Abstract
T lymphocytes express two Src tyrosine kinases, Lck and Fyn. While thymocyte and T cell subsets are largely normal in fyn−/− mice, animals lacking Lck have impaired T cell development. Here, it is shown that Fyn is required for the rapid burst of interleukin (IL)-4 and IL-13 synthesis, which occurs promptly after T cell receptor activation. The lack of cytokine induction in fyn mutant mice is due to a block in natural killer (NK) T cell development. Studies using bone marrow chimeras indicate that the defect behaves in a cell-autonomous manner, and the lack of NK T cells is probably not caused by inappropriate microenvironmental cues. Both NK T cells and conventional T cells express similar levels of Lck, implying that Fyn and Lck have distinct roles in regulating NK T cell ontogeny. The fyn mutation defines the first signaling molecule that is selectively required for NK T cell, but not for T lymphocyte or NK cell development.
Keywords: signal transduction, mouse mutants, Lck, β2-microglobulin, CD1
The Src family of tyrosine kinases plays an important role in the transduction of signals necessary for the development or activation of various cell types (for a review, see reference 1). T lymphocytes and NK cells express mainly two Src kinase family members, Fyn and Lck. By analyzing mouse mutants, Fyn has been found to have a role in regulating calcium flux and proliferation induced by ligation of the TCR or Thy-1, especially in single positive thymocytes 2 3. These defects do not appear to compromise T cell development, as normal numbers of T cell subsets are present in the thymus and periphery of fyn-deficient animals, and T cell function is largely normal. In contrast, mice deficient in lck have a block in T cell development, resulting in reduced numbers of immature CD4+CD8+ double positive as well as mature single positive (CD4+ or CD8+) T lymphocytes 4. Although each kinase appears to have distinct functions during T cell ontogeny, Fyn also can partially compensate for loss of Lck, since mice deficient in both fyn and lck have a profound block in T cell development at the double negative stage, similar to recombination activating gene–deficient (RAG−/−) mice 5 6. Interestingly, mice deficient in either fyn or lck alone exhibit normal numbers of γ/δ intraepithelial lymphocytes (IELs), whereas fyn/lck double mutants have few γ/δ IELs 7. This suggests that Fyn and Lck have redundant functions during γ/δ IEL development, but only partially overlapping roles in α/β T cell development. Moreover, the double mutant mice also have normal NK cell development and function 5. These observations raise the question of whether development of NK T cells, a unique lymphoid population that has characteristics of both NK cells and T lymphocytes, will be dependent on Src kinases as well.
NK T cells comprise ∼0.5% of the thymus and 1% of the spleen (for a review, see reference 8). The majority of NK T cells express a TCR with a restricted Vα chain (Vα14Jα281) and have biased Vβ usage, >50% Vβ8 9 10 11. The expression of typical NK markers such as NKR-P1C, members of the Ly49 family, and IL-2Rβ demonstrates their affiliation to the NK lineage. These cells are known to secrete large amounts of cytokines, especially IL-4 and IFN-γ after TCR cross-linking 12 13. In fact, in the first hours after in vivo activation of the TCR, NK T cells are the major source of IL-4 14. There is also evidence to suggest that NK T cells may play a role in a variety of other immune functions, including tumor surveillance, autoimmunity, and responses to glucosyl phosphatidylinositol (GPI)-linked antigens 8 15.
Although the ontogeny of NK T cells remains poorly understood, they do have some unique developmental requirements. Unlike conventional T cells, which use classical MHC expressed by thymic epithelium for selection, murine NK T cells recognize the nonclassical MHC-like molecule, CD1d, expressed on bone marrow–derived cells (for a review, see reference 8). Consequently, the targeted disruption of the CD1.1 gene abrogates NK T cell development 16 17 18. The thymus is the major site of NK T cell development, as demonstrated by the ability to develop NK T cells in thymic organ culture and the drastic reduction in NK T cells in thymectomized mice 19 20. Despite this, other studies indicate that at least a proportion of NK T cells can develop extrathymically 21 22. While NK cells and T lymphocytes have distinct developmental requirements, NK T cells have characteristics of both. For example, T cells and NK T cells require CD3ζ and pre–TCR-α 23 24, but like NK cells, NK T cell development is also dependent on IL-2Rβ 25. Although the signaling requirements for NK T cell development remain poorly defined, some evidence suggests that it may differ from conventional T cells. The Ras/Raf/Mek/MAP kinase cascade is not necessary for NK T cell ontogeny, but is required for positive selection of conventional T cells 26. Therefore, at least some receptor-distal signaling pathways are used differently between T and NK T cells.
In this study, we demonstrate the necessity of the Fyn tyrosine kinase in NK T cell development. fyn mutant mice fail to produce the initial burst of IL-4 or IL-13 after CD3 cross-linking in vivo or in vitro. This is due to an absence of NK T cells in the thymus and periphery as assessed by flow cytometric analysis and reverse transcription (RT)-PCR for the canonical Vα14Jα281 TCR. This appears to be a cell-autonomous defect rather than microenvironmental effect, since NK T cells do not arise when fyn mutant bone marrow is injected into irradiated wild-type hosts. Finally, NK T cells express both Lck and Fyn, suggesting a specific role for Fyn in NK T cell ontogeny that cannot be compensated for by Lck. This is the only mutation reported to date involved with signal transduction that specifically abolishes NK T cell development, leaving all other lymphoid lineages intact.
Materials and Methods
Mice.
The fyn mutation 2 is maintained on a mixed (129 × B6) genetic background, but mice were homozygous for the NKR-P1C allele recognized by the NK1.1 antibody. In some experiments, fyn mutants that had been backcrossed 10 generations to C57Bl/6 were also used. Mice on the C57Bl/6 background that were deficient for either β2-microglobulin (β2M) or Lck were purchased from The Jackson Laboratory. C57Bl/6 and 129Sv mice were bred on site. All mice used in these studies were from 7 to 12 wk of age and were maintained under American Association of Accreditation of Laboratory Animal Care (AALAC) and institutional approved guidelines.
Antibodies and Flow Cytometry.
Thymocyte and splenocyte suspensions were filtered through mesh to obtain single cell cultures. Spleen suspensions were also depleted of red blood cells by treating with 0.14 M ammonium chloride. Liver lymphocytes were obtained as detailed elsewhere 27. The following antibodies were used for flow cytometric analysis: anti–TCR-β–FITC (H57-597), anti-NK1.1–PE (PK 136), Fc block (2.4G4), anti-CD4–PE (GK1.5), anti-Ly9.1–FITC (30C7), and anti–heat stable antigen (HSA)–biotin (M1/69) from PharMingen, and Tricolor-strepavidin from Caltag. Three-color immunofluorescence analysis was performed using a FACScan™ flow cytometer (Becton Dickinson) and analyzed using CELLQuest™ software.
Quantitative RT-PCR.
The Vα14Jα281 rearrangement was detected after RT of 5 μg total RNA using random hexamers and Superscript II reverse transcriptase (GIBCO BRL). Quantitative PCR was performed using the LightCycler (Roche Molecular Biochemicals). In brief, 2 μl of a 1:4 dilution of the RT reaction was amplified in the following reaction mixture: 67 mM Tris, pH 8.8, 16.6 mM ammonium sulfate, 6.7 mM magnesium chloride, 5 mM β-mercaptoethanol, 0.01% gelatin, 10% DMSO, 1 mM dNTPs, 100 ng each primer, 1.5 U Taq, and a 1:20,000 dilution of SYBR green gel stain (Roche Molecular Biochemicals). A standard curve was made using dilutions of an RT reaction from C57Bl/6 splenocytes. The relative value for each sample was then calculated using the LightCycler software. To control for variations in the RT reaction, all PCR reactions were normalized for hypoxanthine phosphoribosyltransferase (HPRT) expression. The following primers were used: HPRT sense, GTAATGATCAGTCAACGGGGGAC; HPRT antisense, CCAGCAAGCTTGCAACCTTAACCA; Vα14 5′, CTAAGCACAGCACGCTGCACA; J281 3′, CAGGTATGACAATCAGCTGAGTCC 9.
RNase Protection.
To induce cytokines in vivo, mice were injected intravenously through the retroorbital sinus with either 5 μg anti-CD3 (2C11) or PBS. After 1.5 h, the mice were killed, splenocytes were isolated, and RNA was purified. For the in vitro studies, T cells were enriched for by passing splenocytes over a nylon wool column. The suspensions averaged 80–90% T cells. Aliquots of 20 × 106 cells were stimulated in a 2-ml volume with 5 μg/ml anti-CD3 (2C11) and 1 ng/ml PMA or 1 ng/ml PMA and 500 nM ionomycin at 37°C. After 2 h, RNA was isolated and 7.5 μg of RNA was analyzed using the RiboQuant RNase protection assay system (PharMingen), probe set m-CK1. The samples were separated on a 5% acrylamide/urea gel and dried at 80°C for 60 min. The gels were then exposed on a PhosphorImager® (Molecular Dynamics) or directly onto film.
Cell Sorting and Western Blot.
Thymocytes from five C57Bl/6 mice were cultured in 6-well dishes at 30 × 106 cells/ml in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 25 IU/ml penicillin, 25 μg/ml streptomycin, 50 mM β-mercaptoethanol, 11 μg/ml sodium pyruvate, 3.6 μg/ml asparagine, 0.6 μg/ml folic acid, 11.2 μg/ml arginine, and 150 U/ml human recombinant (hr)IL-2 (Roche Molecular Biochemicals) plus 1% conditioned media from the IL-7–producing cell line, J558. After 5 d, dead cells were removed by centrifugation through Lympholyte-M (Accurate Chemical, Inc.). The cells were then cultured at 2 × 105 cells/ml in media with 150 U/ml hrIL-2. After 3 d, the cells were harvested and used for fluorescent sorting after staining with anti-NK1.1–PE and anti–TCR-β–FITC. Protein lysates were resolved by 8% SDS-PAGE, then transferred to polyvinylidene difluoride membrane (NEN). The membrane was blocked using TNB buffer (30 mM Tris, pH 7.6, 75 mM NaCl, and 3% bovine serum albumin) overnight at 4°C. Polyclonal anti-Fyn (Santa Cruz Biotechnology) or anti-Lck (Upstate Biotechnology) was added at concentrations suggested by the manufacturers and incubated for 1 h. The membrane was then incubated with 0.5 μCi/ml iodinated protein A for 1 h at room temperature, and washed with TBST (30 mM Tris, pH 7.6, 75 mM NaCl, and 0.2% Tween 20) for 1 h followed by autoradiography.
Radiation Chimeras.
6-wk-old 129Sv mice received 700 rads whole body irradiation from a cesium source. 5 × 106 C57Bl/6 strain bone marrow cells (Fyn, β2M, or wild-type), plus 1 × 106 129Sv bone marrow cells were then injected intravenously through the retroorbital sinus. Thymus and spleen were harvested after 10–12 wk and analyzed by flow cytometry for the presence of NK T cells. The relative contribution of C57Bl/6 and 129Sv cells to the hematopoietic lineages was assessed by monitoring Ly9.1 expression, which is present only on 129Sv-derived cells.
Results
Impaired IL-4 Synthesis in fyn Mutants.
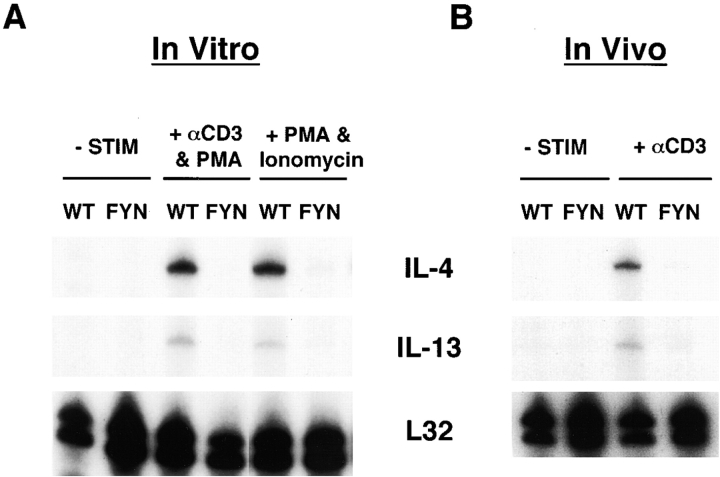
Since fyn mutant mice synthesize reduced amounts of IL-2 after cross-linking of the TCR 2, we measured the induction of T cell–produced cytokines, IL-4 and IL-13, after TCR engagement to determine whether their production was also impaired. Splenic T cells from naive wild-type and fyn mutants were isolated and stimulated in vitro with anti-CD3∈ plus PMA for 2 h. No IL-4 RNA was detected in the fyn mutant cultures (Fig. 1 A), but was present in the wild-type cells. Additionally, induction of IL-13, which shares some common regulatory elements and functions with IL-4 (for a review, see reference 28), also does not occur in the mutant cells. Longer incubation with antibody still failed to elicit IL-4 or IL-13 in the mutant cultures (data not shown). To confirm the in vitro data, naive mice were injected with anti-CD3∈ antibody to induce cytokine production. Analysis of spleens from treated mice again revealed that no IL-4 or IL-13 RNA was induced in the fyn mutants, whereas they were readily detectable in wild-type mice (Fig. 1 B). To determine if the failure in IL-4 production was due to defective activation through the TCR, T cells were treated in vitro with ionomycin and PMA to bypass the requirement for surface receptors. Splenic T cells from wild-type mice produced abundant amounts of IL-4 and IL-13 RNA, but again none was induced in fyn-deficient T cells (Fig. 1 A). One interpretation of these data is that Fyn may be required for proper development of the T cell subset(s) capable of rapid IL-4/IL-13 production.
Figure 1.
Rapid induction of IL-4 or IL-13 RNAs after CD3 cross-linking fails to occur in fyn mutants. (A) Splenocytes from fyn mutant (FYN) or wild-type (WT) mice were stimulated with 5 μg/ml anti-CD3 (2C11) and 1 ng/ml PMA or 1 ng/ml PMA and 500 nM ionomycin for 2 h in vitro. RNA was harvested, and an RNase protection assay specific for IL-4 and IL-13 was performed. L32 RNA expression was also determined as a loading control (−STIM). (B) Wild-type and fyn − / − mice were injected intravenously with PBS or 5 μg of anti-CD3 antibody. The spleen was harvested 1.5 h later, and IL-4/IL-13 RNA induction was measured by RNase protection assay. Shown are representative data from three separate experiments.
fyn Mutants Have Highly Reduced Numbers of NK T Cells.
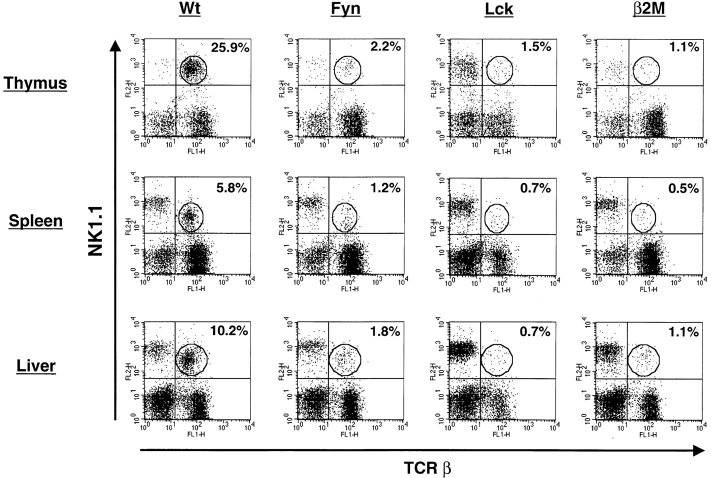
The rapid accumulation of IL-4 after TCR engagement has been shown to originate mostly from the NK T cell subset 14. The lack of IL-4 induction in fyn mutants suggests that this cell type may be missing or nonfunctional. To address this, lymphocytes were isolated from thymus, spleen, or liver, and examined for the presence of NK T cells by flow cytometric analysis (Fig. 2). Relative to wild-type animals, the number of NK T cells in fyn−/− mice was reduced 10-fold in the thymus and >5-fold in the spleen and liver. This approached the limits of detection under the conditions used. The NK T cell number was compared with β2M-deficient mice, since this mutation prevents NK T cell development 19 29. In all tissues examined, the fyn mutant had roughly twice as many NK T cells as β2M mice. These data indicate that loss of Fyn significantly disrupts NK T cell development.
Figure 2.
Fyn and Lck are both required for proper development of NK1.1+TCR-β+ cells. Thymocytes, splenocytes, and liver lymphocytes were stained with HSA, TCR-β, and NK1.1. After gating on HSAlow cells, two-color TCR-β vs. NK1.1 plots were constructed. The percentage of cells positive for both NK1.1 and TCR (NK T cells) in the circled gate is displayed in the upper right quadrant of each plot. The data shown are representative from 10 C57Bl/6 (B6, Wt), 12 Fyn (129 × B6 hybrid or B6), 4 Lck (B6), and 5 β2M (B6) mice analyzed in 5 separate experiments.
There Is a Cell-intrinsic Component to the Requirement of Fyn in NK T Cell Development.
Since NK T cells are selected by CD1d, it was determined that fyn mutant mice still express normal levels of CD1d in the thymus (data not shown). This indicates that the fyn mutation does not disrupt NK T cell development by affecting CD1d surface expression. However, it remains a formal possibility that CD1d or the stromal environment may be altered in the mutant and prevent maturation of NK T cells. For example, CD1d uses a tyrosine-based motif for entering the endocytic pathway required for loading antigen and subsequent presentation to NK T cells 30; perhaps phosphorylation by Fyn is required for targeting to endosomes. To determine whether Fyn is required in accessory cells of the microenvironment or directly in NK T cells, radiation chimeras were constructed. Since NK T cells are selected by bone marrow–derived cells rather than thymic epithelia, it was necessary to mix bone marrows from wild-type 129Sv mice with either wild-type C57Bl/6 or mice on the C57Bl/6 background that were mutant for fyn or β2M, then inject into irradiated 129Sv mice. Cells derived from the 129Sv bone marrow should provide the appropriate environmental cues necessary for C57Bl/6 wild-type or mutant cells to develop into NK T cells. The 129Sv strain was selected for two reasons. First, the 129Sv strain does not express the NK1.1 marker, so any NK1.1+TCR-α/β+ (NK T) cells detected must be C57Bl/6 derived. Second, the 129Sv and C57Bl/6 strains express different alleles of the Ly9 cell surface marker, and the relative contributions of the two types of bone marrows to the chimera can then be determined by flow cytometry.
Thymocytes from each group were analyzed for the presence of NK T cells by flow cytometry. Irradiated mice which received either wild-type or β2M mutant bone marrow developed NK T cells (Fig. 3 A). Since β2M is required for stable surface expression of CD1, this result indicated that the CD1d present on wild-type 129Sv cells was able to complement the defect and allow the β2M-deficient lymphocytes to undergo selection and form NK T cells. In contrast, mice receiving the mixture of 129Sv and fyn mutant bone marrow had no detectable NK T cells. Because the wild-type 129Sv–derived cells were unable to rescue NK T cell development in the fyn mutant chimera, it is likely that this is a cell-autonomous defect in the NK T cell or progenitor. Fig. 3 B shows that all of the chimeras contain C57Bl/6-derived thymocytes at similar levels. Chimerism extended to the periphery, since spleens from all reconstituted mice contained C57Bl/6-derived NK cells and T lymphocytes as well (data not shown). This indicates that the lack of NK T cells in the chimeras made from fyn mutants is not due to poor colonization by fyn mutant bone marrow.
Figure 3.
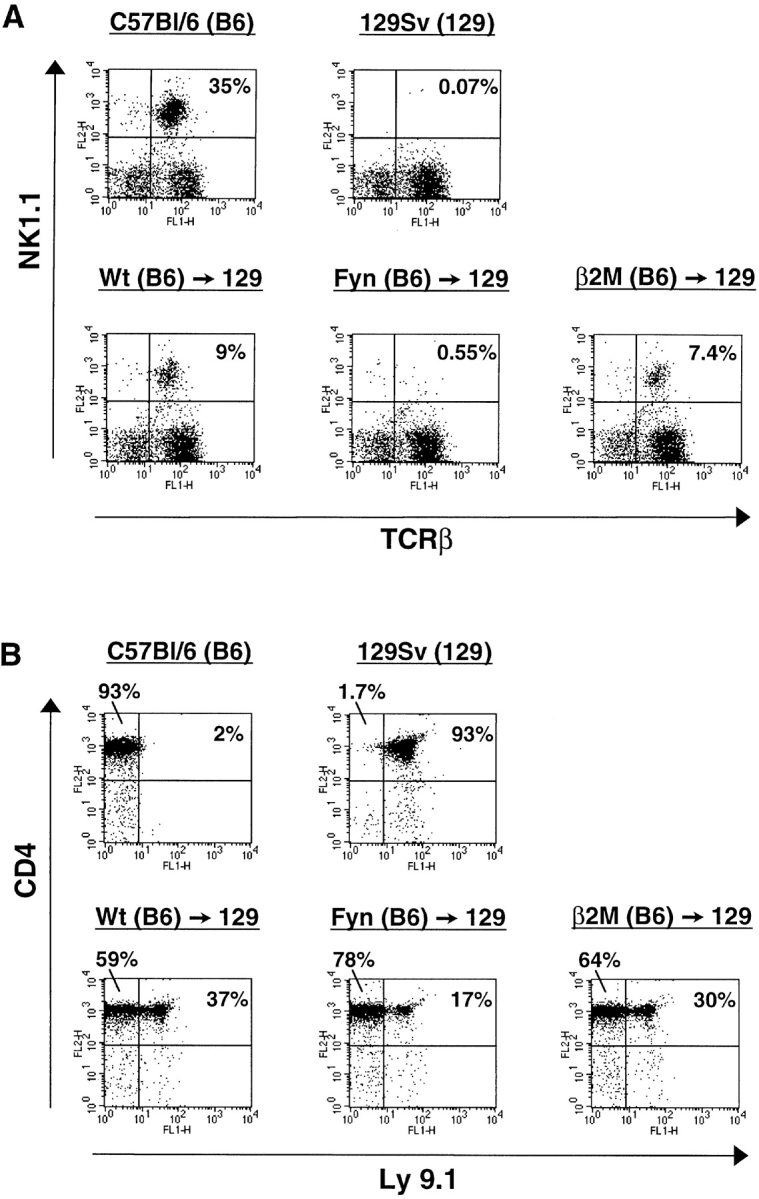
The fyn mutation may function in a cell-autonomous manner during NK T cell development. Irradiated mice were injected with a mix of wild-type 129Sv (NK1.1−; Ly9.1+) and wild-type or mutant C57Bl/6 (NK1.1+; Ly9.1−) derived bone marrow. (A) Reconstitution of NK T cells in the thymus by the different bone marrows was assessed by flow cytometry as in Fig. 2. NK T cells developed from wild-type (Wt) and β2M mutant bone marrow, but not fyn mutant bone marrow. (B) The relative contribution of 129Sv- and C57Bl/6-derived lymphoid cells in the thymus was determined by comparing Ly9.1 expression on CD4+ thymocytes by flow cytometry. All the radiation chimeras contain a mix of CD4+ thymocytes derived from the two strains. The data presented are representative of two independent experiments (chimeras: wild-type, n = 7; Fyn, n = 7; β2M, n = 4).
Both Fyn and Lck Are Required in NK T Cells.
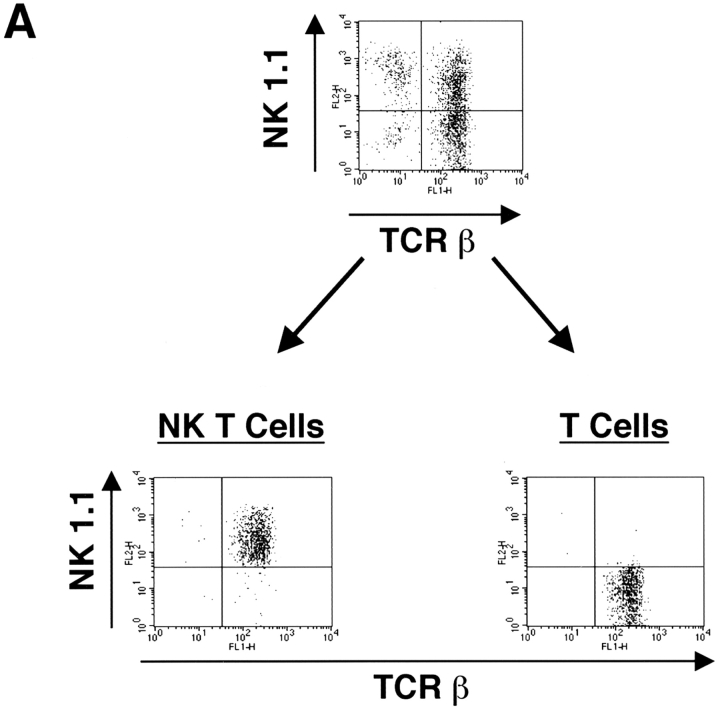
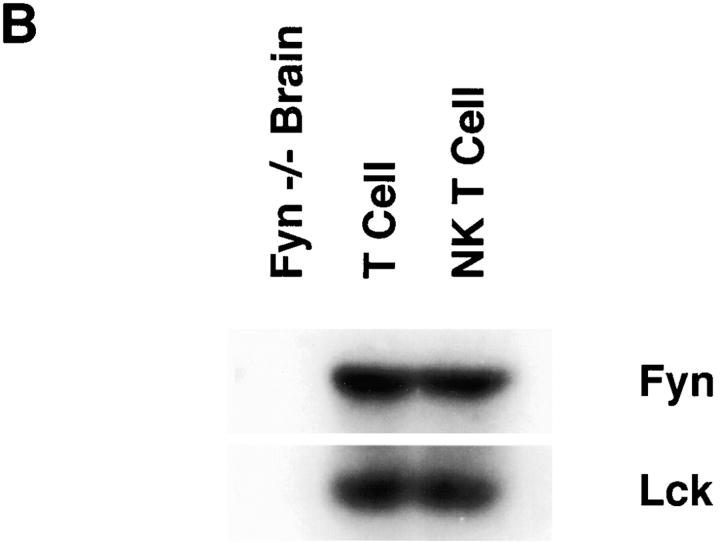
Lck appears to be the predominant Src family member required for conventional T cell development. However, from studies using transgenic mice overexpressing Fyn, and mice with mutations in both fyn and lck, it is clear that Fyn can partially compensate for Lck and allow development of double positive and mature single positive T cells, though at a much reduced efficiency 5 6. One possibility as to why Fyn is required for NK T cell development is that Lck may not be expressed during NK T cell development or in mature NK T cells, leaving Fyn as the only Src family member required for a later expansion or survival of NK T cells. Alternatively, Fyn may have a specific role that Lck cannot compensate for. To distinguish between these two possibilities, the expression of Lck and Fyn was determined in NK T cells. Both conventional and NK T cells were purified from thymocyte cultures, then examined for Lck and Fyn expression by Western blot analysis. As shown in Fig. 4, equivalent amounts of Lck and Fyn are present in both NK T cells and conventional T cells. This suggests that Fyn and Lck may have nonredundant roles in NK T cell ontogeny, although it is still formally possible that Lck may not be expressed in some progenitor. This seems unlikely, since Lck is expressed throughout normal T cell development as well as in mature T cells and NK cells 31. These data demonstrate that Lck is expressed in NK T cells as well.
Figure 4.
Both conventional T cells and NK T cells express similar levels of Lck and Fyn. (A) Thymocytes were cultured from C57Bl/6 mice to enrich for NK T cells. The cultures were sorted by FACS® into NK1.1+TCR-β+ (NK T cell) and NK1.1−TCR-β+ (T cell) populations. (B) These populations as well as brain extract from a fyn mutant mouse as a negative control were then assayed for Fyn and Lck expression by Western blot analysis.
To assess whether Lck is required for NK T cell development, lck mutant mice were examined for the presence of this population by flow cytometry (Fig. 2). The levels of NK T cells were reduced 10–20-fold relative to wild-type mice, and were equivalent to β2M mutant mice. Thus, both Fyn and Lck are required during NK T cell development. NK T cells are thought to progress through the pre-TCR stage of T cell development due to their requirement for pre–TCR-α 24. Since Lck is required for proper pre-TCR signaling, it is expected that lack of Lck should have an adverse effect on NK T cell development.
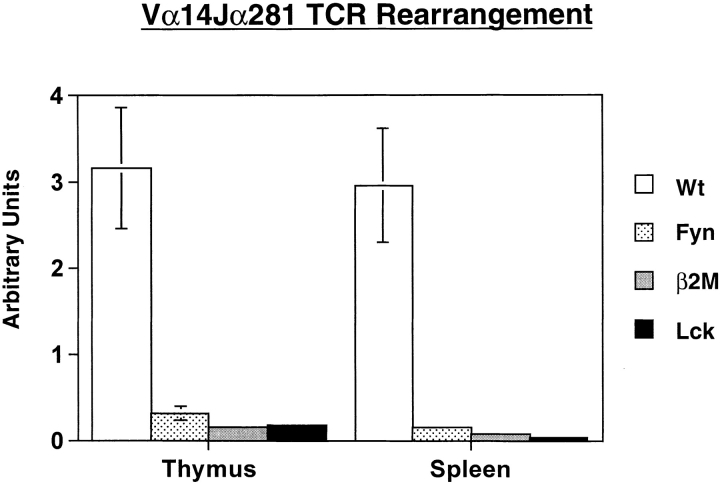
NK T cell ontogeny has been largely defined by various mouse mutants. Mice lacking CD1 or β2M have an early block in development as determined by a lack of cells using the invariant Vα14Jα281 TCR, as well as cells that express NK cell markers in conjunction with the TCR. It has been shown that the common cytokine receptor γ chain is required for NK1.1 expression and IL-4 secretion, but not for selection of Vα14Jα281-positive cells 32. To gauge where the block in development lies in fyn − / − and lck − / − mice, this rearrangement was assayed for by quantitative RT-PCR using the LightCycler quantitative PCR machine (Fig. 5). Low levels of this rearrangement were observed in all of the mutant animals due to nonproductive or random rearrangements as demonstrated by detectable Vα14Jα281 rearrangements in NK T cell–deficient mice (e.g., β2M mutants). The usage of this specific rearrangement is reduced 10- and 20-fold in fyn − / − thymus and spleen, respectively. In lck mutant mice, this rearrangement is decreased >20-fold in both the thymus and spleen, similar to the levels observed in β2M mice. These data, combined with the flow cytometric analyses, suggest that the lck mutation leads to an early block similar to that seen in β2M mice. However, the amount of the invariant rearrangement present in fyn−/− mice was about twofold higher than that found in either the lck − / − or β2M − / − mice, confirming the flow cytometric data. This suggests that lack of Fyn leads to drastic though incomplete block in NK T cell development. Fyn may have a role in an early selection event or later expansion occurring after TCR rearrangement. Regardless, it is clear that Fyn is required for more than just NK1.1 upregulation and IL-4 secretion.
Figure 5.
Quantitative RT-PCR for the canonical Vα14Jα281 rearrangement used by NK T cells. RNA was harvested from thymocytes and splenocytes, then subjected to quantitative RT-PCR analysis. The data presented have been normalized for HPRT expression (wild-type [Wt], n = 5; Fyn, n = 5; β2M, n = 4; Lck, n = 4).
These studies demonstrate the requirement of the two Src family members, Fyn and Lck, in NK T cell ontogeny. Additionally, these results indicate that the rapid pulse of IL-13 synthesis occurring after TCR ligation is most likely due to NK T cells. While conventional T cell ontogeny is highly dependent on Lck 4 33 34, the NK T cell subset is the first lymphocyte population that also demonstrates a strict requirement for Fyn during development. These experiments underscore a novel role for Fyn in regulating NK T cells, especially since this kinase is not required in pre-TCR signaling, positive selection, and negative selection of conventional T cells 2 3 35. This suggests that NK T cells use unique signals in their selection or expansion process. The fyn mutation defines the first intracellular signaling molecule that is selectively required for NK T cell, but not for conventional T lymphocyte or NK cell development. These and future studies will give insights into where this unique lineage diverges from conventional T cell development.
Acknowledgments
We thank Dr. C. Guidos for supplying some of the Lck mutant mice for this study, and Dr. E. Heber-Katz for critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health, the Council for Tobacco Research, and the Arthritis Foundation. P.L. Stein is a recipient of an Arthritis Foundation Investigator Award.
References
- Lowell C.A., Soriano P. Knockouts of Src-family kinasesstiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- Stein P.L., Lee H.M., Rich S., Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Appleby M.W., Gross J.A., Cooke M.P., Levin S.D., Qian X., Perlmutter R.M. Defective T cell receptor signalling in mice lacking the thymic isoform of p59fyn . Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- Molina T.J., Kishihara K., Siderovski D.P., van Ewijk W., Narendran A., Timms E., Wakeham A., Paige C.J., Hartmann K.U., Veillette A. Profound block in thymocyte development in mice lacking p56lck . Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- van Oers N.C., Lowin-Kropf B., Finlay D., Connolly K., Weiss A. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- Groves T., Smiley P., Cooke M.P., Forbush K., Perlmutter R.M., Guidos C.J. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- Page S.T., van Oers N.S., Perlmutter R.M., Weiss A., Pullen A.M. Differential contribution of Lck and Fyn protein tyrosine kinases to intraepithelial lymphocyte development. Eur. J. Immunol. 1997;27:554–562. doi: 10.1002/eji.1830270229. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Rivera M.G., Park S., Roark J. Mouse CD1-specific NK1 T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Lantz O., Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y., Kanno R., Ito T., Higashio K., Taniguchi M. Predominant expression of invariant Vα14+TCRα chain in NK1.1+ T cell populations. Int. Immunol. 1995;7:1157–1160. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- Ohteki T., MacDonald R. Stringent Vβ requirement for the development of NK1.1+ T cell receptor-α/β+ cells in mouse liver. J. Exp. Med. 1996;183:1277–1280. doi: 10.1084/jem.183.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H., Arase N., Nakagawa K., Goog R., Onoe K. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur. J. Immunol. 1993;23:307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Lin B., Hardy R.R. Murine thymic CD4+ T cell subsetsa subset (Thy0) that secretes diverse cytokines and overexpresses the Vβ8 T cell receptor gene family. J. Exp. Med. 1992;176:269–274. doi: 10.1084/jem.176.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Paul W.E. CD4+ NK1.1+ T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., McConville M.J., Hansen D., Campbell A.S., Fraser-Reid B., Grusby M.J., Tachado S.D. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Chiu N.M., Mandal M., Wang N., Wang C.R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Mendiratta S.K., Martin W.D., Hong S., Boesteanu A., Joyce S., Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- Smiley S.T., Kaplan M.H., Grusby M.J. Immunoglobulin E production in the absence of interleukin-4 secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Killeen N., Littman D., Schwartz R.H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- Hammond K., Cain W., Driel I., Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int. Immunol. 1998;10:1491–1499. doi: 10.1093/intimm/10.10.1491. [DOI] [PubMed] [Google Scholar]
- Kikly K., Dennert G. Evidence for extrathymic development of TNK cells. NK1+ CD3+ cells responsible for acute marrow rejection are present in thymus-deficient mice. J. Immunol. 1992;149:403–412. [PubMed] [Google Scholar]
- Sato K., Ohtsuka K., Hasegawa K., Yamagiwa S., Watanabe H., Asakura H., Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J. Exp. Med. 1995;182:759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H., Ono S., Arase N., Park S.Y., Wakizaka K., Watanabe H., Ohno H., Saito T. Developmental arrest of NK1.1+ T cell antigen receptor (TCR)-α/β+ T cells and expansion of NK1.1+ TCR-γ/δ+ T cell development in CD3ζ-deficient mice. J. Exp. Med. 1995;182:891–895. doi: 10.1084/jem.182.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo J.P., Rodewald H. In vivo roles of receptor tyrosine kinases and cytokine receptors in early thymocyte development. Curr. Opin. Immunol. 1998;10:196–207. doi: 10.1016/s0952-7915(98)80249-5. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Ho S., Suzuki H., Mak T.W., Ohashi P.S. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J. Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- Alberola-Ila J., Hogquist K.A., Swan K.A., Bevan M.J., Perlmutter R.M. Positive and negative selection invoke distinct signaling pathways. J. Exp. Med. 1996;184:9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Ohtsuka K., Kimura M., Ikarashi Y., Ohmori K., Kusumi A., Ohteki T., Seki S., Abo T. Details of an isolation method for hepatic lymphocytes in mice. J. Immunol. Methods. 1992;146:145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- Chomarat P., Banchereau J. Interleukin-4 and interleukin-13their similarities and discrepancies. Int. Rev. Immunol. 1998;17:1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- Coles M.C., Raulet D.H. Class I dependence of the development of CD4+ CD8− NK1.1+ thymocytes. J. Exp. Med. 1994;180:395–399. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y., Jayawardena J., Weiss A., Lee D., Park S., Dautry-Varsat A., Bendelac A. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 1999;189:103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszowy M.W., Leuchtmann P.L., Veillette A., Shaw A.S. Comparison of p56lck and p59fyn protein expression in thymocyte subsets, peripheral T cells, NK cells, and lymphoid cell lines. J. Immunol. 1995;155:4236–4240. [PubMed] [Google Scholar]
- Lantz O., Sharara L.I., Tilloy F., Andersson A., Di Santo J.P. Lineage relationships and differentiation of NK T cellsintrathymic selection and IL-4 production in the absence of NKR-P1 and Ly49 molecules. J. Exp. Med. 1997;185:1395–1401. doi: 10.1084/jem.185.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S.J., Abraham K.M., Nakayama T., Singer A., Perlmutter R.M. Inhibition of T-cell receptor β-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Anderson S.J., Perlmutter R.M., Mak T.W., Tonegawa S. An activated lck transgene promotes thymocyte development in RAG-1 mutant mice. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Utting O., Teh S.J., Teh H.S. T cells expressing receptors of different affinity for antigen ligands reveal a unique role for p59fyn in T cell development and optimal stimulation of T cells by antigen. J. Immunol. 1998;160:5410–5419. [PubMed] [Google Scholar]