Abstract
T cell differentiation relies on pre–T cell receptor (TCR) and TCR signaling events that take place at successive steps of the pathway. Here, we show that two of these T cell differentiation checkpoints are regulated by Ikaros. In the absence of Ikaros, double negative thymocytes can differentiate to the double positive stage without expression of a pre-TCR complex. Subsequent events in T cell development mediated by TCR involving transition from the double positive to the single positive stage are also regulated by Ikaros. Nonetheless, in Ikaros-deficient thymocytes, the requirement of pre-TCR expression for expansion of immature thymocytes as they progress to the double positive stage is still maintained, and the T cell malignancies that invariably arise in the thymus of Ikaros-deficient mice are dependent on either pre-TCR or TCR signaling. We conclude that Ikaros regulates T cell differentiation, selection, and homeostasis by providing signaling thresholds for pre-TCR and TCR.
Keywords: thymocyte, selection, signaling, homeostasis, malignancy
Differentiation along the T cell pathway proceeds in a series of steps that result in the successive development of double negative (CD4−CD8−), double positive (CD4+ CD8+), and single positive (CD4+ or CD8+) thymocytes. Progression from the late double negative to the double positive stage of differentiation depends on rearrangement and expression of the TCR β chain as part of the pre-TCR complex 1 2. In mice homozygous for a mutation in the recombinase activating gene 1 (RAG-1−/−)1 that prevents TCR-β chain rearrangement, thymocyte differentiation arrests at the double negative stage 3. Rearrangement of the TCR α chain gene and its subsequent expression as part of the TCR complex promotes maturation of a double positive to a single positive thymocyte. Mice with mutations in the TCR-α locus have no single positive thymocytes or mature T cells in the periphery 4.
For a double positive thymocyte to become a single positive mature T cell that can be exported to the periphery, it must pass through at least two TCR-mediated differentiation events designated positive and negative selection 5 6. During negative selection, a double positive thymocyte whose TCR interacts with high affinity to a self-antigen/MHC complex is signaled to die by apoptosis. Positive selection, on the other hand, ensures the exclusive maturation of T cells whose TCRs have the appropriate affinity for MHC class I or class II and self-antigen. There are two major models, an instructive and a stochastic, to explain positive selection 2. According to the instructive model, double positive thymocytes whose TCR shows specificity for MHC class I/self-antigen downregulate CD4 expression to become mature CD8 T cells, whereas those that show specificity for MHC class II/self-antigen downregulate CD8 expression to become mature CD4 T cells. According to the stochastic model, the double positive thymocyte arbitrarily downregulates either the CD4 or CD8 coreceptor, independent of TCR specificity. It is then signaled to survive only if the correct choice of coreceptor expression was made—CD4 for a class II–restricted TCR and CD8 for a class I–restricted TCR.
We have previously shown that Ikaros proteins are essential for T cell commitment and homeostasis. Mice homozygous for an Ikaros null mutation lack all identifiable T cells and their precursors during fetal development 7. Postnatally, a reduced number of T cell precursors are detected in the thymus which give rise to TCR-α/β lineage cells. In addition, in Ikaros null mice, thymocyte profiles are skewed towards CD4 T cells and precursors in transition to this phenotype. Ikaros null−/− thymocytes and peripheral T cells display augmented proliferative responses when triggered via their TCR ex vivo and undergo aberrant clonal expansions in vivo 7. Given that Ikaros exists in stable complexes with other family members, a second Ikaros mutation was engineered that generates exclusively non-DNA binding dominant negative proteins. Mice homozygous for this mutation display a more severe phenotype than their null homozygous counterparts. They lack all T cell precursors during fetal and adult life 8. In mice heterozygous for the Ikaros dominant negative mutation (DN+/−), lymphoid populations appear normal through the first month of age, but thymocytes and peripheral T cells display augmented proliferative responses after TCR stimulation 9. Phenotypic changes in the thymocyte compartment are detected between 2 and 3 mo of age. Expansion of the intermediate double positive and single positive thymocyte populations is observed, and these mice invariably develop aggressive T cell leukemias and lymphomas 9.
In this study, we delineate the role of Ikaros during T cell differentiation. We show that when levels of Ikaros activity are severely reduced (as in the DN+/− mice) or absent (as in the null−/− mice), the number of T cell precursors is decreased and their relative distribution within the double negative subsets is altered. In addition, Ikaros-deficient double negative thymocytes can differentiate to the double positive and CD4 single positive stages in the absence of a pre-TCR complex. Interestingly, this transition occurs in the absence of the normally concomitant proliferative expansion. Furthermore, Ikaros-deficient double positive thymocytes transit to the single positive stage without an appropriate TCR–coreceptor combination. Finally, we show that expression of a pre-TCR or TCR complex is required for the transformation of Ikaros-deficient thymocytes.
Materials and Methods
Mice.
Ikaros DN+/− and null+/− mice of a mixed background (C57BL/6 × SV129) were bred against RAG-1 −/− (gift of E. Spanopoulou, Mount Sinai School of Medicine, New York, NY), TCR-α 2/− and TCR-β 2/− (The Jackson Laboratory), and F5 (gift of D. Kioussis, Medical Research Council, The National Institute for Medical Research, London, UK) mice of the C57BL/6 background. DN+/− and null+/− daughters who were heterozygous for the RAG, TCR-α, and TCR-β mutations were then backcrossed with their RAG −/−, TCR-α 2/−, and TCR-β 2/− fathers. Colonies were expanded by intercrossing of littermates.
Cytofluorometry of T Lymphocyte Cell Populations.
Cells from the thymus, spleen, lymph nodes, and bone marrow were prepared and analyzed for expression of surface differentiation antigens as described previously 8 9. All antibodies using for stainings were from PharMingen. Flow cytometric analyses and cell sorting were performed using a FACscan™ (Becton Dickinson) and high speed MoFlo sorter (Cytomation, Inc.), respectively.
Preparation of Triple Negative Thymocytes.
Thymocytes were prepared as described previously 8. Mice were 4–8 wk of age. Thymocytes from two to six mice were pooled before depletion. Each pool was stained with the following antibodies provided as hybridoma supernatants for complement-mediated depletion: anti-CD4 (GK1.5) and anti-CD8 (3.168.8). In some experiments, purification was performed as described elsewhere 10. In brief, the lightest 30% of thymocytes was selected by a density cut procedure, and all adherent cells were removed by culture for 1 h at 37°C. Cells bearing CD3, CD4, CD8, CD2, CD25, B220, Mac-1, Gr-1, Ter119, and class II MHC were removed by depletion using Dynabeads (Dynal). In some experiments, remaining double negative cells were further purified by removal of CD4+ and CD8+ cells using the VarioMacs™ (Miltenyi Biotec) or sorted using the MoFlo™ sorter (Cytomation, Inc.).
Reverse Transcriptase PCR Analysis.
RNA and cDNA were prepared as described previously 11 12. The relative concentration of cDNAs for each sample was determined with primers that would amplify cDNA of the housekeeping gene, hypoxanthine-guanine phosphoribosyltransferase (HPRT). Adjusted amounts of cDNAs were amplified with primers derived from sequences corresponding to the first exon of the constant region of the TCR α chain gene 13. [α-32P]ATP and [α-32P]CTP (0.5 μCi each) were included in each PCR reaction. Cycling conditions were: 95°C for 45 s, 60°C for 45 s, and 72°C for 1 min for 35 cycles. The two sets of primers used were: HPRT 5′, TGG CCC TCT GTG TGC TCA AG; HPRT forward, CAC AGG ACT AGA ACA CCT GC; Cα1, CCC AGA ACC TGC TGT GTA C; and Cα2, TGA ACT GGG GTA GGT GGC. PCR products were electrophoresed through an 8% polyacrylamide gel, which was then dried and exposed to autoradiography film. Exposures shown here are for <12 h for HPRT, lanes 1–5, and TCR-α, lanes 3–5, and for 30 h for TCR-α, lanes 1 and 2.
Cell Cycle Analysis.
Cells were prestained with FITC-conjugated mAbs against CD25 (double negative cells) or TCR-γ/δ (Ikaros DN+/− × TCR-β 2/− cells) (both from PharMingen), and then fixed in 95% ethanol. Fixed cells were resuspended in a staining solution consisting of 10 μg/ml propidium iodide and 250 μg/ml RNase A, and incubated at 37°C for 30 min. Flow cytometric analyses were performed on a FACscan™ (Becton Dickinson).
PCR Analysis of TCR γ Chain Rearrangements.
Analysis was carried out as reported previously 14.
Results
T Cell Precursors in Ikaros Null and DN+/− Mice.
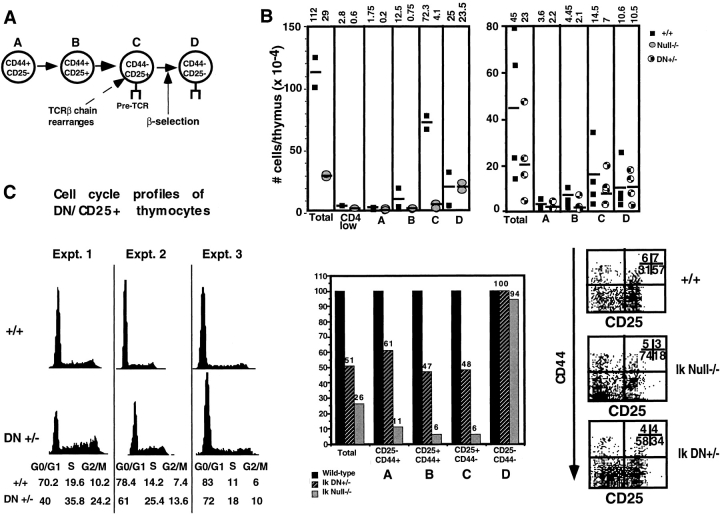
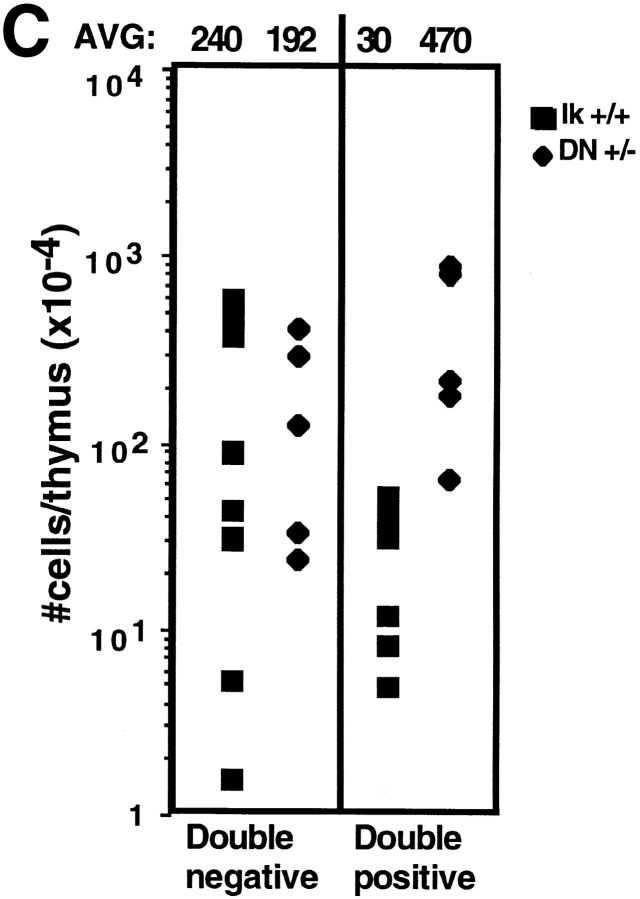
To delineate the role of Ikaros in T cell differentiation, we analyzed T cell precursor populations within the thymi of young Ikaros null mice, which have a polyclonal thymocyte repertoire. The earliest identifiable T cell precursors express low levels of the CD4 coreceptor and can give rise to T, B, and thymic dendritic cells 15. The number of CD4low precursors in the Ikaros null thymus was 4.7-fold less than that present in the wild-type thymus (Fig. 1 B, CD4low). This decrease in numbers was also observed through the next four stages of T cell development, identified in part by cell surface expression of CD44 and CD25 (together designated as the double negative stage). The maturational sequence of these precursors is (A) CD44+CD25− to (B) CD44+CD25+ to (C) CD44−CD25+ to (D) CD44−CD25− (16 17 18; Fig. 1 A). Ikaros null thymi contain an average 3.9-fold fewer double negative thymocytes, 88% of which do not express CD25 (Fig. 1 B), whereas in the wild-type thymus, only 30–40% of these cells are CD25−. As expected, the decrease in absolute numbers of double negative precursors is most dramatic within the CD25+ population (stages B and C), which is 6% of wild-type. In contrast, the numbers of CD25− stage D precursors are similar to those seen in the wild-type (Fig. 1 B).
Figure 1.
Differentiation profiles of early T cell precursors in the absence of Ikaros. (A) Schematic of differentiation events within the double negative T cell precursor stage in the thymus. (B) Numbers and staining profiles of double negative T cell precursor subsets in Ikaros wild-type, DN+/−, and null−/− thymi. Data from six independent experiments are shown: two comparing wild-type (+/+) and Ikaros null−/− double negative populations, and four comparing wild-type and Ikaros DN+/− double negative populations. Horizontal lines indicate the average absolute number of precursors that fall into each subset, also illustrated in the bar graph (y-axis is the ratio of double negative cells that fall into each subset compared with wild-type, which is considered as 100%). Numbers shown in representative FACS® profiles denote the relative percentage of cells that fall into each quadrant. Ik, Ikaros. (C) Histograms showing cell cycle profiles of double negative (DN) thymocytes. DN thymocytes were stained with anti-CD25–FITC, ethanol fixed, then stained with propidium iodide/RNase. Propidium iodide staining profiles (DNA content) of CD25+ cells from each genotype for three representative experiments are shown. The percentage of cells containing DNA contents placing them in G0/G1, S, or G2/M are shown below. Expt., experiment.
A similar trend was observed when thymocyte precursors were analyzed in mice heterozygous for the Ikaros DN mutation in which, due to the expression of a dominant negative interfering Ikaros isoform, there is a severe reduction in Ikaros activity. Thymi from young Ikaros DN+/− mice (1–2 mo of age) with polyclonal thymocyte repertoires contain, on average, twofold fewer double negative thymocytes, the majority of which, as seen in the Ikaros null thymi, do not express CD25 (Fig. 1 B). A decrease to 48% of wild-type numbers is observed in the number of CD25+ cells. Taken together, these studies show that reduction in levels of Ikaros activity leads to a decrease in numbers of the earliest thymic T cell precursors (stages A–C). However, this decrease is not observed in the most mature of these immature precursors (stage D).
The developmental stage at which thymocytes receive a pre-TCR signal necessary for their proliferative expansion and further differentiation is the stage directly preceding stage D, which is composed of CD25+ thymocytes (stage C, Fig. 1 A; reference 19). In the Ikaros DN+/− thymus, there is an increase in the percentage of CD25+ cells in the S/G2/M phases of the cell cycle (average 41 vs. 23% for DN+/− and +/+ thymocytes, respectively; Fig. 1 C). Therefore, the less dramatic reduction in precursor numbers observed in stage D is possibly due to their accelerated maturation through stage C as a result of pre-TCR–mediated hyperresponsiveness to differentiation signals provided by the microenvironment, or to a greater proliferative expansion that occurs as cells transit from stage C to D.
Ikaros-deficient Thymocytes Progress from the Double Negative to the Double Positive Stage without Pre-TCR Signaling.
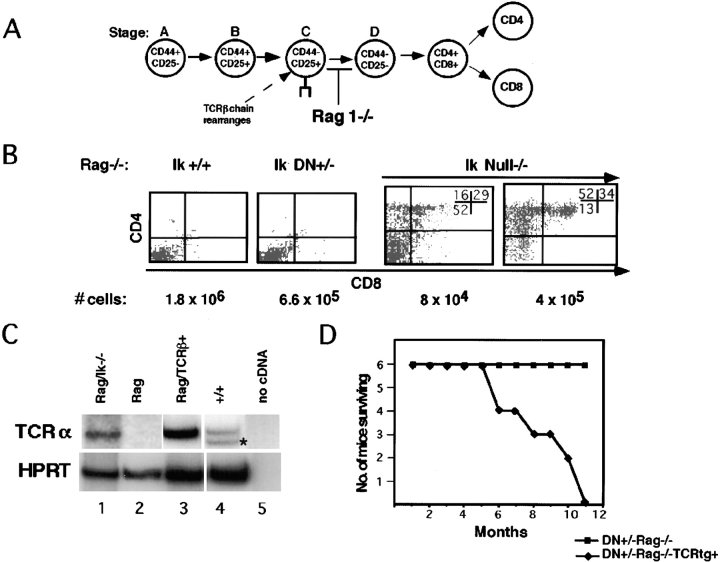
To investigate the role of Ikaros in the transition from a double negative to a double positive thymocyte, mice homozygous for an Ikaros null mutation were bred onto a genetic background with a mutation in RAG-1 (RAG-1 −/−). Thymocyte differentiation in RAG-1 −/− mice is unable to proceed beyond the double negative C stage (CD44−CD25+) due to lack of TCR β chain rearrangement (Fig. 2 A; reference 3). Unexpectedly, in Ikaros null−/− × RAG-1 −/− thymi, thymocytes expressing CD4/CD8 and CD4 were present (Fig. 2 B, Ik Null−/−). By reverse transcriptase (RT)-PCR analysis, it can be shown that at least some of these thymocytes express TCR-α germline transcripts, providing further evidence that they have developmentally progressed (Fig. 2 C, lane 1). As wild-type thymocytes transit from double negative to the double positive stage, there is a dramatic proliferative expansion that results in a double positive to double negative ratio of ∼80:1. In the absence of Ikaros, this ratio is <3:1. Therefore, in the absence of Ikaros activity, T cell differentiation, as evidenced by expression of the CD4 and CD8 coreceptor(s), proceeds in the absence of pre-TCR signaling, but without the accompanying dramatic proliferative expansion. The observed decrease in cellularity of the Ikaros null−/− × RAG −/− thymi is likely due to the reduction in numbers of thymic progenitors in these mice and to the lack of proliferative expansion as double negative cells proceed to the double positive stage, as discussed above.
Figure 2.
Thymocyte development proceeds in the absence of pre-TCR signaling in the absence of Ikaros. (A) Schematic depicting block in differentiation that occurs in RAG-1 −/− thymocytes. (B) Representative FACS® profiles showing staining patterns of thymocytes in RAG −/− mice with the following Ikaros (Ik) genotypes: +/+, DN+/−, and null−/−. Thymocytes were stained with anti-CD4–PE and anti-CD8α–FITC. Numbers shown in FACS® profiles denote percentage of cells that fall into each quadrant. Cellularity of the thymus is shown below the profiles. (C) RT-PCR analysis to analyze expression of TCR-α germline transcripts in thymi of the following animals: Ikaros null−/− × RAG −/− (lane 1), Ikaros +/+ × RAG −/− (lane 2), Ikaros +/+, TCR β chain transgenic × RAG −/− (lane 3), wild-type (lane 4), and no cDNA control (lane 5). As expected, lane 2 shows the absence of germline transcripts in the absence of pre-TCR expression and development to the double positive stage, whereas lanes 3 and 4 show the normal expression of these transcripts when pre-TCR expression allows development to the double positive stage. Lane 1 shows that in the absence of Ikaros, thymocytes can express TCR-α germline transcripts in the absence of pre-TCR expression. The lower band present in lane 4 (denoted by an asterisk) represents message from rearranged TCR α chain genes. (D) Graph depicting morbidity due to lymphomagenesis in a cohort of six Ikaros DN+/− × RAG −/− mice vs. six Ikaros DN+/−, TCR transgene+ × RAG −/− mice. tg, transgene.
Ikaros DN+/− × RAG-1 −/− thymi have, on average, 50% fewer thymocyte precursors than their RAG-1 −/− counterparts (Fig. 2 B, Ik DN+/−). The decrease in cellularity is most dramatic at the stage where pre-TCR is initially expressed (stage C, CD44−CD25+; data not shown), as observed with the DN+/− double negative populations. In contrast to the phenotype observed in Ikaros null−/− × RAG-1 −/− thymocytes, in the majority of the cases, Ikaros DN+/− × RAG-1 −/− thymocytes do not progress past the double negative stage of differentiation (Fig. 2 B, Ik DN+/−). Therefore, the reduced levels of Ikaros present in these thymocyte precursors suffice to provide a barrier to differentiation.
Ikaros DN+/− mice develop leukemias and lymphomas with 100% penetrance within 3 mo of life. Interestingly, when a cohort of DN+/− × RAG −/− mice was followed for 11 mo, no lymphomagenesis was observed (Fig. 2 C). When a transgene expressing a class I–restricted TCR (F5; reference 18) was bred onto the DN+/− × RAG −/− background, thereby restoring T cell differentiation from the double through to the CD8 single positive stage (see Fig. 8 A), the transformation phenotype could be restored (Fig. 2 C). Therefore, expression of a functional pre-TCR or TCR complex at subsequent stages of differentiation is necessary for Ikaros deficiency to have a destabilizing effect on T cell homeostasis.
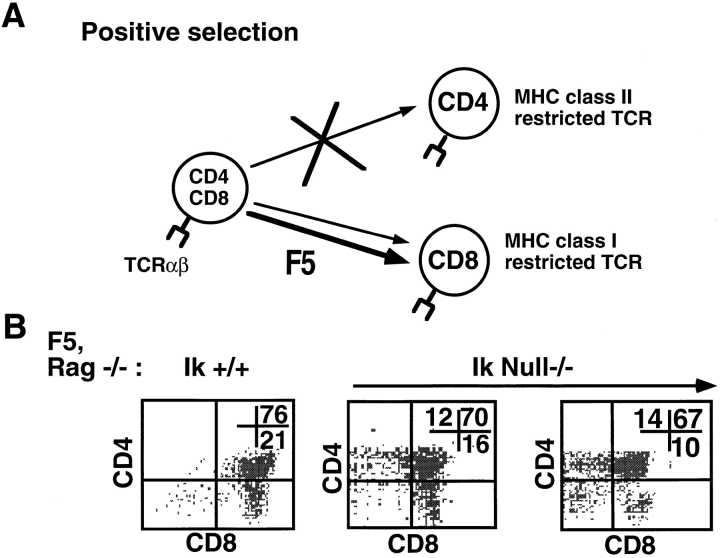
Figure 8.
Inappropriate differentiation to CD4 T cells in the presence of a class I–restricted TCR. (A) Schematic depicting positive selection in F5 TCR × RAG −/− transgenic mice. (B) Representative FACS® profiles showing staining patterns of wild-type and Ikaros (Ik) null−/− thymocytes on the F5, RAG −/− genetic background. Thymocytes were stained with anti-CD4–PE, anti-CD8α–Cychrome, and anti–TCR-β–FITC. Shown are staining profiles of cells that express high levels of TCR. Numbers shown in FACS® profiles denote percentage of cells that fall into each quadrant.
These studies implicate Ikaros as a critical regulator of the pre-TCR–mediated checkpoint in T cell differentiation known as β-selection. In the absence of Ikaros, signals received through engagement of a pre-TCR are no longer necessary for differentiation from a double negative to a double positive and CD4 single positive thymocyte. This progression occurs in the absence of proliferative expansion, thereby uncoupling two critical events in T cell differentiation.
Ikaros Is Required for Proper Differentiation and Homeostasis of Double Positive Thymocytes.
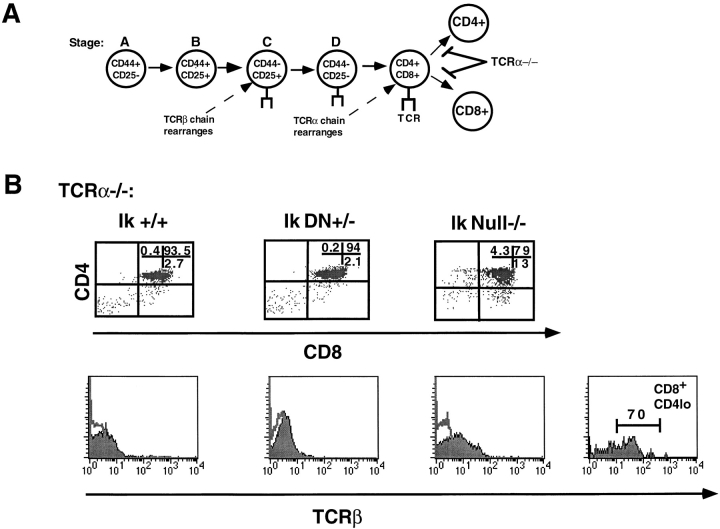
To delineate the role of Ikaros during the transition from the double positive to the single positive stage of thymocyte differentiation, the Ikaros null−/− and DN+/− mutations were bred onto a TCR-α 2/− genetic background. Thymocytes that lack expression of TCR-α arrest at the double positive stage due to their inability to transit through positive selection (Fig. 3 A; reference 4). However, in Ikaros null−/− × TCR-α 2/− thymi, cells are detected that have downregulated expression of CD4 or CD8 coreceptor (Fig. 3 B, Ik Null−/−). Therefore, these thymocytes have the phenotype of transitional-stage intermediates, a population that arises upon the combinatorial engagement of a TCR of the appropriate specificity and its coreceptor. However, these intermediate populations never fully mature into CD4 and CD8 single positive T cells and are not observed in the periphery.
Figure 3.
Developmental progression from double positive stage is deregulated in the absence of Ikaros. (A) Schematic depicting block in differentiation that occurs in TCR-α 2/− thymocytes. (B) Representative FACS® profiles showing staining patterns of thymocytes in young TCR-α 2/− mice with the following Ikaros (Ik) genotypes: +/+, null−/−, and DN+/−. Thymocytes were stained with anti-CD4–PE and anti-CD8α–FITC. Numbers shown in FACS® profiles denote percentage of cells that fall into each quadrant. Histogram profiles depict staining with anti–TCR β chain (solid histograms) and an isotype control antibody (outlined histograms). Two TCR β chain staining histograms are shown for the Ikaros null−/− × TCR-α 2/− thymocytes: one depicting the staining profile of total thymocytes (far left) and the other, the staining profile of the CD8+CD4lo thymocytes exclusively (far right). The expression of TCR β chain on the surface of these CD8+ thymocytes that do not express high levels of CD4 confirms that they are not immature single positives (ISPs).
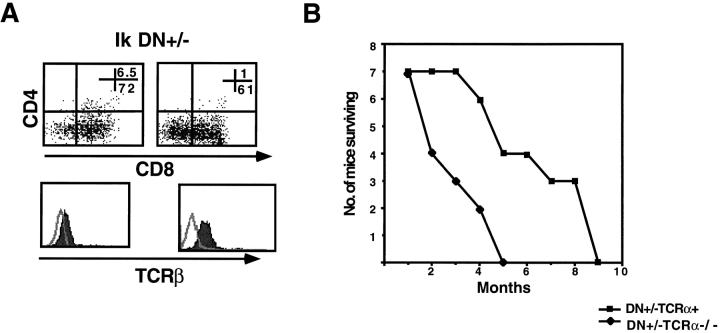
Thymi from young (up to 1 mo of age) Ikaros DN+/− × TCR-α 2/− mice are indistinguishable phenotypically from their TCR-α 2/− counterparts, indicating no obvious differentiation defects (Fig. 3 B, Ik DN+/−). However, shortly thereafter, abnormal thymic profiles are observed. In many animals, CD8+ cells are seen in the thymus, indicating inappropriate downregulation of the CD4 coreceptor (Fig. 4 A, anti-CD4 and CD8 stainings). These CD8+ cells express the TCR β chain on their cell surface, suggesting that they belong to the TCR-α/β cell lineage (data not shown). Cells of this phenotype are also observed in the periphery where they undergo dramatic expansions resulting in splenomegaly and lymphadenopathy (data not shown). In fact, between 2 and 4 mo of age, Ikaros DN+/− × TCR-α 2/− mice succumb to leukemias and lymphomas with 100% penetrance. Disease development takes about half the time in these mice compared with their Ikaros DN+/− × TCR-α 1/− littermates, which do not have a block in T cell differentiation (Fig. 4 B).
Figure 4.
Homeostasis of double positive thymocytes is deregulated in the absence of Ikaros. (A) Representative FACS® profiles showing staining patterns of thymocytes in two older Ikaros DN+/− × TCR-α 2/− mice with lymphomas. Thymocytes were stained with anti-CD4–PE and anti-CD8α–FITC. Numbers shown in FACS® profiles denote percentage of cells that fall into each quadrant. Histogram profiles depict staining with anti–TCR β chain (solid histograms) and an isotype control antibody (outlined histograms). (B) Graph denoting increased kinetics of morbidity due to lymphomagenesis in a cohort of seven Ikaros DN+/− × TCR-α 2/− mice compared with seven of their Ikaros DN+/− × TCR-α 1 counterparts.
Therefore, in the absence of all Ikaros activity, thymocytes can progress in differentiation to the transitional intermediate stage (CD4loCD8hi and CD4hiCD8lo) in the absence of TCR-mediated signals. Nevertheless, their further differentiation to the mature single positive stage is prohibited. On the other hand, when levels of Ikaros are severely reduced (Ikaros DN+/− × TCR-α 2/−), a block in T cell differentiation at the double positive, pre-TCR+ stage promotes an even more rapid development of leukemias and lymphomas.
Normal Differentiation along the γ/δ T Cell Lineage Requires Ikaros.
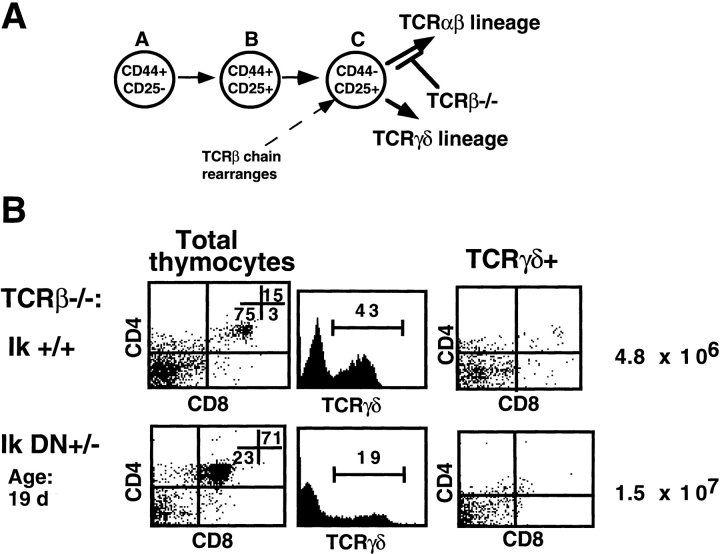
We next examined the effects of the Ikaros mutation on differentiation of the γ/δ T cell lineage. Block in α/β T cell differentiation caused by lack of expression of the TCR β chain is observed at the late double negative precursor stage (stage C; Fig. 5 A). However, this differentiation block does not affect differentiation of thymocyte precursors along the γ/δ T cell pathway 4. Analysis of the role of Ikaros in TCR-γ/δ lineage differentiation and homeostasis was studied using the Ikaros DN+/− mutation, since only a very small subset of TCR-γ/δ lineages, those found in the mucosal epithelia, develops on the Ikaros null−/− genetic background.
Figure 5.
Deregulated development of the TCR-γ/δ lineage in the absence of Ikaros. (A) Schematic depicting block in differentiation which occurs in TCR-β 2/− thymocytes. (B) Representative FACS® profiles showing staining patterns of thymocytes in young TCR-β 2/− mice with the following Ikaros (Ik) genotypes: +/+ and DN+/−. Thymocytes were stained with anti-CD4–PE, anti-CD8α–Cychrome, and anti–TCR-γδ–FITC. Numbers shown in FACS® profiles denote percentage of cells that fall into each quadrant. “Total thymocytes” shows profiles of total thymocyte populations, whereas “TCRγδ+” only shows profiles of cells that stain positively for TCR-γ/δ. (C) Numbers of double positive and double negative cells per thymus in 3–6-wk-old Ikaros (Ik) wild-type and DN+/− mice on the TCR-β 2/− genetic background. Average number (AVG) of precursors (×10−4) that fall into each subset is shown at the top of the graph.
The thymi of Ikaros wild-type × TCR-β 2/− mice are hypocellular, and the majority of thymocytes are double negative, with the percentage of double positive thymocytes varying between animals. Mature TCR-γ/δ+ thymocytes are CD4−CD8−, and therefore fall within the double negative population (Fig. 5 B). Thymi from young (up to 1 mo of age) Ikaros DN+/− × TCR-β 2/− mice contain two- to threefold more cells due to an increase in the numbers of double positive, TCR-γ/δ2 thymocytes (Fig. 5B and Fig. c). In the majority, these double positive cells do not express TCR-γ/δ in either Ikaros wild-type or Ikaros DN+/− × TCR-β 2/− thymi (Fig. 5 B, anti–TCR-γ/δ staining). Evidence exists that these double positive thymocytes may represent TCR-α/β lineage precursors that can develop due to the presence of functional TCR-γ and TCR-δ gene rearrangements 20. They normally cannot expand and are arrested in development due to the inability to express a pre-TCR. Upon reduction in Ikaros activity, larger numbers of these cells may indicate increased commitment into the α/β lineage or an increased ability of these TCR-α/β lineage precursors to survive in the absence of normally required survival signals mediated by a pre-TCR. Alternatively, this expanded population may represent TCR-γ/δ lineage precursors not normally observed due to the vast overrepresentation of TCR-α/β lineage precursors in a wild-type thymus. However, this does not seem likely, since we do not observe an increase in the numbers of mature TCR-γ/δ+ cells in these young animals.
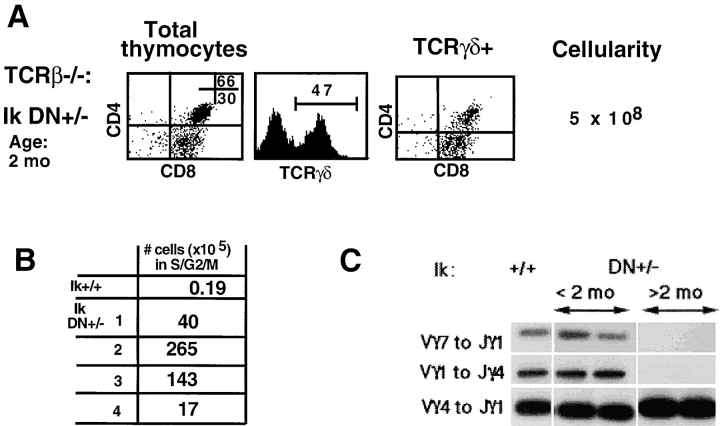
Thymi from older animals contain up to 200-fold more cells than their Ikaros wild-type × TCR-β 2/− counterparts, again due to a dramatic increase in the number of double positive cells (an average 67-fold higher than Ikaros wild-type × TCR-β 2/− thymi; Fig. 6 A). This increase in thymic cellularity in older Ikaros DN+/− × TCR-β 2/− mice led us to investigate whether these cells have become transformed. Therefore, the cell cycle profiles of thymocytes from TCR-β 2/− animals with normal and reduced (Ik DN+/−) levels of Ikaros were examined. An average 400-fold increase in the number of cycling cells was observed in thymi of Ikaros DN+/− × TCR-β 2/− mice (Fig. 6 B). The majority of cycling cells express TCR-γ/δ (data not shown), suggesting a deregulation of growth among the normally quiescent thymic γ/δ T cells. In addition, in many cases these TCR-γ/δ+ cells also express CD8 (Fig. 6 A), a cellular phenotype not observed in normally differentiating thymic TCR-γ/δ lineage cells. An increase in numbers of TCR-γ/δ cells, with the same phenotype as the cycling cells observed in the thymus, is also observed in the periphery of Ikaros DN+/− × TCR-β 2/− animals, as determined by analysis of spleen and lymph node cell populations (data not shown). These cell populations are mono- or oligoclonal as shown by a PCR analysis of V to J rearrangements at the TCR γ chain locus (Fig. 6 C), suggesting the outgrowth of a malignant clone(s). This evidence clearly shows that γ/δ T cells also depend on Ikaros activity for homeostasis.
Figure 6.
Ikaros is required for homeostasis of TCR-γ/δ lineage cells. (A) Representative FACS® profiles showing staining patterns of thymocytes in older Ikaros (Ik) DN+/− × TCR-β 2/− mice. Thymocytes were stained with anti-CD4–PE, anti-CD8α–Cychrome, and anti–TCR-γδ–FITC. Numbers shown in FACS® profiles denote percentage of cells that fall into each quadrant. “Total thymocytes” shows profiles of total thymocyte populations, whereas “TCRγδ+” only shows profiles of cells that stain positively for TCR-γ/δ. (B) Thymocytes were ethanol fixed and then stained with propidium iodide/RNase. Cell cycle profiles were analyzed by FACS® analysis. The table shows percentage of cells from each genotype that contained >2 N DNA content, indicating that they were in the S or G2/M phase of the cell cycle. (C) PCR analysis was performed with primers to amplify rearrangements at the TCR γ chain locus. These three rearrangements were chosen because they are predominant in the adult mouse thymus (reference 14). As shown, although all three rearrangements are readily seen in Ikaros wild-type and young DN+/− × TCR-β 2/− thymi, indicating a polyclonal TCR-γ/δ repertoire, only a single rearrangement, in these cases Vγ4 to Jγ1, is observed in the older DN+/− × TCR-β 2/− thymocyte populations, suggesting a monoclonal outgrowth.
Transition from the Double Positive to the Single Positive Stage Is Deregulated in the Absence of Ikaros.
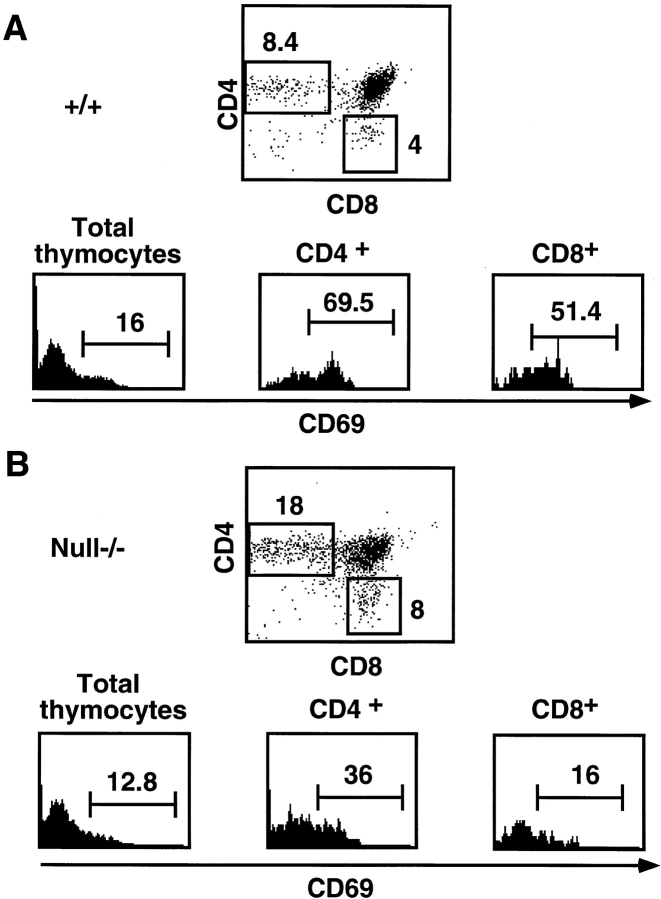
Differentiation of a double positive thymocyte to a CD4 or CD8 single positive T cell depends on TCR specificity. CD4 and CD8 T cells arise from double positive cells with a TCR specificity for an MHC class II/self-antigen complex and an MHC class I/self-antigen complex, respectively. This differentiation process is known as positive selection, and thymocytes that are positively selected upregulate the CD69 activation marker as a consequence of productive ligation of the TCR complex 21. Ikaros null−/− thymocyte profiles are heavily skewed towards CD4 single positive T cells and intermediates in transition to this phenotype 7. Although all Ikaros null−/− single positive thymocytes express high levels of TCR-α/β, the majority do not express CD69, suggesting that they may undergo this differentiation step without being positively selected (Fig. 7). Therefore, in the absence of Ikaros, differentiation through the checkpoint imposed by positive selection may be occurring in the absence of signals delivered upon engagement of an appropriate TCR–coreceptor complex. Alternatively, positive selection may be occurring at an accelerated rate due to lowered differentiation thresholds, as we have shown occurs during the process of β-selection in Ikaros-deficient thymocytes.
Figure 7.
Differentiation to the single positive stage occurs without positive selection signals in the absence of Ikaros. (A and B) Representative FACS® profiles showing staining patterns of wild-type (+/+) and Ikaros (Ik) null−/− thymocytes. Thymocytes were stained with anti-CD4–PE, anti-CD8α–Cychrome, and anti-CD69–FITC. Numbers shown in FACS® profiles denote percentage of cells that fall into each quadrant. Histograms show staining levels with anti-CD69–FITC. Numbers indicate percentage of CD69+ cells within the total, CD4 single positive, and CD8 single positive thymocyte populations as listed above the histograms.
To clarify the effects of Ikaros on the double to single positive transition as regulated by the process of positive selection, we introduced a transgene expressing a class I MHC–restricted TCR (F5) onto the Ikaros null−/− × RAG-1 −/− background. The F5 TCR is expressed on all thymocytes and, in Ikaros wild-type mice, results in the exclusive positive selection of CD8 T cells (Fig. 8 A; reference 22). However, Ikaros null−/−, F5 × RAG-1 −/− thymocytes differentiate into both CD8 and CD4 T cells, showing approximately equal percentages of each population (Fig. 8 B). However, these CD4 T cells do not appear in the periphery, indicating that they have not undergone final selection steps required for their exportation.
Discussion
These studies delineate the role of Ikaros in the development of the α/β and γ/δ T cell lineages. Ikaros proteins set thresholds that must be overcome by signaling through the pre-TCR and TCR complexes for T cell differentiation to occur. In the absence of Ikaros activity, these thresholds are reset to a lower level, allowing progression from the double negative through the double positive to the single positive stage to occur without the appropriate pre-TCR and TCR signaling. Thus, it appears that the existence of distinct TCR signaling thresholds that control the outcome of T cell differentiation pathways relies on the Ikaros family of nuclear factors.
Thymocytes that do not express a pre-TCR complex (i.e., RAG-1 −/−) arrest their development at an early precursor stage (double negative, CD44−CD25+). Pre-TCR signaling is required for progression to the next stage of differentiation where both CD4 and CD8 coreceptors are expressed (double positive stage; reference 23). It is also required for the proliferative expansion of thymocytes during this transition. However, in the absence of Ikaros activity, thymocyte precursors can transit from the double negative to the double and CD4 single positive stages without the signaling events provided by pre-TCR and TCR engagement. However, this differentiation event occurs without the normally occurring proliferative expansion, resulting in a highly hypocellular thymus. Thus, lack of Ikaros activity in thymocyte precursors uncouples the process of differentiation from that of proliferation, allowing the one to occur in the absence of the other. Interestingly, the preferential “development” of CD4 lineage cells observed in Ikaros null−/− × RAG −/− thymi correlates with the phenotype previously reported for Ikaros null−/− thymi without the RAG mutation 7. Therefore, we hypothesize that Ikaros plays a central role in establishing thresholds for differentiation that give rise first to double positive, and subsequently to CD4 single positive thymocytes.
In support of this hypothesis, we have shown that within the double negative thymocyte population, reduction of Ikaros activity also causes an apparent increase in the rate of differentiation to the double positive stage, possibly by lowering thresholds of pre-TCR signaling. In Ikaros-deficient mice, there is a decrease in both the absolute and relative numbers of late double negative precursors (CD25+, stage C), a greater percentage of which are in cell cycle. However, normal numbers of stage D precursors are observed. Therefore, precursors may be transitioning through the CD25+ stage more rapidly, leading to the decrease in the size of this precursor compartment and, in addition, undergoing a more dramatic proliferative expansion such that normal numbers of stage D precursors are generated.
In mature T cells, Ikaros is a negative regulator of TCR-mediated proliferative responses 24. Progressive reduction of Ikaros activity in peripheral T cells results in a progressive increase in the TCR-mediated proliferative response. In addition, reduction of Ikaros activity allows T cells to proliferate in response to lower levels of signaling that normally do not support proliferation. In a similar fashion, Ikaros may provide negative regulation for pre-TCR–mediated differentiation. In the absence of Ikaros, differentiation can proceed in the absence of pre-TCR, and in the presence of pre-TCR may proceed at a faster rate.
Double positive thymocytes expressing TCR undergo a process known as positive selection as they differentiate to the CD4 and CD8 single positive stages. Using a TCR transgenic model system, we have shown that, in the absence of Ikaros, CD4+TCR+ thymocytes are produced without the signals provided by a class II MHC–restricted TCR. Ikaros null−/− mice with a polyclonal thymocyte repertoire also show a significant increase in the relative percentage of CD4 single positive thymocytes. The majority of single positive cells in the Ikaros null−/− thymus do not express the CD69 activation marker, an event indicative of positive selection. Therefore, transition from the double to the CD4 single positive stage is occurring in the absence of selection, possibly as a result of lowered signaling thresholds. Alternatively, lower thresholds of signaling at the double positive TCR+ stage may allow for faster transition through the CD69+ stage. The CD4+ T cells that develop in the Ikaros null−/−, F5 × RAG-1 −/− thymus are not found in the periphery, indicating that the final step in the selection process is not taking place and, therefore, that Ikaros activity is not required for regulating this final step of T cell maturation which occurs in the thymus.
We have shown that thymocytes express the highest levels of Ikaros 11, and it is within this population that transformation occurs with 100% penetrance when these levels are reduced 7 9. However, Ikaros-deficient thymocytes that lack expression of a receptor linked to TCR signaling pathways are refractory to transformation. Therefore, when levels of Ikaros activity are reduced, expression of a pre-TCR, TCR-α/β, or TCR-γ/δ is necessary to destabilize homeostasis and trigger proliferative events that eventually lead to malignancy. We have recently shown that when primary Ikaros-deficient T cells proliferate in response to engagement of the TCR complex, they consistently show chromosome instability 24. Perhaps this same instability arises after receptor engagement within the thymocyte population. Supporting this hypothesis, we have observed that when Ikaros-deficient thymocytes are arrested in differentiation at a stage that is targeted for dramatic proliferative expansion (pre-TCR+ stage), malignancies arise with increased kinetics. Expression of an active recombinase machinery in immature thymocytes may greatly contribute to the chromosomal aberrations mediated by decreased levels of Ikaros activity during their proliferation.
In conclusion, we have provided evidence that Ikaros activity is required at several distinct steps of thymocyte differentiation. One function of Ikaros is to regulate the transit through pre-TCR and TCR-mediated differentiation checkpoints by providing signaling thresholds, while the second function is to control proliferation in response to pre-TCR and TCR engagement on immature thymocytes. We have strong evidence that Ikaros is a critical nuclear effector of multiple signaling pathways downstream of TCR complex engagement in mature peripheral T cells (i.e., Src protein tyrosine kinases, mitogen-activated protein kinase, calcineurin [24]). Many of these same pathways are used within the thymocyte population to regulate proliferation and differentiation 23 25 26. How Ikaros activity is modulated by these signaling pathways to effect its function will be the focus of future research.
Acknowledgments
We thank Shuwei Jiang for cell sorting, and Taj Pathan for DNA analysis of mice.
The transgenic and other research were supported by National Institutes of Health grant R01 AI38342-03 to K. Georgopoulos, and by a core grant from the Cutaneous Biology Research Center (Shiseido Co., Ltd.). K. Georgopoulos is a Scholar of the Leukemia Society of America; S. Winandy is a recipient of a King Trust Research Award from the Medical Foundation; and L. Wu was supported by a fellowship from the International Union against Cancer.
Footnotes
1used in this paper: DN, Ikaros dominant negative mutation; HPRT, hypoxanthine-guanine phosphoribosyltransferase; RAG, recombinase activating gene; RT, reverse transcriptase
J.-H. Wang's current address is Bristol-Myers Squibb, P.O. Box 4000, Princeton, NJ 08453-4000.
References
- Godfrey D.I., Kennedy J., Mombaerts P., Tonegawa S., Zlotnik A. Onset of TCR-β gene rearrangement and role of TCR-β expression during CD3−CD4−CD8− thymocyte differentiation. J. Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R., Herrup K., Tonegawa S., Papaioannou V. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A., Rudnicki M., Iacomini J., Itohara S., Lafaille J., Wang L., Ichikawa Y., Jaenisch R., Hooper M., Tonegawa S. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages [published erratum at 360:491] Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Fehling H.J. Structure and function of the pre-T cell receptor. Annu. Rev. Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- Wang J.-H., Nichogiannopoulou A., Wu L., Sun L., Sharpe A.H., Bigby M., Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K., Bigby M., Wang J.-H., Molnár Á., Wu P., Winandy S., Sharpe A. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- Winandy S., Wu P., Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83:289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Ismaili J., Antica M., Wu L. CD4 and CD8 expression and T cell antigen receptor gene rearrangement in early intrathymic precursor cells. Eur. J. Immunol. 1996;26:731–737. doi: 10.1002/eji.1830260402. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K., Moore D., Derfler B. Ikaros, an early lymphoid restricted transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Marshall B., Schulz R., Zhou M., Mellor A. Alternative splicing and hypermutation of a nonproductively rearranged TCR alpha-chain in a T cell hybridoma. J. Immunol. 1999;162:871–877. [PubMed] [Google Scholar]
- Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A.R., Hooper M.L., Farr A., Tonegawa S. T cell receptor δ gene mutant miceindependent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Ardavin C., Wu L., Li C., Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- Pearse M., Wu L., Egerton M., Wilson A., Shortman K., Scollay R. A murine early thymocyte developmental sequence is marked by transient expression of the interleukin 2 receptor. Proc. Natl. Acad. Sci. USA. 1989;86:1614–1618. doi: 10.1073/pnas.86.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D., Zlotnik A. Control points in early T-cell development. Immunol. Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Kennedy H., Suda T., Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- Hoffman E.S., Passoni L., Crompton T., Leu T.M.J., Schatz D.G., Koff A., Owen M.J., Hayday A.C. Productive T-cell receptor β chain gene rearrangementcoincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- Livak F., Wilson A., MacDonald H.R., Schatz D.G. αβ lineage committed thymocytes can be rescued by the γδ T cell receptor (TCR) in the absence of TCR β chain. Eur. J. Immunol. 1997;27:2948–2958. doi: 10.1002/eji.1830271130. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Matzinger P., Seder R., Paul W., Schwartz R. Activation events during thymic selection. J. Exp. Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamalaki C., Elliott J., Norton T., Yannoutsos N., Townsend A.R., Chandler P., Simpson E., Kioussis D. Positive and negative selection in transgenic mice expressing a T-cell receptor specific for influenza nucleoprotein and endogenous superantigen. Dev. Immunol. 1993;3:159–174. doi: 10.1155/1993/98015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling H.J., von Boehmer H. Early αβ T cell development in the thymus of normal and genetically altered mice. Curr. Opin. Immunol. 1997;9:263–275. doi: 10.1016/s0952-7915(97)80146-x. [DOI] [PubMed] [Google Scholar]
- Avitahl N., Winandy S., Friedrich C., Jones B., Ge Y., Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10:333–343. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- Saito T., Watanabe N. Positive and negative thymocyte selection. Crit. Rev. Immunol. 1998;18:359–370. doi: 10.1615/critrevimmunol.v18.i4.40. [DOI] [PubMed] [Google Scholar]
- Aberola-Ila J., Takaki S., Kerner J.D., Perlmutter R. Differential signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]