Abstract
The acquired immune responses are crucial to the survival of Yersinia-infected animals. Mice lacking T cells are sensitive to Yersinia infection, and a humoral response to Yersinia can be protective. Diverse mechanisms for Yersinia to impair and evade the host innate immune defense have been suggested, but the effects of Yersinia on lymphocytes are not known. Here, we demonstrate that after a transient exposure to Y. pseudotuberculosis, T and B cells are impaired in their ability to be activated through their antigen receptors. T cells are inhibited in their ability to produce cytokines, and B cells are unable to upregulate surface expression of the costimulatory molecule, B7.2, in response to antigenic stimulation. The block of lymphocyte activation results from the inhibition of early phosphorylation events of the antigen receptor signaling complex. Through the use of Y. pseudotuberculosis mutants, we show that the inhibitory effect in both T cells and B cells is dependent on the production of Yersinia outermembrane protein (Yop) H, a tyrosine phosphatase. Our results suggest a mechanism by which the pathogenic bacteria may modulate a wide range of T and B cell–mediated immune responses.
Keywords: T cell, B cell, Yersinia pseudotuberculosis, bacterial pathogenesis, YopH
An animal host must possess a well-developed adaptive immune response as well as robust innate immunity to clear most microbial pathogens. Pathogenic bacteria employ diverse mechanisms to avoid the innate immune defense system 1 2. Several bacterial pathogens reprogram the course of endosomal development to prevent fusion with lysosomal elements, creating a privileged niche within host cells. Many bacteria produce surface components to resist phagocytosis and complement killing, while others possess enzymes that inactivate components of the host defense system. Known mechanisms by which bacteria affect the adaptive immune response are sparse. Mycobacterium tuberculosis has been reported to inhibit antigen presentation by macrophages through an unknown mechanism 3. Clearly, the adaptive immune response remains essential for host defense of bacterial pathogens, including the pathogenic members of the genus Yersinia 4 5.
The genus Yersinia includes the causative agent of bubonic plague, Y. pestis, as well as the common enteric pathogens, Y. enterocolitica and Y. pseudotuberculosis. Infection with Y. pseudotuberculosis and Y. enterocolitica generally causes gastroenteritis and lymphadenitis. However, in some susceptible individuals, infection is associated with the development of reactive arthritis 6 7. All three Yersinia species have a tropism for lymphatic tissue. In animal model systems, Y. pseudotuberculosis and Y. enterocolitica reach the intestinal tract, enter through the M cells of the Peyer's patches, and encounter the host cellular elements 8. Several days after infection, Yersinia are found in the mesenteric lymph nodes and, subsequently, in the spleen and liver. Unlike most bacterial enteropathogens that breach the epithelial barrier, such as Shigella and Salmonella species, Yersinia are not found intra–cellularly but, rather, are seen firmly fixed to the host cell surface 9.
An essential key to Yersinia's pathogenicity is the presence of a 70-kb plasmid (pYV) 10 11. This plasmid, found in all three pathogenic Yersinia species, encodes a type III secretion system and several temperature- and calcium-regulated virulence proteins or effector molecules, the Yersinia outermembrane proteins (Yops). Yersinia also express several adhesion molecules that allow the bacteria to preferentially attach to different classes of integrin receptors on the surface of epithelial, fibroblast, macrophage, and lymphocyte cell lines. Direct cell contact, in combination with the type III secretion machinery, allows Yersinia to translocate the Yops from the cytoplasm of the bacteria directly into the cytoplasm of a host cell to modify host cell function(s). At least six virulence proteins are thought to be active in the host cell: YopH, YopE, YopJ/P, YopO, YopM, and YopT. The effects of these Yops have been studied primarily in macrophages and epithelial cell lines 10 11. YopH, a tyrosine phosphatase, was reported to dephosphorylate p130cas, p125FAK, and paxillin, all tyrosine-phosphorylated proteins found in the focal adhesion (FA) complexes. YopH activity was found to cause the disassembly of FA, which impairs the entry of the bacteria into HeLa cells or their phagocytosis by macrophages 12 13 14. Additionally, YopH may function cooperatively with YopE in inhibiting phagocytosis by neutrophils 15. YopE activity is associated with depolymerization of the host cell cytoskeleton, thus preventing ingestion of the bacteria 16. YopJ/P, in certain conditions, induces apoptosis in macrophages in vitro and in vivo 17 18 19. In other conditions, YopJ/P can affect nuclear factor κB–mediated signal transduction in macrophages through an unknown mechanism and, subsequently, inhibit TNF-α production 20 21 22. Although the functions of YopO, which has homology to serine/threonine kinases 23, and YopT are not clear, it has been shown that both YopO and YopT can perturb the cytoskeleton of epithelial cells in the absence of YopE 24 25. YopM has homology to von Willebrand factor, suggesting that it may play a role in plasma clotting 26 27. Thus, Yersinia produce a spectrum of virulence factors which when injected into host cells may alter host cell antiphagocytic function and, sometimes, host cell viability.
Although these observations suggest possible mechanisms for Yersinia to impair the host innate immune system, the effect of Yersinia on components of the adaptive immune system is not clear. This is despite the fact that acquired immune responses are crucial to the survival of infected animals and the observed link between Yersinia infection and autoimmunity. During the natural course of Yersinia infection, the bacteria almost certainly encounter lymphocytes as they colonize and multiply extracellularly in Peyer's patches, mesenteric lymph nodes, spleen, and liver 5 8. Indeed, T cells as well as macrophages are present in Yersinia-induced lesions in the spleen and liver 28. In vitro studies indicate that Yersinia can bind both T and B lymphocytes, presumably via the integrins on the lymphocytes 29 30. Thus, T and B lymphocytes are potential targets for the bacteria in vivo.
In this study, we found that Y. pseudotuberculosis can directly interfere with T and B cell antigen receptor–mediated activation and that the lymphocyte inhibitory effects are dependent on the production of YopH, the tyrosine phosphatase. The presence of YopH in T and B cells results in hypophosphorylation of almost all tyrosine-phosphorylated components associated with the antigen receptor signaling complex after receptor activation. Consequently, T cells, transiently exposed to Yersinia, were unable to flux calcium and produce cytokines. Likewise, primary B cells, transiently exposed to Y. pseudotuberculosis, were unable to upregulate the costimulatory molecule, B7.2, in response to antigen stimulation. As a result, a wide range of T and B cell–mediated immune responses may be profoundly affected during infection. These observations suggest a novel way by which Yersinia can incapacitate the host adaptive immune response. Thus, Yersinia seems to have evolved to produce effectors that are specifically designed for the different cell types that the bacteria encounter in the course of an infection.
Materials and Methods
Cell Lines, Y. pseudotuberculosis Strains, and Growth Conditions.
5C.C7 T cell lines derived from 5C.C7 TCR transgenic mice (specific for moth cytochrome c [MCC]/I-Ek; reference 31) were maintained in RPMI supplemented with l-glutamine, nonessential amino acids, sodium pyruvate, penicillin/streptomycin, and 10% fetal bovine serum. T cell lines were stimulated every 2 wk with irradiated splenocytes and MCC peptide (88–103). 2 d after the addition of peptide and splenocytes, the medium was supplemented with 5 U/ml of IL-2. Jurkat T cells, MCC9 T cell hybridoma (specific for MCC/I-Ek 32), and CH27 were grown in the RPMI medium using 10% bovine calf serum. Primary B cell cultures were maintained in RPMI supplemented with 3% fetal bovine serum.
Wild-type and mutant Y. pseudotuberculosis 33 were initially grown overnight to plateau phase in 2× YT at 26°C. Cells were diluted 1:50 into 2× YT supplemented with 20 mM sodium oxalate and 20 mM magnesium chloride and grown for 2 h at 26°C, then shifted to 37°C for 2 h. Bacterial numbers were determined by OD600 = 1 × 109 bacteria/ml.
Lymphocyte Infection and Activation.
MCC9 T cells, extensively washed with antibiotic-free medium, were exposed to the specified Y. pseudotuberculosis strain at a multiplicity of infection (MOI) of 10 for 1 h at 37°C. Gentamicin (final concentration 100 μg/ml) was added to kill the bacteria followed by T cell activation assays. T cells in 96-well plates were activated by either MCC peptide (88–103) complexed with glycosyl phosphatidylinositol–linked I-Ek 34, 1 × 105 CH27 cells and MCC peptide, or 1 μM ionomycin and 25 ng/ml PMA in 200 μl of supplemented RPMI medium. After overnight incubation at 37°C, 100 μl of medium was assayed for IL-2 production by standard sandwich ELISA (JES6-1A12, JES6-5H4; PharMingen) and streptavidin-europium detection (Wallac). For calcium flux, experiments were carried out by exposing 5C.C7 T cells to Y. pseudotuberculosis at an MOI of 50 for 1 h. Cells were loaded with the calcium-sensitive dye, Fura-2 (Molecular Probes), washed, and stimulated by an MCC peptide–loaded, confluent layer of I-Ek–expressing Chinese hamster ovary cells, and monitored as described previously 35.
Jurkat T cells were exposed to specified Y. pseudotuberculosis for 1 h at an MOI of 50 at 37°C, followed by the addition of anti-CD3 (OKT3 ascites, 1:100). After 5 min, cells were spun down at 4°C and lysed with cytoplasmic extract buffer (1% NP-40, 150 mM NaCl, 10 mM Tris [pH 8.0], 2 mM sodium orthovanadate, 10 mM sodium fluoride, 5 mM β-glycerol phosphate, 5 mM dinitrophenyl phosphate, 100 μg/ml leupeptin, 100 μg/ml pepstatin, and 1 μM PMSF). Nuclei were removed by centrifugation at 4°C. The cytoplasmic extract (1 × 106 cell equivalents) was diluted into 2× reducing Laemmli buffer and run on 10% SDS-PAGE. Phosphotyrosine proteins were detected by Western blotting, using the unconjugated antiphosphotyrosine antibody, 4G10 (Upstate Biotechnology), followed by an anti–mouse Ig–horseradish peroxidase conjugate (P0260; Dako) for detection. Blots were developed with enhanced chemiluminescence (Amersham Pharmacia Biotech). Each blot was subsequently stripped and reprobed for β-actin. CD3ζ immunoprecipitations were conducted as described 36.
B cells were isolated from MD4 anti–hen egg lysozyme (HEL) Ig transgenic mouse spleens by negative depletions with anti-Thy1, anti-CD4, anti-CD8, and anti–Mac-1 (Caltag) as described 37. Cells were >98% B220+ as assessed by FACS®. Anti-HEL B cells were exposed to wild-type or mutant Y. pseudotuberculosis at an MOI of 50 for 1 h at 37°C, followed by the addition of HEL protein (500 ng/ml). After 3, 10, and 30 min, the B cells were lysed with cytoplasmic extract buffer, nuclei were removed by centrifugation, and 1 × 106 cell equivalents were diluted into 2× reducing Laemmli buffer and run on 10% SDS-PAGE. Phosphotyrosine proteins were detected by Western blot as described above.
For B7.2 upregulation, freshly isolated anti-HEL Ig B cells were first incubated at 37°C with the specified Y. pseudotuberculosis at an MOI of 20. After 1 h, gentamicin was added to a final concentration of 100 μg/ml. Splenic B cells from anti-HEL Ig transgenic or C57BL/6 mice were stimulated with HEL (500 ng/ml) or anti-IgM (3 μg/ml; Jackson ImmunoResearch Laboratories), respectively, for 12 h at 37°C. B cells were then stained for FACS® analysis using anti-B220–FITC (RA3-6B2; PharMingen) and anti-B7.2–PE (GL1; PharMingen). Propidium iodide was included to assess cell viability.
Results
Wild-type Y. pseudotuberculosis Inhibits Antigen-specific T Cell Activation in a YopH-dependent Manner.
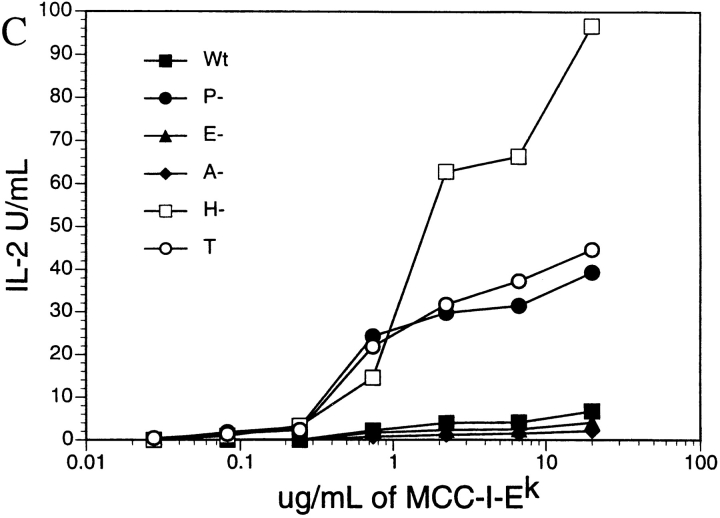
The development of a specific immune response is essential to resolve Yersiniosis. Since T cells are integral components of the specific immune response, we investigated whether Yersinia infection could significantly alter T cell activity by monitoring their cytokine production in response to antigenic activation. In the initial experiment, we preexposed either the APC, CH27, an I-Ek–expressing B cell lymphoma, or MCC9, an MCC/I-Ek specific T cell hybridoma, to either wild-type Yersinia or a Yersinia lcr mutant. The lcr mutant possesses the virulence plasmid, pYV, but is unable to produce or secrete Yops due to a mutation in a Yop regulatory locus 38 39. After a 1-h incubation with Yersinia at an MOI of 10, the cells were treated with gentamicin (to kill the bacteria) and then mixed with MCC peptide and the appropriate untreated cells to achieve antigen activation. As shown in Fig. 1, in both cases, IL-2 production was significantly decreased after exposure to wild-type Yersinia but not to the lcr Yersinia mutant. This indicates that one or more secreted Yop(s) was responsible for the observed cytokine inhibition. Interestingly, the Yersinia inhibitory effect on cytokine production is much more pronounced when T cells were preexposed to Yersinia, suggesting that Yersinia's effect is greater on T cells than on CH27 cells. However, to definitively test if the bacteria were directly affecting T cells, we needed to exclude the possibility that, when the two cell types are mixed together, the bacteria interact with the other cell type before gentamicin killing takes effect. Therefore, plate-bound I-Ek/MCC peptide (88–103) protein complex, instead of CH27 cells, was used in subsequent T cell activation assays. MCC9 T cells exposed to wild-type, but not a virulence plasmid–deficient (pYV−) or an lcr mutant (data not shown) Yersinia, showed a dramatic reduction in IL-2 production compared with unexposed controls (Fig. 1 C). These data suggest that one or more components of the Y. pseudotuberculosis virulence plasmid can directly inhibit T cell activation.
Figure 1.

Y. pseudotuberculosis suppresses T cell antigen–specific IL-2 production in a YopH-dependent manner. IL-2 production of T cell activation assays with (A) MCC9 or (B) CH27 preexposed to either wild-type (Wt), YopH mutant (H−), Lcr mutant (Lcr−) Yersinia, or medium alone (T); or with (C) MCC9 T cells activated by plate-bound MCC/I-Ek after the T cells were preexposed to wild-type (Wt), YopH mutant (H−), YopE mutant (E−), YopO/YopJ mutant (A−), pYV− (P−) Yersinia, or medium alone (T). At least three independent experiments were conducted, and representative result is shown.
To determine which Yop(s) are necessary for the suppression of IL-2 production, several Yersinia Yop mutants were screened in the T cell activation assay (Fig. 1 C). YopO/YopJ- and YopE-deficient bacteria, but not YopH-deficient bacteria, were able to inhibit IL-2 production as efficiently as wild-type bacteria. In addition, the YopH mutant was unable to inhibit IL-2 production when MCC9 was stimulated by APCs (Fig. 1A and Fig. B). These results suggest that YopH is necessary for the inhibition of cytokine production in T cells. Similarly, exposure to wild-type, but not pYV-cured or YopH-deficient mutant, Yersinia inhibited the MCC/I-Ek–specific production of IL-3, IL-4, and IFN-γ by T cell lines derived from the 5C.C7 TCR transgenic mice (data not shown). Cell viability, as assayed by propidium iodide staining and trypan blue exclusion, did not significantly change as a result of exposure to Yersinia in any of the above experiments (data not shown). These data suggest that YopH may target a common component in cytokine activation pathways.
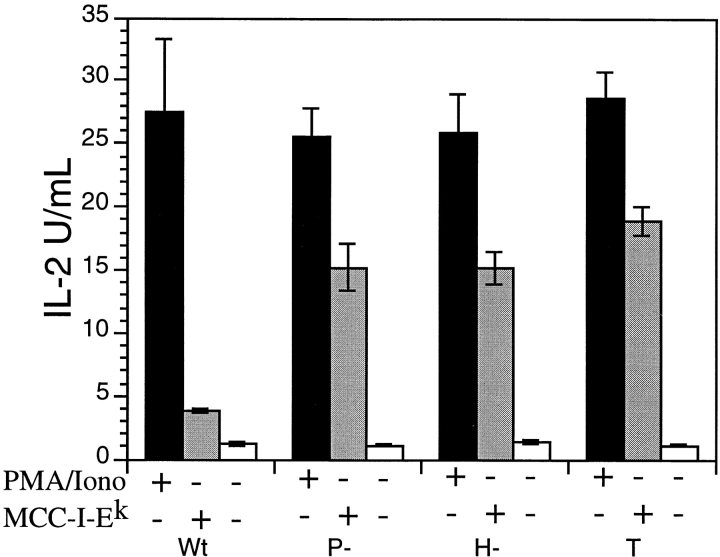
The YopH Inhibitory Effect Is Upstream of Calcium Flux and Protein Kinase C Activation.
YopH has tyrosine phosphatase activity, and T cell activation through the antigen receptor is known to require the rapid phosphorylation of the tyrosine kinases, CD3ζ chain, and linker/adaptor molecules 40. The phosphorylation and clustering of these molecules are critical to T cell activation, and these events precede the activation of protein kinase C as well as the rapid changes in intracellular calcium concentration accompanying T cell activation. Protein kinase C activation and the intracellular calcium flux are, in turn, required for induction of cytokine production. As shown in Fig. 2, the YopH inhibitory effect can be reversed by the addition of PMA/ionomycin, indicating that the YopH-mediated cytokine suppression was upstream of protein kinase C activation and calcium flux. Consistent with this supposition, the ability of Yersinia-exposed 5C.C7 T cells to flux calcium in response to antigen was impaired in a YopH-dependent manner (data not shown). These results suggest that the effect of YopH is largely limited to early tyrosine phosphorylation events in T cell signaling.
Figure 2.
The inhibitory effect on T cell activation can be overridden by PMA and ionomycin. IL-2 production from MCC9 T cells exposed to wild-type (Wt), pYV− (P−), or YopH− (H−) Yersinia, or medium alone (T) for 1 h and incubated overnight with PMA and ionomycin (PMA/Iono) or 60 μg/ml of plate-bound MCC/I-Ek.
YopH Inhibits the T Cell Tyrosine Phosphorylation Signal Cascade.
The biochemistry of antigen receptor signaling has been well characterized in the human T cell Jurkat line (for a review, see reference 40). Hence, we evaluated whether the induction of the tyrosine phosphorylation cascade in response to TCR cross-linking by the mAb OKT3 is altered when Jurkat cells are exposed to Yersinia. As shown in Fig. 3, many tyrosine-phosphorylated proteins induced after TCR cross-linking, including CD3ζ, were severely reduced or absent in T cells exposed to wild-type or YopE-deficient, but not pYV− or YopH-deficient, mutant Yersinia compared with the sham-exposed T cells. These results suggest that YopH targets some of the earliest initiators of the T cell signaling complex.
Figure 3.
YopH inhibits tyrosine phosphorylation of the TCR signaling complex. (A) Antiphosphotyrosine blot of resting (−) and OKT3-activated (+) T cell lysates from Jurkat T cells exposed to either wild-type (Wt), YopE− (ΔE), YopH− (ΔH), or pYV− (P−) Y. pseudotuberculosis. Arrows indicate the heavy and light chains of OKT3. (B) Blots were stripped and reprobed for actin. (C) Antiphosphotyrosine blot of immunoprecipitated CD3ζ from extracts.
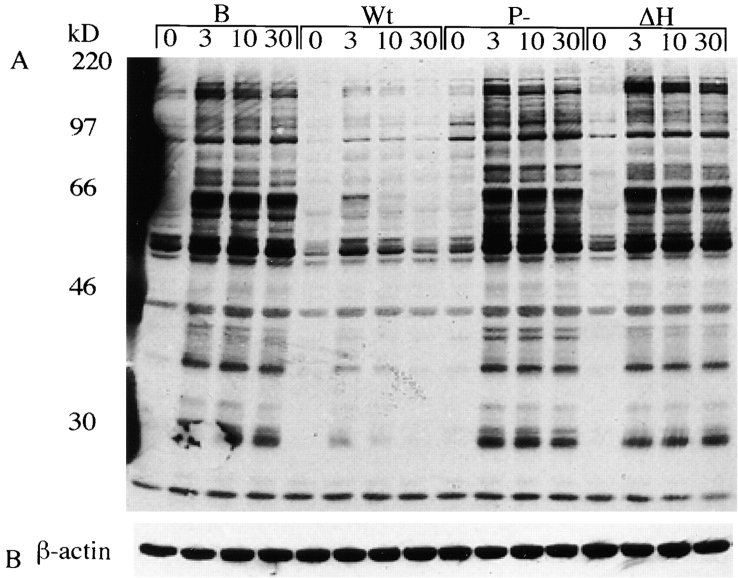
YopH Inhibits B Cell Antigen–specific Responses.
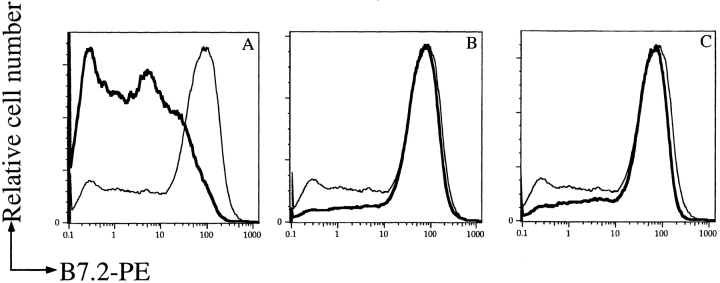
The antigen receptor on B lymphocytes (BCR) consists of the membrane-bound Ig and associated Igα and Igβ molecules. Tyrosine phosphorylation of Igα and Igβ is required for the initiation of the tyrosine phosphorylation signaling cascade after antigen binding 41. Because of the signaling similarities between T cells and B cells, we tested whether the initial signaling events after BCR stimulation are affected by exposure to Y. pseudotuberculosis. As in T cells, we found that exposure to wild-type Yersinia interfered with the induction of the early tyrosine phosphorylation signaling cascade in response to antigen receptor engagement in a YopH-dependent manner (Fig. 4). Anti-HEL Ig transgenic B cells were exposed to wild-type, pYV−, or YopH-deficient Yersinia and activated with HEL. B cells exposed to wild-type but not pYV− or YopH-deficient Yersinia showed a drastic reduction in the surface expression of B7.2 (Fig. 5) and CD69 (data not shown). Similarly, the induction of B7.2 and CD69 on splenic B cells isolated from C57BL/6 mice in response to anti-IgM cross-linking was also impaired when the B cells were exposed to wild-type but not pYV− or YopH-deficient Yersinia (data not shown). This effect was not due to Yersinia-induced cell death since the number of live cells, as assessed by propidium iodide, was not significantly different among samples (data not shown).
Figure 4.
Yersinia inhibits tyrosine phosphorylation of the BCR signaling complex in a YopH-dependent manner. (A) Antiphosphotyrosine blot of cell extracts from splenic B cells from anti-HEL transgenic mice. B cells were incubated with medium alone (B), wild-type (Wt), YopH− (ΔH), or pYV− (P−) Yersinia for 1 h, then activated with HEL protein (500 ng/ml) for the indicated times. (B) Blots were stripped and reprobed for actin.
Figure 5.
YopH suppresses B cells antigen–specific B7.2 upregulation. Splenic B cells from anti-HEL Ig transgenic mice were exposed to either wild-type (A), pYV− (B), or YopH− (C) Y. pseudotuberculosis before the addition of HEL protein. After 12 h, cells were stained with FITC-conjugated anti-B220, PE-conjugated B7.2, or propidium iodide, and analyzed by FACS®. Only B220+ and propidium iodide–negative cells (live) were included in the analysis. Bold lines represent expression of B7.2 on Yersinia-exposed, HEL-activated cells. Thin lines represent B7.2 expression on unexposed, HEL-activated B cells. At least three independent experiments were conducted, and a representative plot is shown.
Discussion
In this study, we have examined the interaction between Yersinia and the adaptive immune system by monitoring the response of T and B lymphocytes to antigen after a brief exposure to Yersinia. Our results provide evidence that the Y. pseudotuberculosis tyrosine phosphatase, YopH, inhibits the signaling cascades associated with T and B cell antigen receptor activation. This is one of the first examples of a bacterial pathogen directly manipulating lymphocyte signaling and activation. Continuation of this inhibitory effect may explain why Yersinia infection, in some cases, is characterized by a chronic infection of lymphatic organs.
The consequences of the YopH dephosphorylation activity may affect a wide range of T and B cell–mediated immune responses, including cytokine production. In fact, Yersinia infection dramatically suppresses the induction of IFN-γ and TNF-α production in some mouse strains 42 43. When these cytokines are replaced exogenously, the mice are better able to survive infection. Since infection with an lcr − Yersinia triggers IFN-γ and TNF-α production in infected mice, one or more Yops are likely responsible for the suppression of cytokines. Recent experiments suggest that YopJ/P suppresses TNF-α production in macrophages 20 21 22, whereas we found that IFN-γ production was reduced in T cells infected with Yersinia. Thus, the inhibition in cytokine production observed after infection with wild-type Yersinia may be due to the combined action of YopH and YopJ/P on different cell types.
The pathological significance of our finding is not limited to a bacteria-induced suppression of cytokine production. The development of a T cell–mediated autoimmune disease, reactive arthritis, has long been associated with Y. pseudotuberculosis and Y. enterocolitica infection in genetically susceptible individuals. Inappropriate T cell signaling has been postulated as a factor in the development of autoimmune diseases. Thus, T cells exposed even briefly to wild-type Y. pseudotuberculosis may signal aberrantly, if at all, for a significant period of time. If a stimulus is delivered to the TCR during a partially responsive period, the T cell may respond inappropriately, leading to the development of abnormal immune responses. In addition, complete T cell activation requires a signal delivered through TCR–peptide/MHC complex and the engagement of costimulatory molecules such as CD28/B7.2. A failure to induce normal levels of B7.2 on APCs has been associated with the induction of T cell nonresponsiveness 44. Although many other factors are likely to be involved in the development of reactive arthritis, we believe that the ability of Yersinia to disrupt TCR and BCR signaling cascades may contribute to this condition in some individuals.
What Proteins Are Target(s) of YopH in T and B Cells?
It has been suggested that the catalytic domain of YopH at the COOH-terminal end of the molecule selectively targets tyrosine-phosphorylated sites that contain the D/EpYxxP motif 45. In addition, a domain in the NH2-terminal region of YopH may be required for the efficient recognition of substrates. This substrate-binding domain exhibits a ligand specificity that is similar to that of the Crk Src homology 2 domain 13. Although the target(s) of YopH in lymphocytes is not known, we observed that most of the tyrosine kinases and adapter/linker proteins that are normally phosphorylated after T and B cell antigen receptor stimulation were either not phosphorylated or rapidly dephosphorylated in the presence of YopH. The three known YopH targets identified in epithelial and Hela cells, paxillin, p125FAK, and p130cas 12 13 14, and its closely related homologue, p105casL, are also found in lymphocytes 46. Further, p105casL, associated with the cell membrane and clustered integrins, is known to be phosphorylated after TCR ligation 47. Thus, as p105casL may be involved in both TCR and integrin signaling, it is tempting to speculate that, in addition to the CD3ζ-ZAP70 in T cells or the Igα/Igβ pathway in B cells, a second pathway involving paxillin-p125FAK-p105casL contributes to lymphocyte activation. An abrogation of p105casL phosphorylation may have a significant inhibitory effect on T cell function. Alternatively, YopH may also target one member or members of the known T cell signaling cascade, including protein tyrosine kinases such as ZAP70 and Syk and tyrosine-phosphorylated adapter/linker proteins such as linker for activation of T cells (LAT), B cell linker protein (BLNK), and SH2 domain–containing leukocyte protein of 76 kD (SLP-76), which all contain sequences similar to the optimum YopH target consensus sequence. It is possible that a rapid dephosphorylation of these proteins may destabilize the TCR complex, which may lead to its disassembly and exposure of most or all components of the TCR complex, including CD3ζ, to phosphatases. We are currently characterizing the cellular distribution and protein targets of YopH in lymphocytes to distinguish among these possibilities.
What Cells Are Targets of Yops during Infection?
The Yops play a crucial role in Yersinia virulence. Most past experiments have focused on the effects of these Yops in epithelial cells and macrophages. Yet, our work clearly demonstrates the rapid and essential role of YopH in inhibiting T cell and B cell signaling. Importantly, the redistribution and reorientation of the cytoskeleton are necessary early events for lymphocyte activation 48. Yet, both YopE and YopO, which have been shown to disrupt actin filaments leading to alterations in the cytoskeleton of both phagocytes and epithelial cells 16 49, have no demonstrable effect in our assays. Furthermore, although YopJ/YopP induces apoptosis in macrophages 17 19, neither primary B cells, T cell lines, or T cell hybridomas appear susceptible to this virulence factor. YopH activity has been shown to mediate several effects in epithelial cells as well as macrophages, including: inhibition of Fc receptor–mediated oxidative burst in macrophages 50, inhibition of neutrophil functions 51 52, FA disassembly in epithelium cells, and inhibition of phagocytosis in conjunction with YopE. These effects are all very different from the one we report here. Thus, it appears that different Yops target different host cell types involved in defense against Yersinia infection. By the same token, the enteropathogenic Yersinia harbor several different adhesion molecules that mediate their attachment to host cells. There is experimental evidence to suggest that some of the adhesins are better adapted to deliver specific Yops to certain host cells types. Invasin, which targets β1 integrins 53 54, is essential for Peyer's patch translocation across the epithelial barrier, but not as efficient for delivering Yops to macrophages. Perhaps the plasmid-mediated adhesin, YadA, suffices in delivering Yops into macrophages. Taken together, it appears that Yersinia have evolved to produce effectors that are specifically designed for the different cell types that the bacteria encounter in the course of an infection.
The study of pathogen–host interactions provides a fascinating insight into the strategies used by microbes to manipulate normal host functions to their own benefit. The Yops are part of a complex secretion–translocation apparatus that responds to host cell cues and results in the delivery of an array of proteins into the host cell cytoplasm. Homologues of the secretory arm of this complex, the type III secretion system, have been found in a growing number of pathogenic gram-negative microorganisms. It is likely that other pathogenic microorganisms secrete molecules analogous to Yops to alter host cell function and cause immune suppression or an alteration in the immune response to the benefit of the microbe. It is instructive to see, for example, that Salmonella species have two fully functional type III secretory systems, one involved in entry and the other involved in intracellular replication, that are essential for virulence. Moreover, one of these Salmonella type III secretory pathways delivers an effector protein, SptP/SptA, into the host cell cytoplasm. SptP/SptA has a COOH-terminal phosphatase domain similar to that found in YopH and an NH2-terminal domain that is homologous to the YopE cytotoxin 55 56. Curiously, infection with Salmonella can also trigger reactive arthritis in humans. Similarly, Shigella and Chlamydia and other pathogens have chromosomal islands containing type III secretory and effector homologues, and these bacteria are likewise associated with the development of autoimmune disease subsequent to infection. Is this the result of an underlying common strategy employed by many bacteria to alter the host immune response? Regardless, studying the molecular mechanisms of bacterial infection and host immune responses will reveal additional subtleties of bacterial pathogenicity and provide new information about the development of immune response.
Acknowledgments
We thank Jay Boniface and Dan Lyons (Stanford University) for the soluble MCC/I-Ek complex, Christoph Wülfing for help with the calcium flux experiments, Richard Glynne for the induction of B7.2 and CD69 on B cells in response to anti-IgM, Arthur Weiss (University of California, San Francisco, CA) for anti-CD3ζ antibody 6B10.2, Pat Jones (Stanford University) for the 5C.C7 T cell line, Diane Mathis and Christoph Benoist (Institut de Génétique et Biologie Moléculaire et Cellulaire, Strasbourg, France) for the MCC9 T cells; and Mark Davis, John Xu, Denise Monack, and Tim McDaniel for helpful discussions.
This work is supported by grants from the National Institutes of Health (to Y. Chien), the Medical Scientist Training Program of the National Institutes of Health (to T. Yao and J.I. Healy), the American Cancer Society (PF-4477 to J. Mecsas), and the Defense Advanced Research Projects Agency (to J. Mecsas and S. Falkow). The content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsements should be inferred.
Footnotes
Abbreviations used in this paper: BCR, B cell antigen receptor; FA, focal adhesion; HEL, hen egg lysozyme; MCC, moth cytochrome c; MOI, multiplicity of infection; Yop, Yersinia outermembrane protein.
J. Mecsas and J.I. Healy contributed equally to this work.
References
- Finlay B., Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- Sherris, J., and K. Ryan. 1994. Sherris Medical Microbiology. 3rd ed. K. Ryan, editor. Appleton & Lange, East Norwalk, CT. 890 pp.
- Pancholi P., Mirza A., Bhardwaj N., Steinman R.M. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993;260:984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- Autenrieth I.B., Tingle A., Reske-Kunz A., Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in micecharacterization of murine T-cell clones specific for Y. enterocolitica . Infect. Immun. 1992;60:1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth I.B., Vogel U., Preger S., Heymer B., Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 micecomparison of time course, histomorphology, and immune response. Infect. Immun. 1993;61:2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuikova K., Pokrovski V. Clinical and immune peculiarities of pseudotuberculous polyarthritis against a background of chronic opisthorchiasis. Br. J. Rheumatol. 1998;37:341–342. doi: 10.1093/rheumatology/37.3.341. [DOI] [PubMed] [Google Scholar]
- Toivanen A., Granfors K., Lahesmaa-Rantala R., Leino R., Stahlberg T., Vuento R. Pathogenesis of Yersinia-triggered reactive arthritisimmunological, microbiological and clinical aspects. Immunol. Rev. 1985;86:47–70. doi: 10.1111/j.1600-065x.1985.tb01137.x. [DOI] [PubMed] [Google Scholar]
- Autenrieth I.B., Firsching R. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocoliticaan ultrastructural and histological study. J. Med. Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- Simonet M., Richard S., Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G.R., Wolf-Watz H. The Yersinia Yop virulona bacterial system for subverting eukaryotic cells. Mol. Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- Cornelis G.R., Boland A., Boyd A.P., Geuijen C., Iriarte M., Neyt C., Sory M.P., Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C., Carballeira N., Wolf-Watz H., Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D.S., Montagna L.G., Zitsmann S., Bliska J.B. Identification of an amino-terminal substrate-binding domain in the Yersinia tyrosine phosphatase that is required for efficient recognition of focal adhesion targets. Mol. Microbiol. 1998;29:1263–1274. doi: 10.1046/j.1365-2958.1998.01014.x. [DOI] [PubMed] [Google Scholar]
- Black D.S., Bliska J.B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel K., Roggenkamp A., Schubert S., Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect. Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Forsberg A., Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D.M., Mecsas J., Ghori N., Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D.M., Mecsas J., Bouley D., Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S.D., Boland A., Sory M.P., van der Smissen P., Kerbourch C., Finlay B.B., Cornelis G.R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schesser K., Spiik A.K., Dukuzumuremyi J.M., Neurath M.F., Pettersson S., Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expressionYopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- Boland A., Cornelis G.R. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L.E., Hobbie S., Galan J.E., Bliska J.B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- Galyov E.E., Hakansson S., Forsberg A., Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- Hakansson S., Galyov E.E., Rosqvist R., Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol. Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- Iriarte M., Cornelis G.R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- Leung K.Y., Reisner B.S., Straley S.C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 1990;58:3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K.Y., Straley S.C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb alpha. J. Bacteriol. 1989;171:4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth I.B., Hantschmann P., Heymer B., Heesemann J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in miceevidence for the involvement of T lymphocytes. Immunobiology. 1993;187:1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- Lundgren E., Carballeira N., Vazquez R., Dubinina E., Branden H., Persson H., Wolf-Watz H. Invasin of Yersinia pseudotuberculosis activates human peripheral B cells. Infect. Immun. 1996;64:829–835. doi: 10.1128/iai.64.3.829-835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arencibia I., Suarez N.C., Wolf-Watz H., Sundqvist K.G. Yersinia invasin, a bacterial beta1-integrin ligand, is a potent inducer of lymphocyte motility and migration to collagen type IV and fibronectin. J. Immunol. 1997;159:1853–1859. [PubMed] [Google Scholar]
- Fazekas de St. Groth B., Patten P.A., Ho W.Y., Rock E.P., Davis M. An analysis of T cell receptor-ligand interaction using a transgenic antigen model for T cell tolerance and T cell receptor mutagenesis. In: Alt F.W., Vogel A.H., editors. Molecular Mechanisms of Immunological Self-Recognition. Academic Press; San Diego: 1992. pp. 123–127. [Google Scholar]
- Nakano N., Rooke R., Benoist C., Mathis D. Positive selection of T cells induced by viral delivery of neopeptides to the thymus [published erratum at 279:151] Science. 1997;275:678–683. doi: 10.1126/science.275.5300.678. [DOI] [PubMed] [Google Scholar]
- Mecsas J., Raupach B., Falkow S. The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol. 1998;28:1269–1281. doi: 10.1046/j.1365-2958.1998.00891.x. [DOI] [PubMed] [Google Scholar]
- Wettstein D.A., Boniface J.J., Reay P.A., Schild H., Davis M.M. Expression of a class II major histocompatibility complex (MHC) heterodimer in a lipid-linked form with enhanced peptide/soluble MHC complex formation at low pH. J. Exp. Med. 1991;174:219–228. doi: 10.1084/jem.174.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfing C., Rabinowitz J.D., Beeson C., Sjaastad M.D., McConnell H.M., Davis M.M. Kinetics and extent of T cell activation as measured with the calcium signal. J. Exp. Med. 1997;185:1815–1825. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers N.S., Teh S.J., Irving B.A., Tiong J., Weiss A., Teh H.S. Production and characterization of monoclonal antibodies specific for the murine T cell receptor zeta chain. J. Immunol. Methods. 1994;170:261–268. doi: 10.1016/0022-1759(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Healy J.I., Dolmetsch R.E., Timmerman L.A., Cyster J.G., Thomas M.L., Crabtree G.R., Lewis R.S., Goodnow C.C. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- Sarker M.R., Sory M.P., Boyd A.P., Iriarte M., Cornelis G.R. LcrG is required for efficient translocation of Yersinia Yop effector proteins into eukaryotic cells. Infect. Immun. 1998;66:2976–2979. doi: 10.1128/iai.66.6.2976-2979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman T., Hakansson S., Forsberg A., Norlander L., Macellaro A., Backman A., Bolin I., Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosisevidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- Reth M., Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- Autenrieth I.B., Heesemann J. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med. Microbiol. Immunol. (Berl.). 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- Nakajima R., Brubaker R.R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield E.A., Nguyen K.A., Kuchroo V.K. CD28/B7 costimulationa review. Crit. Rev. Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Thieme-Sefler A.M., Maclean D., McNamara D.J., Dobrusin E.M., Sawyer T.K., Dixon J.E. Substrate specificity of the protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA. 1993;90:4446–4450. doi: 10.1073/pnas.90.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi M., Tachibana K., Sato T., Iwata S., Nojima Y., Morimoto C. Structure and function of Cas-L, a 105-kD Crk-associated substrate-related protein that is involved in β1 integrin–mediated signaling in lymphocytes. J. Exp. Med. 1996;184:1365–1375. doi: 10.1084/jem.184.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H., Mimura T., Morino N., Hamasaki K., Nakamoto T., Hirai H., Morimoto C., Yazaki Y., Nojima Y. Ligation of the T cell antigen receptor induces tyrosine phosphorylation of p105CasL, a member of the p130Cas-related docking protein family, and its subsequent binding to the Src homology 2 domain of c-Crk. Eur. J. Immunol. 1997;27:2113–2117. doi: 10.1002/eji.1830270840. [DOI] [PubMed] [Google Scholar]
- Monks C.R., Freiberg B.A., Kupfer H., Sciaky N., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Holmstrom A., Rosqvist R., Wolf-Watz H., Forsberg A. Virulence plasmid-encoded YopK is essential for Yersinia pseudotuberculosis to cause systemic infection in mice. Infect. Immun. 1995;63:2269–2276. doi: 10.1128/iai.63.6.2269-2276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J.B., Black D.S. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 1995;63:681–685. doi: 10.1128/iai.63.2.681-685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S., Friedlander A., McDowell D., Weeks J., Tobery S. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathog. 1998;24:185–196. doi: 10.1006/mpat.1997.0188. [DOI] [PubMed] [Google Scholar]
- Andersson K., Magnusson K.E., Majeed M., Stendahl O., Fallman M. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 1999;67:2567–2574. doi: 10.1128/iai.67.5.2567-2574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R.R., Voorhis D.L., Falkow S. Identification of invasina protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Isberg R.R., Leong J.M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Arricau N., Hermant D., Waxin H., Popoff M.Y. Molecular characterization of the Salmonella typhi StpA protein that is related to both Yersinia YopE cytotoxin and YopH tyrosine phosphatase. Res. Microbiol. 1997;148:21–26. doi: 10.1016/S0923-2508(97)81896-7. [DOI] [PubMed] [Google Scholar]
- Kaniga K., Uralil J., Bliska J.B., Galan J.E. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium . Mol. Microbiol. 1996;21:633–641. doi: 10.1111/j.1365-2958.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]