAATATGCGGAAAGC-3′, and antisense, 5′-AGTTGTCCCATTCGTCATTCC-3′; rat iNOS: sense, 5′-TGACCTGAAAGAGGAAAAGGAC-3′, and antisense, 5′-CCAGTTTTTGATCCTCACGTG-3′. The PCR reaction was optimized for each primer pair. PCR was performed over a range of cycles 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 to ensure that amplification occurred in the linear range, and equal starting amounts of each sample were used.
rAd Vectors and Gene Transduction of Rodent Islets.
The rAd vector expressing A20 (rAd.A20) was a gift from Dr. V. Dixit (Department of Molecular Oncology, Genentech, Inc., South San Francisco, CA); the control vector expressing β-galactosidase (rAd.β-gal) was a gift from Dr. R. Gerard (Department of Biochemistry, University of Texas, Southwestern Medical Center, Dallas, TX). Islets were infected with rAd vectors immediately after isolation as described previously for other cell types 28. After infection, islets were cultured for an additional 24 h before being used for further experiments. For all experiments (unless otherwise stated), 200 islets were cultured in 500 μl of media in 24-well tissue culture plates.
Analysis of A20 Protein Expression and Islet Viability after rAd Infection.
Expression of A20 protein after rAd.A20 gene transduction in islets was determined by Western blotting using standard techniques. A20 protein expression was detected with a polyclonal A20 antiserum (A20-NT) raised against an NH2 terminus peptide sequence of human A20 (IRERTPEDIFKPTN). Islet viability after viral infection was assessed by staining with propidium iodide (10 μg/ml) and calcein-AM (2 μM; Molecular Probes), then determined by two-color fluorescence microscopy.
Flow Cytometric Analysis of Apoptosis.
Islet cultures were stimulated with recombinant murine IL-1β (10 U/ml) and recombinant rat IFN-γ (300 U/ml) (R&D Systems) for 40 h. Islets were then harvested, dispersed, fixed in 70% ethanol, and suspended into DNA staining buffer (PBS, pH 7.4, containing 0.1% Triton X-100, 0.1 mM EDTA, 50 μg/ml propidium iodide, 50 μg/ml RNase A). Islet DNA content was analyzed on a FACScan™ using CELLQuest™ acquisition software (Becton Dickson Immunocytometry Systems). Islets with a normal DNA content (≥2 N) were scored as viable, whereas islets with a hypodiploid DNA content (<2 N, termed A°) were scored as apoptotic. To exclude debris and apoptotic cell-free fragments, all events with an FL-2 area profile below that of chicken erythrocyte nuclei were excluded from analysis.
Determination of iNOS Protein Expression.
To determine the effects of A20 expression on iNOS protein induction, islets were stimulated with IL-1β (10 U/ml) for 24 h. iNOS protein expression was determined by Western blotting using the polyclonal anti-iNOS Ab, N-20 (Santa Cruz).
Determination of NO (Nitrite) Generation.
Culture media were analyzed for NO levels (measured as nitrite) by adding 50 μl of Griess reagent (equal volume of 1% sulfanilamide in 0.1 M HCl and 0.1% N-[-1-naphthyl-ethylenediamine dihydrochloride]) to 50 μl of culture media. Nitrite concentration was determined by spectrophotometry (560 nM) from a standard curve (0–200 μM) derived from NaNO2. NO data are expressed as mean ± SD [nitrite] in μM per 200 islets.
Analysis of the Role of NO in Cytokine-induced Apoptosis.
To examine whether NO could directly induce apoptosis, islets were treated for 24 h with the NO donors S-nitrosoglutathione (GSNO) or N-(2-aminoethyl)-N-(2-hydroxy-2-nitrosohydrazino-1,2-ethylenediamine (NONOate) over a range of concentrations (0.001–10 mM). To determine the role of NO in cytokine-induced apoptosis, islets were treated with IL-1β (10 U/ml) and IFN-γ (300 U/ml) in the presence or absence of the NOS inhibitor l-N 5-(1-iminoethyl) ornithine, dihydrochloride (L-NIO) used at the optimal concentration of 500 μM. The extent of islet apoptosis and NO generation was determined as described above.
Transient Transfection of the Murine β Cell Line, β-TC3.
β-TC3 cells 29 were plated at a density of 1.5 × 106 cells/well into 6-well tissue culture plates and transfected 24 h later using the Lipofectamine-Plus reagent (GIBCO BRL) with 1 μg total DNA. Specifically, β-TC3 cells were transfected with 0.6 μg of the iNOS reporter (pGLH/H2; containing 1,755 bp of the murine iNOS promoter linked to a luciferase gene [30]), a gift of Dr. W.J. Murphy (Wilkinson Laboratory of the Kansas Cancer Institute, University of Kansas Medical Center, Kansas City, KS); 0.3 μg of an expression plasmid containing the human A20 gene (pcDNA3/HA-A20) or the control empty plasmid pcDNA3; and 0.1 μg of a β-gal reporter (driven by the CMV promoter), used to correct for transfection efficiency. 24 h after transfection, cells were stimulated with IL-1β (100 U/ml) for 36 h. These conditions were shown to be optimal in preliminary experiments (data not shown). Luciferase and β-gal activity were assessed as described 27. Data are expressed as relative luciferase activity according to the formula: luciferase light units/β-gal light units × 100.
Electrophoretic Mobility Shift Assay.
To determine the effect of A20 overexpression on the transcription factor NF-κB, islets (1,000 islets/1 ml media in 24-well tissue culture plates) were stimulated with IL-1β (100 U) for 1 h. Islet nuclei were recovered by an isoosmotic/NP-40 lysis procedure, and nuclear proteins were extracted as described 31. DNA binding reactions were performed by incubating 5 μg of nuclear proteins with 1 μg of poly(dI-dC) and 105 cpm of radiolabeled NF-κB consensus oligonucleotide, 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ (Promega Corp.). For competition assays, 1.75 pmol of either unlabeled NF-κB or an unrelated oligonucleotide was added to the reaction mixture. Supershift analysis was conducted by adding 0.1 μg of Ab specific for p50/NF-κB1, p65/RelA, Rel-B, c-Rel, or Ets-1 (Santa Cruz) to the reaction 1 h before the addition of radiolabeled oligonucleotide. The DNA binding reactions were resolved on a 6% polyacrylamide gel and analyzed by autoradiography.
Determination of IκBα Degradation.
The effect of A20 expression on IκBα protein degradation was determined by Western blot analysis, after treatment with IL-1β (100 U/ml) for 0, 15, and 60 min. IκBα protein expression was detected using the polyclonal anti-IκBα Ab, C-20 (Santa Cruz).
Statistical Analysis.
All statistical analysis was conducted using the alternate Welch's method.
Results
A20 Is Induced in Islets of Langerhans in Response to Inflammatory Stimuli.
We first examined if A20 was expressed constitutively in islets and whether A20 expression could be induced by cytokine stimulation. No or weak constitutive A20 mRNA was detected in rat and human islets as analyzed by reverse transcription (RT)-PCR (Fig. 1, a and b). A20 mRNA was rapidly induced (within 1–2 h) in both rat and human islets after IL-1β stimulation (Fig. 1, a and b). Rat β insulinoma cells (Rin5F) could also be induced to rapidly express A20 mRNA after IL-1β stimulation, indicating that β cells specifically express A20 (Fig. 1 c). The identity of the A20 PCR product was confirmed by sequence analysis (data not shown). Our data demonstrate that A20 is an early response gene in cytokine-activated islets.
Figure 1.
A20 mRNA is induced in human and rat islets after stimulation with IL-1β. After isolation, 500–1,000 islets were cultured overnight and then stimulated with IL-1β (100 U/ml). A20 mRNA expression was determined by RT-PCR in (a) human islets, 1 h after stimulation; (b) rat islets, 2 h after stimulation; and (c) rat insulinoma cells (Rin5F), 1 and 2 h after stimulation. A20 mRNA was rapidly induced after IL-1β activation in both species. media, no IL-1β stimulation; TC, template control without cDNA.
rAd-mediated Gene Transfer of A20 to Rat Islets of Langerhans Achieves High Level of Expression without Toxic Effects.
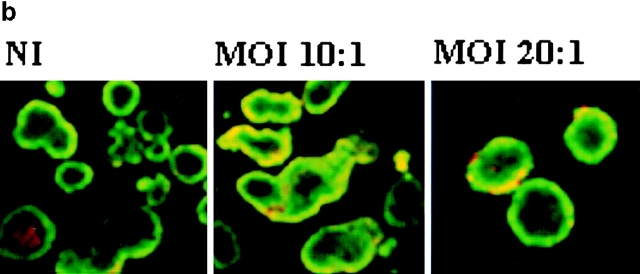
To study the function of A20 in islets, we overexpressed A20 by rAd-mediated gene transfer. Islets infected with a recombinant β-gal adenovirus (rAd.β-gal) were used as controls. Islets infected in vitro with a rAd carrying the A20 transgene (rAd.A20) expressed high levels of A20 protein (Fig. 2 a). In vitro–infected islets showed normal morphology and viability in culture (Fig. 2 b). Infection with greater multiplicity (MOI; e.g., ≥30:1) led to significant toxicity in our system (data not shown) and hence MOIs in the range of 1–20:1 were used.
Figure 2.
rAd–mediated gene transfer induces high A20 expression in rat islets without toxic effects. (a) To determine the level of A20 expression after gene transduction, rat islets infected with rAd.A20 (MOI 1:1, 10:1, and 20:1) were cultured for 24 h and assessed for expression of A20 protein by Western blotting with the polyclonal Ab, A20-NT. Controls were noninfected islets (1.) and rAd.β-gal–infected islets (2.). (b) Noninfected islets (NI) and rAd.A20 (MOI 10:1 and 20:1)–infected islets were cultured for 48 h and assessed for cell viability by staining with calcein-AM (2 μM) and propidium iodide (10 μg/ml). Viable cells stain green, whereas necrotic and apoptotic cells label red. Islets infected with rAd.A20 express high levels of the transgene and show normal morphology with the absence of central necrosis.
To test the function of islets after infection with rAd, 500 freshly isolated islets were infected in vitro with rAd.β-gal (MOI 10:1) for 1 h at 37°C. Islets were then washed and transplanted under the kidney capsule of B6AF1 mice rendered diabetic by intraperitoneal injection of streptozotocin (160 mg/kg) 7–14 d before the day of transplantation 32. All transplanted animals were normoglycemic (glucose levels 76–116 mg/dl) by day 4–5 after transplantation. This result indicates that adenoviral infection of islets per se does not alter their function.
A20 Overexpression Protects Islets from Cytokine-induced Apoptosis.
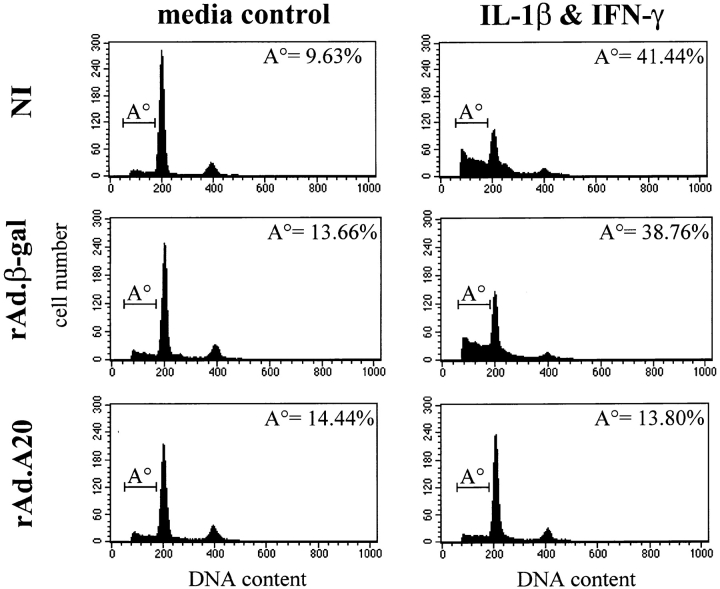
Previous work has demonstrated that A20 is an early response gene that protects cells against cytokine-mediated cytotoxicity 25 26. The proinflammatory cytokine IL-1β is cytotoxic to β cells and represents a significant mediator of β cell apoptosis in IDDM, especially in combination with IFN-γ 10. Therefore, we examined whether A20 would protect islets against IL-1β– and IFN-γ–mediated toxicity. IL-1β and IFN-γ used at the optimal dose of 10 and 300 U/ml, respectively, induced a significant percentage of apoptosis in rat islets after 40 h in culture (Fig. 3). This percentage (mean ± SD, n = 4 independent experiments) reached 57.58 ± 16.51 and 55.08 ± 18.35% in both noninfected and control rAd.β-gal–infected islets, respectively (P < 0.01, n = 4), as evaluated by FACS® analysis of DNA content (Fig. 3). In contrast, rAd.A20-infected islets were protected from IL-1β– and IFN-γ–mediated apoptosis; the percentage of apoptosis in these islets was not significantly different (P = 0.714, n = 4) from that observed in non–cytokine-activated control islets (Fig. 3). These data demonstrate that A20 protects islets from cytokine-mediated apoptosis.
Figure 3.
A20 protects rat islets against cytokine-induced apoptosis. Noninfected (NI), rAd.β-gal–, and rAd.A20-infected islets were cultured in the presence or absence of IL-1β (10 U/ml) and IFN-γ (300 U/ml) for 40 h, and the percentage of apoptotic cells was determined by flow cytometry. The percentage of apoptosis in each treatment (given in upper right corner) was calculated by analysis of the percentage of events in the subdiploid region (termed A°; where DNA content <2 N) from the FL-2 area histogram (total of 10,000 events collected). The data presented are representative of four independent experiments conducted. Results demonstrate that expression of A20 in islets protects them from cytokine-mediated apoptosis.
A20-mediated Protection from Apoptosis Correlates with Suppression of NO Production.
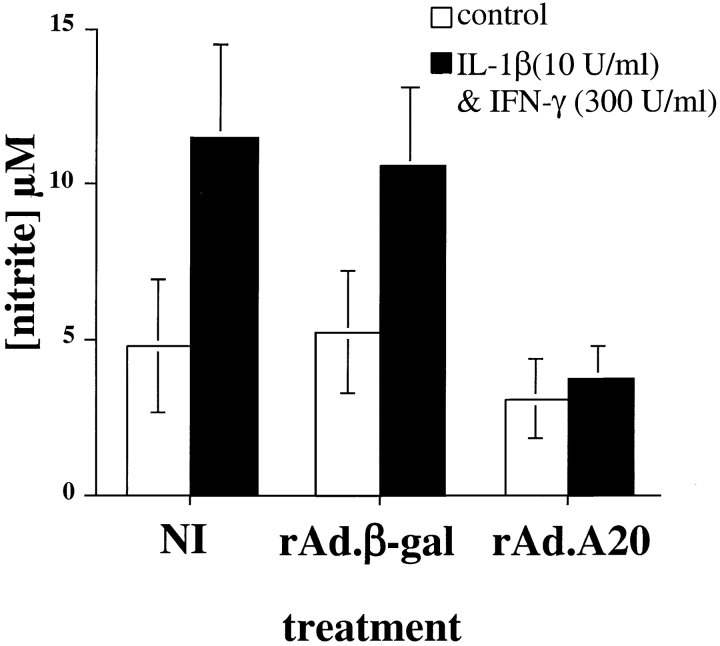
There is substantial evidence that free radical generation, such as release of NO and peroxynitrites, mediates the proapoptotic effects of cytokines on islets 9 13 14. Therefore, we examined the levels of NO released in the culture medium of noninfected, rAd.β-gal–, and rAd.A20-infected islets 40 h after cytokine stimulation. Noninfected and rAd.β-gal–infected islets produced equally high levels of NO after stimulation with IL-1β and IFN-γ (Fig. 4). In contrast, NO production in rAd.A20-infected islets was totally suppressed (P < 0.0001, n = 4) compared with noninfected and rAd.β-gal–infected islets and was not significantly different (P = 0.099, n = 4) from background levels observed in non–cytokine-activated groups (Fig. 4). Thus, the percentage of islets undergoing apoptosis for each treatment correlated with their production of NO.
Figure 4.
A20 inhibits production of NO by cytokine-activated rat islets. Noninfected (NI), rAd.β-gal–, and rAd.A20-infected islets were cultured in the presence or absence of IL-1β (10 U/ml) and IFN-γ (300 U/ml) for 40 h, and NO levels were determined in the culture medium. There was no significant difference in IL-1β–stimulated NO production by rAd.β-gal–infected islets compared with noninfected islets (P = 0.315). However, NO production was totally abrogated in A20-expressing islets compared with noninfected or rAd.β-gal–infected islets (P < 0.0001). Nitrite levels (μM/200 islets) are the mean ± SD of triplicate determinations, pooled from four independent experiments.
IL-1β– and IFN-γ–induced Apoptosis Is Mediated by NO.
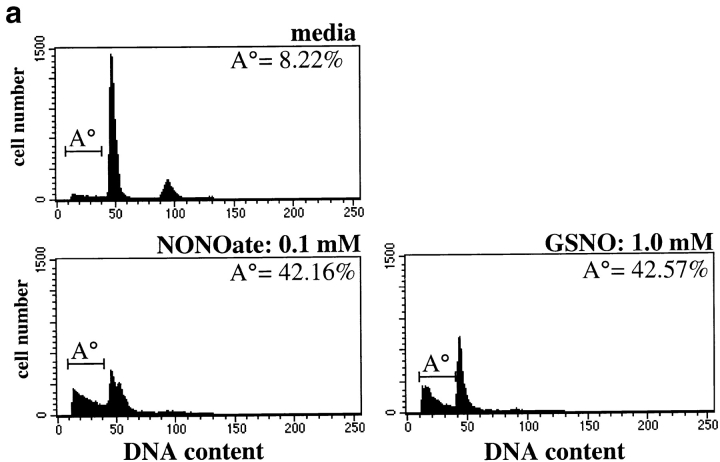
Our data demonstrate that A20 can protect islets from cytokine-induced apoptosis. Furthermore, they show that the antiapoptotic effect of A20 correlates with suppression of cytokine-induced NO production, suggesting that A20 is protecting islets through effects on NO generation. This hypothesis is in accordance with data from the literature showing that NO is a key mediator of cytokine-induced islet cytotoxicity 9 14 33. To determine whether the antiapoptotic effect of A20 was a direct result of its ability to suppress NO production, we examined the role of NO in cytokine-induced apoptosis of islets. We first examined if NO could directly induce apoptosis in rat islets. Rat islets were cocultured with one of two NO donors, NONOate or GSNO, at various concentrations ranging from 0.01 μM to 10 mM. 16 h later, islets were examined for induction of apoptosis (Fig. 5 a). Both NONOate and GSNO, in a dose-dependent manner, induced significant levels of apoptosis in rat islets. However, NONOate was 10-fold more potent than GSNO due to its higher release of NO in the medium (Fig. 5 a, and data not shown). Given that NO is able to directly induce apoptosis in rat islets, we next examined whether NO was the agent responsible for islet apoptosis after cytokine stimulation. The NOS inhibitor L-NIO (500 μM) was added to cytokine-stimulated islets. Islets stimulated with IL-1β and IFN-γ underwent apoptosis and generated high levels of NO (Fig. 5 b). In contrast, islets stimulated with IL-1β and IFN-γ in the presence of L-NIO were completely protected from apoptosis (P < 0.001, n = 3), and NO generation was suppressed to below background levels (P < 0.01, n = 3; Fig. 5 b). Taken together, these data demonstrate that NO is the central mediator of cytokine-induced islet apoptosis.
Figure 5.
NO mediates islet apoptosis induced by IL-1β and IFN-γ. (a) NO donors induce apoptosis in rat islets. Islets were left untreated or were stimulated with GSNO (1.0 mM) or NONOate (0.1 mM) for 16 h, and the percentage of apoptotic cells was determined by flow cytometry. The percentage of apoptotic events was calculated as described and is given in the upper right corner. Data are from a representative experiment of three independent experiments conducted. (b) The l-arginine analogue L-NIO inhibits both apoptosis and NO generation in rat islets. Islets were cultured in the presence or absence of IL-1β (10 U/ml) and IFN-γ (300 U/ml) for 40 h with or without L-NIO (2.2 μM), and the percentage of apoptosis for each condition was measured by flow cytometry. Data from three independent experiments were pooled and are given as the percentage of apoptosis (mean ± SD). NO production (mean ± SD, [nitrite] μM) was measured in the culture medium from each condition and is given in the chart. Suppression of NO production correlated with protection from apoptosis.
A20 Inhibits Cytokine-induced iNOS Upregulation in Islets through Inhibition of inos Gene Transcription.
To clarify the mechanism(s) by which A20 was suppressing NO production, we examined the effects of A20 overexpression on iNOS protein expression, steady state mRNA levels, and regulation of gene transcription. For these and subsequent experiments, islets were stimulated with IL-1β alone, as IFN-γ by itself had little or no effect on NO induction (data not shown).
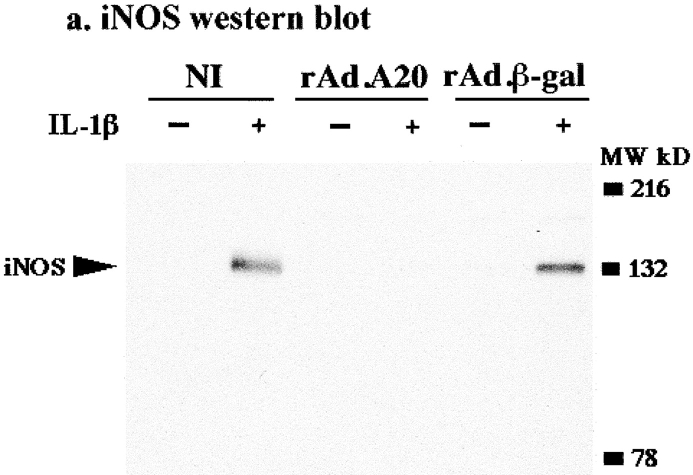
We examined whether A20 overexpression would modulate the induction of iNOS protein after cytokine stimulation. Noninfected and rAd.β-gal–infected islets expressed high levels of iNOS protein 24 h after activation with IL-1β (Fig. 6 a). These data are in accordance with previous studies demonstrating that in islets, cytokine treatment results in de novo production of iNOS mRNA and protein 34. In contrast, IL-1β–mediated upregulation of iNOS protein was totally suppressed in A20-expressing islets (Fig. 6 a). Accordingly, NO generation after IL-1β stimulation was highly suppressed (≥90%) in A20-expressing islets compared with the significant NO levels detected in noninfected and rAd.β-gal infected islets (data not shown).
Figure 6.
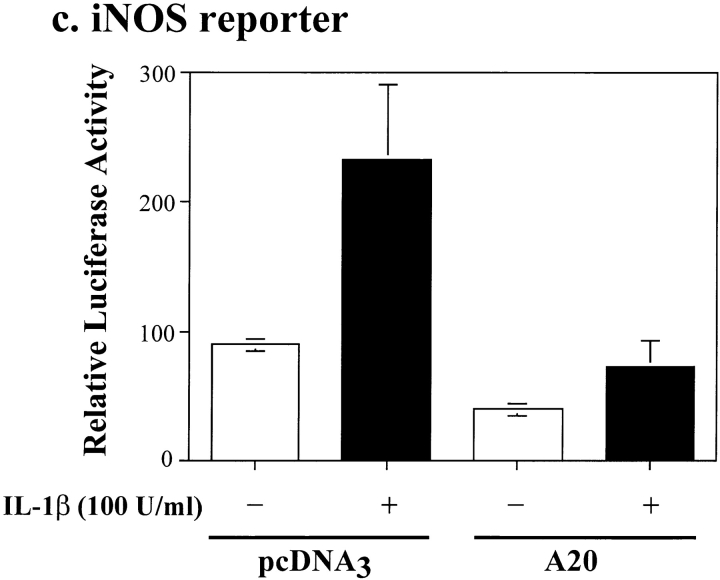
A20 inhibits de novo induction of inos, an NF-κB–dependent gene in rat islets. (a) Induction of iNOS protein in A20-expressing islets. Noninfected (NI), rAd.β-gal–, and rAd.A20-infected islets were cultured in the presence or absence of IL-1β (10 U/ml) for 24 h, and iNOS protein levels were assessed by Western blot analysis. The 130-kD iNOS protein was revealed using the polyclonal Ab, N-20, and its presence is indicated by the arrow. iNOS protein was not detected in A20-expressing islets after IL-1β stimulation. Data are from a representative experiment of three independent experiments conducted. (b) Induction of iNOS steady state mRNA levels in A20-expressing islets. rAd.β-gal– and rAd.A20-infected islets were cultured in the presence or absence of IL-1β (100 U/ml) for 6 h, and both iNOS and β-actin steady state mRNA levels were analyzed by RT-PCR. Upregulation of iNOS mRNA was suppressed in A20-expressing islets. TC, template control. (c) Induction of an iNOS reporter in A20-expressing islets. β-TC3 cells were transiently cotransfected with a luciferase reporter construct containing the iNOS promoter and a human A20 expression plasmid (A20). Control cells were transfected with the iNOS reporter and the control construct pcDNA3 (pcDNA3). 24 h later, β-TC3 cells were stimulated with IL-1β (100 U/ml) for 36 h, and relative luciferase activity (normalized to β-gal light units) was determined as described in Materials and Methods. A20 inhibited IL-1β–induced activation of the iNOS reporter (P < 0.0001). Data (expressed as relative luciferase activity [mean ± SEM]) are representative of five independent experiments conducted in triplicate.
To determine the underlying mechanism by which A20 was suppressing iNOS protein upregulation, we examined, by RT-PCR analysis, iNOS steady state mRNA levels after IL-1β activation. No iNOS mRNA was detected in nonstimulated islets, whereas iNOS transcript was induced 5 h after IL-1β stimulation in both noninfected and rAd.β-gal–infected islets (Fig. 6 b). In contrast, no iNOS mRNA was detected in rAd.A20-infected islets (Fig. 6 b).
It has been established that induction of iNOS mRNA expression by IL-1β is regulated at the transcription level 30 34 35. Therefore, we questioned whether the inhibitory effect of A20 on inos gene upregulation occurred at the level of gene transcription. To address this possibility, β-TC3 cells were cotransfected with a murine iNOS reporter 30 and a human A20 expression plasmid or the control plasmid, pcDNA3. β-TC3 cells were stimulated with IL-1β (100 U/ml) for 36 h after transfection, and luciferase values were calculated as described in Materials and Methods. As shown in Fig. 6 c, IL-1β stimulation resulted in a significant two- to threefold induction of the iNOS reporter in the pcDNA3-transfected β-TC3 cells (mean fold induction ± SD, 2.23 ± 0.747; P < 0.0001, n = 5). In contrast, IL-1β induction of the iNOS reporter in A20-expressing β-TC3 cells was totally suppressed (P < 0.0001, n = 5) to the extent that there was no difference relative to background levels in pcDNA3-transfected β-TC3 cells (P = 0.75, n = 5). Interestingly, A20 overexpression also significantly reduced the basal (nonstimulated) iNOS reporter activity by ∼50% (P < 0.005, n = 5) compared with β-TC3 cells transfected with pcDNA3.
A20 Inhibits NF-κB Activation at a Level Upstream of IκBα Degradation.
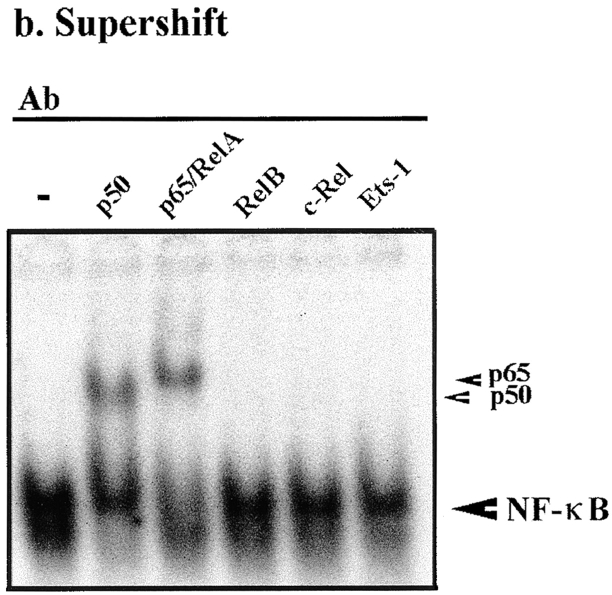
Our data indicate that A20 can suppress the IL-1β–dependent activation of the inos gene. Previous reports have implicated the transcription factor NF-κB as an essential component of this activation 34 36. Therefore, we examined whether A20 was suppressing inos transcription via modulation of NF-κB activation. To check whether A20 expression was altering NF-κB translocation to the nucleus, we performed electrophoretic mobility shift assays (EMSAs) using nuclear extracts isolated from noninfected, rAd.β-gal–, and rAd.A20-infected islets after IL-1β stimulation (Fig. 7 a). A slow migrating complex, binding to an NF-κB consensus sequence, was observed in noninfected and rAd.β-gal–infected islets 1 h after stimulation with IL-1β (Fig. 7 a, arrow). In contrast, this complex was not detected in nuclear extracts from rAd.A20-infected islets after IL-1β stimulation. This complex was resolved by supershift analysis to comprise the p50 and p65 NF-κB subunits (Fig. 7 b). The fastest migrating band was not affected by any treatment and most likely represents a nonspecific protein interaction. These data show that A20 inhibits, in islets, the translocation of NF-κB to the nucleus.
Figure 7.

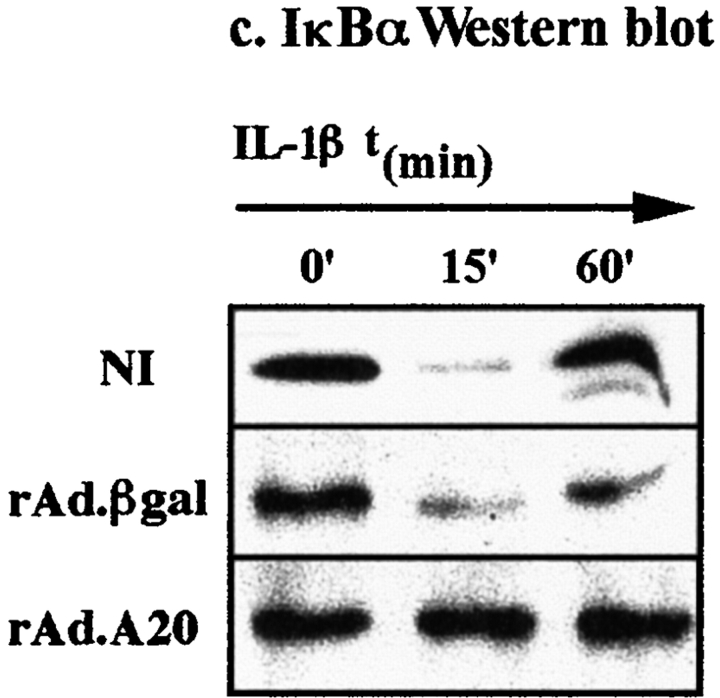
A20 inhibits NF-κB activation in rat islets, at a level upstream of IκBα degradation. (a) NF-κB activation in A20-expressing islets. Noninfected (NI), rAd.β-gal–, and rAd.A20-infected islets were cultured in the presence or absence of IL-1β (100 U/ml) for 1 h, and the presence of nuclear binding proteins for an NF-κB consensus sequence was determined by EMSA. A slow migrating complex binding to an NF-κB oligonucleotide was detected in nuclear extracts from noninfected and rAd.β-gal–infected islets after IL-1β treatment (arrow). No complex was observed in A20-expressing islets after IL-1β stimulation. (b) Supershift analysis of nuclear extracts from noninfected islets stimulated with IL-1β (100 U/ml) for 1 h was performed to determine the identity of the NF-κB complex. Nuclear extracts were incubated with 0.1 μg of polyclonal Ab directed against p50, p65/RelA, Rel-B, c-Rel, or Ets-1. Small arrows indicate supershifted complexes. The induced NF-κB binding complex comprised p50 and p65 subunits. (c) IκBα degradation in A20-expressing islets. Noninfected, rAd.β-gal–, and rAd.A20-infected islets were stimulated with IL-1β (100 U/ml) for the indicated times, and IκBα degradation in the cytoplasm was assessed by Western blot analysis. IL-1β induced a rapid transient decrease in IκBα protein levels in noninfected and rAd.β-gal–infected islets, whereas no degradation of IκBα was observed in A20-expressing islets. The data shown are from a representative experiment of three independent experiments performed.
Degradation of the natural inhibitor of NF-κB, IκBα, in response to IL-1β is a prerequisite for NF-κB translocation 37 38. We next examined whether expression of A20 in islets was affecting the degradation of IκBα in response to IL-1β. Western blot analysis of cytoplasmic extracts from noninfected and rAd.β-gal–infected islets showed that IκBα was rapidly degraded within 15 min after IL-1β stimulation (Fig. 7 c). In contrast, expression of A20 in islets totally inhibited the degradation of IκBα observed after IL-1β stimulation (Fig. 7 c). To ascertain that expression of A20 in islets was not simply delaying IκBα degradation, we examined IκBα levels at several time points after IL-1β stimulation (e.g., 20, 30, 45, and 60 min). No IκBα degradation was observed at any of these time points (Fig. 7 c, and data not shown).
Discussion
IDDM is an autoimmune disease characterized by the specific destruction of β cells in islets of Langerhans 3. Cumulative evidence suggests that apoptosis of the β cell is a critical component of IDDM at both the initiation and effector phases of the disease 5. Transplantation of islets of Langerhans represents a potential cure for IDDM, but here again the success of this treatment is hampered by destruction of the islets and loss of β cells to apoptosis 23. β cell apoptosis can be triggered by both nonspecific inflammatory reactions and specific immune responses 3 21. One potential solution to overcome the susceptibility of β cells to apoptosis is the use of gene therapy to express genes that may impart protective properties on islets, thus enabling successful transplantation 24 39.
Little is currently known about the expression of cytoprotective genes in β cells and the molecular basis of their susceptibility to apoptosis. Recent reports demonstrated that islets constitutively express the prototypic antiapoptotic molecule Bcl-2, the stress-related heat-shock protein HSP70, and several free radical scavenging enzymes such as manganese superoxide dismutase (MnSOD) and catalase 40. Despite expression of these proteins, β cells remain particularly sensitive to apoptosis when challenged with additional cellular stress 41. This is in part explained by their lower expression of constitutive cytoprotective genes 41. With this perspective in mind, we questioned whether islets are able to mount a protective response to inflammation. In this report, we examined whether β cells could be induced to express the antiapoptotic protein A20. A20 was originally described as a TNF-α–inducible 7-Zn finger protein in endothelial cells 25. Its expression can also be induced in response to a variety of inflammatory stimuli, such as LPS, CD40 ligation, the LMP1 protein of EBV, and the Tax protein of HIV 42 43 44 45. The rapid induction of A20 mRNA by these diverse stimuli requires the activation of the transcription factor NF-κB. Two κB binding elements map within the A20 promoter and are essential for its expression 46. Here we show that expression of A20 is rapidly induced in β cells in response to IL-1β. This is the first report showing the induced expression of the antiapoptotic gene A20 in β cells. Further, our data show that IL-1β induces the activation of NF-κB in islets, which concurs with its ability to upregulate the expression of A20. The rapid kinetics of A20 expression in islets suggests that, as in endothelial cells, it may be a component of their physiological protective response to injury 47.
Having established that A20 is a rapid response gene in β cells, we examined whether A20 maintained its antiapoptotic function in islets. Expression of A20 in islets by means of an rAd protects them from apoptosis induced by IL-1β and IFN-γ. The protective effect of A20 against IL-1β– and IFN-γ–induced apoptosis is critical given the central role of IL-1β in β cell dysfunction and destruction during IDDM 9 48. IL-1β inhibits glucose-dependent insulin secretion, impairs glucokinase synthesis, and induces cell death by apoptosis 49 50. Inhibition of IL-1β using neutralizing mAbs prevents diabetes progression in NOD mice 51. The pathway by which IL-1β mediates β cell destruction and toxicity has recently been clarified. IL-1β is produced by activated resident macrophages within the islets 48 21 52 53. Once produced, IL-1β acts directly and selectively upon β cells to induce iNOS, leading to the production of high and sustained levels of NO and to a lesser extent superoxide 12 54. NO directly induces apoptosis of β cells and is the mediator of the multiple toxic effects of IL-1β on β cells 55 56 57. We confirmed the apoptotic potential of NO in our system with the NO donors GSNO and NONOate, which rapidly induced apoptosis of rat islets. Furthermore, the addition of the NOS inhibitor L-NIO to cytokine-activated islets prevented both NO production and apoptosis. These data demonstrate that endogenously generated NO is the mediator of cytokine-induced islet apoptosis in our system.
The central role of NO in cytokine-mediated β cell toxicity prompted us to examine whether the protective effect of A20 in islets was associated with modulation of NO levels. We found that expression of A20 in islets abrogated NO production in response to cytokines. Taken together with our data showing that pharmacologic suppression of NO production also protects from cytokine-induced apoptosis, these data establish the suppression of NO production as one mechanism by which A20 protects islets 58. The suppression of NO production by A20 could also impact on T cell–dependent β cell destruction. Indeed, NO facilitates T cell–dependent killing via upregulation of Fas on human islets 15 59. Ongoing work in our laboratory is aiming at determining whether expression of A20 in islets will also protect β cells against T cell–mediated cytotoxicity via the perforin/granzyme or the Fas/FasL pathway.
The mechanism by which A20 suppresses cytokine-induced NO production is shown to be via inhibition of IL-1β–induced iNOS mRNA and protein expression. Expression of iNOS protein in islets is regulated by de novo transcription of the inos gene 30 34 35. We reasoned that the absence of iNOS protein and mRNA after cytokine stimulation points to a blockade at the level of transcription. Indeed, we found that A20 suppresses IL-1β–induced activation of a murine iNOS reporter, indicating that A20 was regulating iNOS expression at the level of gene transcription. Since NF-κB is the major transcription factor responsible for de novo activation of inos transcription by inflammatory stimuli including IL-1β, we examined the effect of A20 overexpression on NF-κB activation 60. We found that A20 suppresses the activation of the transcription factor NF-κB in islets. Expression of other NF-κB–dependent proinflammatory genes involved in IDDM, such as intercellular adhesion molecule 1 (ICAM-1), are also expected to be blunted by A20, thereby adding to the beneficial effect of A20 as a gene therapy tool 61 62. We have previously shown that A20 has a dual antiapoptotic and antiinflammatory function in primary endothelial cells 28. This dual function is clearly maintained in islets, suggesting that inhibition of NF-κB activation by A20 is an important component of the natural physiological role of A20. The effect of A20 seems specific to NF-κB and is not a result of a toxic effect of A20 on the transcription machinery. Indeed, A20 overexpression had no effect on IFN-γ–mediated MHC class I upregulation (data not shown), a process requiring the activation of the transcription factors signal transducer and activator of transcription 1α (STAT-1α) and IFN regulatory factor 1 (IRF-1) 63 64.
NF-κB is a ubiquitous transcription factor constitutively expressed in the cytoplasm in an inactive form associated to an inhibitory protein termed IκBα 37 38. Cellular activation by inflammatory stimuli such as IL-1β results in the phosphorylation and subsequent degradation of IκBα, thus allowing NF-κB to translocate into the nucleus and activate target genes such as inos 37 38. Therefore, we examined what effect A20 had on IκBα degradation. Our data demonstrate that A20 interferes with NF-κB activation at a level upstream of the kinase cascade leading to IκBα degradation, as no IκBα degradation was observed in A20-expressing islets after IL-1β stimulation. Several potential targets for A20 within the IL-1β–stimulated cascade leading to NF-κB activation have been reported. Yeast double hybrid studies have demonstrated that A20 interacts with TNF receptor–associated factor (TRAF)-1/2, TRAF-6, and the adapter proteins 14-3-3 65 66 66a. The interaction of A20 with 14-3-3 proteins is interesting given the potential involvement of 14-3-3 (via their interaction with c-raf) in multiple signaling cascades leading to NF-κB activation 67. In addition, IL-1β–mediated activation of NF-κB requires TRAF-6 and the IL-1 receptor–associated kinase IRAK 68 69 70. Therefore, TRAF-6 is also a likely point where A20 intercepts the IL-1β signaling cascade. Interactions between A20 and TRAF-6 or 14-3-3 in islets are currently being studied in our laboratory.
In addition, data in the literature show that IL-1β–induced NF-κB activation and inos mRNA induction can be suppressed in islets by antioxidants such as pyrrolidine dithiocarbamate (PDTC) 34. Moreover, NF-κB is a redox-sensitive transcription factor, as indicated by the fact that NF-κB activation can be induced by H2O2 or, conversely, NF-κB nuclear translocation is blocked by antioxidants such as PDTC 71 72. The potential for A20 to interfere at the oxidative step in NF-κB activation is currently being tested. Interestingly, several studies have addressed the protective potential of antioxidants in islets by overexpressing free radical scavenging enzymes 41 73 74 75. The overexpression of MnSOD in an engineered β cell resulted in selective protection from IL-1β–induced cytotoxicity as well as a reduction in cytokine-induced NO generation 75. In addition, transgenic expression of the antioxidant thioredoxin in β cells of NOD mice reduced the incidence of spontaneous diabetes and protected from streptozotocin-induced diabetes 76. Interestingly, thioredoxin has been shown to inhibit NF-κB by interfering with a redox-sensitive step required for its activation 77 78. Thus, in the model of Hotta et al. 76, the protective effect of thioredoxin may involve inhibition of NF-κB activation, given the role of NF-kB activation in NO generation and islet destruction 36 54 79. Together, these data illustrate a novel concept whereby protection of the target (in this case, β cells) would offer a potent therapeutic strategy to inhibit disease occurrence even in the presence of the effector mechanisms (cellular and soluble mediators). This approach might constitute an alternative to systemic modulation of the immune system as currently practiced using diverse immunosuppressants, such as costimulation blockade 80 81 82 83. Along with this approach, other antiapoptotic genes such as bcl-2 have been proposed as gene therapy tools to protect islets from cytokine-mediated apoptosis. Expression of Bcl-2 in a murine β cell line did provide modest protection from cytokine-mediated apoptosis 84 85. Interestingly, bcl genes have, like A20, antiinflammatory properties through blockade of transcription factors, such as NF-κB in endothelial cells 86 87 88. We are currently testing whether they maintain this dual function in islets and could synergize with A20 to protect β cells. However, in contrast to A20, Bcl-2 is expressed constitutively in islets and is not induced upon cytokine activation (data not shown). We propose that constitutively expressed antiapoptotic proteins such as Bcl-2 may function to protect cells from baseline cellular stress, whereas induced cytoprotective proteins such as A20 protect cells from greater stress caused by inflammatory reactions 47. We suggest that A20 could be a more relevant gene therapy candidate for protection of β cells against the additional stress encountered in the setting of transplantation and autoimmunity. Future experiments will determine the efficacy of A20 in both islet transplant and autoimmune diabetes models.
Acknowledgments
We thank Dr. Deborah Stroka for cloning of the HA-A20 construct; Drs. Jerome Mahiou, Arun Sharma, Anne Z. Badrichani, and Robert H. Harrington for helpful advice regarding the transfection of β-TC3 cells; and Dr. Karl Stuhlmeier for helpful comments and advice with the EMSA experiments. We also acknowledge Dr. Gordon C. Weir, Dr. Susan Bonner-Weir, and Jennifer Lock for providing rodent islets, helpful advice, and discussion.
This research is supported by National Institutes of Health grant 1PO1DK53087/01 awarded to C. Ferran and in part by the Juvenile Diabetes Foundation International through the Juvenile Diabetes Foundation Center for Islet Transplantation at Harvard Medical School. This is manuscript no. 791 from our laboratories.
Footnotes
1used in this paper: β-gal, β-galactosidase; EMSA, electrophoretic mobility shift assay; GSNO, S-nitrosoglutathione; IDDM, insulin-dependent diabetes mellitus; IκBα, inhibitor of NF-κB; iNOS, inducible NO synthase; L-NIO, l-N5-(1-iminoethyl) ornithine, dihydrochloride; MnSOD, manganese superoxide dismutase; MOI, multiplicity of infection; NF-κB, nuclear factor κB; NO, nitric oxide; NOD, nonobese diabetic; NONOate, N-(2-aminoethyl)-N-(2-hydroxy-2-nitrosohydrazino-1,2-ethylenediamine; rAd, recombinant adenovirus; RT, reverse transcription; TRAF, TNF receptor–associated factor
Shane T. Grey, Immunobiology Research Center, Harvard Medical School, Beth Israel Deaconess Medical Center, 99 Brookline Ave., Boston, MA 02215. Phone: 617-632-0859; Fax: 617-632-0880; E-mail: sgrey@caregroup.harvard.edu
References
- Benoist C., Mathis D. Cell death mediators in autoimmune diabetes—no shortage of suspects. Cell. 1997;89:1–3. doi: 10.1016/s0092-8674(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Andre I., Gonzalez A., Wang B., Katz J., Benoist C., Mathis D. Checkpoints in the progression of autoimmune diseaselessons from diabetes models. Proc. Natl. Acad. Sci. USA. 1996;93:2260–2263. doi: 10.1073/pnas.93.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delovitch T.L., Singh B. The nonobese diabetic mouse as a model of autoimmune diabetesimmune dysregulation gets the NOD. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- Cameron M.J., Meagher C., Delovitch T.L. Failure in immune regulation begets IDDM in NOD mice. Diab. Metab. Rev. 1998;14:177–185. doi: 10.1002/(sici)1099-0895(199806)14:2<177::aid-dmr209>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Mauricio D., Mandrup-Poulsen T. Apoptosis and the pathogenesis of IDDMa question of life and death. Diabetes. 1998;47:1537–1543. doi: 10.2337/diabetes.47.10.1537. [DOI] [PubMed] [Google Scholar]
- Kurrer M.O., Pakala S.V., Hanson H.L., Katz J.D. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D., Odermatt B., Seiler P., Zinkernagel R.M., Mak T.W., Hengartner H. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J. Exp. Med. 1997;186:989–997. doi: 10.1084/jem.186.7.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervonsky A.V., Wang Y., Wong F.S., Visintin I., Flavell R.A., Janeway C.A., Jr., Matis L.A. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Arnush M., Heitmeier M.R., Scarim A.L., Marino M.H., Manning P.T., Corbett J.A. IL-1 produced and released endogenously within human islets inhibits beta cell function. J. Clin. Invest. 1998;102:516–526. doi: 10.1172/JCI844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmeier M.R., Scarim A.L., Corbett J.A. Interferon-γ increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J. Biol. Chem. 1997;272:13697–13704. doi: 10.1074/jbc.272.21.13697. [DOI] [PubMed] [Google Scholar]
- Xie Q., Nathan C. The high-output nitric oxide pathwayrole and regulation. J. Leukocyte Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- Xia Y., Roman L.J., Masters B.S., Zweier J.L. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J. Biol. Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- Kaneto H., Fujii J., Seo H.G., Suzuki K., Matsuoka T., Nakamura M., Tatsumi H., Yamasaki Y., Kamada T., Taniguchi N. Apoptotic cell death triggered by nitric oxide in pancreatic β-cells. Diabetes. 1995;44:733–738. doi: 10.2337/diab.44.7.733. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M., Dypbukt J.M., Brune B., Nicotera P. Interleukin-1β-induced nitric oxide production activates apoptosis in pancreatic RINm5F cells. Exp. Cell Res. 1994;213:172–177. doi: 10.1006/excr.1994.1187. [DOI] [PubMed] [Google Scholar]
- Stassi G., Maria R.D., Trucco G., Rudert W., Testi R., Galluzzo A., Giordano C., Trucco M. Nitric oxide primes pancreatic beta cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J. Exp. Med. 1997;186:1193–1200. doi: 10.1084/jem.186.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura T., Kato I., Kimura N., Nakazawa T., Yonekura H., Takasawa S., Okamoto H. Transgenic mice overexpressing type 2 nitric-oxide synthase in pancreatic β cells develop insulin-dependent diabetes without insulitis. J. Biol. Chem. 1998;273:2493–2496. doi: 10.1074/jbc.273.5.2493. [DOI] [PubMed] [Google Scholar]
- Reckard C.R., Stuart F.P., Schulak J.A. Immunologic comparisons of isolated pancreatic islet and whole-organ allografts. Transplant. Proc. 1979;11:563–566. [PubMed] [Google Scholar]
- Ballinger W. ProceedingsIsolation and transplantation of islets of Langerhans in rat and monkey. Ann. R. Coll. Surg. Engl. 1976;58:327–330. [PMC free article] [PubMed] [Google Scholar]
- Lacy P.E., Davie J.M. Transplantation of pancreatic islets. Annu. Rev. Immunol. 1984;2:183–198. doi: 10.1146/annurev.iy.02.040184.001151. [DOI] [PubMed] [Google Scholar]
- Weir G.C., Bonner-Weir S. Islet transplantation as a treatment for diabetes. J. Am. Optom. Assoc. 1998;69:727–732. [PubMed] [Google Scholar]
- Bottino R., Fernandez L.A., Ricordi C., Lehmann R., Tsan M.F., Oliver R., Inverardi L. Transplantation of allogeneic islets of Langerhans in the rat livereffects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316–323. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- Davalli A.M., Scaglia L., Zangen D.H., Hollister J., Bonner-Weir S., Weir G.C. Early changes in syngeneic islet graftseffect of recipient's metabolic control on graft outcome. Transplant. Proc. 1995;27:3238–3239. [PubMed] [Google Scholar]
- Davalli A.M., Scaglia L., Zangen D.H., Hollister J., Bonner-Weir S., Weir G.C. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- Efrat S. Prospects for gene therapy of insulin-dependent diabetes mellitus. Diabetologia. 1998;41:1401–1409. doi: 10.1007/s001250051085. [DOI] [PubMed] [Google Scholar]
- Opipari A.J., Boguski M.S., Dixit V.M. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J. Biol. Chem. 1990;265:14705–14708. [PubMed] [Google Scholar]
- Opipari A.J., Hu H.M., Yabkowitz R., Dixit V.M. The A20 zinc finger protein protects cells from TNF cytotoxicity. J. Biol. Chem. 1992;267:12424–12427. [PubMed] [Google Scholar]
- Cooper J.T., Stroka D.M., Brostjan C., Palmetshofer A., Bach F.H., Ferran C. A20 blocks endothelial cell activation through a NF-κB-dependent mechanism. J. Biol. Chem. 1996;271:18068–18073. doi: 10.1074/jbc.271.30.18068. [DOI] [PubMed] [Google Scholar]
- Ferran C., Stroka D.M., Badrichani A.Z., Cooper J.T., Wrighton C.J., Soares M., Grey S.T., Bach F.H. A20 inhibits NF-κB activation in endothelial cells without sensitizing to tumor necrosis factor-mediated apoptosis. Blood. 1998;91:2249–2258. [PubMed] [Google Scholar]
- D'Ambra R., Surana M., Efrat S., Starr R.G., Fleischer N. Regulation of insulin secretion from β-cell lines derived from transgenic mice insulinomas resembles that of normal β-cells. Endocrinology. 1990;126:2815–2822. doi: 10.1210/endo-126-6-2815. [DOI] [PubMed] [Google Scholar]
- Lowenstein C.J., Alley E.W., Raval P., Snowman A.M., Snyder S.H., Russell S.W., Murphy W.J. Macrophage nitric oxide synthase genetwo upstream regions mediate induction by interferon γ and lipopolysaccharide. Proc. Natl. Acad. Sci. USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer R.B., Herzog N.K. Isolation of intact nuclei for nuclear extract preparation from a fragile B-lymphocyte cell line. Biotechniques. 1995;19:192–195. [PubMed] [Google Scholar]
- O'Brien B.A., Harmon B.V., Cameron D.P., Allan D.J. Beta-cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin model. J. Pathol. 1996;178:176–181. doi: 10.1002/(SICI)1096-9896(199602)178:2<176::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Corbett J.A., Lancaster J.R., Jr., Sweetland M.A., McDaniel M.L. Interleukin-1β-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1β-induced inhibition of insulin secretion. J. Biol. Chem. 1991;266:21351–21354. [PubMed] [Google Scholar]
- Kwon G., Corbett J.A., Rodi C.P., Sullivan P., McDaniel M.L. Interleukin-1β-induced nitric oxide synthase expression by rat pancreatic β-cellsevidence for the involvement of nuclear factor κB in the signaling mechanism. Endocrinology. 1995;136:4790–4795. doi: 10.1210/endo.136.11.7588208. [DOI] [PubMed] [Google Scholar]
- Lorsbach R.B., Murphy W.J., Lowenstein C.J., Snyder S.H., Russell S.W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-γ and lipopolysaccharide. J. Biol. Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- Taylor B.S., de Vera M.E., Ganster R.W., Wang Q., Shapiro R.A., Morris S.M., Jr., Billiar T.R., Geller D.A. Multiple NF-κB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J. Biol. Chem. 1998;24:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- Baeuerle P.A., Baltimore D. IκBa specific inhibitor of the NF-κB transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Traenckner E.B., Pahl H.L., Henkel T., Schmidt K.N., Wilk S., Baeuerle P.A. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Fejer G., Brownlee M., Horwitz M.S. Prolonged survival of pancreatic islet allografts mediated by adenovirus immunoregulatory transgenes. Proc. Natl. Acad. Sci. USA. 1995;92:6947–6951. doi: 10.1073/pnas.92.15.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh N., Margulis B., Borg L.A., Wiklund H.J., Saldeen J., Flodstrom M., Mello M.A., Andersson A., Pipeleers D.G., Hellerstrom C. Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic isletsimplications for the pathogenesis of insulin-dependent diabetes mellitus. Mol. Med. 1995;1:806–820. [PMC free article] [PubMed] [Google Scholar]
- Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- Hu X., Yee E., Harlan J.M., Wong F., Karsan A. Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood. 1998;92:2759–2765. [PubMed] [Google Scholar]
- Sarma V., Lin Z., Clark L., Rust B.M., Tewari M., Noelle R.J., Dixit V.M. Activation of the B-cell surface receptor CD40 induces A20, a novel zinc finger protein that inhibits apoptosis. J. Biol. Chem. 1995;270:12343–12346. doi: 10.1074/jbc.270.21.12343. [DOI] [PubMed] [Google Scholar]
- Laherty C.D., Hu H.M., Opipari A.W., Wang F., Dixit V.M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating NF-κB. J. Biol. Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- Laherty C.D., Perkins N.D., Dixit V.M. Human T cell leukemia virus type I Tax and phorbol 12-myristate 13-acetate induce expression of the A20 zinc finger protein by distinct mechanisms involving nuclear factor κB. J. Biol. Chem. 1993;268:5032–5039. [PubMed] [Google Scholar]
- Krikos A., Laherty C.D., Dixit V.M. Transcriptional activation of the tumor necrosis factor α-inducible zinc finger protein, A20, is mediated by κB elements. J. Biol. Chem. 1992;267:17971–17976. [PubMed] [Google Scholar]
- Bach F.H., Hancock W.W., Ferran C. Protective genes expressed in endothelial cellsa regulatory response to injury. Immunol. Today. 1997;18:483–486. doi: 10.1016/s0167-5699(97)01129-8. [DOI] [PubMed] [Google Scholar]
- Arnush M., Scarim A.L., Heitmeier M.R., Kelly C.B., Corbett J.A. Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J. Immunol. 1998;160:2684–2691. [PubMed] [Google Scholar]
- Ling Z., Chen M.C., Smismans A., Pavlovic D., Schuit F., Eizirik D.L., Pipeleers D.G. Intercellular differences in interleukin 1β-induced suppression of insulin synthesis and stimulation of noninsulin protein synthesis by rat pancreatic β-cells. Endocrinology. 1998;139:1540–1545. doi: 10.1210/endo.139.4.5894. [DOI] [PubMed] [Google Scholar]
- Ma Z., Landt M., Bohrer A., Ramanadham S., Kipnis D.M., Turk J. Interleukin-1 reduces the glycolytic utilization of glucose by pancreatic islets and reduces glucokinase mRNA content and protein synthesis by a nitric oxide-dependent mechanism. J. Biol. Chem. 1997;272:17827–17835. doi: 10.1074/jbc.272.28.17827. [DOI] [PubMed] [Google Scholar]
- Cailleau C., Diu-Hercend A., Ruuth E., Westwood R., Carnaud C. Treatment with neutralizing antibodies specific for IL-1β prevents cyclophosphamide-induced diabetes in nonobese diabetic mice. Diabetes. 1997;46:937–940. doi: 10.2337/diab.46.6.937. [DOI] [PubMed] [Google Scholar]
- Jun H.S., Santamaria P., Lim H.W., Zhang M.L., Yoon J.W. Absolute requirement of macrophages for the development and activation of β-cell cytotoxic CD8+ T-cells in T-cell receptor transgenic NOD mice. Diabetes. 1999;48:34–42. doi: 10.2337/diabetes.48.1.34. [DOI] [PubMed] [Google Scholar]
- Jun H.S., Yoon C.S., Zbytnuik L., van Rooijen N., Yoon J.W. The role of macrophages in T cell–mediated autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 1999;189:347–358. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch A., Suarez-Pinzon W.L., Sorensen O., Bleackley R.C. Inducible nitric oxide synthase (iNOS) in pancreatic islets of nonobese diabetic miceidentification of iNOS-expressing cells and relationships to cytokines expressed in the islets. Endocrinology. 1996;137:2093–2099. doi: 10.1210/endo.137.5.8612552. [DOI] [PubMed] [Google Scholar]
- Corbett J.A., Sweetland M.A., Wang J.L., Lancaster J.R., Jr., McDaniel M.L. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc. Natl. Acad. Sci. USA. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney C.A., Pavlovic D., Hoorens A., Pipeleers D.G., Eizirik D.L. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology. 1997;138:2610–2614. doi: 10.1210/endo.138.6.5204. [DOI] [PubMed] [Google Scholar]
- Wilson G.L., Patton N.J., LeDoux S.P. Mitochondrial DNA in beta-cells is a sensitive target for damage by nitric oxide. Diabetes. 1997;46:1291–1295. doi: 10.2337/diab.46.8.1291. [DOI] [PubMed] [Google Scholar]
- Corbett J.A., McDaniel M.L. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992;41:897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- Loweth A.C., Williams G.T., James R.F., Scarpello J.H., Morgan N.G. Human islets of Langerhans express Fas ligand and undergo apoptosis in response to interleukin-1β and Fas ligation. Diabetes. 1998;47:727–732. doi: 10.2337/diabetes.47.5.727. [DOI] [PubMed] [Google Scholar]
- Xie Q.W., Kashiwabara Y., Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Yang X.D., Michie S.A., Mebius R.E., Tisch R., Weissman I., McDevitt H.O. The role of cell adhesion molecules in the development of IDDMimplications for pathogenesis and therapy. Diabetes. 1996;45:705–710. doi: 10.2337/diab.45.6.705. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Gage A., Montag A., Rothlein R., Thistlethwaite J., Bluestone J. Inhibition of transplant rejection by pretreatment of xenogeneic pancreatic islet cells with anti-ICAM-1 antibodies. Transplantation. 1994;58:681–689. [PubMed] [Google Scholar]
- Gao J., Morrison D.C., Parmely T.J., Russell S.W., Murphy W.J. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-γ and lipopolysaccharide. J. Biol. Chem. 1997;272:1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- Martin E., Nathan C., Xie Q.W. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong-Song H., Rothe M., Goeddel D.V. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF-1/TRAF-2 and inhibits NF-κB activation. Proc. Natl. Acad. Sci. USA. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenz C., Dixit V.M. 14-3-3 proteins associate with A20 in an isoform-specific manner and function both as chaperone and adapter molecules. J. Biol. Chem. 1996;271:20029–20034. doi: 10.1074/jbc.271.33.20029. [DOI] [PubMed] [Google Scholar]
- Heyninck K., Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-κD activation at the level of TRAF6. FEBS Lett. 1999;442:147–150. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- Tzivion G., Luo Z., Avruch J. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature. 1998;394:88–92. doi: 10.1038/27938. [DOI] [PubMed] [Google Scholar]
- Cao Z., Henzel W.J., Gao X. IRAKa kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- Cao Z., Xiong J., Takeuchi M., Kurama T., Goeddel D.V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- Lomaga M.A., Yeh W.C., Sarosi I., Duncan G.S., Furlonger C., Ho A., Morony S., Capparelli C., Van G., Kaufman S. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferran C., Millan M.T., Csizmadia V., Cooper J.T., Brostjan C., Bach F.H., Winkler H. Inhibition of NF-κB by pyrrolidine dithiocarbamate blocks endothelial cell activation. Biochem. Biophys. Res. Commun. 1995;214:212–223. doi: 10.1006/bbrc.1995.2277. [DOI] [PubMed] [Google Scholar]
- Kubisch H.M., Wang J., Bray T.M., Phillips J.P. Targeted overexpression of Cu/Zn superoxide dismutase protects pancreatic β-cells against oxidative stress. Diabetes. 1997;46:1563–1566. doi: 10.2337/diabetes.46.10.1563. [DOI] [PubMed] [Google Scholar]
- Benhamou P.Y., Moriscot C., Richard M.J., Beatrix O., Badet L., Pattou F., Kerr-Conte J., Chroboczek J., Lemarchand P., Halimi S. Adenovirus-mediated catalase gene transfer reduces oxidant stress in human, porcine and rat pancreatic islets. Diabetologia. 1998;41:1093–1100. doi: 10.1007/s001250051035. [DOI] [PubMed] [Google Scholar]
- Hohmeier H.E., Thigpen A., Tran V.V., Davis R., Newgard C.B. Stable expression of manganese superoxide dismutase (MnSOD) in insulinoma cells prevents IL-1β–induced cytotoxicity and reduces nitric oxide production. J. Clin. Invest. 1998;101:1811–1820. doi: 10.1172/JCI1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta M., Tashiro F., Ikegami H., Niwa H., Ogihara T., Yodoi J., Miyazaki J. Pancreatic β cell–specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J. Exp. Med. 1998;188:1445–1451. doi: 10.1084/jem.188.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D.Y., Chae H.Z., Rhee S.G., Jeang K.T. Regulatory role for a novel human thioredoxin peroxidase in NF-κB activation. J. Biol. Chem. 1997;272:30952–30961. doi: 10.1074/jbc.272.49.30952. [DOI] [PubMed] [Google Scholar]
- Kim I.Y., Stadtman T.C. Inhibition of NF-κB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc. Natl. Acad. Sci. USA. 1997;94:12904–12907. doi: 10.1073/pnas.94.24.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien B.A., Harmon B.V., Cameron D.P., Allan D.J. Apoptosis is the mode of β-cell death responsible for the development of IDDM in the nonobese diabetic (NOD) mouse. Diabetes. 1997;46:750–757. doi: 10.2337/diab.46.5.750. [DOI] [PubMed] [Google Scholar]
- Lenschow D.J., Zeng Y., Thistlethwaite J.R., Montag A., Brady W., Gibson M.G., Linsley P.S., Bluestone J.A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Benhamou P.Y., Moriscot C., Badet L., Halimi S. Strategies for graft immunomodulation in islet transplantation. Diab. Metab. 1998;24:215–224. [PubMed] [Google Scholar]
- Kendall D.M., Robertson R.P. Pancreas and islet transplantation in humans. Diab. Metab. 1996;22:157–163. [PubMed] [Google Scholar]
- Stratta R.J. Immunosuppression in pancreas transplantationprogress, problems and perspective. Transpl. Immunol. 1998;6:69–77. doi: 10.1016/s0966-3274(98)80020-8. [DOI] [PubMed] [Google Scholar]
- Iwahashi H., Hanafusa T., Eguchi Y., Nakajima H., Miyagawa J., Itoh N., Tomita K., Namba M., Kuwajima M., Noguchi T. Cytokine-induced apoptotic cell death in a mouse pancreatic β-cell lineinhibition by Bcl-2. Diabetologia. 1996;39:530–536. doi: 10.1007/BF00403299. [DOI] [PubMed] [Google Scholar]
- Liu Y., Rabinovitch A., Suarez-Pinzon W., Muhkerjee B., Brownlee M., Edelstein D., Federoff H.J. Expression of the bcl-2 gene from a defective HSV-1 amplicon vector protects pancreatic β-cells from apoptosis. Hum. Gene Ther. 1996;7:1719–1726. doi: 10.1089/hum.1996.7.14-1719. [DOI] [PubMed] [Google Scholar]
- Shibasaki F., Kondo E., Akagi T., McKeon F. Suppression of signaling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- Stroka D.M., Badrichani A.Z., Bach F.H., Ferran C. Overexpression of A1, an NF-κB-inducible anti-apoptotic bcl gene, inhibits endothelial cell activation. Blood. 1999;93:3803–3810. [PubMed] [Google Scholar]
- Badrichani A.Z., Stroka D.M., Bilbao G., Curiel D.T., Bach F.H., Ferran C. Bcl-2 and Bcl-XL serve an anti-inflammatory function in endothelial cells through inhibition of NF-κB. J. Clin. Invest. 1999;103:543–553. doi: 10.1172/JCI2517. [DOI] [PMC free article] [PubMed] [Google Scholar]