Abstract
CD4 T helper (Th) type 1 and Th2 cells have been identified in the airways of asthmatic patients. Th2 cells are believed to contribute to pathogenesis of the disease, but the role of Th1 cells is not well defined. In a mouse model, we previously reported that transferred T cell receptor–transgenic Th2 cells activated in the respiratory tract led to airway inflammation with many of the pathologic features of asthma, including airway eosinophilia and mucus production. Th1 cells caused inflammation with none of the pathology associated with asthma. In this report, we investigate the role of Th1 cells in regulating airway inflammation. When Th1 and Th2 cells are transferred together into recipient mice, there is a marked reduction in airway eosinophilia and mucus staining. To address the precise role of Th1 cells, we asked (i), Are Th2-induced responses inhibited by interferon (IFN)-γ? and (ii) Can Th1 cells induce eosinophilia and mucus in the absence of IFN-γ? In IFN-γ receptor−/− recipient mice exposed to inhaled antigen, the inhibitory effects of Th1 cells on both airway eosinophilia and mucus production were abolished. In the absence of IFN-γ receptor signaling, Th1 cells induced mucus but not eosinophilia. Thus, we have identified new regulatory pathways for mucus production; mucus can be induced by Th2 and non-Th2 inflammatory responses in the lung, both of which are inhibited by IFN-γ. The blockade of eosinophilia and mucus production by IFN-γ likely occurs through different inhibitory pathways that are activated downstream of Th2 cytokine secretion and require IFN-γ signaling in tissue of recipient mice.
Keywords: asthma, T helper cell type 1, T helper cell type 2, IFN-γ, mucus
Asthma is a chronic inflammatory disorder of the airways that is characterized by intermittent episodes of airway obstruction and wheezing. Activation of CD4 Th2 cells in the respiratory tract is now believed to be responsible, in part, for pathogenesis in this disease. CD4 Th2 cells secreting IL-4, IL-5, and IL-13 have been identified in the airways of asthmatics 1 2 3. Th2 cytokines produced in the respiratory tract lead to airway eosinophilia, high levels of serum IgE, and mast cell activation 4, all believed to contribute to the pathological consequences: airway hyperresponsiveness (AHR),1 epithelial damage, and mucus hypersecretion.
Th1 cells have also been identified in the airways of asthmatics, but their function is not clear 5 6. One theory is that Th1 cells protect against asthma 7. This is supported by studies in allergic patients showing an increase in IFN-γ levels after successful immunotherapy 8 9 10. IFN-γ–dominated immune responses to viral or mycobacterial infection in childhood are associated with a reduced incidence of asthma 11 12. And, after infection with Bacillus Calmette-Guérin, mice exhibited a reduction in eosinophilic airway inflammation and AHR 13. Other studies suggest that Th1 cells might potentiate the inflammatory response in asthma due to the proinflammatory effects of Th1 cytokines 5 14. A shift in balance of cytokines from a dominant Th2 response to a Th1 response may relieve or resolve symptoms, or, alternatively, worsen existing disease. The precise mechanisms by which Th1 cells effect Th2 cell responses in the lung have not been well characterized.
To investigate the role of Th1 or Th2 cells in airway disease, we developed a model in which highly skewed populations of Th1 or Th2 cells were activated in the respiratory tract with inhaled antigen 15 16. We showed that activation of Th2 cells leads to airway eosinophilia, AHR, and mucus hypersecretion, features consistent with human asthma. In contrast, when activated in the lungs, Th1 cells caused inflammation without the pathophysiologic characteristics of asthma. We now address how Th1 cells regulate inflammation in asthma. Using TCR-transgenic Th1 and Th2 cells generated in vitro and transferred into recipient mice, we dissect the mechanisms by which Th1 cells inhibit Th2-induced eosinophilia and mucus production.
Materials and Methods
Mice.
DO11.10 mice, which are transgenic for the TCR recognizing OVA peptide 323–339 (pOVA323–339) 17, were provided to us on a BALB/c background by Ken Murphy (Washington University, St. Louis, MO) and were bred in our facilities. IL-4–deficient BALB/c mice (The Jackson Laboratory) were bred in our facilities. Cells were transferred into 6–12-wk-old BALB/c mice (The Jackson Laboratory) or IFN-γR−/− mice (provided by J. Aguet, Molecular Biology Institute, Zurich, Switzerland; backcrossed six generations onto BALB/c).
Generation of Th1 or Th2 Cells.
To generate Th1 or Th2 cells from DO11.10 mice, CD4 T cells were isolated by negative selection as previously described 18 using mAbs to CD8 (clone 53-6.72, clone 2.43; reference 19), class II MHC I-Ad, and anti-Ig–coated magnetic beads (Collaborative Research, Inc.). Naive CD4 T cells were further isolated from this population by positive selection with a biotinylated anti–L-selectin antibody (Mel-14; PharMingen) and streptavidin microbeads using MACS™ (Miltenyi Biotec). Syngeneic T cell–depleted splenocytes were used as APCs and prepared by negative selection using antibodies to CD4 (GK1.5; reference 20), anti-CD8, anti-Thy1 21, and treatment with rabbit complement. APCs were mitomycin-C treated. To generate Th1 cells, cultures contained pOVA323–339 at 5 μg/ml, IL-12 at 5 ng/ml (Genetics Institute), IL-2 at 10 U/ml (Collaborative Research, Inc.), and anti–IL-4 (11B11; reference 22) at inhibitory concentration. To generate Th2 cells, cultures contained pOVA323–339 at 5 μg/ml, IL-4 at 200 U/ml (Collaborative Research, Inc.), IL-2 at 10 U/ml, and anti–IFN-γ (XMG1.2; reference 23) at inhibitory concentration. All cultures were set up in flasks containing equal numbers of CD4 T cells and APCs at a final concentration of 5 × 105 cells/ml and were maintained for 4 d.
Transfer of Cells and Aerosol Administration of OVA.
Cultured Th1- or Th2-like cells were harvested after 4 d and washed with PBS, and cells were injected intravenously into syngeneic recipients. 1 d after transfer of cells, mice were challenged with inhaled 1% OVA in PBS as previously described 15 for 20 min daily for a total of seven days over a period of nine days (four consecutive days exposed, two days rested, and three consecutive days exposed). Control mice received inhaled OVA only. Mice were analyzed 24 h after the final exposure to antigen.
Cytokine Assays.
At the time of transfer, an aliquot of Th1- or Th2-like cells was retained for restimulation. 5 × 105 CD4 T cells/ml and 5 × 105 freshly isolated BALB/c APCs per milliliter were cultured with pOVA (5 μg/ml). Supernatants were collected after 48 h. Bronchoalveolar lavage (BAL) cells obtained from individual mice were restimulated in vitro at 2 × 106 cells/ml in the presence of pOVA (5 μg/ml). IFN-γ, IL-4, and IL-5 levels from cell supernatants were determined by ELISA (Endogen). Assays were standardized with recombinant IFN-γ, IL-5, IL-10 (Endogen), and IL-4 (Collaborative Research, Inc.). The lower limit of sensitivity for each of the ELISAs was 0.6 ng/ml (IFN-γ), 5 pg/ml (IL-4), 0.010 ng/ml (IL-5), and 200 pg/ml (IL-10). For BAL fluid cytokines, mice were exposed to aerosolized OVA on days 1 and 2, BAL was performed on day 3, and fluid was analyzed by ELISA for cytokines IL-4, IL-13 (R & D Systems, Inc.), IL-5 (Endogen), and IFN-γ (Biosource International, Inc.).
FACS™ Analysis.
Cell populations were stained with anti-CD4 (Quantum Red-L3T4; Sigma Chemical Co.), the biotinylated anticlonotypic antibody KJ1-26 24, and FITC–avidin D (Vector Labs.). KJ1-26 is specific for the transgenic TCR in the DO11.10 mice. Th1 and Th2 cell populations transferred were >97% CD4 and KJ1.26 positive. After a period of inhalational exposure, BAL cells were analyzed by FACS™ using these antibodies.
BAL.
BAL was performed by cannulation of the trachea and lavage with 1 ml of PBS. Total cell counts were performed, cytospin preparations of BAL cells were stained with Dif-Quik (Baxter Healthcare Corp.), and differentials were performed based on morphology and staining characteristics.
Lung Histology.
Lungs were prepared for histology by perfusing the animal via the right ventricle with 20 ml of PBS. Lungs were then inflated with 1.0 ml of fixative instilled through a tracheostomy tube. Samples for paraffin sectioning were formalin fixed and sectioned in the coronal plane at 5 μm, ensuring that central airways were visible. Sections were stained with hematoxylin and eosin and periodic acid-Schiff (PAS). Histological mucus index (HMI) was performed as previously described on PAS-stained sections and is equivalent to the linear percent of epithelium positive for mucus 15 25. This index was calculated for each mouse lung, and then the mean HMI was calculated for each experimental group.
Results
Th1 Cells Inhibit Th2-induced Eosinophilia and Mucus Production.
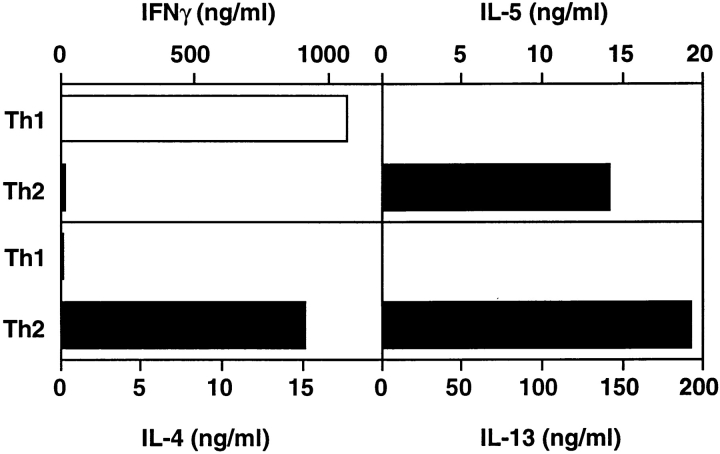
We previously showed that TCR-transgenic Th1 or Th2 cells generated in vitro and transferred into recipient mice are recruited to the lung after inhaled antigen challenge and retain their polarized cytokine profile in vivo, and that both Th1 and Th2 cells induce airway inflammation 15. Th2 cells stimulate inflammation with lymphocytes and eosinophils and induce airway epithelial mucus production (Table ). Th1 cell activation leads to lymphocytic inflammation, but Th1 cells fail to stimulate eosinophilia or mucus production. It was unclear if the differences in Th1 and Th2 cell effects resulted from Th1 cells lacking the cytokines necessary to induce eosinophilia and mucus or if Th1 cells actively inhibited these processes. To investigate the mechanism by which Th1 cells failed to stimulate eosinophilia and mucus production and if Th1 cells could inhibit Th2-induced inflammation, we performed mixing experiments by transferring both Th1 and Th2 cells together into recipient mice. Th1 and Th2 cells were each generated in vitro from CD4 T cells isolated from TCR-transgenic, DO11.10 mice that were cultured with APCs, pOVA323–339, and polarizing cytokines as previously described 15. At the time of transfer, Th1 cells secrete high levels of IFN-γ and no IL-4 and IL-5, whereas Th2 cells produce very low levels of IFN-γ but high levels of IL-4 and IL-5 (Fig. 1).
Table 1.
Effects of Th1 or Th2 Cell Activation in the Respiratory Tract
| Cells transferred | BAL cells (predominant) | Airway epithelial mucus |
|---|---|---|
| Th1 | Neutrophils | − |
| Th2 | Eosinophils | + |
DO11.10 Th1 or Th2 cells were transferred into wild-type BALB/c recipient mice and exposed to inhaled OVA (reference 15). BAL differentials were performed on cytospin samples, and lung sections were analyzed for mucus staining.
Figure 1.
Cytokine production by Th1 or Th2 cells. At the time of transfer into recipient mice, in vitro–generated DO11.10 CD4 Th1 or Th2 cells were cultured with APCs in the presence of pOVA323–339. Supernatants were collected after 48 h, and cytokine ELISAs were performed. One experiment is shown and is representative of five experiments.
Mice received transfer of Th1, Th2, or a mixture of Th1 and Th2 cells (Th1 + Th2) and were exposed to inhaled antigen. Airway eosinophilia, which is consistently present after transfer of Th2 cells and exposure to inhaled OVA, is markedly reduced when Th1 + Th2 cells are transferred (Fig. 2). The inhibitory effects of Th1 cells on airway eosinophilia are dependent on the number of Th1 cells transferred, as when fewer Th1 cells are transferred into the mice, more eosinophils are present in the BAL. When equal numbers of Th1 and Th2 cells were transferred together and mice were exposed to inhaled antigen, eosinophils were consistently inhibited sevenfold or greater in experiments that employed transfer of a range of different cell numbers (106–5 × 106 cells). Mice exposed to inhaled OVA after no cells were transferred or after Th1 cells were transferred did not exhibit BAL eosinophilia.
Figure 2.
Effects of Th1 cells on airway eosinophilia. Th1, Th2, or Th1 + Th2 cells (numbers shown) were transferred into BALB/c recipient mice, and mice were exposed to inhaled OVA for 7 d. 24 h later, mice were killed and BAL was performed. Total cell and differential counts were performed on cells recovered from BAL in individual mice. Data represents mean number of BAL eosinophils (±SEM) (n = 4 mice per group). One experiment is shown and is representative of four experiments. Statistical significance was determined by unpaired Student's t test. *P < 0.05 Th2 versus Th1 (1.25) + Th2 (2.5); ‡ P < 0.002 Th2 versus Th1 (2.5) + Th2 (2.5).
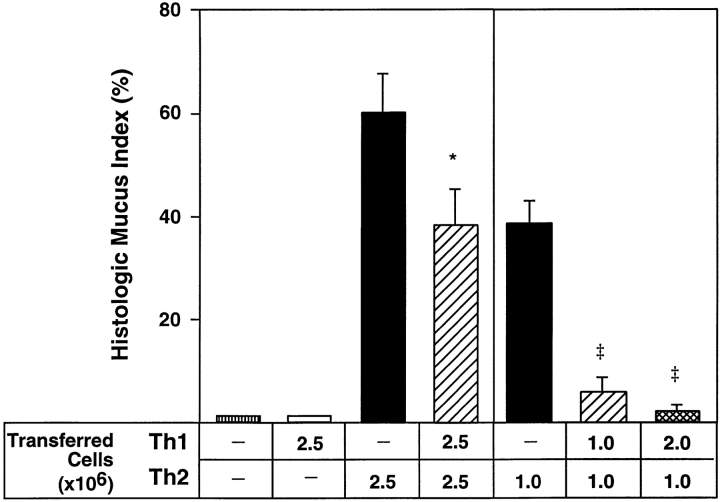
Th2 cells induce a marked increase in mucus staining in the bronchial epithelium, but when Th1 + Th2 cells are transferred, there is a reduction in airway epithelial mucus staining (Fig. 3). This inhibition of mucus staining in the airway epithelium is modest when airway inflammation is severe (after transfer of 2.5 × 106 cells). When fewer Th2 cells are transferred into recipient mice, resulting in less inflammation, mucus staining is markedly inhibited by equal numbers of Th1 cells (Fig. 3). When two times the number of Th1 cells are transferred with Th2 cells (2:1), there is complete inhibition of mucus staining. Thus, Th1 cells inhibit Th2-induced mucus in a dose-dependent fashion. Mice that were exposed to inhaled OVA and received transfer of no cells or Th1 cells showed mucus staining in <5% of bronchial epithelial cells. Therefore, when Th1 and Th2 cells are transferred together into recipient mice, Th1 effects dominate, resulting in the inhibition of Th2-induced airway eosinophilia and mucus production.
Figure 3.
Effects of Th1 cells on airway mucus production. Th1, Th2, or Th1 + Th2 cells (numbers shown) were transferred into BALB/c recipient mice, and mice were exposed to inhaled OVA for 7 d. 24 h later, mice were killed. Mucus staining was assessed on PAS-stained lung sections. Data represents mean HMI (±SEM) (n = 4 mice per group). One experiment is shown and is representative of four experiments. Statistical significance was determined by unpaired Student's t test. *P < 0.05 Th2 versus Th1 (2.5) + Th2 (2.5). ‡ P < 0.0004 Th2 versus Th1 (1.0) + Th2 (1.0) or Th1 (2.0) + Th2 (1.0).
IFN-γ– and IL-4–producing Cells Are Present in the Airways after Transfer of Th1 and Th2 Cells, but Inflammation Is Not Increased.
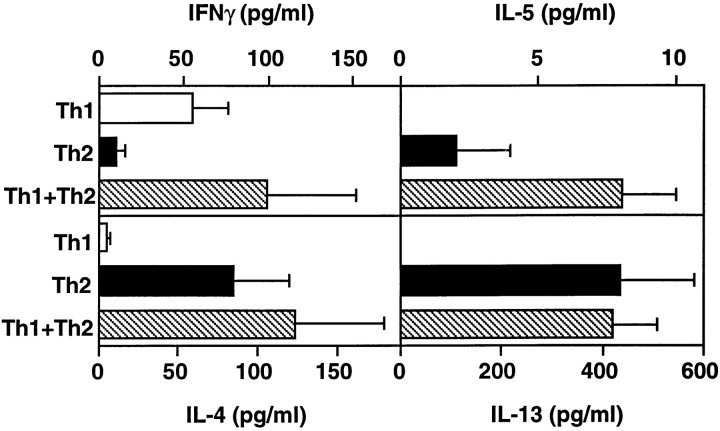
It was possible that Th1 cell inhibition of Th2-induced inflammation resulted from decreased Th2 cell activity, either by blocking recruitment of cells to the lung or by direct suppression of Th2 cell activation. To determine if Th2 cells were active in the lungs of mice after transfer of Th1 + Th2 cells, we examined BAL fluid recovered after transfer of cells and exposure to inhaled OVA. In mice that received Th1 + Th2 cells and inhaled OVA, IL-4, IL-5, and IL-13 were present in the BAL fluid at levels comparable to those in BAL fluid recovered from mice that were exposed to OVA after transfer of Th2 cells alone (Fig. 4). IFN-γ levels were similar in mice that received Th1 + Th2 cells or Th1 cells alone and inhaled OVA. BAL cells recovered from mice after exposure to inhaled OVA and transfer of Th1 + Th2 cells produced IL-4, IL-5, IL-13, and IFN-γ (data not shown), again indicating that both the transferred, OVA-responsive DO11 Th1 and Th2 cells are present in the respiratory tract. These data suggest that Th1 inhibition of Th2-induced inflammation does not result from inhibition of Th2 cell activation in the respiratory tract but at steps downstream of cytokine secretion.
Figure 4.
Cytokine levels in BAL fluid after inhaled antigen exposure. After transfer of Th1, Th2, or Th1 + Th2 (2.5 × 106 cells from each population) and exposure to inhaled antigen, BAL fluid was collected and cytokines were measured by ELISA. The mean cytokine level (±SEM) is shown (n = 3 mice per group).
In mice that received Th1 + Th2 cells and inhaled OVA, airway inflammation, as measured by the total number of BAL cells recovered from mice, was not increased when compared with mice that received Th2 cells and inhaled OVA (Table ). This was independent of the number of transferred cells, as shown. These data indicate that cotransfer of Th2 and Th1 cells, doubling the number of transferred cells, does not lead to increased airway inflammation yet results in suppression of airway eosinophilia and mucus induction.
Table 2.
Airway Inflammation in Mice after Inhaled Antigen Challenge
| Cells transferred | Total BAL cells | BAL cell differential | ||||
|---|---|---|---|---|---|---|
| Type | Number | Eosinophils | PMNs | Lymphocytes | Macrophages | |
| ×106 | ×105 | % (±SE) | % (±SE) | % (±SE) | % (±SE) | |
| Experiment 1 | ||||||
| Th1 | 5 | 5.7 (1.7) | 0 (0) | 21 (2) | 37 (7) | 42 (6) |
| Th2 | 5 | 5.3 (0.4) | 35 (9) | 3 (1) | 37 (4) | 25 (6) |
| Th1 + Th2 | 5 + 5 | 5.9 (1.2) | 3 (1) | 23 (1) | 39 (5) | 35 (4) |
| Experiment 2 | ||||||
| Th1 | 2.5 | 2.9 (0.4) | 0 (0) | 37 (3) | 43 (4) | 20 (2) |
| Th2 | 2.5 | 8.3 (1.9) | 36 (2) | 5 (1) | 31 (3) | 28 (2) |
| Th1 + Th2 | 2.5 + 2.5 | 3.5 (0.5) | 1 (0) | 23 (3) | 44 (1) | 32 (3) |
DO11.10 Th1, Th2, or Th1 + Th2 cells were transferred into wild-type BALB/c recipient mice, and mice were exposed to inhaled OVA. Total cell and differential counts were performed on BALs. Cell counts are shown (±SE).
Eosinophilia Is Dependent on Th2 Cells and Is Inhibited by IFN-γ.
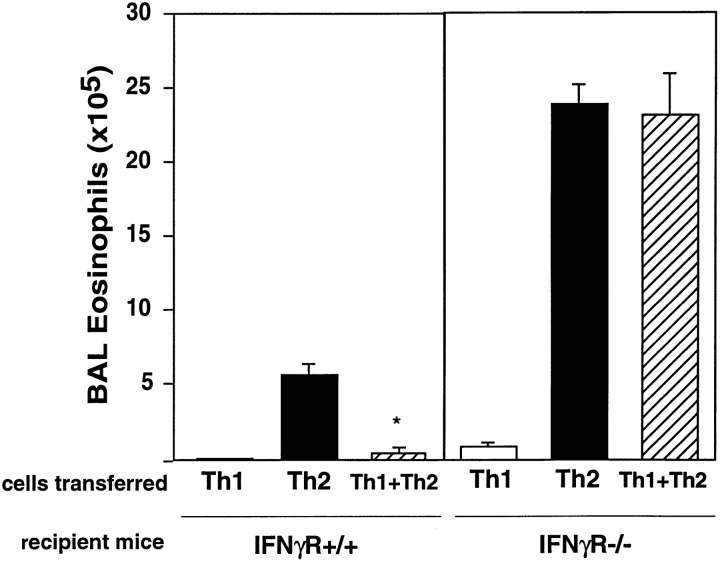
To determine the mechanism by which Th1 cells inhibit Th2-induced inflammatory responses, we investigated the role of the Th1 cytokine IFN-γ, as IFN-γ had been shown previously to have inhibitory effects on Th2 cell functions in vivo, including inhibition of CD4 T cell migration, eosinophilia, and IgE production 26 27 28 29. We transferred DO11 Th1, Th2, or Th1 + Th2 cells into IFN-γR+/+ or IFN-γR−/− mice. Although the transferred DO11 Th1 cells were able to secrete IFN-γ at the time of transfer, IFN-γR−/− recipient mice were unable to respond to IFN-γ. After exposure to inhaled OVA, IFN-γR−/− mice that received Th1 + Th2 cells showed marked airway eosinophilia, with levels of eosinophils equivalent to those in IFN-γR−/− mice that received transfer of Th2 cells and inhaled OVA (Fig. 5). Thus, Th1 cells no longer inhibited Th2-induced eosinophilia in the absence of IFN-γ signaling. IFN-γR+/+ mice that received transfer of the same population of Th1 + Th2 cells had 18-fold fewer eosinophils in the BAL. After transfer of Th2 cells and exposure to inhaled antigen, IFN-γR−/− recipients had four times as many eosinophils in the BAL than IFN-γR+/+ recipients, indicating that even during heavily skewed Th2 responses, the inhibitory effects of IFN-γ on airway eosinophilia are present. IFN-γR−/− mice that received Th1 cells and inhaled OVA or mice that received inhaled OVA alone did not exhibit significant eosinophilic airway inflammation. Therefore, the induction of airway eosinophilia requires activated Th2 cells and can be inhibited by IFN-γ secreted by Th1 cells.
Figure 5.
Airway eosinophilia induced in IFN-γR−/− mice. Th1, Th2, or Th1 + Th2 cells (2.5 × 106 cells from each population) were transferred into IFN-γR+/+ (BALB/c) or IFN-γR−/− recipient mice, and mice were exposed to inhaled OVA. Total cell and differential counts were performed on cells recovered from BAL in individual mice. Data represents mean number of BAL eosinophils (±SEM) (n = 4 mice per group). One experiment is shown and is representative of two experiments. Statistical significance was determined by unpaired Student's t test. *P < 0.0001 Th2 versus Th1 + Th2.
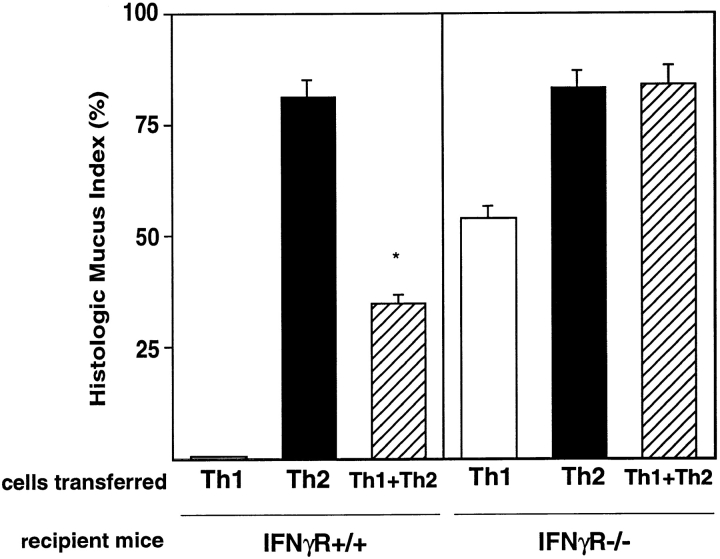
Mucus Production Is Not Inhibited by Th1 Cells in IFN-γR−/− Mice.
When Th1 + Th2 cells are transferred into IFN-γR−/− mice and mice are exposed to inhaled OVA, mucus staining is present at levels similar to mucus staining in IFN-γR−/− mice that received Th2 cells alone (Fig. 6). Th1 cell inhibition of Th2-induced mucus is no longer present in IFN-γR−/− mice, suggesting that IFN-γ inhibits mucus production by airway epithelial cells. Yet when Th1 cells were transferred into IFN-γR−/− mice and mice were exposed to inhaled OVA, mucus was also induced (Fig. 6), indicating that Th1 cells are capable of inducing mucus when IFN-γ signaling is absent. IFN-γ not only inhibits Th2-induced mucus production, but the absence of mucus in IFN-γR+/+ animals that received Th1 cells (Fig. 3) appears to be a result of active inhibition by IFN-γ. These data suggest that Th2 cells are not essential for mucus induction.
Figure 6.
Airway mucus production in IFN-γR−/− mice. Th1, Th2, or Th1 + Th2 cells (2.5 × 106 cells from each population) were transferred into IFN-γR+/+ (BALB/c) or IFN-γR−/− recipient mice, and mice were exposed to inhaled OVA. Mucus staining was assessed on PAS-stained lung sections. Data represents mean HMI (±SEM) (n = 4 mice per group). One experiment is shown and is representative of two experiments. Statistical significance was determined by unpaired Student's t test. *P < 0.0004 Th2 versus Th1 + Th2.
It was still possible that the induction of mucus production by DO11 Th1 cells in IFN-γR−/− mice was a result of Th2 cytokine activation, as blockade of IFN-γ during a Th1 cell response can induce the cell population to produce IL-4 30. To test if the cytokine pattern of the DO11.10 Th1 population had shifted in vivo, we harvested BAL cells from mice that received Th1 cells and inhaled OVA and restimulated the cells in vitro with pOVA323–339. BAL cells recovered from IFN-γR−/− recipient mice secreted high levels of IFN-γ and minimal IL-4, IL-5, and IL-13, similar to the levels of cytokines secreted by BAL cells recovered from IFN-γR+/+ recipient mice (Table ). Thus, Th1 cells, after recruitment and activation in IFN-γR−/− mice, remain polarized to Th1 yet are capable of stimulating mucus production.
Table 3.
Cytokine Production by BAL Cells Recovered from Mice after Transfer of Th1 Cells
| Transferred cells | Recipient mice | IFN-γ | IL-4 | IL-13 | IL-5 |
|---|---|---|---|---|---|
| ng/ml | ng/ml | ng/ml | ng/ml | ||
| Th1 | IFN-γR+/+ | 2,138 | 0 | 0.3 | 0 |
| Th1 | IFN-γR−/− | 1,084 (425) | 0 | 0.6 (0.3) | 0 |
BAL cells were recovered from mice after transfer of 2.5 × 106 Th1 cells and exposure to inhaled OVA. Cells were restimulated in vitro with pOVA323–339 for 48 h, and cytokine ELISAs were performed on supernatants. In IFN-γR−/− mice, mean cytokine production is shown (±SE) (n = 3 mice per group). In IFN-γR+/+ recipient mice, BAL cells from three mice were pooled.
In summary, airway epithelial mucus production and airway eosinophilia are stimulated by different mechanisms; recruitment of eosinophils to the airway requires Th2 cells, whereas mucus can be induced by both Th1 and Th2 cells, as long as IFN-γR signaling is absent. Despite different mechanisms of induction, both airway eosinophilia and mucus production can be inhibited by activation of Th1 cells producing IFN-γ.
Inhibition of Airway Mucus and Eosinophilia Requires IFN-γR in Recipient Mice.
Th1 and Th2 cytokines are both present after transfer of Th1 + Th2 cells and exposure to inhaled OVA (Fig. 4), suggesting that the downregulatory effects of Th1 on Th2 cells are not through direct inhibition of Th2 cell activation. The mechanism of action of Th1 cells is precisely defined in transfer experiments using IFN-γR−/− recipient mice (Fig. 5 and Fig. 6). In IFN-γR−/− mice, the transferred DO11.10 Th2 cells express the IFN-γR and are capable of responding to IFN-γ secreted by Th1 cells. If Th1 cells were directly blocking Th2 cell function, then inhibition of eosinophilia and mucus production would persist in IFN-γR−/− mice. Yet the inhibitory effects of Th1 cells on Th2 cell–induced eosinophilia and mucus production are completely abolished in IFN-γR−/− mice. These studies suggest that IFN-γ produced by Th1 cells inhibits Th2-induced eosinophilia and mucus production despite ongoing Th2 cytokine secretion, through effects requiring IFN-γR in recipient mice. As different inflammatory pathways stimulate airway eosinophilia and mucus production, it is likely that IFN-γ inhibits these processes through distinct mechanisms that are induced in target tissue.
Discussion
Recent studies have established that CD4 Th2 cells and their cytokines initiate an inflammatory response in the respiratory tract with many features of asthma. In contrast, Th1 cells lead to inflammation but exhibit none of the asthmatic pathology. As both Th1 and Th2 cells have been identified in the lungs of asthmatic patients, we investigated whether Th1 cells could regulate allergic airway pathology. In this report, we show that coactivation of Th1 and Th2 cells in the lung leads to a dominance of Th1 effects, inhibiting both airway eosinophilia and mucus production. Th1 cells, through the production of IFN-γ, inhibit these Th2-induced effects, not by regulating Th2 cell activity, as previously suggested, but by blocking downstream pathways induced by Th2 cytokines. Furthermore, the marked inhibitory effects of Th1 cells occur without an increase in airway inflammation. Thus, it appears that Th1 cells block critical pathologic changes that contribute significantly to morbidity and mortality in asthma. Our data show that IFN-γ inhibits airway eosinophilia even during polarized Th2-type responses, indicating that Th1 cells in the airways of asthmatics may be active in controlling disease.
In addition, these studies are the first to show that the development of inflammatory pathology in asthma can be differentially controlled. Whereas airway eosinophilia depends on the presence of activated Th2 cells in the lung, mucus can be induced by different types of inflammatory infiltrates as long as IFN-γR signaling is blocked. Th1 cells fail to stimulate mucus because IFN-γ inhibits its production. Th1 cells do not induce eosinophilia, most likely due to a lack of IL-5 31.
Th1 responses have been proposed to protect against asthma. This theory is based on Th2-dominant lymphocyte populations in the airways of asthmatics and evidence that Th1 responses protect in asthmatics and in animal models of asthma 2 7 11 12 13. Th1 cells may inhibit Th2 cell function at different stages in the effector response. Th1 cells, through the production of IFN-γ, have been shown to inhibit Th2 cell cytokine production and Th2 cell proliferation in vitro 32 33. In mice, the Th1 cytokine IFN-γ has inhibitory effects on Th2-induced airway eosinophilia and AHR. When administered before inhaled antigen challenge, IFN-γ reduced the number of CD4 T cells in the respiratory tract 26 28 29 or reduced Th2 cytokine secretion 26 34. These effects may result from inhibition of Th2 cell recruitment by IFN-γ. Once Th2 cells are present in the respiratory tract, IFN-γ suppresses the resolution of Th2-induced inflammation, as shown in IFN-γR−/− mice that had prolonged eosinophilia and Th2 cytokine production 35. In this report, we show another role of IFN-γ in the regulation of Th2 responses. Th1 cell production of IFN-γ blocks Th2-induced inflammatory pathways downstream of cytokine production. This may occur by direct effects on eosinophils and epithelial cells or through an intermediate cell derived from the recipient mice. This mechanism of inhibition is of potential importance in asthmatic airways, where Th2 cells are chronically present, as we show that Th1 cells can inhibit Th2 cell effects while Th2 cells are actively secreting cytokines.
The inhibitory pathways induced by Th1 cells require more than a few days for induction. In our studies, the inhibitory effects of Th1 cells were seen in mice exposed to antigen over 9 d. These effects were not observed when we killed mice after just 2 d of antigen challenge: BAL eosinophilia was similar after transfer of Th2 cells or Th1 + Th2 cells and inhaled antigen (data not shown). It is possible that a short period of antigen exposure explains, in part, why other investigators did not observe a similar reduction in eosinophilia after recruitment and activation of Th1 and Th2 cells in the respiratory tract 36 37. This delay in inhibition of eosinophilia and mucus production by Th1 cells may point to a biological pathway that requires either time or stimulation with higher levels of IFN-γ to induce inhibition.
In defining the functional effects of Th1 cells on Th2-mediated airway inflammation, our studies provide insights into potential mechanisms governing symptom control in conventional allergy immunotherapy. These mechanisms need to be analyzed, as we have shown, by assessing the lymphocyte populations present at sites of inflammation where cytokines exert their effects. Studies of grass pollen immunotherapy for atopic skin disease and allergic rhinitis showed a reduction in late phase responses and eosinophil accumulation in the skin and nasal mucosa. These effects were associated with increased IFN-γ–expressing cells, yet cells positive for IL-4 and IL-5 were unchanged 9 10. In addition, persistent Th2 cytokine production with increased IFN-γ–producing cells may explain why IgE levels and skin prick testing were not reduced, but IgG1/IgG4 levels increased in successful immunotherapy of pollen-allergic individuals 38. Increasing the population of activated Th1 cells at sites of allergic inflammation should be a focus of new techniques of immunotherapy, as IFN-γ can exert its effects despite ongoing Th2 cell activation. These effects may be sustained, as there is evidence that potent, long-term stimulation with Th1 cytokines can shift a Th2-predominant population toward Th1 39 40 41.
Increasing Th1 cell activation during immunotherapy in asthma bears the potential risk of increasing inflammation. Successful immunotherapy for atopic skin disease and rhinitis in allergic patients has not borne out these concerns 10 41. In our studies and others, airway inflammation was not increased when both Th1 and Th2 cells were recruited to the lung in wild-type recipient mice 42. This may relate to normal regulation of T cell proliferation that is typically present in intact, immunocompetent mice 43. Hansen et al. 42 showed that Th1 and Th2 cells transferred into SCID mice, which lack these regulatory elements, leads to unrestrained proliferation and overwhelming airway and parenchymal inflammation. Furthermore, reducing airway eosinophilia leads to a reduction in tissue damage and cellular infiltration 44 45 46. Blocking recruitment of eosinophils to the airway results in a reduction in proinflammatory factors that potentiate inflammation. Thus, as these studies show, the net effect of recruiting Th1 cells to the lung is that total inflammation is relatively unchanged.
Airway inflammation has long been associated with excess mucus production. In chronic bronchitis, cystic fibrosis, and asthma, mucus hypersecretion is associated with different characteristic immune responses in the lung. Unlike asthmatics, patients with cystic fibrosis or chronic bronchitis typically do not show activated Th2 cells in their airways. In a model of asthma, we recently showed that Th2 cells stimulate airway mucus production 15. Mucus induction requires IL-4Rα but is independent of IL-4, IL-5, eosinophils, and mast cells 47. Other recent studies showed that IL-13 and IL-4 are important mediators in mucus production 48 49 50 51 52. Here we also show that Th1 cells stimulate mucus production in the absence of IFN-γR signaling. The neutrophil-predominant inflammatory response in these mice and the relationship of neutrophilia with mucus hypersecretion in cystic fibrosis and chronic bronchitis suggests that neutrophils may be involved in mucus hyperproduction. Neutrophil elastase is a potent mucus secretagogue 53, yet the ability of this enzyme to stimulate increased mucin production has not been determined. We have shown that mucus induction is not due to a shift of the transferred Th1 cell population to a Th2 phenotype in IFN-γR−/− recipient mice. There still remains the possibility that very low levels of IL-13 secreted by Th1 cells stimulate mucus production in IFN-γR−/− mice. In summary, we have shown that mucus can be induced by an inflammatory response that is not dominated by production of IL-13 and IL-4.
Although different inflammatory responses stimulate mucus production, Th1 cells, through production of IFN-γ, inhibit mucus induced by both Th1 and Th2 cells. Inhibitory pathways for mucus have not been previously demonstrated. IFN-γ has been shown to have inhibitory effects on some epithelial functions. In gastric epithelial cells, mucus secretion was inhibited by IFN-γ 53. IFN-γ also inhibited growth of a human bronchial epithelial cell line and reduced barrier function and chloride secretion in intestinal epithelial cells 54 55. Beyond these limited studies, the inhibitory effects of IFN-γ on airway epithelium are not known. Interestingly, mucus production is not a feature of Th1-mediated pulmonary diseases in humans. Mycobacterium tuberculosis infection and sarcoidosis are diseases in which IFN-γ–producing CD4 T cells have been identified in the lung biopsies and in BAL 56 57 58. It is possible that the lack of mucus production in these conditions results from IFN-γ suppression. IFN-γ has many proinflammatory effects in the lung, most notably on macrophages, activating production of reactive nitrogen and oxygen species. The inhibitory effects of IFN-γ could therefore be through the production of inhibitory mediators by inflammatory cells or by direct effects on goblet cells. These studies establish the first known natural inhibitor of mucus production, one that can be active in different inflammatory settings.
In summary, using a transfer system that we developed to study the role of CD4 Th1 and Th2 cells in airway disease, we have defined two different pathways by which Th1 cells can regulate airway inflammation. Th1 cells, through an effect mediated by IFN-γR, block the recruitment of eosinophils to the airway and inhibit airway epithelial mucus production. In the absence of IFN-γR, Th1 cells induce mucus production but do not stimulate airway eosinophilia. Thus, Th1 cells have differential effects on stimulating these inflammatory responses. The inability of Th1 cells to stimulate eosinophilia likely results from a lack of IL-5. The mechanism by which mucus production is stimulated by Th1 cells in the absence of IFN-γR signaling is not yet known. As we learn how these inflammatory responses are regulated, we will identify new targets for directed immunotherapy for asthma and mucus hypersecretion.
Acknowledgments
The authors would like to thank A. Ray for helpful discussion and P. Ranney and H. MacLeod for technical assistance.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grants K08-HL03308 (to L. Cohn), R01-HL54450 (to K. Bottomly), and P50-HL56389 (to K. Bottomly, L. Cohn, and R.J. Homer) and the Yale Cancer Center.
Footnotes
1used in this paper: AHR, airway hyperresponsiveness; BAL, bronchoalveolar lavage; HMI, histologic mucus index; PAS, periodic acid-Schiff
References
- Huang S.K., Xiao H.Q., Kleine-Tebbe J., Paciotti G., Marsh D.G., Lichtenstein L.M., Liu M.C. IL-13 expression at the sites of allergen challenge in patients with asthma. J. Immunol. 1995;155:2688–2694. [PubMed] [Google Scholar]
- Robinson D.S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A.M., Corrigan C., Durham S.R., Kay A.B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Walker C., Bode E., Boer L., Hansel T.T., Blaser K., Virchow J.J. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am. Rev. Respir. Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. Immunologic basis of antigen-induced airway induced hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- Krug N., Madden J., Redington A.E., Lackie P., Dhukanovic R., Schauer U., Holgate S.T., Frew A.J., Howarth P.H. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am. J. Respir. Cell Mol. Biol. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- Cembrzynska-Nowak M., Szklarz E., Inglot A.D., Teodorczyk-Injeyan J.A. Elevated release of tumor necrosis factor-alpha and interferon-gamma by bronchoalveolar leukocytes from patients with bronchial asthma. Am. Rev. Respir. Dis. 1993;147:291–295. doi: 10.1164/ajrccm/147.2.291. [DOI] [PubMed] [Google Scholar]
- Holt P.G. A potential vaccine strategy for asthma and allied atopic diseases during early childhood. Lancet. 1994;344:456–458. doi: 10.1016/s0140-6736(94)91776-0. [DOI] [PubMed] [Google Scholar]
- Jutel M., Pichler W.J., Skrbic D., Urwyler A., Dahinden C., Muller U.R. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J. Immunol. 1995;154:4187–4194. [PubMed] [Google Scholar]
- Durham S.R., Ying S., Varney V.A., Jacobson M.R., Sudderick R.M., Mackay I.S., Kay A.B., Hamid Q.A. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-gamma. J. Allergy Clin. Immunol. 1996;97:1356–1365. doi: 10.1016/s0091-6749(96)70205-1. [DOI] [PubMed] [Google Scholar]
- Varney V.A., Hamid Q.A., Gaga M., Ying S., Jacobson M., Frew A.J., Kay A.B., Durham S.R. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J. Clin. Invest. 1993;92:644–651. doi: 10.1172/JCI116633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa T., Enomoto T., Shimazu S., Hopkin J.M. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- Shaheen S.O., Aaby P., Hall A.J., Barker D.J., Heyes C.B., Shiell A.W., Goudiaby A. Measles and atopy in Guinea-Bissau. Lancet. 1996;347:1792–1796. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- Erb K.J., Holloway J.W., Sobeck A., Moll H., Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J. Exp. Med. 1998;187:561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman M.J., Sampath D., Castro M., Look D.C., Jayaraman S. The one-two of T helper cellsdoes interferon-gamma knock out the Th2 hypothesis for asthma? Am. J. Respir. Cell Mol. Biol. 1996;14:316–318. doi: 10.1165/ajrcmb.14.4.8600934. [DOI] [PubMed] [Google Scholar]
- Cohn L., Homer R.J., Marinov A., Rankin J., Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cellsa critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn L., Tepper J.S., Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J. Immunol. 1998;161:3813–3816. [PubMed] [Google Scholar]
- Murphy K.M., Heimberger A.B., Loh D.Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+ TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Levin D., Constant S., Pasqualini T., Flavell R., Bottomly K. Role of dendritic cells in the priming of CD4+ T lymphocytes to peptide antigen in vivo. J. Immunol. 1993;151:6742–6750. [PubMed] [Google Scholar]
- Ledbetter J.A., Herzenberg L.A. Xenogenic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol. Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Dialynas D.P., Wilde D.B., Marrack P., Pierres A., Wall K.A., Havran W., Otten G., Loken M.R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, L3T4a, recognized by a monoclonal antibody GK1.5expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol. Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Jones B. Evidence that the Thy-1 molecule is a target for T cell mitogenic antibody against brain-associated antigens. Eur. J. Immunol. 1983;13:678–684. doi: 10.1002/eji.1830130813. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W.E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Cherwinski H.M., Schumacher J.H., Brown K.D., Mosmann T.R. Two types of mouse helper T cell cloneIII. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J. Exp. Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Shimonkevitz R., Hannum C., Haskins K., Kappler J. The major histocompatibility complex–restricted antigen receptor on T cells. J. Exp. Med. 1983;158:1635–1646. doi: 10.1084/jem.158.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E.R. Sterologic Methods 1979. Academic Press; London: pp. 9–159 [Google Scholar]
- Li X.-M., Chopra R.K., Chou T.-Y., Schofield B.H., Wills-Karp M., Huang S.-K. Mucosal IFN-γ gene transfer inhibits pulmonary allergic responses in mice. J. Immunol. 1996;157:3216–3219. [PubMed] [Google Scholar]
- Lack G., Renz H., Saloga J., Bradley K.L., Loader J., Leung D.Y., Larsen G., Gelfand E.W. Nebulized but not parenteral IFN-gamma decreases IgE production and normalizes airway function in a murine model of allergen sensitization. J. Immunol. 1994;5:2546–2554. [PubMed] [Google Scholar]
- Iwamoto I., Nakajima H., Endo H., Yoshida S. Interferon γ regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J. Exp. Med. 1993;177:573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Iwamoto I., Yoshida S. Aerosolized recombinant interferon-gamma prevents antigen-induced eosinophil recruitment in mouse trachea. Am. Rev. Respir. Dis. 1993;148:1102–1104. doi: 10.1164/ajrccm/148.4_Pt_1.1102. [DOI] [PubMed] [Google Scholar]
- Murray J.S., Madri J., Pasqualini T., Bottomly K. Functional CD4 T cell subset interplay in an intact immune system. J. Immunol. 1993;150:4270–4276. [PubMed] [Google Scholar]
- Sher A., Coffman R.L., Hieny S., Scott P., Cheever A.W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni . Proc. Natl. Acad. Sci. USA. 1990;87:61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T.F., Joyce J., Fitch F.W. Antiproliferative effect of IFN-γ in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-γ. J. Immunol. 1989;143:15–21. [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V.M., Mosmann T.R., Vitetta E.S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J. Exp. Med. 1988;168:543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett S.H., O'Hearn D.J., Li X., Huang S., Finkelman F.D., Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J. Exp. Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle A.J., Tsuyuki S., Bertrand C., Huang S., Aguet M., Alkan S.S., Anderson G.P. Mice lacking the IFN-γ receptor have an impaired ability to resolve a lung eosinophilic inflammatory response associated with a prolonged capacity of T cells to exhibit a Th2 cytokine profile. J. Immunol. 1996;156:2680–2685. [PubMed] [Google Scholar]
- Li L., Xia Y., Nguyen A., Feng L., Lo D. Th2-induced eotaxin expression and eosinophilia coexist with Th1 responses at the effector stage of lung inflammation. J. Immunol. 1998;161:3128–3135. [PubMed] [Google Scholar]
- Randolph D.A., Carruthers C.J., Szabo S.J., Murphy K.M., Chaplin D.D. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J. Immunol. 1999;162:2375–2383. [PubMed] [Google Scholar]
- Gehlhar K., Schlaak M., Becker W., Bufe A. Monitoring allergen immunotherapy of pollen-allergic patientsthe ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin. Exp. Allergy. 1999;29:497–506. doi: 10.1046/j.1365-2222.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Nabors G.S., Afonso L.C., Farrell J.P., Scott P. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and pentostam. Proc. Natl. Acad. Sci. USA. 1995;92:3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung V.P., Gieni R.S., Umetsu D.T., DeKruyff R.H. Heat-killed Listeria monocytogenes as an adjuvant converts established murine Th2-dominated immune responses into Th1-dominated responses. J. Immunol. 1998;161:4146–4152. [PubMed] [Google Scholar]
- Durham S.R., Walker S.M., Varga E.M., Jacobson M.R., O'Brien F., Noble W., Till S.J., Hamid Q.A., Nouri-Aria K.T. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- Hansen G., Berry G., DeKruyff R.H., Umetsu D.T. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape K.A., Kearney E.R., Khoruts A., Mondino A., Merica R., Chen Z.M., Ingulli E., White J., Johnson J.G., Jenkins M.K. Use of adoptive transfer of T-cell-antigen-receptor-transgenic T cell for the study of T-cell activation in vivo. Immunol. Rev. 1997;156:67–78. doi: 10.1111/j.1600-065x.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Hogan S.P., Koskinen A., Mathaei K.I., Young I.G., Foster P.S. Interleukin-5-producing CD4+ T cells play a pivotal role in aeroallergen-induced eosinophilia, bronchial hyperreactivity, and lung damage in mice. Am. J. Respir. Crit. Care Med. 1998;157:210–218. doi: 10.1164/ajrccm.157.6.mar-1. [DOI] [PubMed] [Google Scholar]
- Huffnagle G.B., Boyd M.B., Street N.E., Lipscomb M.F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J. Immunol. 1998;160:2393–2400. [PubMed] [Google Scholar]
- Kung T.T., Stelts D.M., Zurcher J.A., Adams I.G.K., Egan R.W., Kreutner W., Watnick A.S., Jones H., Chapman R.W. Involvement of IL-5 in a murine model of allergic pulmonary inflammationprophylactic and therapeutic effect of an anti-IL-5 antibody. Am. J. Respir. Cell Mol. Biol. 1995;13:360–365. doi: 10.1165/ajrcmb.13.3.7654390. [DOI] [PubMed] [Google Scholar]
- Cohn L., Homer R.J., MacLeod H., Mohrs M., Brombacher F., Bottomly K. Th2-induced airway mucus production is dependent on IL-4Rα, but not on eosinophils. J. Immunol. 1999;162:6178–6183. [PubMed] [Google Scholar]
- Dabbagh K., Takeyama K., Lee H.M., Ueki I.F., Lausier J.A., Nadel J.A. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 1999;162:6233–6237. [PubMed] [Google Scholar]
- Grunig G., Warnock M., Wakil A.E., Venkayya R., Brombacher F., Rennick D.M., Sheppard D., Mohrs M., Donaldson D.D., Locksley R.M. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J.A., Picarella D.E., Geba G.P., Temann A., Prasad B., DiCosimo B., Tarallo A., Stripp B., Whitsett J., Flavell R.A. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lunglymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc. Natl. Acad. Sci. USA. 1996;93:7821–7825. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M., Luyimbazi J., Xu X., Schofield B., Neben T.Y., Karp C.L., Donaldson D.D. Interleukin-13central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Homer R., Wang Z., Chen Q., Geba G., Wang J., Zhang Y., Elias J. Transgenic expression of IL-13 in murine lung causes airway inflammation, mucus hypersecretion, subendothelial fibrosis, eotaxin production and airways hyperresponsiveness to methacholine. J. Clin. Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer R., Christensen T.G., Lucey E.C., Stone P.J., Snider G.L. Quantitative study of secretory cell metaplasia induced by human neutrophil elastase in the large bronchi of hamsters. J. Lab. Clin. Med. 1985;105:635–640. [PubMed] [Google Scholar]
- Holmgren J., Fryklund J., Larsson H. Gamma-interferon-mediated down-regulation of electrolyte secretion by intestinal epithelial cellsa local immune mechanism? Scand. J. Immunol. 1989;30:499–503. doi: 10.1111/j.1365-3083.1989.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Madara J.L., Stafford J. Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I.M., Andersen P., Boom W.H. T cell response to Mycobacterium tuberculosis . J. Infect. Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- Garlepp M.J., Rose A.H., Dench J.E., Robinson B.W. Clonal analysis of lung and blood T cells in patients with sarcoidosis. Thorax. 1994;49:577–585. doi: 10.1136/thx.49.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron A., Bonay M., Kambouchner M., Lecossier D., Riquet M., Soler P., Hance A., Tazi A. Cytokine patterns in tuberculous and sarcoid granulomascorrelations with histopathologic features of the granulomatous response. J. Immunol. 1997;159:3034–3043. [PubMed] [Google Scholar]