Abstract
Complement is part of the innate immune system and one of the first lines of host defense against infections. Its importance was evaluated in this study in virus infections in mice deficient either in soluble complement factors (C3−/−, C4−/−) or in the complement signaling complex (complement receptor [CR]2−/−, CD19−/−). The induction of the initial T cell–independent neutralizing immunoglobulin (Ig)M antibody response to vesicular stomatitis virus (VSV), poliomyelitis virus, and recombinant vaccinia virus depended on efficient antigen trapping by CR3 and -4–expressing macrophages of the splenic marginal zone. Neutralizing IgM and IgG antibody responses were largely independent of CR2-mediated stimulation of B cells when mice were infected with live virus. In contrast, immunizations with nonreplicating antigens revealed an important role of B cell stimulation via CR2 in the switch to IgG. The complement cascade was activated after infection with VSV via the classical pathway, and active complement cleavage products augmented the effector function of neutralizing IgM and IgG antibodies to VSV by a factor of 10–100. Absence of the early neutralizing antibody responses, together with the reduced efficiency of neutralizing IgM in C3−/− mice, led to a drastically enhanced susceptibility to disease after infection with VSV.
Keywords: complement, CD19, antiviral immunity, T cell independence, neutralizing antibodies
Thus, complement enhances the immunogenicity of various viruses mainly by promoting antigen trapping to CR-expressing macrophages in the marginal zones of secondary lymphoid organs, a process that leads to activation of B cells independent of T cell help; therefore, the T cell–independent activation of virus-neutralizing epitope–specific B cells is largely dependent on complement.
The importance of complement in protection against bacterial infection has been extensively documented; complement deficiencies are often associated with bacterial infections 1 2. Different possibilities of virus interacting with the complement system have been described in vitro 3 4, and complement components have been shown to enhance the specific antibody response to different model antigens 5 6 7 8. These results indicate an important link between innate and acquired immunity 9 10. In viral infections, C3 and C4 may directly coat a virion and thereby prevent infection of target cells or lead to direct lysis of the virus 11. In addition, complement components can bind and lyse virus-infected cells 12. Several viruses have evolved strategies to evade complement lysis either by using complement receptors (CRs)1 and complement control proteins as viral receptors or by producing complement-blocking or -modulating molecules 3 13.
In various experimental systems using mAbs to complement components or soluble CRs, impaired immune responses to T cell–dependent (TD) antigens have been described 14 15 16 17. More recently, immunization of mice deficient in soluble complement components or in CRs with low doses of different model antigens have confirmed these observations 1 18 19 20. For example, C3d coupled to hen egg lysozyme (HEL) is 1,000–10,000-fold more immunogenic than HEL alone 21. Two mechanisms to explain the increase in immunogenicity have been proposed. First, opsonization of the antigen by complement enhances the targeting of antigen to follicular dendritic cells (FDCs), which express CD35 (CR1) and CD21 (CR2), leading to more efficient antigen presentation to B cells and germinal center (GC) formation 22 23. Second, a direct enhancement of B cell receptor signal transduction has been proposed, a concept called “dual antigen recognition” 6 8: CR2 is expressed on B cells and forms a complex with CD19 and TAPA-1 (target of antiproliferative antibody 1); however, CR2 does not possess an intracellular domain and therefore, after binding of C3b and C3d, CR2 probably signals via CD19. As a consequence, active cleavage products of C3 bound to antigen induce the cross-linking of the B cell receptor with the coreceptor complex and lower the threshold for B cell activation. Mice deficient in soluble complement components or in CR1 and CR2 are able to initiate GC formation after immunization, but these GCs are reduced in number and size 18 19. Antigen concentration on FDCs is drastically reduced in CD21/CD35-deficient mice. In addition, as CD21–CD21L (ligand) interaction has been shown to provide signals for GC B cells to become memory B cells 24, a reduction in antigen persistence on FDCs and a decrease in GC B cell survival may influence long-term B cell memory, although this has not yet been formally demonstrated.
The role of complement components in the initiation of a protective neutralizing antibody response to viral infections in vivo has remained largely unexplored. We therefore studied the interaction of various cytopathic and noncytopathic viruses with the complement system using the recently generated mice deficient in the central complement component of both activation pathways (C3−/−; reference 1), deficient in a component of the classical pathway (C4−/−; reference 20), or with defects in the receptor signaling complex (CD19−/−, reference 25; CR2−/−, reference 18). It is noteworthy that murine CR1 and CR2 are alternative transcripts of the CR2 gene. Therefore, inactivation of the CR2 gene in CR2−/− mice led to a deficiency in both CR1 and CR2 18.
Many bacteria and viruses activate B cells independent of Th cells. They can be divided into two groups 26: T cell–independent type 1 (TI-1) antigens activate B cells without the need of second signals, either in a polyclonal (prototype LPS) or antigen-specific fashion (several viruses such as vesicular stomatitis virus [VSV]; reference 27); in contrast, TI-2 antigens need residual noncognate T cell help for activation of B cells (i.e., bacterial polysaccharides, poliomyelitis virus, recombinant vaccinia virus). We analyzed infection of mice with VSV, a close relative of rabies virus and a member of the Rhabdoviridae 28 29. VSV is in mice a largely neurotropic, highly cytopathic virus that causes paralysis and death if it reaches neuronal tissues 28 30. Recovery from primary infection and resistance against reinfection depends virtually exclusively on neutralizing antibodies and not on cytotoxic T cells 31. As for infections with many cytopathic viruses (e.g., polio, influenza, and rhabdoviruses), recovery from infection is crucially determined by initial distribution of the virus after systemic spread and early protective defense mechanisms during the first few hours after infection 28.
Materials and Methods
Mice.
The generation of C3−/−, C4−/−, CR2−/−, and CD19−/− mice has been described previously 1 18 20 25. C57BL/6 and 129Sv mice were purchased from the Institute for Laboratory Animals (Veterinary Hospital, Zurich, Switzerland). C57BL/6 and (C57BL/6 × 129Sv)F1 were used as controls. Experiments were done in a conventional mouse house facility, and mice were used at 6–12 wk of age.
Virus and Measurement of VSV Titers.
VSV Indiana (VSV-IND; Mudd-Summers isolate) and VSV New Jersey (VSV-NJ; Pringle isolate) were originally received from Dr. D. Kolakovsky (University of Geneva, Switzerland) and were grown on BHK21 cells. Lymphocytic choriomeningitis virus (LCMV)-WE was originally obtained from Dr. F. Lehmann Grube (Heinrich Pette Institute, Hamburg, Germany) and was propagated on L929 fibroblast cells. Poliovirus stock solutions of serotype II were obtained from the Swiss Serum and Vaccine Institute (Bern, Switzerland). Inactivated poliovirus vaccine containing all three major serotypes (Salk) was purchased from BERNA, Switzerland. Recombinant baculoviruses expressing the glycoprotein of VSV (VSV G) and the nucleoprotein of LCMV (LCMV NP) were gifts from Dr. D.H.L. Bishop (NERC Institute of Virology, Oxford, UK). They were derived from nuclear polyhedrosis virus and were grown at 28°C in Spodoptera frugiperda cells in spinner cultures 32.
VSV titers in different organs were analyzed by a plaque-forming assay. 1:10 serial dilutions of organ homogenates were incubated on a vero cell monolayer in 24-well plates for 1 h at 37°C in an atmosphere with 5% CO2. Overlay with methylcellulose, incubation, and staining of plaques was similarly done as described for the neutralization assay.
VSV and Poliomyelitis Virus Neutralization Assay.
Serum of immunized mice was prediluted 40-fold in MEM containing 2% FCS. Serial twofold dilutions were mixed with equal volumes of VSV (500 pfu/ml) and incubated for 90 min at 37°C in an atmosphere with 5% CO2. 100 μl of the serum–virus mixture was transferred onto vero cell monolayers in 96-well plates and incubated for 1 h at 37°C. The monolayers were overlaid with 100 μl DMEM containing 1% methylcellulose and incubated for 24 h at 37°C. The overlay was flicked off, and the monolayer was fixed and stained with 0.5% crystal violet. The highest dilution of serum that reduced the number of plaques by 50% was taken as titer. To determine IgG titers, undiluted serum was pretreated with an equal volume of 0.1 mM β-ME in saline. Poliovirus neutralization assays were performed similarly, but samples were prediluted 1:20.
LCMV NP–specific ELISA.
We used an ELISA with the following steps: (a) coating with baculovirus-derived LCMV NP (1 μg/ml); (b) blocking with 2% BSA (Fluka AG) in PBS; (c) addition of 10-fold–prediluted sera, titrated 1:3 over 12 dilution steps; (d) detection with IgM- or IgG-specific horseradish peroxidase–labeled goat anti–mouse antibodies (0.5 μg/ml; Southern Biotechnology Associates, Inc.); and (e) addition of substrate ABTS (2.2′-azino-bis-[3-ethylbenzthiazoline-6-sulfonate]; Boehringer Mannheim) and H2O2 (Fluka AG). Plates were coated overnight at 4°C; all other incubations were done for 60–90 min at room temperature (RT). Between incubations, plates were washed three times with PBS containing 0.05% Tween-20. OD was measured at 405 nm in an ELISA reader, and antibody titers were determined as the serum dilutions yielding an absorption of twice background levels.
Enzyme-linked Immunospot Assay for VSV-specific Antibody-forming Cells.
Antibody-forming cell (AFC) frequencies were determined as described 33. In brief, 25 square-well polystyrene plates were coated with purified VSV-IND (≈1011 pfu/ml). On the next day, plates were blocked with 2% BSA in PBS for 2 h. Titrated amounts of single-cell suspensions were added in 2% MEM and incubated for 5 h at 37°C. After washing with PBS–Tween, goat anti–mouse IgM or IgG antibody (2 μl/ml; EY Labs.) was added, and plates were incubated for 2 h at 37°C. After washing with PBS–Tween, alkaline phosphatase–labeled donkey anti–goat antibody (1 μg/ml; Jackson ImmunoResearch Labs, Inc.) was added, and plates were incubated overnight at RT. The next day, plates were washed, and the substrate solution (5-bromo-4-chloro-3-indolyl phosphate at 1 μg/ml in 0.6% agarose) was added to develop blue color spots.
In Vivo CD4+ T Cell Depletion.
Mice were treated intraperitoneally on days 3 and 1 before infection with 1 mg of anti-CD4 mAb YTS191.1 34. This treatment completely abrogates the switch from IgM to IgG and depletes CD4+ T helper cells to below detection level by FACS™ analysis (not shown).
Immunohistochemistry.
Freshly removed organs were immersed in HBSS and snap frozen in liquid nitrogen. 5-μm-thick tissue sections were cut in a cryostat, placed on siliconized glass slides, air dried, fixed with acetone for 10 min, and stored at −70°C. For staining of cell differentiation markers, rehydrated sections were incubated with rat mAbs against marginal zone macrophages (ERTR-9; reference 35) and against marginal zone metallophils (MOMA-1; Biomedicals). Primary rat antibodies were revealed by sequential incubation with goat antibodies to rat Igs (Caltag Labs.) and alkaline phosphatase–labeled donkey antibodies to goat Igs (Jackson ImmunoResearch Labs., Inc.). Dilutions of secondary antibodies were made in TBS containing 5% normal mouse serum. Incubations were done at RT for 30 min; TBS was used for all washing steps. Alkaline phosphatase was visualized using naphthol AS-BI (6-bromo-2-hydroxy-3-naphtholic acid-2-methoxy anilide) phosphate and new fuchsin as substrate. Endogenous alkaline phosphatase was blocked by levamisole. Color reactions were performed at RT for 15 min with reagents from Sigma Chemical Co. Sections were counterstained with hemalum, and coverslips were mounted with glycerol and gelatin. Staining for VSV antigen was done as described 36.
Results
A Role for Complement in IgG Antibody Responses against Nonreplicating Viral Antigens but Not against Replicating Virus.
Primary neutralizing antibody responses against VSV or recombinant VSV G protein and ELISA binding antibodies against LCMV NP were assayed (Fig. 1) in C3−/− and control mice. The early (day 2–6) IgM response to VSV was completely TI-1; thereafter, VSV induced a rapid and strong TD neutralizing IgG response starting around day 6–7 after infection, reaching a plateau level after 3 wk 31. VSV G on the membranes of cells infected with a recombinant vaccinia virus expressing VSV G (Vacc VSV G) and baculovirus-expressing VSV G protein have been shown to be TI-2 antigens 27. In contrast, IgM and IgG antibodies to LCMV NP (an internal viral antigen) are strictly dependent on Th cells.
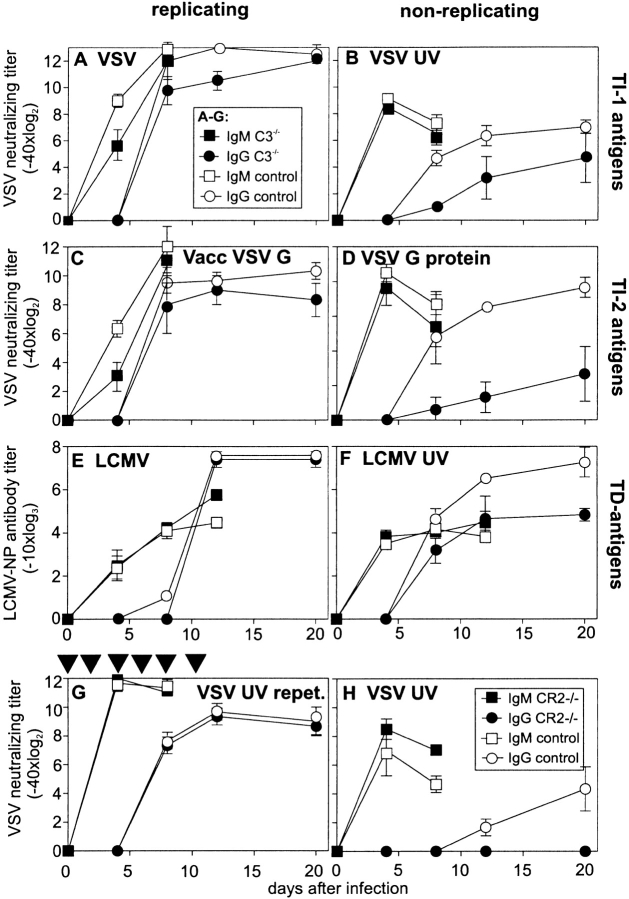
Figure 1.
Antibody responses to replicating and nonreplicating TI-1, TI-2, and TD viral antigens. C3−/− (▪, •) and control mice (□, ○) ([C57BL/6 × 129Sv]F1 or C57BL/6) were immunized with (A) 2 × 106 pfu live VSV i.v., (B) 2 × 108 pfu VSV UV-inactivated i.v., (C) 2 × 106 pfu Vacc VSV G, or (D) 10 μg baculovirus VSV G protein i.v. Neutralizing antibody titers were assessed at the time points indicated. In the experiment shown, three out of five C3−/− mice died between day 8 and 12 after infection with VSV (Table ). Antibody titers in the dying mice were not different from those in surviving mice. After immunization with (E) 200 pfu LCMV-WE or (F) UV-inactivated purified LCMV-WE (∼20 μg protein), LCMV NP–specific antibodies were determined in an ELISA on baculovirus-derived LCMV NP–coated plates. Titers are shown as dilutions leading to OD 405 nm twice over background. (G) C3−/− and control mice were immunized every other day until day 12 with 2 × 108 pfu UV-inactivated VSV, and neutralizing antibody titers were measured. (H) CR2−/− and control mice were immunized once with 2 × 108 pfu UV-inactivated VSV, and VSV-IND–neutralizing antibody titers were assessed at the time points indicated. All results (A–H) are given as mean ± SD of three mice per group. Experiments were repeated twice with similar results.
After infection with the two replicating viruses expressing the VSV G as TI antigen (VSV, 2 × 106 pfu i.v., Fig. 1 A; and Vacc VSV G, 2 × 106 pfu i.v., Fig. 1 C), a comparable antibody response was observed in wild-type and C3−/− mice; the initial TI IgM response was, surprisingly, somewhat delayed in C3−/− mice, whereas the TD switch to IgG was comparable in C3−/− and control mice. In contrast, after immunization with nonreplicating viral antigens (2 × 108 pfu i.v. UV-inactivated VSV, Fig. 1 B; and 10 μg i.v. baculovirus-derived VSV G protein, Fig. 1 D), a reduction in the TD IgG antibody responses by a factor of ∼8 against inactivated VSV and a factor of ∼60–100 against VSV G protein was observed. It has been shown previously that for immunization with nonreplicating antigens, higher amounts of antigen had to be used to induce an antibody response comparable to that after infection with 2 × 106 pfu live VSV 37. In this study, we used 2 × 108 pfu equivalents of UV-inactivated VSV (100 times more than live VSV, Fig. 1 B) and 10 μg baculovirus-derived VSV G protein (corresponding to ∼105 times more VSV G protein than expressed on 2 × 106 pfu of live VSV, Fig. 1 D). This comparison was based on virus particles counted by electron microscopy and on one virus particle expressing ∼1,300 VSV G proteins in its envelope 29. With a high initial antigen dose, the early IgM response could be restored in C3−/− mice (Fig. 1B and Fig. D). Interestingly, the capacity of C3−/− mice to mount TD IgM and IgG antibodies against LCMV NP after infection with 200 pfu LCMV i.v. was similar to that of controls (Fig. 1 E). Immunization with UV-inactivated LCMV subcutaneously (300 μl of purified LCMV in CFA corresponding to ∼20 μg of protein) also revealed an ∼10-fold reduction of the IgG titer (Fig. 1 F).
The reduced IgG titers after immunization with nonreplicating viral antigens confirm earlier results with model TD antigens (bacteriophage ΦX174, sheep red blood cells) in C3−/− and CR2−/− mice 1 18 19. In contrast, during infections with live virus, where efficient T cell help and/or antigen persistence is provided (Fig. 1A, Fig. C, and Fig. E), switching to IgG is here shown to be largely complement independent. The influence of antigen dose and antigen persistence in vivo on the antibody response was analyzed in C3−/− and control mice that were immunized repetitively every other day with 2 × 108 pfu UV-inactivated VSV (Fig. 1 G). Repetitive injections of high doses of nonreplicating VSV particles induced comparable IgG titers in C3−/− and control mice. This indicates, first, that B cells of C3−/− mice are comparably functional in vivo and second, that the influence of complement on the specific immune response probably reflects the importance of antigen dose and persistence. The reduced IgG titers in C3−/− mice may be a consequence of an impaired interaction of antigen-bound complement cleavage products with CD21/CD35. To test this hypothesis, the immune response of CR2−/− mice to viral antigens was analyzed (Fig. 1 H). The blocked switch to IgG in CR2−/− mice after immunization with 2 × 108 pfu UV-inactivated VSV confirms the importance of the CD21–CD21L interaction in the enhancement of B cell stimulation and/or antigen trapping on FDCs in response to nonreplicating antigen. In contrast, the IgG response after infection with live viruses in C3−/− (Fig. 1A, Fig. C, and Fig. E) and CR2−/− (not shown) was within normal ranges.
Increased Susceptibility of C3−/− Mice to Paralytic Disease after Infection with VSV.
Recovery of mice from primary or secondary VSV infection depends mainly on the neutralizing antibody response and on IFN-α 31 38 39. Most of the C3−/− mice infected with 2 × 106 pfu died within 8–10 d after infection despite a quite normal antibody response. VSV infects neuronal tissue and causes paralysis and death after infection of the spinal cord and the brain. A titration of the infectious dose of VSV revealed an increased susceptibility to VSV by at least a factor of 100–1,000 (Table ) in complement-deficient mice. Whereas six of nine C3−/− mice infected intravenously with 105 pfu died, only one of six control animals died after infection with 107 pfu VSV. High VSV titers were found in the brains of paralyzed mice (Table ), implying infection of the central nervous system by VSV as cause of disease and death. In contrast, no increased susceptibility to VSV was observed after infection of CR2−/− mice (not shown).
Table 1.
Susceptibility of C3−/− and Control Mice to Infection with VSV
| Infectious dose injected | Survival of animals in each group | VSV brain titers (log10) (numbers of mice tested) | ||
|---|---|---|---|---|
| C3−/− | Controls | C3−/− | Controls | |
| 107 pfu | 0/6 | 5/6 | 5.3 ± 1.7 (6) | 6.1 (1) |
| <1.7 (5) | ||||
| 105 pfu | 3/9 | 6/6 | 6.3 ± 2.7 (6) | <1.7 (6) |
| 103 pfu | 3/3 | 3/3 | <1.7 (3) | <1.7 (3) |
A number of factors may have contributed to this drastic increase in susceptibility to disease (as a comparison, the LD50 of antibody-deficient μMT mice is ∼103 pfu VSV; reference 40) may be explained as follows: First, the observed delay in the neutralizing IgM antibody response may allow VSV to reach its target tissue, the brain, before a sufficiently high neutralizing antibody titer is mounted. Second, complement components may directly—or indirectly via antibodies—inactivate VSV and reduce peripheral infection. Third, opsonization with complement could lead to a more efficient recruiting of the virus to secondary lymphoid organs and therefore an enhanced clearance of virus from the circulation. These possibilities are analyzed in the following sections.
No Thymus-independent Activation of B Cells in C3−/− Mice.
The observation that the early (day 2–6) TI IgM responses against VSV and Vacc VSV G were reduced whereas the TD IgM response after infection with LCMV was normal was unexpected, as earlier studies with nonreplicating model antigens had suggested that mainly TD antibody responses were enhanced by complement-mediated stimulation of the B cell coreceptor complex (CR2–CD19–TAPA-1) 5 6 7 8 10.
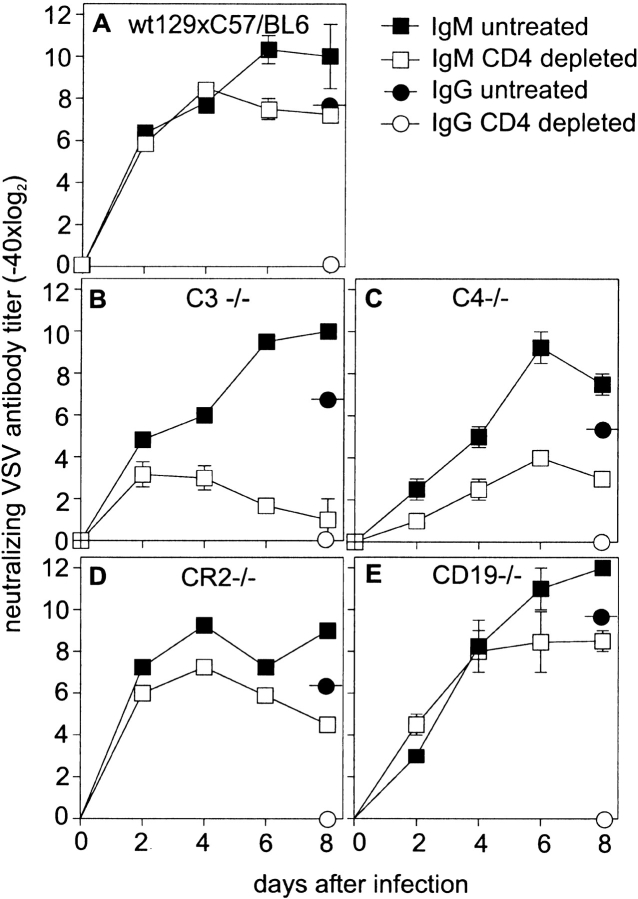
Analysis of B cell responses in mice depleted of CD4+ T cells revealed that the IgM response was reduced in C3−/− by a factor of 250 compared with control mice on day 6 after infection with 2 × 106 pfu VSV i.v. (Fig. 2 B compared with Fig. 1 A), i.e., in the absence of C3, the normally TI antibody response was almost completely blocked. Neutralizing IgG (distinguished from IgM by reduction with 0.1 M β-ME, an unequivocal means of destroying IgM; reference 41) on day 8 after immunization was at least two titer steps lower than total Ig, indicating that the antibodies measured until day 8 largely represented neutralizing IgM. The efficiency of the CD4+ T cell depletion protocol was verified by FACS™ analysis of the CD4+ T cell counts in the blood (not shown). In addition, no switch to IgG was observed in mice treated with anti-CD4+ antibodies, indicating that depletion of T cell help in vivo was efficient (Fig. 2A–E). To assess the pathway of complement activation and the mechanisms by which complement contributes to Th cell–independent B cell activation, we analyzed the early neutralizing IgM response to VSV in CD4+ T cell–depleted C4−/− (Fig. 2 C), CR2−/− (Fig. 2 D), and CD19−/− mice (Fig. 2 E) and compared it to the antibody response in equally treated control mice (Fig. 2 A). Similar to C3−/− mice, the early neutralizing IgM antibody response to VSV was delayed in C4−/− mice and depended largely on T cell help. This indicated that early during VSV infection, the complement cascade was probably activated via the classical pathway. In contrast, mice deficient in the CR1 and -2 (CR2−/−, Fig. 2 D) or the coreceptor component CD19 (Fig. 2 E) mounted early neutralizing IgM titers that were independent of T cell help. Therefore, the effect of complement on the early antibody response could not be explained by the lack of stimulation via the B cell coreceptor complex. In an earlier study, complement inactivation using cobra venom factor (CVF) could not reveal an influence of complement on the IgM responses against VSV 42. However, although inactivation using CVF may be efficient for serum complement components, it was shown to be rather inefficient for locally produced complement components in secondary lymphoid organs 43.
Figure 2.
T cell dependence of VSV-specific IgM antibody responses. Control (C57BL/6 × 129Sv)F1 (A), C3−/− (B), C4−/− (C), CR2−/− (D), and CD19−/− (E) were infected with 2 × 106 pfu VSV. Total VSV-neutralizing Ig was assessed at the time points indicated. Mice were either depleted of CD4+ T cells (two 200-μl administrations of anti-CD4 antibody YTS191.1, on day 3 and day 1) or left untreated. Efficiency of CD4+ T cell depletion was checked by FACS™. Neutralizing IgG (circles) was assessed on day 8 (reduction with β-ME) and was always at least two titer steps lower than total Ig, indicating that antibody titers up to day 8 represent IgM. One of three comparable experiments is shown.
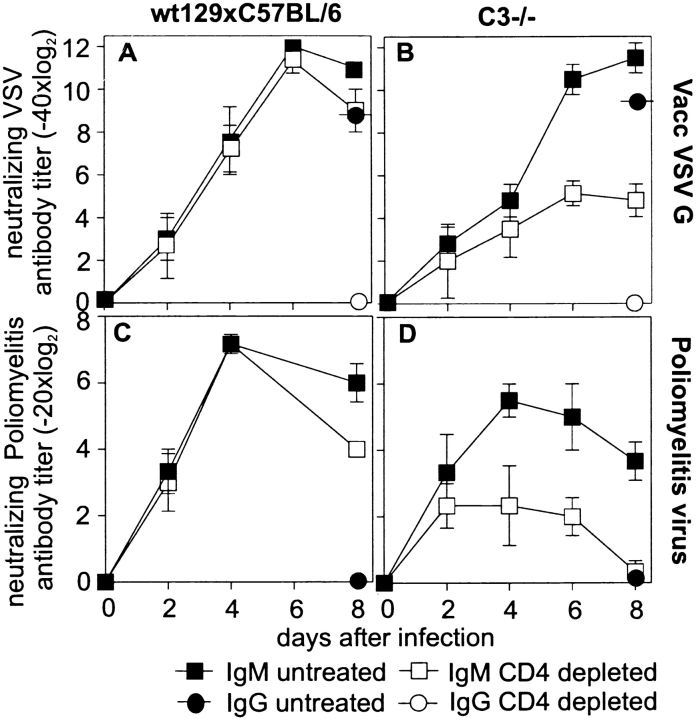
To analyze whether the impaired TI antibody response in C-deficient mice was unique to VSV or was a general phenomenon, we studied antibody responses of C3−/− and control mice after immunization with Vacc VSV G (Fig. 3A and Fig. B) or poliomyelitis virus (Fig. 3C and Fig. D). IgM responses to both of these TI-2 antigens were also largely dependent on T cell help in C3−/− mice.
Figure 3.
TD IgM antibody responses to recombinant vaccinia virus and poliomyelitis virus. C3−/− and control mice (either depleted of CD4+ T cells or left untreated) were immunized with 2 × 106 pfu Vacc VSV G (A and B), and neutralizing antibody titers were assessed every other day. Neutralizing IgG (circles) was determined 8 d after immunization and was always at least two titer steps lower than total Ig, indicating that antibody titers until day 8 represent IgM. CD4+ T cell–depleted and untreated control (C) or C3−/− (D) mice were immunized with 500 μl of inactivated polio vaccine (Salk), and polio virus (serotype II)-specific neutralizing antibodies were assessed until day 8. No switch to IgG was observed after single immunization with polio vaccine. The experiment was repeated twice with comparable results.
Complement Recruits Viral Antigen to the Splenic Marginal Zone.
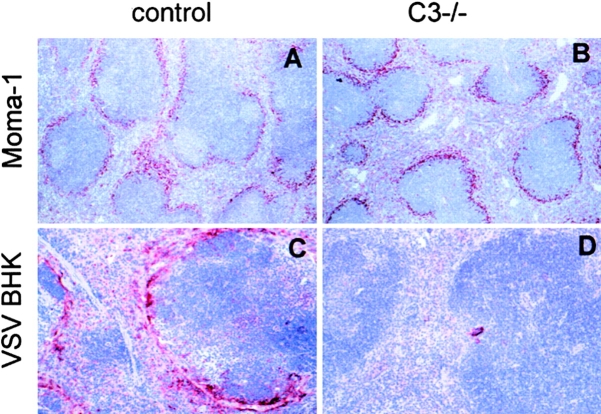
Because the TI activation of B cells was not dependent on CR1 or -2, the effect may be mediated by CR3 (CD11b, CD18) or CR4 (CD11c, CD18) 44. These receptors are mainly expressed on macrophages. Immunohistological analysis after immunization with VSV antigen showed a high concentration of VSV antigen accumulated in the splenic marginal zone in control (C57BL/6 × 129Sv)F1 mice (Fig. 4 C). In contrast, this local concentration was virtually absent in C3−/− mice (Fig. 4 D). The staining of the marginal zone macrophages with MOMA-1 (specific for metallophilic macrophages of the marginal zone; Fig. 4A and Fig. B) and ERTR-9 (specific for marginal zone macrophages; not shown) confirmed that C3−/− mice possessed a normal marginal zone structure. Interestingly, VSV titers in the spleen 6 h after infection with 2 × 108 pfu VSV were comparable in C3−/− and control mice (Table ). This difference may be explained by the fact that immunohistochemically, only high concentrations of antigen can be stained. In contrast, under conditions where antigen is distributed more diffusely in the spleen, as in C3−/− mice, no intensive immunohistological signal can be detected. Thus, complement mainly influenced the specific targeting of the antigen to marginal zone macrophages but less influenced the general uptake of virus in the spleen.
Figure 4.
Recruitment of viral antigen to the spleen. The spleens of naive (C57BL/6 × 129Sv)F1 (A, control) and C3−/− (B) mice were stained with MOMA-1 (specific for metallophilic macrophages of the marginal zone). Control and C3−/− mice were immunized intraperitoneally with viral antigen derived from disrupted VSV-infected BHK cells. 1 d later, VSV antigen was stained on spleen sections as described in Materials and Methods (original magnification: A and B, 125; C and D, 250). One of two comparable experiments is shown.
Table 2.
Protective Capacity of Neutralizing Antibodies after Infection with VSV or LCMV in C3−/− and (C57BL/6 × 129Sv)F1 Control Mice
| Antibody | Class | Dose | Antibody titer | Mouse | Virus titer in the spleen | Δ Fold increase in virus titer |
|---|---|---|---|---|---|---|
| Infection with 2 × 108 pfu VSV i.v.: | ||||||
| – | – | – | – | C3−/− | 6.0 ± 0.1 | <2 |
| Control | 5.8 ± 0.1 | |||||
| M4C11H12 | IgM | 10 μg | 150 | C3−/− | 5.9 ± 0.3 | 500 |
| Control | 3.2 ± 0.6 | |||||
| H2F1C1 | IgM | 10 μg | 6,600 | C3−/− | 4.7 ± 0.1 | 30 |
| Control | 3.2 ± 0.2 | |||||
| C3C5B9 | IgG1,k | 10 μg | 175 | C3−/− | 6.2 ± 0.7 | 80 |
| Control | 4.3 ± 0.4 | |||||
| E5D9H5 | IgG1,k | 10 μg | 14,580 | C3−/− | 4.6 ± 0.2 | 50 |
| Control | 2.9 ± 0.7 | |||||
| Polyclonal | IgM | 500 μl | 40,960 | C3−/− | 4.4 ± 0.1 | 20 |
| Control | 3.1 ± 0.1 | |||||
| Infection with 200 pfu LCMV i.v.: | <2 | |||||
| – | – | – | – | C3−/− | 6.6 ± 0.4 | |
| Control | 6.7 ± 0.1 | <2 | ||||
| H25 | IgM | 500 μl | 20,480 | C3−/− | 3.7 ± 0.1 | |
| Control | 3.9 ± 0.7 | |||||
| Infection with 2 × 106 pfu LCMV i.v.: | ||||||
| – | – | – | – | C3−/− | 7.4 ± 0.5 | <2 |
| Control | 7.1 ± 0.1 | |||||
| KL25 | IgG | 100 μg | C3−/− | 6.9 ± 0.4 | 5 | |
| Control | 6.2 ± 0.1 | |||||
Complement Augments the Protective Capacity of Neu-tralizing Antibodies.
The only slightly impaired antibody response in C3−/− mice was in contrast to the massively increased mortality after infection with VSV. We therefore tested the protective capacity of a panel of seven monoclonal IgM and five monoclonal IgG VSV-specific neutralizing antibodies after transfer into C3−/− or control mice 45. The results obtained with the antibodies with the highest and the lowest in vitro neutralization titers are shown in Table . Viral titers in the spleens of C3−/− and control mice 6 h after infection with 2 × 108 pfu VSV were similar, ∼6 log10, and comparable titers were also obtained in peripheral organs (liver and kidney; data not shown). Injection of neutralizing IgM or IgG antibodies 20–30 min before VSV infection reduced viral organ titers in control mice by 100–1,000-fold but only reduced titers in C3−/− mice ∼10-fold (Table ). The augmentation of the neutralizing capacity of mAbs by complement was confirmed with the administration of pooled, complement-inactivated polyclonal IgM of day 4 VSV immune mice 20–30 min before infection with 2 × 108 pfu VSV (Table ). In contrast, the neutralization of noncytopathic LCMV with IgM or IgG mAbs seems to be largely independent of complement in vivo (Table ). These results differ from earlier in vitro observations that suggested a direct role of complement in protection against LCMV 46.
In summary, the VSV-neutralizing antibodies are more efficient in vivo in the presence of an intact complement system.
Antiviral B Cell Memory Is Not Complement Dependent.
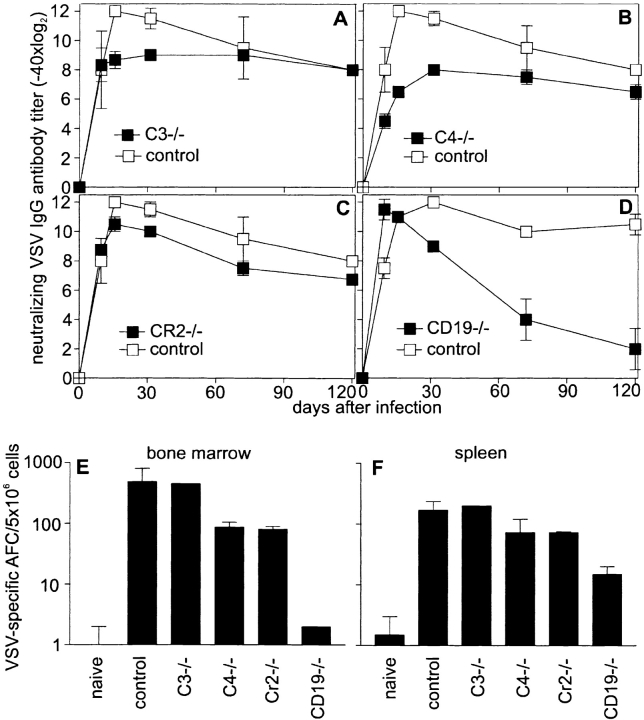
Complement has been shown to be involved in targeting antigen to CD21 and CD35 on FDCs in GCs, where the survival of GC B cells is dependent on the expression of CRs 23 24 47. To assess antiviral B cell memory, C3−/−, C4−/−, CR2−/−, or CD19−/− mice were infected with 2 × 106 pfu VSV, and antibody titers were followed up to 150 d. C3−/−, C4−/−, and CR2−/− mice maintained IgG antibody titers comparably to control mice (Fig. 5A–C). In contrast, CD19−/− mice lost memory antibody titers within 90 d (Fig. 5 D), confirming earlier results 33. On day 120 after the initial infection, the number of AFCs was assessed in the bone marrow (Fig. 5 E) and spleen (Fig. 5 F). C3−/− and control mice had similar numbers of AFCs in the bone marrow and spleen, a finding that correlated with the observed antibody titers. The AFCs in C4−/− and CR2−/− mice in the bone marrow were reduced by 80–90% and in the spleen by ∼50% compared with the number of AFCs present in control mice. The AFCs in CD19−/− mice were reduced by >99.9% in the bone marrow and the spleen, confirming earlier results analyzing B cell memory in CD19−/− mice 33. The reduced numbers of AFCs in C4−/− and CR2−/− mice by a factor of 5 or 2 in spleen or bone marrow, respectively, were still sufficient to maintain long-term antibody titers after immunization with VSV. However, in CD19−/− mice, where AFCs are reduced by a factor of 100–1,000, neutralizing antibody titers could not be maintained. B cell memory was also assessed in C3−/− mice 150 d after infection with Vacc VSV G, VSV G protein, and LCMV. Long-term antibody titers after these different immunization protocols were comparably maintained in C3−/− and control mice (not shown).
Figure 5.
Long-term antibody and B cell memory in complement-deficient mice. C3−/− (A), C4−/− (B), CR2−/− (C), and CD19−/− (D) mice were immunized with 2 × 106 pfu VSV, and long-term antibody titers were compared with controls. Three of six C3−/− and two of five C4−/− animals died between day 8 and 12 after immunization. Antibody titers in surviving and dying mice were comparable until day 8. Antibody titers in surviving animals were followed up to day 120. 120 d after infection, VSV-specific AFCs in the spleen (F) and bone marrow (E) were assessed in an enzyme-linked immunospot assay. Results are given as mean ± SD of three mice per group. Experiments were repeated twice with comparable results.
Discussion
Complement may be involved in a viral infection in various ways 3, and its importance for host protection is documented here by the increased susceptibility of C3−/− mice to a model infection with the cytopathic virus VSV. VSV activates the complement cascade via the classical pathway, i.e., antibodies initially activate the C1 convertase or, as described for some retroviruses, the classical pathway is directly activated by virus-infected cells independent of antibodies 48. Natural IgM antibodies to VSV are present in the serum of antigen-inexperienced mice and can bind to virus and activate the complement cascade 49 50. Complement may then directly augment the efficiency of antibodies to neutralize VSV. Early in vitro studies had shown that the active cleavage product C3b bound to VSV and was incorporated into its surface and, thereby, may have prevented infection of target cells 50. Also, a role of complement has been demonstrated in in vitro studies with various viruses including LCMV and HIV 4 51 52, but in extension of earlier in vitro experiments 51, in this study we could not detect a direct influence of complement on the severity of LCMV infection in vivo.
A surprising effect of complement was found here on viruses that elicit an antibody response independently of T cell help. Several viruses have been shown to be TI antigens, e.g., influenza, polio, rabies virions, and others 53. After infection with a cytopathic virus such as VSV, early defense mechanisms are crucial to prevent infection of neuronal tissue. Complement does not seem to directly influence early distribution of VSV, as C3−/− and control mice have comparable titers of VSV in the spleen and in peripheral organs 6 h after infection. Nevertheless, complement-coated virus is targeted more efficiently to CR-expressing cells. This effect has been extensively analyzed and documented for CD21 and CD35 expressed on FDCs 23 47, suggesting an influence on antigen persistence in GCs that may have an impact on long-term B cell memory. After infection with VSV, LCMV, and different recombinant antigens, we did not observe any impairment in B cell memory in mice deficient in complement components or CRs, although AFCs in C4−/− and CR2−/− mice were reduced by a factor of 2–5. The binding of antigen IgG complexes on FDC seems to be sufficient to maintain long-term B cell memory after a viral infection 47. The observations that C3−/−, C4−/−, and CR2−/− mice maintained B cell memory, whereas CD19−/− mice had a drastic reduction in IgG antibody titers and AFCs 90–150 d after infection remains unexplained. So far, it was assumed that CD19 signaled solely after binding of C3d to CR2, as no specific ligand for CD19 is known 5 33. However, the different phenotype of CD19−/− and CR2−/− mice is best explained by an intrinsic role of CD19 in BCR signaling. Alternatively, there might be a ligand for CD19 that is independent of the CR. In a different set of experiments, B cell memory to another infectious virus, human herpes simplex type 1, that replicates only to a very limited extent in mice, particularly when injected subcutaneously (a condition probably more comparable to immunization with UV-inactivated VSV), was found to be classical pathway and CD21 dependent 53a. Thus, in addition to antigen dose and replication capacity, the antigenic structure and the route of infection might be important determinants of whether and to what extent complement is required for humoral responses.
Early on during a viral infection, a sufficient antigen concentration on CR3– and -4–expressing macrophages in the splenic marginal zone seems to be crucial to elicit an early, TI B cell response. Marginal zone macrophages have been shown to be important for the induction of TI-2 antibody responses to nonreplicating model antigens 54. This mechanism causes a very efficient early IgM response necessary for survival of the infection. Any delay in the early neutralizing antibody response may allow the virus to reach neuronal tissue before an efficient neutralizing antibody response is mounted.
Our results suggested that lowering the threshold of B cell activation by binding to the B cell coreceptor (CR2, CD19, TAPA-1) does not seem to play a major role after infection with live viral antigens. However, signaling via the B cell coreceptor enhanced the IgG response when nonreplicating antigens were used. This difference may be explained by nonspecific inflammatory reactions and cytokine secretion plus prolonged antigen persistence during infections with live virus that may compensate for the lack of costimulation via CR2/CD19 55.
In conclusion, during a viral infection, complement is an important link to adaptive immunity: it helps to recruit antigen to marginal zone macrophages and stimulate B cells of the marginal zone and thereby enhances specific TI antibody responses. Thus, TI IgM antibody responses are complement dependent.
Acknowledgments
We would like to thank Norbert Wey for photographs.
This work was supported by the Swiss National Science Foundation (grant no. 31-50900.97 to R.M. Zinkernagel) and the Kanton Zurich.
Footnotes
1used in this paper: AFC, antibody-forming cell; CRs, complement receptors; FDCs, follicular dendritic cells; G, glycoprotein; GC, germinal center; L, ligand; LCMV, lymphocytic choriomeningitis virus; NP, nucleoprotein; RT, room temperature; TD, T cell–dependent; TI, T cell–independent; VSV, vesicular stomatitis virus
References
- Wessels M.R., Butko P., Ma M., Warren H.B., Lage A.L., Carroll M.C. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.M., Gallin J.I. Evaluation of the patient with recurrent bacterial infections. Annu. Rev. Med. 1998;49:185–199. doi: 10.1146/annurev.med.49.1.185. [DOI] [PubMed] [Google Scholar]
- Lachmann P.J., Davies A. Complement and immunity to viruses. Immunol. Rev. 1997;159:69–77. doi: 10.1111/j.1600-065x.1997.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Stoiber H., Clivio A., Dierich M.P. Role of complement in HIV infection. Annu. Rev. Immunol. 1997;15:649–674. doi: 10.1146/annurev.immunol.15.1.649. [DOI] [PubMed] [Google Scholar]
- Carter R.H., Fearon D.T. CD19lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- Van Noesel C.J., Lankester A.C., Van Lier R.A. Dual antigen recognition by B cells. Immunol. Today. 1993;14:8–11. doi: 10.1016/0167-5699(93)90316-d. [DOI] [PubMed] [Google Scholar]
- Tedder T.F., Zhou L.J., Engel P. The CD19/CD21 signal transduction complex of B lymphocytes. Immunol. Today. 1994;15:437–442. doi: 10.1016/0167-5699(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Fearon D.T., Carter R.H. The CD19/CR2/TAPA-1 complex of B lymphocyteslinking natural to acquired immunity. Annu. Rev. Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- Fearon D.T., Locksley R.M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Carroll M.C. The role of complement and complement receptors in induction and regulation of immunity. Annu. Rev. Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- Berry D.M., Almeida J.D. The morphological and biological effects of various antisera on avian infectious bronchitis virus. J. Gen. Virol. 1968;3:97–102. doi: 10.1099/0022-1317-3-1-97. [DOI] [PubMed] [Google Scholar]
- Sissons J.G., Oldstone M.B., Schreiber R.D. Antibody-independent activation of the alternative complement pathway by measles virus-infected cells. Proc. Natl. Acad. Sci. USA. 1980;77:559–562. doi: 10.1073/pnas.77.1.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade R., Barel M., Ehlin-Henriksson B., Klein G. gp140, the C3d receptor of human B lymphocytes, is also the Epstein-Barr virus receptor. Proc. Natl. Acad. Sci. USA. 1985;82:1490–1493. doi: 10.1073/pnas.82.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M.B. Role of complement in induction of the allergic response. Nat. New Biol. 1972;237:157–159. doi: 10.1038/newbio237157a0. [DOI] [PubMed] [Google Scholar]
- Heyman B., Wiersma E.J., Kinoshita T. In vivo inhibition of the antibody response by a complement receptor–specific monoclonal antibody. J. Exp. Med. 1990;172:665–668. doi: 10.1084/jem.172.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebell T., Ahearn J.M., Fearon D.T. Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991;254:102–105. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- Gustavsson S., Kinoshita T., Heyman B. Antibodies to murine complement receptor 1 and 2 can inhibit the antibody response in vivo without inhibiting T helper cell induction. J. Immunol. 1995;154:6524–6528. [PubMed] [Google Scholar]
- Ahearn J.M., Fischer M.B., Croix D., Goerg S., Ma M., Xia J., Zhou X., Howard R.G., Rothstein T.L., Carroll M.C. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- Molina H., Holers V.M., Li B., Fung Y., Mariathasan S., Goellner J., Strauss-Schoenberger J., Karr R.W., Chaplin D.D. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M.B., Ma M., Goerg S., Zhou X., Xia J., Finco O., Han S., Kelsoe G., Howard R.G., Rothstein T.L. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- Dempsey P.W., Allison E.D., Akkaraju S., Goodnow C.C., Fearon D.T. C3d of complement as a molecular adjuvantbridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- Papamichail M., Gutierrez C., Embling P., Johnson P., Holborow E.J., Pepys M.B. Complement dependence of localisation of aggregated IgG in germinal centres. Scand. J. Immunol. 1975;4:343–347. doi: 10.1111/j.1365-3083.1975.tb02635.x. [DOI] [PubMed] [Google Scholar]
- Klaus G.G., Humphrey J.H., Kunkl A., Dongworth D.W. The follicular dendritic cellits role in antigen presentation in the generation of immunological memory. Immunol. Rev. 1980;53:3–28. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Fischer M.B., Goerg S., Shen L., Prodeus A.P., Goodnow C.C., Kelsoe G., Carroll M.C. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- Rickert R.C., Rajewsky K., Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- Mond J.J., Lees A., Snapper C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Hengartner H., Zinkernagel R.M. T helper cell-independent neutralizing B cell response against vesicular stomatitis virusrole of antigen patterns in B cell induction? Eur. J. Immunol. 1995;25:3445–3451. doi: 10.1002/eji.1830251236. [DOI] [PubMed] [Google Scholar]
- Baer G.M., Bellini W.J., Fishbein D.B. Rhabdoviruses. In: Fields B.N., Knipe D.M., editors. Virology. Raven Press; New York: 1990. [Google Scholar]
- Wagner R.R. The Rhabdoviruses 1987. Plenum Press; New York: pp. 1–544 [Google Scholar]
- Christian A.Y., Barna M., Bi Z., Reiss C.S. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol. 1996;9:195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Hengartner H., Zinkernagel R.M. Neutralizing anti-viral B cell responses. Annu. Rev. Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R.D., Overton H.A., Bishop D.H. Baculovirus expression vectorsthe requirements for high level expression of proteins, including glycoproteins. J. Gen. Virol. 1987;68:1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Fehr T., Rickert R.C., Odermatt B., Roes J., Rajewsky K., Hengartner H., Zinkernagel R.M. Antiviral protection and germinal center formation, but impaired B cell memory in the absence of CD19. J. Exp. Med. 1998;188:145–155. doi: 10.1084/jem.188.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T.P., Cobbold S.P., Waldmann H., Aguet M., Zinkernagel R.M. Functional analysis of T lymphocyte subsets in antiviral host defense. J. Immunol. 1987;138:2278–2281. [PubMed] [Google Scholar]
- van Vliet E., Melis M., van Ewijk W. Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J. Histochem. Cytochem. 1985;33:40–44. doi: 10.1177/33.1.3880783. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Kündig T.M., Kalberer C.P., Hengartner H., Zinkernagel R.M. How many specific B cells are needed to protect against a virus? J. Immunol. 1994;152:4235–4241. [PubMed] [Google Scholar]
- Bachmann M.F., Kündig T.M., Kalberer C.P., Hengartner H., Zinkernagel R.M. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B-cell responses. J. Virol. 1993;67:3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Steinhoff U., Müller U., Schertler A., Hengartner H., Aguet M., Zinkernagel R.M. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J. Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A.R., Nansen A., Anderson C., Johansen J., Marker O., Christensen J.P. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus. Int. Immunol. 1997;9:1757–1766. doi: 10.1093/intimm/9.11.1757. [DOI] [PubMed] [Google Scholar]
- Scott D.W., Gershon R.K. Determination of total and mercaptoethanol-resistant antibody in the same serum sample. Clin. Exp. Immunol. 1970;6:313–316. [PMC free article] [PubMed] [Google Scholar]
- Fehr T., Bachmann M.F., Bluethmann H., Kikutani H., Hengartner H., Zinkernagel R.M. T-independent activation of B cells by vesicular stomatitis virusno evidence for the need of a second signal. Cell. Immunol. 1996;168:184–192. doi: 10.1006/cimm.1996.0065. [DOI] [PubMed] [Google Scholar]
- Carroll M.C. The role of complement and complement receptors in induction and regulation of immunity. Annu. Rev. Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- Brown E.J. Complement receptors and phagocytosis. Curr. Opin. Immunol. 1991;3:76–82. doi: 10.1016/0952-7915(91)90081-b. [DOI] [PubMed] [Google Scholar]
- Roost H.-P., Bachmann M.F., Haag A., Kalinke U., Pliska V., Hengartner H., Zinkernagel R.M. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc. Natl. Acad. Sci. USA. 1995;92:1257–1261. doi: 10.1073/pnas.92.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R.M., Jr., Zinkernagel R.M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977;268:646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]
- Tew J.G., Kosco M.H., Burton G.F., Szakal A.K. Follicular dendritic cells as accessory cells. Immunol. Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Ebenbichler C.F., Thielens N.M., Vornhagen R., Marschang P., Arlaud G.J., Dierich M.P. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J. Exp. Med. 1991;174:1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobet R., Cerny A., Rüedi E., Hengartner H., Zinkernagel R.M. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp. Cell Biol. 1988;56:175–180. doi: 10.1159/000163477. [DOI] [PubMed] [Google Scholar]
- Beebe D.P., Cooper N.R. Neutralization of vesicular stomatitis virus (VSV) by human complement requires a natural IgM antibody present in human serum. J. Immunol. 1981;126:1562–1568. [PubMed] [Google Scholar]
- Welsh R.M. Host cell modification of lymphocytic choriomeningitis virus and Newcastle disease virus altering viral inactivation by human complement. J. Immunol. 1977;118:348–354. [PubMed] [Google Scholar]
- Takefman D.M., Sullivan B.L., Sha B.E., Spear G.T. Mechanisms of resistance of HIV-1 primary isolates to complement-mediated lysis. Virology. 1998;246:370–378. doi: 10.1006/viro.1998.9205. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Zinkernagel R.M. Virus structure, antibody response and virus serotypes. Immunol. Today. 1996;17:553–558. doi: 10.1016/s0167-5699(96)10066-9. [DOI] [PubMed] [Google Scholar]
- Da Costa X.J., Brockman M.A., Alicot E., Ma M., Fischer M.B., Zhou X., Knipe D.M., Carroll M.C. Humoral response to herpes simplex virus is complement dependent. Proc. Natl. Acad. Sci. USA. 1999;In press doi: 10.1073/pnas.96.22.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting A.M.J., De Rover Z., Kraal G., van Rooijen N. Humoral immune responses against particulate bacterial antigens are dependent on marginal metallophilic macrophages in the spleen. Scand. J. Immunol. 1996;43:398–405. doi: 10.1046/j.1365-3083.1996.d01-54.x. [DOI] [PubMed] [Google Scholar]
- Ramshaw I., Ruby J., Ramsay A., Ada G., Karupiah G. Expression of cytokines by recombinant vaccinia virusesa model for studying cytokines in viral infections in vivo. Immunol. Rev. 1992;127:157–182. doi: 10.1111/j.1600-065x.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Seiler P., Brundler M.A., Zimmermann C., Weibel D., Bruns M., Hengartner H., Zinkernagel R.M. Induction of protective cytotoxic T cell responses in the presence of high titers of virus-neutralizing antibodiesimplications for passive and active immunization. J. Exp. Med. 1998;187:649–654. doi: 10.1084/jem.187.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler P., Kalinke U., Rulicke T., Bucher E.M., Bose C., Zinkernagel R.M., Hengartner H. Enhanced virus clearance by early inducible lymphocytic choriomeningitis virus-neutralizing antibodies in immunoglobulin-transgenic mice. J. Virol. 1998;72:2253–2258. doi: 10.1128/jvi.72.3.2253-2258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]