Abstract
In Streptococcus pneumoniae, the two-component signaling system MicAB was previously shown to contribute to repression of competence when oxygen is limited. In virulent strains expressing the serotype 2 and 6 capsule, mutation of the MicB kinase reduced the lag period of growth when cultures were switched from an aerobic to anaerobic atmosphere. After intranasal challenge of mice, the micB::km mutation decreased virulence, as shown by the absence of symptoms and by a lower level of recovery of CFU from lungs and blood. It is proposed that MicAB is involved in the adaptive response of the bacteria to changes in oxygen level during the course of infection.
Streptococcus pneumoniae is an aerotolerant anaerobic human pathogen. During exponential growth in rich medium, central metabolism is essentially fermentative (4), despite NADH oxidase (1) and pyruvate oxidase (11), which reduce molecular substrate to H2O and H2O2, respectively. Implication of these oxidases in virulence (1, 11) indicates that oxidative metabolism is required for full virulence of S. pneumoniae. In vitro, oxidative metabolism also is involved in the control of genetic exchange via the regulation of competence for genetic transformation (1): competence is repressed under oxygen limitation (5).
Integration of cellular redox status with competence occurs by phosphotransfer through the two-component systems (TCS) CiaRH and ComCDE (5-7). A third TCS, MicAB, contributes to repression of competence under oxygen limitation (7). Mutational analysis led to the proposal that the response regulator MicA is essential for bacterial growth, whereas the active form of the kinase, MicB, is not essential in vitro (7, 9, 12). MicB is the single protein in S. pneumoniae carrying the PAS signature (9).
PAS domains are conserved motifs present in proteins that sense light or the redox and energetic status of the cell (2). Mutation of the PAS domain in MicB of S. pneumoniae abolishes the kinase activity of the recombinant protein and allows expression of competence under microaerobic conditions, suggesting that MicB phosphorylation both is dependent on PAS and constitutes one response to oxygen concentration (7).
The requirement for this TCS in growth and in regulation of competence in vitro prompted us to investigate its role in the fate of pneumococci in vivo. The effect on in vivo growth and virulence of a mutation that annuls MicB kinase activity has been evaluated. Aerobic growth and anaerobic growth in vitro were studied for both serotype 2 and 6 pneumococcal micB::km mutants and their isogenic parents, and the virulence of these strains was determined with an in vivo model of pneumonia and bacteremia.
The S. pneumoniae strains were a serotype 2 strain, D39 (NCTC 7466; National Collection of Type Cultures, London, United Kingdom), and a serotype 6B strain, S6 (10). Isogenic derivatives of these strains with the micB::km mutation were obtained by transformation of the wild-type strains with pPT12::Km, using 100 μg of competence-stimulating peptide per ml (7). Transformants were selected with 40 μg of kanamycin per ml and verified by PCR (7). Bacteria were confirmed as pneumococci by Gram staining, catalase test, α-hemolysis on blood agar, and optochin sensitivity, and serotypes were determined by the Quellung reaction. All pneumococci used in these studies were first passaged through mice and subsequently stored as described previously (3, 8). When required, bacteria were thawed and resuspended in phosphate-buffered saline (PBS).
For in vitro growth experiments, pneumococci were cultured overnight at 37°C in brain heart infusion (BHI) broth containing 20% (vol/vol) heat-inactivated fetal calf serum. Medium for micB::km mutants contained 90 μg of kanamycin per ml. The next day, samples were centrifuged, and pellets were resuspended in fresh BHI-fetal calf serum (with or without antibiotic), adjusted to an optical density at 500 nm of 0.2. An anaerobic atmosphere was generated by using BBL GasPak envelopes (Becton Dickinson, Paramus, N.J.) and verified with BBL anaerobic indicator strips (Becton Dickinson). Each in vitro growth experiment was repeated twice, with each time point sample within one experiment done in triplicate.
Female MF1 outbred mice, weighing 30 to 35 g (Harlan Olac, Bichester, United Kingdom), were anesthetized with halothane (Fluothane) before administration into the nostrils of 50 μl of PBS containing 106 CFU of S. pneumoniae (wild type or mutant) (3, 8). The inoculum doses were confirmed by colony counting on blood agar before and after administration of the dose. At preselected time intervals following infection, blood was collected from preselected groups of mice before they were killed by cervical dislocation. The lungs were removed separately into 10 ml of sterile distilled water and weighed. Lungs then were homogenized, and viable counts were determined as previously described (8). It was verified that these mice did not have detectable levels of anti-type 2 or anti-type 6 antibodies. Data were analyzed by analysis of variance.
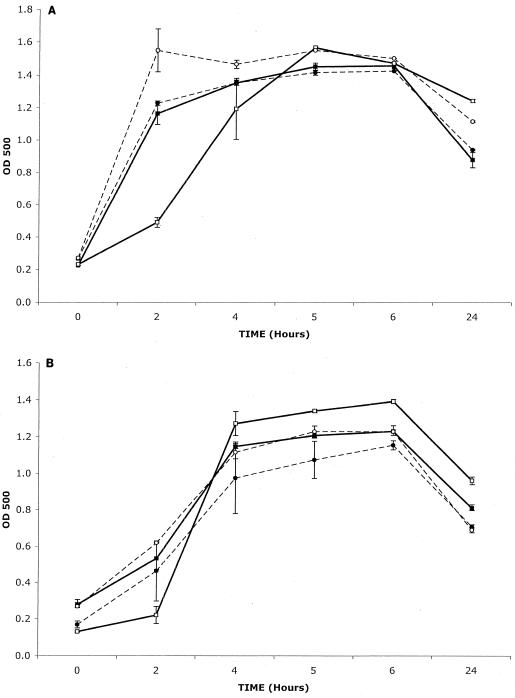
Kanamycin-resistant transformants resulting from allelic exchange between the chromosomal micB and plasmid micB::km alleles were obtained at significant levels (1 to 3% of the transformed population), indicating that the mutation did not affect the colony-forming capacity of the transformed bacteria in rich medium in air. In rich liquid medium in air, the growth of both micB::km mutants was the same as that of the wild-type strains, and the mutants grew similarly and without a lag. In contrast, both wild-type strains showed a lag phase under anaerobic conditions (Fig. 1), and this lag phase was absent in strains carrying the micB::km mutation. This suggests that MicB was involved in growth repression in cultures shifted from aerobiosis to anaerobiosis.
FIG. 1.
Growth in brain heart infusion broth of wild-type (▪, aerobic; □, anaerobic) and micB::km mutant (•, aerobic; ○, anaerobic) CFU of S. pneumoniae D39 (A) and S6 (B). n = 3 for each time point; error bars indicate standard error of the mean. OD 500, optical density at 500 nm.
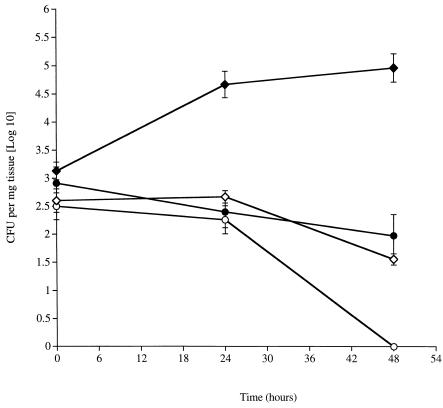
The role of MicB in pneumococcal virulence during pneumonia was investigated. At 48 h following intranasal infection, significantly higher counts (P < 0.05) of both wild-type pneumococci than the corresponding micB::km mutant were recovered from the lungs (Fig. 2). Counts of wild-type D39 also were significantly higher (P < 0.05) than that of the micB::km mutant at 24 h.
FIG. 2.
Time course of change in numbers of CFU of wild-type S. pneumoniae D39 and S6 (⧫ and •, respectively) and D39 and S6 carrying the micB::km mutation (◊ and ○, respectively) in the lungs of MF1 mice infected intranasally with 106 CFU. n = 5 mice per each time point; error bars indicate standard error of the mean.
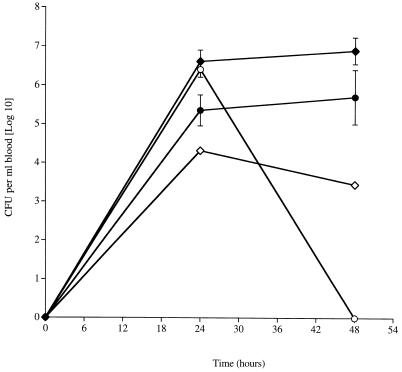
Significantly fewer mutant (P < 0.05) pneumococci of either serotype were recovered from the blood after 48 h than the wild type (Fig. 3). Indeed, the S6 micB::km mutant was not detected in the blood at this time. As with the lungs, there were also significantly smaller numbers of the D39 micB::km mutant at 24 h postinfection (P < 0.05).
FIG. 3.
Time course of change in numbers of CFU of wild-type S. pneumoniae D39 and S6 (⧫ and •, respectively) and D39 and S6 carrying the micB::km mutation (◊ and ○, respectively) in the blood of MF1 mice infected intranasally with 106 CFU. n = 5 mice per each time point; error bars indicate standard error of the mean.
After infection with the wild-type strain D39 or S6, mice showed progressively worsening symptoms of disease after 24 h postinfection, and by 48 h, all mice showed clear signs of disease (very lethargic or moribund). However, mice challenged with either micB::Km mutant showed no symptoms of disease at any time over the 48 h after infection, indicating a significant attenuation of virulence due to the mutation even at high bacteremia.
The results presented highlight the influence of the genetic background on the impact of micB::km mutation on virulence. Indeed, it has been reported that MicB mutation had no influence on the virulence of a serotype 3 strain (12). At this point, we do not have enough information to explain the reason for the strain differences.
We suggest that in some S. pneumoniae strains, MicB phosphorylation is required for the expression of virulence factors unrelated directly to growth in vivo, because the in vitro data show that basic mechanisms for aerobic or anaerobic growth are not affected adversely by the absence of MicB. The requirement for MicB in vivo suggests that even in the lungs pneumococci experience changes in their oxygen environment and enter a microaerobic compartment. The difference between strains in the timing of the influence of MicB may reflect the timing of the entry of the bacteria into this compartment. Since these microaerobic compartments are more likely to be found intracellularly or within the lung parenchyma, rather than within the alveoli, the different timing of the MicB influence may be reflecting the invasive capacity of the strains. Alternatively, it may indicate differences in timing of pathological changes in the lung resulting in oxygen limitation, following infection with the different strains.
Acknowledgments
The Wellcome Trust supported work in Leicester. Université Paul Sabatier supported work in Toulouse. J.E. was supported by an Aventis postdoctoral fellowship.
Editor: A. D. O'Brien
REFERENCES
- 1.Auzat, I., S. Chapuy-Regaud, G. Le Bras, D. Dos Santos, A. D. Ogunniyi, I. Le Thomas, J. R. Garel, J. C. Paton, and M. C. Trombe. 1999. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol. Microbiol. 34:1018-1028. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, C. E., S. Elsen, and T. H. Bird. 1999. Mechanisms of redox control of gene expression. Annu. Rev. Microbiol. 53:495-523. [DOI] [PubMed] [Google Scholar]
- 3.Canvin, J. R., A. P. Marvin, M. Sivakumaran, J. C. Paton, G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 172:119-123. [DOI] [PubMed] [Google Scholar]
- 4.Chapuy-Regaud, S., F. Duthoit, L. Malfroy-Mastrorillo, P. Gourdon, N. D. Lindley, and M.-C. Trombe. 2001. Competence regulation by oxygen availability and by Nox is not related to specific adjustment of central metabolism by Streptococcus pneumoniae. J. Bacteriol. 183:2957-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Echenique, J. R., S. Chapuy-Regaud, and M. C. Trombe. 2000. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol. Microbiol. 36:688-696. [DOI] [PubMed] [Google Scholar]
- 6.Echenique, J. R., and M. C. Trombe. 2001. Competence modulation by the NADH oxidase of Streptococcus pneumoniae involves signal transduction. J. Bacteriol. 183:768-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echenique, J. R., and M. C. Trombe. 2001. Competence repression under oxygen limitation through the two-component MicAB signal transducing system in Streptococcus pneumoniae and involvement of the PAS domain of MicB. J. Bacteriol. 183:4599-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange, R., C. Wagner, A de Saizieu, N. Flint, J. Molnos, M. Stieger, P. Caspers, M. Kamber, W. Keck, and K. E. Amrein. 1999. Domain organisation and molecular characterisation of 13 two-component systems identified by genome sequencing of Streptococcus pneumoniae. Gene 237:223-234. [DOI] [PubMed] [Google Scholar]
- 10.Palmen, R., A. D. Ogunniyi, P. Berroy, S. Larpin, J. C. Paton, and M. C. Trombe. 1999. Insertional mutation of orfD of the DCW cluster of Streptococcus pneumoniae. Microb. Pathog. 27:337-348. [DOI] [PubMed] [Google Scholar]
- 11.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 12.Throup, J. P., K. K. Koretke, A. P. Bryant, K. A. Ingraham, A. F. Chalker, Y. Ge, A. Marra, N. G. Wallis, J. R. Brown, D. J. Holmes, M. Rosenberg, and M. K. Burnham. 2000. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35:566-576. [DOI] [PubMed] [Google Scholar]