Abstract
We have analyzed the presence of immature and mature dendritic cells (DCs) within adenocarcinoma of the breast using immunohistochemistry. Immature DCs were defined by expression of CD1a-, Langerin-, and intracellular major histocompatibility complex class II–rich vesicles. Mature DCs were defined by expression of CD83 and DC-Lamp. Breast carcinoma cells were defined by morphology and/or cytokeratin expression. We demonstrate two levels of heterogeneity of DCs infiltrating breast carcinoma tissue: (a) immature CD1a+ DCs, mostly of the Langerhans cell type (Langerin+), were retained within the tumor bed in 32/32 samples and (b) mature DCs, CD83+DC-Lamp+, present in 20/32 samples, are confined to peritumoral areas. The high numbers of immature DCs found in the tumor may be best explained by high levels of macrophage inflammatory protein 3α expression by virtually all tumor cells. Confirming the immature/mature DC compartmentalization pattern, in vitro–generated immature DCs adhere to the tumor cells, whereas mature DCs adhere selectively to peritumoral areas. In some cases, T cells are clustering around the mature DCs in peritumoral areas, thus resembling the DC–T cell clusters of secondary lymphoid organs, which are characteristic of ongoing immune reactions.
Keywords: breast cancer, dendritic cells, tumor immunity, MHC class II, chemokines
Dendritic cells (DCs)1 constitute a complex system of cells uniquely able to induce primary immune responses 1 2 3 4. DC progenitors in the bone marrow give rise to circulating precursors that home to the tissue, where they reside as immature cells with high phagocytic capacity. Upon tissue damage, DCs capture Ag and subsequently migrate to the lymphoid organs, where they select rare Ag-specific T cells, thereby initiating immune responses. During migration and within the secondary lymphoid organs, DCs undergo maturation from Ag-capturing cells to APCs. DCs present Ag to CD4+ T cells 5, which in turn regulate the immune effectors, including Ag-specific effectors such as CD8+ T cells and B cells as well as nonspecific effectors such as macrophages, eosinophils, and NK cells.
It is currently believed that three subsets of human DCs exist: two of myeloid origin and one of lymphoid origin. These subsets differ with respect to their biological function. Whereas both myeloid subsets, i.e., epidermal Langerhans cells (LCs) and interstitial (dermal) DCs (intDCs), induce the proliferation of naive T cells, only intDCs are able to induce the differentiation of naive B cells in vitro 6 7. Furthermore, in contrast to LCs, intDCs display potent and long-lasting phagocytic capacity 7. DC subsets can also skew Th responses toward either the Th1 or Th2 pathway. DCs derived from CD14+ blood precursors were thus found to induce Th1 differentiation 8. On the contrary, DCs derived from lineage-negative CD4+IL-3Rα+ plasmacytoid precursors induce Th2 polarization, further strengthening the notion of functional heterogeneity between DC subsets 8 9.
Tumor immunity can be viewed as a three-step process that includes (i) presentation of tumor-associated Ags (TAAs), (ii) selection and activation of TAA-specific T cells as well as non-Ag–specific effectors, and (iii) homing of TAA-specific T cells to the tumor site and elimination of tumor cells 10 11 12 13. Tumors may escape immune surveillance due to alterations at each of these steps. Thus, by release of factors such as IL-6, IL-10, M-CSF, and vascular endothelial growth factor, tumors can prevent DC differentiation and/or APC function 14 15. Indeed, tumor-associated DCs are usually of a low allostimulatory capacity, particularly if isolated from the progressing metastatic lesions, as in malignant melanoma or blood from patients with advanced breast cancer 16 17 18.
Recent findings challenge the concept that breast cancer is a “nonimmunogenic” tumor: (a) T cells and antibodies specific for breast cancer–associated Ags, such as her-2/neu and cdr2, have been detected in some patients 19 20 21, and (b) tumor-infiltrating lymphocytes from breast cancer tissue give rise to T cell lines responsive to autologous tumor 22.
Given the pivotal role of DCs in the induction of immunity, surprisingly few studies have addressed the in situ status of DCs in breast carcinoma with immature CD1a+ DCs observed in close association with tumor cells 23 24 25. The conceptual progress in the field of DC physiology and the availability of novel markers differentiating DC subsets have led us to reevaluate DC infiltration in breast carcinoma tissue. Here, we report two levels of heterogeneity of DCs infiltrating breast carcinoma tissue: first, immature DCs, mostly of the LC type, are retained in all samples within the tumor beds; second, mature DCs, when present, are confined to peritumoral areas.
Materials and Methods
Patient Characterization and Sample Acquisition.
Tissue samples obtained at surgery were immediately embedded in OCT and then frozen and stored at −80°C until further processed. All tumor samples were received as coded specimens, and for patient confidentiality, numbers corresponding to the order received in the laboratory were assigned. Nine normal breast specimens were processed in the same way. The material was collected under the auspices of Institutional Review Board protocol 97-79. The 32 studied cases of breast carcinoma, graded according to Bloom and Richardson, were distributed as follows: ductal carcinoma, high grade III (DC III), n = 17; ductal carcinoma, intermediate grade II (DC II), n = 9; ductal carcinoma, low grade I (DC I), n = 1; mixed lobular carcinoma, n = 3; carcinoma in situ, n = 2. Approximately one-half of the cases presented with metastases to regional lymph nodes. Patients' ages at the time of diagnosis ranged from 23 to 75 yr. See Table for clinicopathological parameters of each case, including age, tumor histological grade, tumor size, lymph node involvement, lymphatic and vascular invasion, estrogen and progesterone receptor status, proliferation index using Ki67 antibody MIB-1, DNA ploidy, and S phase.
Table 1.
Clinicopathological Characteristics of Patients
| Patient | Age | Tumor type/grade | Tumor size | Lymph node metastasis | Lymphatic invasion | Vascular invasion | Estrogen receptor | Progesterone receptor | MIB-1 | DNA index/ploidy | S phase |
|---|---|---|---|---|---|---|---|---|---|---|---|
| yr | cm | % | % | ||||||||
| 1 | 54 | DCIII | 6 | 8/10 | + | − | + | + | ND | 0.9/Hypodiploid | 5.1 |
| 2 | 55 | CIS | 11 | 0/11 | − | − | ND | ND | ND | ND | ND |
| 3 | 35 | DCIII | 6 | 0/13 | − | − | − | − | 20 | 1.8/Aneuploid | 7.7 |
| 4 | 58 | DCIII | 8 | 0/17 | − | − | ND | ND | ND | ND | ND |
| 5 | 57 | DCIII | 4.5 | 2/21 | − | − | ND | ND | ND | ND | ND |
| 6 | 59 | MLC | 2.5 | 1/14 | + | − | ND | ND | ND | ND | ND |
| 7 | 62 | DCIII | 9 | 0/16 | − | − | − | − | ND | 1.6/Aneuploid | 5.3 |
| 8 | 50 | DCIII | 2.5 | 0/21 | + | − | + | − | 50 | 1.9/Aneuploid | ND |
| 9 | 23 | DCIII | 9.1 | 0/11 | + | + | − | − | 90 | ND | ND |
| 10 | 74 | DCIII | 5 | 5/15 | + | − | + | − | ND | 1.8/Aneuploid | 7.5 |
| 11 | 64 | DCIII | 3.7 | 11/14 | + | + | − | − | 90 | 1.0/Diploid | 3.3 |
| 12 | 50 | DCIII | 2.5 | 2/22 | + | − | − | − | ND | 1.6/Aneuploid | 9.5 |
| 13 | 56 | DCII and LC | 3 | 3/17 | − | − | + | + | 10 | 1.0/Diploid | 3.0 |
| 14 | 72 | MLC | 2 | 0/29 | − | − | + | + | ND | 1.0/Diploid | 1.3 |
| 15 | 47 | DCIII | 2.4 | 1/18 | − | − | − | − | ND | 1.9/Tetraploid | 14.5 |
| 16 | 52 | DCII | 3.2 | 0/17 | − | − | + | + | ND | 3.3/Hypertetraploid | 8.9 |
| 17 | 56 | DCIII | 2.7 | 0/18 | + | + | − | − | ND | 1.0/Diploid | 6.0 |
| 18 | 39 | DCII | ND | 1/34 | − | − | + | + | ND | 1.8/Aneuploid | 6.0 |
| 19 | 63 | DCIII | 4.2 | 8/18 | + | − | − | − | ND | 1.5/Aneuploid | 21.1 |
| 20 | 66 | DCII | 8 | 0/14 | − | − | + | + | ND | 1.6/Aneuploid | 10.6 |
| 21 | 39 | DCIII | 2.2 | 1/26 | − | − | − | − | 60–70 | ND | ND |
| 22 | 38 | DCIII | 3.8 | 1/19 | + | − | + | + | ND | 1.0/Diploid | 6.7 |
| 23 | 64 | MLC | 5 | 33/43 | + | − | + | + | 10 | ND | ND |
| 24 | 60 | DCIII | 7.5 | 0/0 | + | − | − | − | ND | 2.6/Hypertetraploid | 30 |
| 25 | 45 | DCI | 0.8 | 0/3 hot LN | + | − | + | + | 5 | ND | ND |
| 26 | 60 | DCII | 1.5 | 5/7 | + | − | + | + | 40 | ND | ND |
| 27 | 75 | DCII and LC | 1.5 | 3/23 | + | − | + | + | 5–10 | ND | ND |
| 28 | 54 | DCIII | 7.0 | 2/4 | + | − | − | − | 15 | 2.1/Tetraploid | 13.2 |
| 29 | 59 | DCII | 6.5 | 4/9 | − | − | + | + | 20 | ND | ND |
| 30 | 35 | CIS | 5.7 | ND | − | − | + | + | 30 | ND | ND |
| 31 | 44 | DCII | 4 | 2/9 | − | − | + | + | 10 | ND | ND |
| 32 | 72 | DCII | 5 | 1/14 | − | − | + | + | 20 | ND | ND |
Histopathological diagnosis of breast carcinoma: CIS, carcinoma in situ; DC, ductal carcinoma; LC, lobular carcinoma; MLC, multifocal lobular carcinoma.
Positive (+) and negative (−) findings.
Immunohistochemistry and Immunofluorescence.
Samples were snap frozen, and serial 5-μm-thick sections were cut. Immunohistochemical staining of acetone-fixed sections was performed by incubation with mAbs recognizing the following molecules: CD1a (IgG2a) and HLA-DR (IgG2a) (DAKO Corp.), Langerin/DCGM4 (IgG1) and DC-Lamp (IgG1) (generated at Schering-Plough Lab.; reference 26), and CD83 (IgG2b), CD80 (IgG1), CD11c (IgG1), CD3 (IgG1), CD4 (IgG1), CD8 (IgG1) (all from Coulter-Immunotech), and CD86 (IgG1; Binding Site), followed by biotinylated goat anti–mouse IgG (DAKO Corp.) and streptavidin–peroxidase (Vector Labs.). The peroxidase was developed by diaminobenzidene tetrahydrochloride (brown color; Vector Labs.), and Mayer hematoxylin (Sigma Chemical Co.) was used as counterstain. In double-step immunohistochemical staining, after an initial blocking with goat serum and BSA, primary antibodies to CD83 or CD11c were followed by goat biotinylated anti–mouse IgG2b, IgG1 (Coulter Immunology), and streptavidin–peroxidase. After another blocking for endogenous biotin with avidin–biotin (Vector Labs.) and BSA, in a secondary step, mAbs to CD4 or DC-Lamp were followed by biotinylated anti–mouse IgG1 (Coulter Immunology) and streptavidin–alkaline phosphatase (Vector Labs.). Rabbit anti–human CD3 (DAKO Corp.) was followed by swine biotinylated anti–rabbit IgG (DAKO Corp.) and streptavidin–alkaline phosphatase. Peroxidase was developed by 3-amino-9 ethylcarbazole (red color; Vector Labs.), and alkaline phosphatase was revealed using Fast Blue as a chromogen (blue color; Vector Labs.). Isotype-matched antibodies (DAKO Corp.) were used as control. For the evaluation of macrophage inflammatory protein (MIP)3α staining, goat polyclonal antibody to MIP3α (R & D Systems, Inc.) was used, followed by biotinylated goat anti–mouse IgG (DAKO Corp.) and biotinylated rabbit anti–goat IgG (DAKO Corp.) and developed using streptavidin–peroxidase (Vector Labs.).
For immunofluorescence, incubation with primary antibodies to CD1a and DC-Lamp was followed by FITC-conjugated goat antibodies to mouse Igs (Molecular Probes, Inc.). In a subsequent secondary step, the rabbit polyclonal anticytokeratin antibody (DAKO Corp.) was followed by biotinylated anti–rabbit IgG (DAKO Corp.) and revealed by streptavidin–Texas Red (Molecular Probes, Inc.). Isotype-matched antibodies were used as a control. Confocal laser scanning microscopy was performed along the x–y axis with a confocal laser scanning microscope (TCS-SP; Leica Inc.) equipped with 20, 40, and 100× oil objectives.
Histological Scoring and Analysis.
Each slide was examined on at least two separate occasions by at least two individuals, including two pathologists. All cell counts were performed using an Olympus Ax-70 epifluorescence photomicroscope at a magnification of 400 (40× objective and 10× eyepiece). Cells displaying membrane staining, cytoplasmic staining, nuclear counterstain, and appropriate morphology were included. The area counted in each section was chosen randomly from a representative field of tumor. For each section, three areas were assessed, and the counts are expressed as the mean number of cells per high power field. In each case, a serial hematoxylin and eosin section was examined for orientation and confirmation of the histological diagnosis. Each case was scored blindly with respect to patient history, presentation, and previous scoring.
Generation of DCs.
DCs were generated from CD34+ hematopoietic progenitors (HPCs) or from CD14+ blood precursors as described previously 6. In brief, cord blood CD34+ HPCs were cultured with GM-CSF (50 ng/ml; Schering-Plough Lab.), stem cell factor (20 ng/ml; Amgen) and TNF (12.5 ng/ml; Genzyme Corp.). DCs were harvested at the stage of immature precursor and used either as a total population or after sorting of CD1a+ CD14− cells on day 7 of culture.
Monocyte-derived DCs were generated by culturing adherent fraction of PBMCs with GM-CSF (100 ng/ml; Schering-Plough Lab.) and IL-4 (50 ng/ml; Genzyme Corp.) 27 28 29. DCs were used on day 7 either as immature CD1ahiCD83−HLA-DRint or after 48 h of activation with CD40 ligand as mature CD1aloCD83+ HLA-DRhi cells.
In Situ Binding Assay.
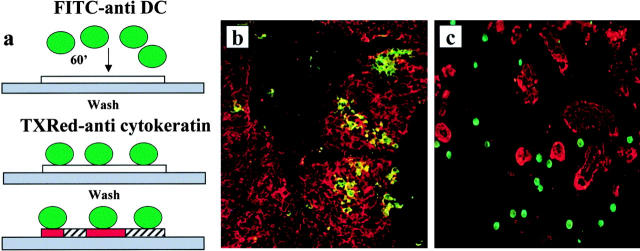
We have used an adapted version of the Stamper-Woodruff assay measuring binding of the lymphocytes to endothelium (see Fig. 3). Immature DCs labeled with CD1a–FITC or mature DCs labeled with HLA-DR–FITC were overlaid onto frozen sections of breast carcinoma and allowed to adhere for 1 h. After stringent washing, tissue sections were indirectly stained for cytokeratin expression. Raji cells were used as a negative control. Binding of DCs to breast carcinoma sections was quantitated in several ways to establish the pattern of cell adherence. First, we determined the density of immature and mature DCs adhering to the breast cancer section at the tumor and stromal site, respectively. Density values were based on the number of FITC-labeled DCs that adhered per 0.2 mm2 (40× objective, high power field). For each section, three areas were assessed (see Table ). Counting the number of DCs that were collected during the washing procedure permitted us to estimate that ∼15% of DCs remained on the tissue section.
Figure 3.
Immature DCs selectively adhere to tumor cells, whereas mature DCs adhere to tumor parenchyma. (a) Schematic presentation of DC binding experiments using a modified version of the Woodruff-Stamper assay. DCs generated in vitro from either CD34+ HPCs or blood monocytes were FITC labeled and overlaid (105 cells) on frozen sections of breast carcinoma tissue. After 60 min at 20°C and stringent washing, the sections were labeled with anticytokeratin antibody (Texas [TX]Red). (b) Immature CD34+, HPC–derived, sorted CD1a+ DCs adhere selectively to cytokeratin-labeled breast cancer cells (20×; patient 17). (c) Mature DCs, generated from blood monocytes and activated by CD40 ligation, adhere selectively to tumor stroma (20×; patient 11). Representative of six experiments using breast carcinoma tissue from six patients.
Table 3.
Binding of Immature and Mature DCs to Breast Cancer Tissue Sections
| Patient | Immature DCs | Mature DCs | ||
|---|---|---|---|---|
| Tumor | Stroma | Tumor | Stroma | |
| 24 | 16 ± 1.5 | 1 ± 0.6 | 1 ± 1.5 | 21 ± 2 |
| 29 | 6 ± 2.5 | 0.7 ± 1 | 6 ± 3 | 39 ± 18 |
| 11 | ND | ND | 0.7 ± 1 | 14 ± 6 |
Quantitation of binding of immature and mature DCs to breast carci-noma tissue sections. Values are mean ± SD of adherent cells per 0.2 mm2 measured within three individual areas in the tissue section.
RNA Isolation and Reverse Transcriptase–PCR.
RNA was prepared from fragments of tumor samples that were snap frozen and stored at −80°C until use using acidified phenol procedure according to the manufacturer's instructions (GIBCO BRL). RNA preparations were treated with RNase-free DNase (37°C for 30 min; Boehringer-Mannheim), digested with proteinase K in 1% SDS, extracted with phenol-chloroform, and precipitated with ethanol. RNA from tonsils was used as positive control. The following primers were used: β-actin, 5′-CTCCTTAATGTCACGCACGATTC-3′ forward and 5′-GTGGGGCGCCCCAGGCACCA-3′ reverse; MIP3α, 5′-TTGCTCCTGGCTGCTTTG-3′ forward and 5′-ACCCTCCATGATGTGCAAG-3′ reverse; MIP3β, 5′-CTGCTGGTTCTCTGGACTTC-3′ forward and 5′-CACACTCACACTCACAACAACAC-3′ reverse. PCR was performed in 35 cycles: 94°C for 1 min, 55°C for 1.5 min, and 72°C for 1.5 min.
Results
We have analyzed the differentiation/maturation status of DCs in frozen sections obtained from 32 patients with adenocarcinoma of the breast using immunohistochemistry. The patients' characteristics and clinicopathological parameters are given in Table . Breast carcinoma cells were identified by morphology and cytokeratin expression.
Immature DCs Are Present within the Tumor.
Immature DCs were characterized by CD1a expression, and the LC subset was characterized by expression of Langerin/DCGM4 (Lang), a recently identified Ag uniquely expressed within LCs 30. In all samples, CD1a+ cells with dendritic morphology were found to infiltrate the tumor beds (Fig. 1 a). The number of CD1a+ immature DCs per high power field (400×) ranged from 1 to 48 cells, with mean and median numbers 12 and 7, respectively (Table ). Although a majority of DCs were CD1a+Lang+ LCs, CD1a+Lang− cells were also found. The immaturity of CD1a+ DCs present within the tumor was further demonstrated by the presence of MHC class II intracellular compartments, as assessed by serial sectioning in confocal microscopy in all CD1a+ DCs examined (Fig. 2 a). Normal breast tissue, i.e., that not infiltrated by tumor, rarely displayed CD1a cells, and five of nine sections were negative, whereas four of nine sections showed less than three CD1a+ cells within the epithelial duct over the entire section (Fig. 1). Thus, the presence of CD1a+ cells within the breast cancer tissue was not solely due to its epithelial origin.
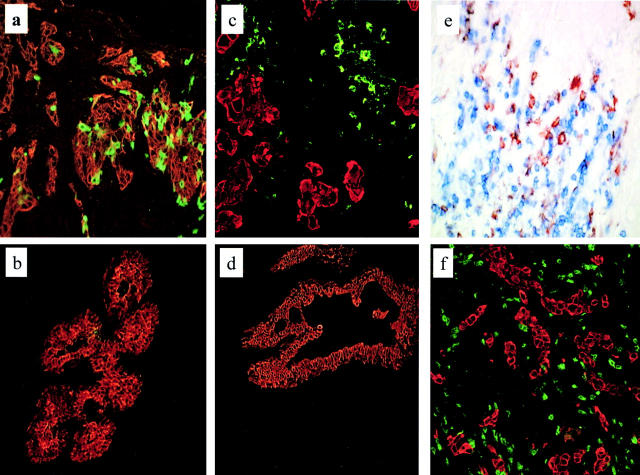
Figure 1.
In breast carcinoma tissue, immature DCs are intratumoral, whereas mature DCs and T cells are peritumoral. Immunofluorescence and immunohistochemistry of frozen sections of breast carcinoma tissue. Breast carcinoma is defined by indirect staining with anticytokeratin antibody (red) or by morphology. Immature DCs were identified by labeling with CD1a mAb, mature DCs were identified by labeling with CD83 or DC-Lamp mAb, and T cells were labeled using CD3 or CD4 mAb. (a) Immature, CD1a+ DCs (green) are retained within the tumor bed (40×). Representative of 32 samples (patient 17). (b) In the normal mammary epithelium, CD1a staining is seldom detected. (c) Mature, DC-Lamp–labeled DCs (green) are selectively localized in peritumoral areas (20×). Pattern representative of 25 samples (patient 11). (d) No mature DCs, as judged by lack of CD83 staining, are present in the normal breast tissue. (e) CD4+ T cells (blue) cluster around CD83+ mature DCs (red; 40×) (patient 6). DC–T cell clusters were seen in three out of five cases. (f) CD3+ T cells (green) are spread within the peritumoral area. Representative of 19 cases (patient 11);in a majority of these cases, T cells were CD8+.
Table 2.
In Situ Phenotype of DCs in Breast Carcinoma Tissue
| Patient | CD1a | DCGM4 | CD83 | CD11c | HLA-DR | CD80 | CD86 |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 0 | 1 | 30 | 45 | 15 | 26 |
| 2 | 1 | 0 | 14 | 38 | 72 | 20 | 32 |
| 3 | 17 | 20 | 8 | 24 | 14 | 11 | 25 |
| 4 | 3 | 0 | 0 | 40 | 51 | 24 | 26 |
| 5 | 4 | 0 | 2 | 24 | 40 | 20 | 22 |
| 6 | 6 | 3 | 11 | 50 | 80 | 15 | 14 |
| 7 | 18 | 11 | 2 | 17 | 52 | 13 | 20 |
| 8 | 1 | 0 | 0 | 30 | 40 | 14 | 36 |
| 9 | 2 | 0 | 7 | 12 | 14 | 9 | 14 |
| 10 | 8 | 13 | 5 | 24 | 22 | 4 | 7 |
| 11 | 6 | 4 | 4 | 55 | 45 | 18 | 26 |
| 12 | 11 | 3 | 0 | 60 | 58 | 26 | 23 |
| 13 | 2 | 2 | 1 | 29 | 60 | 8 | 17 |
| 14 | 12 | 7 | 0 | 30 | 42 | 9 | 16 |
| 15 | 7 | 5 | 2 | 50 | 32 | 8 | 20 |
| 16 | 11 | 3 | 1 | 45 | 50 | 13 | 40 |
| 17 | 48 | 12 | 10 | 39 | 53 | 27 | 42 |
| 18 | 30 | 32 | 28 | 53 | 63 | 22 | 37 |
| 19 | 41 | 17 | 7 | 52 | 59 | 32 | 43 |
| 20 | 10 | 0 | 0 | 24 | 22 | 15 | 10 |
| 21 | 3 | 2 | 0 | 45 | 50 | 25 | 14 |
| 22 | 6 | 0 | 2 | 40 | 12 | 18 | 22 |
| 23 | 10 | 0 | 8 | 35 | 20 | 15 | 15 |
| 24 | 7 | 0 | 12 | 50 | 20 | 15 | 26 |
| 25 | 1 | 1 | 5 | 40 | 30 | 18 | 11 |
| 26 | 4 | 0 | 15 | 25 | 25 | 15 | 23 |
| 27 | 18 | 10 | 1 | 20 | 30 | 20 | 16 |
| 28 | 45 | 10 | 6 | 35 | 40 | 26 | 32 |
| 29 | 5 | 3 | 3 | 58 | 52 | 20 | 32 |
| 30 | 2 | 1 | 2 | 68 | 71 | 18 | 24 |
| 31 | 21 | 15 | 0 | 46 | 38 | 20 | 14 |
| 32 | 28 | 17 | 1 | 50 | 49 | 29 | 32 |
| Mean ± SD | 12 ± 13 | 6 ± 8 | 5 ± 6 | 39 ± 14 | 42 ± 18 | 18 ± 7 | 24 ± 10 |
Values represent the number of cells labeled with antibody recognizing an indicated marker/high power field.
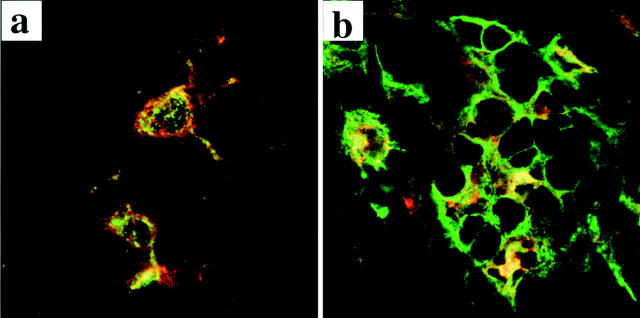
Figure 2.
In breast carcinoma tissue, immature DCs express intracellular MHC class II vesicles, whereas mature DCs express surface MHC class II molecules. Immunofluorescence staining on frozen sections evaluated by confocal microscopy as described in the Fig. 1 legend. (a) MHC class II molecules, stained with directly coupled L243 mAb (green), mostly reside in intracellular compartments in DCs identified by labeling with CD1a mAb (red) within the tumor bed. (b) Mature DCs, identified with lysosome resident marker DC-Lamp (red), express high numbers of cell surface MHC class II molecules (green). This pattern has been observed in three out of three analyzed samples.
Mature DCs Are Localized in the Peritumoral Areas.
We next sought to determine if mature DCs were also infiltrating tumor tissue. Mature DCs were characterized by expression of CD83 and DC-Lamp. Although no CD83+ cells were found in 9/9 samples of normal breast tissue, CD83+ mature DCs could be identified in 20/32 breast cancer samples (the threshold for positivity was established arbitrarily for a density greater than or equal to two cells per high power field). Most interestingly, mature DCs were located in the peritumoral areas surrounding but not penetrating the beds of carcinoma cells (Fig. 1 b). The number of CD83+ mature DCs per high power field (400×) ranged from 0 to 28 cells, with mean and median numbers 5 and 6, respectively (Table ). Staining for DC-Lamp, a highly specific marker of mature DCs 26, confirmed the peritumoral localization of mature DCs in breast cancer tissue (Fig. 1 b). Compared with immature DCs residing within the tumor bed, the mature DC-Lamp+ DCs found in the peritumoral area displayed intense MHC class II labeling mostly located at the cell periphery, attesting to cell surface expression (Fig. 2 b).
The antibodies recognizing HLA-DR, CD80, and CD86 did not reveal DC compartmentalization because their expression pattern also includes macrophages that are scattered throughout the breast cancer tissue in all studied samples. Similarly, CD11c+ labeling, found earlier to define DCs within germinal centers 9, was present in all 32 tumor samples without any apparent compartmentalization.
T Cells Colocalize with Mature DCs in Some Breast Carcinoma Samples.
Because mature DCs can normally be found only in secondary lymphoid organs where they closely interact with Ag-specific T cells, we next analyzed the T cell distribution and activation pattern in breast carcinoma samples. In 19 evaluated samples, CD3+ T cells infiltrated peritumoral areas, where they could be seen either scattered throughout the area in close proximity to tumor cells or clustered (Fig. 1). Double stainings for CD4 and CD83 demonstrated, in three out of five studied cases, T cells clustered around mature DCs in the peritumoral areas (Fig. 1 c). The majority of infiltrating T cells (70–75%) were CD3+CD8+, and a fraction of them (<10%) displayed the early activation markers CD69 and CD25. No T cell proliferation could be seen, as judged by labeling with Ki67 antibody (not shown).
Mature DCs Bind Selectively to Peritumoral Areas.
We assessed the capacity of fluorochrome-labeled DCs to adhere to frozen sections of breast cancer tissue in an adapted version of the Stamper-Woodruff assay measuring binding of lymphocytes to endothelium 31. As illustrated in Fig. 3, immature DCs adhere selectively to the tumor cells and spread upon binding. In contrast, mature DCs, generated by CD40 ligation, adhere selectively to the peritumoral areas, thus confirming the pattern of in situ DC localization (Fig. 3). Both immature and mature DCs adhere to tissue sections that contain infiltrating DCs, as detected immunohistochemically (Table ). Labeled Raji cells did not to bind to any of the tumor sections, further demonstrating the specificity of DC binding.
Immature DC Infiltration Is Associated with High Expression of MIP3α by Tumor Cells.
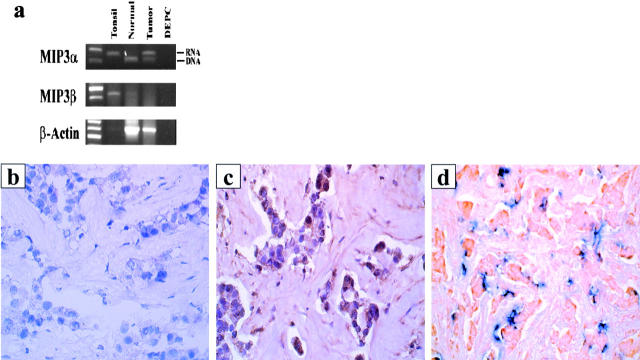
Recent studies demonstrated the expression of CCR6 on immature DCs 32, and the DC ligand MIP3α was identified in epithelia, the site of residence of immature LCs 33. We thus analyzed the expression pattern of MIP3α within breast tumors. First, reverse transcriptase (RT)-PCR analysis of whole breast cancer samples indicated the presence of MIP3α mRNA, whereas no MIP3α mRNA could be amplified from normal breast tissue (Fig. 4 a). PCR analysis of MIP3β expression showed lack of mRNA in the breast cancer tissue, whereas MIP3β was expressed in the tonsillar tissue (Fig. 4 a).
Figure 4.
Breast carcinoma cells express MIP3α mRNA and protein. (a) Expression of MIP3α and MIP3β was determined by RT-PCR using total RNA extracted from fresh breast carcinoma tissue. Immunohistochemistry staining with a control (b) or anti-MIP3α (c) antibody reveals specific MIP3α staining of breast carcinoma cells (20×; patient 28). Representative pattern of MIP3α expression from seven samples. (d) CD1a-labeled immature DCs (blue) colocalize with MIP3α-expressing breast cancer cells (red) (patient 28).
Immunohistochemical analysis of seven breast cancer samples (Table ) further demonstrated the expression of MIP3α protein by the majority of tumor cells and colocalization of immature DCs and MIP3α-producing cells (Fig. 4b–d). Two normal breast specimens did not express the MIP3α protein. Thus, accumulation of immature DCs within the tumor bed may be dependent on the expression of MIP3α by tumor cells.
Table 4.
In Situ DC Phenotype and MIP3α Expression in Breast Carcinoma
| Patient | CD1a+ | CD83+ | MIP3α staining |
|---|---|---|---|
| 16 | 11 | 1 | +++ |
| 25 | 1 | 5 | ++ |
| 28 | 45 | 6 | +++ |
| 29 | 5 | 3 | − |
| 30 | 2 | 2 | + |
| 31 | 21 | 0 | +++ |
| 32 | 28 | 1 | ++ |
Numbers of CD1a- or CD83-expressing cells per high power field.
Discussion
Herein we demonstrate the unique compartmentalization of immature and mature DCs within breast carcinoma tissue. 32/32 tumor samples displayed variable immature DC infiltration. This compartmentalization was further confirmed by the binding of in vitro–generated immature DCs to tumor beds. Tumor-infiltrating immature DCs were heterogeneous, and two populations could be identified, both CD1a+ Langerin+ LCs and non-Langerhans, CD1a+Langerin− cells. Because dermal (interstitial) DCs were found in vitro and in vivo to express CD1a but not Langerin, we currently conclude that CD1a+Langerin− tumor-infiltrating DCs may be intDCs. The pathophysiological significance of this heterogeneity remains to be established. Earlier studies revealed that intDCs generated in vitro from CD34+ HPCs display (a) more potent phagocytic activity than LCs and (b) a unique ability to induce differentiation of naive B cells 6 7.
As a hallmark of immature phenotype, DCs infiltrating the tumor bed expressed compartmentalized intracellular MHC class II molecules 34 35 36. This subcellular distribution of MHC class II molecules changed dramatically in mature peritumoral DCs, where most of the class II molecules were relocated to the cell surface.
The observed numbers of immature CD1a+ DCs in the tumor microenvironment appeared much higher than in normal breast epithelium, suggesting increased homing and infiltration. This may be best explained by the high levels of intratumoral MIP3α, a chemokine that was recently shown to specifically attract immature DCs 33 37 38 39 and is now found to be expressed within the tumor epithelium. The immunohistochemistry analysis with anti-MIP3α is not, unfortunately, precise enough to allow a correlation between the amount of MIP3α within the tumor cells and the numbers of infiltrating immature DCs. The increased numbers of immature DCs could reflect a transient stage due to the high in and out migration or the sequestering of immature DCs within the tumor tissue.
Perhaps the most important finding is the presence, in 20/32 breast carcinoma samples, of mature DCs that were located specifically within the peritumoral areas. This striking compartmentalization was further confirmed by the binding of in vitro–generated mature DCs to peritumoral areas. This indicates that stromal factors are determining the DC adherence. Because mature DCs are only observed in lymphoid organs, where they closely interact with T cells, it is tempting to consider that their presence within the tumor tissue reflects an ongoing immune response, possibly tumor specific. These mature DCs present within the tumor tissue could derive from any of the currently defined DC subsets discussed above. Unfortunately, the tools available now do not allow us to determine the subset origin. Because of recent data showing the role of DC subsets in Th1/Th2 polarization and the induction of different classes of immune response 8, the determination of the origin and immunocompetence of mature, tumor-associated DCs will be of great importance to our understanding of the development of tumor immunity.
The limited number of relatively heterogeneous breast cancer tissue samples analyzed to date does not allow us to establish a prognostic significance of the infiltration of tumor with immature or mature DCs. Such determination will require the analysis of a large number of samples in prospective or retrospective studies. Unfortunately, currently available CD1a and DC-Lamp antibodies do not permit analysis of paraffin-embedded archival material. Therefore, we are now evaluating alternative methodologies for the determination of DC phenotype in archival material and initiating a prospective analysis using frozen tissue samples.
Our observations open a new avenue in tumor immunology. A thorough analysis of the DC system within tumors will provide clues critical to understanding the development of tumor immunity.
Acknowledgments
We are grateful to Drs. Sally Knox and Michael Grant for providing us with samples of breast carcinoma tissue. We thank Dr. Sem Saeland for his comments on the manuscript.
This work was supported by a grant from Baylor Health Care System Foundation.
Footnotes
1used in this paper: DCs, dendritic cells; HPCs, hematopoietic progenitors; int, interstitial; LCs, Langerhans cells; MIP, macrophage inflammatory protein; TAAs, tumor-associated antigens
Presented partly at the 5th International Symposium on Dendritic Cells in Fundamental and Clinical Immunology, Pittsburgh, Pennsylvania, September 23–28, 1998.
References
- Cella M., Sallusto F., Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Hart D.N. Dendritic cellsunique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bell D., Young J.W., Banchereau J. Dendritic cells. Adv. Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- Toes R.E.M., Ossendorp F., Offringa R., Melief C.J.M. CD4 T cells and their role in antitumor immune responses. J. Exp. Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Dezutter-Dambuyant C., de Saint-Vis B., Jacquet C., Yoneda K., Imamura S., Schmitt D., Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+ TNF alpha. J. Exp. Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., de Saint-Vis B., Dezutter-Dambuyant C., Jacquet C., Schmitt D., Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. Adv. Exp. Med. Biol. 1997;417:21–25. doi: 10.1007/978-1-4757-9966-8_4. [DOI] [PubMed] [Google Scholar]
- Rissoan M.C., Soumelis V., Kadowaki N., Grouard G., Briere F., de Waal Malefyt R., Liu Y.J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Grouard G., Durand I., Filgueira L., Banchereau J., Liu Y.J. Dendritic cells capable of stimulating T cells in germinal centers. Nature. 1996;384:364–368. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- Pawelec G., Zeuthen J., Kiessling R. Escape from host-antitumor immunity. Crit. Rev. Oncog. 1997;8:111–141. doi: 10.1615/critrevoncog.v8.i2-3.10. [DOI] [PubMed] [Google Scholar]
- Restifo N.P., Sznol M. Cancer vaccines. In: DeVita V.T. Jr., Hellman S., Rosenberg S.A., editors. CancerPrinciples & Practice of Oncology. 5th ed. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 3023–3043. [Google Scholar]
- Pardoll D.M. Cancer vaccines. Nat. Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- Sogn J.A. Tumor immunologythe glass is half full. Immunity. 1998;9:757–763. doi: 10.1016/s1074-7613(00)80641-x. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D.I., Chen H.L., Girgis K.R., Cunningham H.T., Meny G.M., Nadaf S., Kavanaugh D., Carbone D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- Menetrier-Caux C., Montmain G., Dieu M.C., Bain C., Favrot M.C., Caux C., Blay J.Y. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cellsrole of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- Gabrilovich D.I., Ciernik I.F., Carbone D.P. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell. Immunol. 1996;170:101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D.I., Corak J., Ciernik I.F., Kavanaugh D., Carbone D.P. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin. Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- Enk A.H., Jonuleit H., Saloga J., Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int. J. Cancer. 1997;73:309–316. doi: 10.1002/(sici)1097-0215(19971104)73:3<309::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Darnell R.B. Onconeural antigens and the paraneoplastic neurologic disordersat the intersection of cancer, immunity, and the brain. Proc. Natl. Acad. Sci. USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disis M.L., Cheever M.A. HER-2/neu proteina target for antigen-specific immunotherapy of human cancer. Adv. Cancer Res. 1997;71:343–371. doi: 10.1016/s0065-230x(08)60103-7. [DOI] [PubMed] [Google Scholar]
- Albert M.L., Darnell J.C., Bender A., Francisco L.M., Bhardwaj N., Darnell R.B. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat. Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C.H., Robbins P.F., Sakaguchi K., Appella E., Yannelli J.R., Adema G.J., Miki T., Rosenberg S.A. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.J., Maddox P.H., Jenkins D. CD1a and S100 expression in skin Langerhans cells in patients with breast cancer. J. Pathol. 1991;163:25–30. doi: 10.1002/path.1711630106. [DOI] [PubMed] [Google Scholar]
- Coventry B.J., Austyn J.M., Chryssidis S., Hankins D.A., Harris A. Identification and isolation of CD1a positive putative tumor infiltrating dendritic cells in human breast cancer. Adv. Exp. Med. Biol. 1997;417:571–577. doi: 10.1007/978-1-4757-9966-8_92. [DOI] [PubMed] [Google Scholar]
- Hillebrand E.E., Neville A.M., Coventry B.J. Immunohistochemical localization of CD1a-positive putative dendritic cells in human breast tumors. Br. J. Cancer. 1999;79:940–947. doi: 10.1038/sj.bjc.6690150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint-Vis B., Vincent J., Vandenabeele S., Vanbervliet B., Pin J.J., Ait-Yahia S., Patel S., Mattei M.G., Banchereau J., Zurawski S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Reider D., Heuer M., Ebner S., Kampgen E., Eibl B., Niederwieser D., Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- Palucka K.A., Taquet N., Sanchez-Chapui F., Gluckman J.C. Dendritic cells as the terminal stage of monocyte differentiation. J. Immunol. 1998;160:4587–4596. [PubMed] [Google Scholar]
- Valladeau J., Duvert-Frances V., Dezutter-Dambuyant C., Vincet C., Pin J., Massacrier C., Vincent J., Davoust J., Saeland S. A monoclonal antibody against Langerin, a protein specific of Langerhans cells, is internalized in coated pits and Birbeck granule J. Leukoc. Biol. Suppl. 2 1998. A35(Abstr.) [Google Scholar]
- Woodruff J.J., Katz M., Lucas L.E., Stamper H., Jr. An in vitro model of lymphocyte homing. II. Membrane and cytoplasmic events involved in lymphocyte adherence to specialized high-endothelial venules of lymph nodes. J. Immunol. 1977;119:1603–1610. [PubMed] [Google Scholar]
- Greaves D., Wang W., Dairaghi D., Dieu M., Saint-Vis B., Franz-bacon K., Rossi D., Caux C., McClanahan T., Gordon S. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3α and is highly expressed in human dendritic cells. J. Exp. Med. 1997;186:837–844. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu M.C., Vanderbilt B., Vicari A., Bridon J.M., Oldham E., Ait-Yahia S., Briere F., Zlotnik A., Lebecque S., Caux C. Selective recruitment of immature and mature dendritic cells by distinctive chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartmentdownregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre P., Turley S.J., Gatti E., Hull M., Meltzer J., Mirza A., Inaba K., Steinman R.M., Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Winzler C., Rovere P., Rescigno M., Granucci F., Penna G., Adorini L., Zimmermann V.S., Davoust J., Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor–dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C., Church D., Meyer A., Alouani S., Proudfoot A., Clark-Lewis I., Sozzani S., Mantovani A., Wells T. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3α from lung dendritic cells. J. Exp. Med. 1997;186:825–835. doi: 10.1084/jem.186.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Schaerli P., Loetscher P., Schaniel C., Lenig D., Mackay C., Qin S., Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sozzani S., Allavena P., D'Amico G., Luini W., Bianchi G., Kataura M., Imai T., Yoshie O., Bonecchi R., Mantovani A. Differential regulation of chemokine receptors during dendritic cell maturationa model for their trafficking properties. J. Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]