Abstract
Asthma exacerbations, many of which are virus induced, are associated with airway eosinophilia. This may reflect altered inflammatory response to viruses in atopic individuals. Inhibitory M2 muscarinic receptors (M2Rs) on the airway parasympathetic nerves limit acetylcholine release. Both viral infection and inhalational antigen challenge cause M2R dysfunction, leading to airway hyperresponsiveness. In antigen-challenged, but not virus-infected guinea pigs, M2R dysfunction is due to blockade of the receptors by the endogenous antagonist eosinophil major basic protein (MBP). We hypothesized that sensitization to a nonviral antigen before viral infection alters the inflammatory response to viral infection, so that M2R dysfunction and hyperreactivity are eosinophil mediated. Guinea pigs were sensitized to ovalbumin intraperitoneally, and 3 wk later were infected with parainfluenza. In sensitized, but not in nonsensitized animals, virus-induced hyperresponsiveness and M2R dysfunction were blocked by depletion of eosinophils with antibody to interleukin (IL)-5 or treatment with antibody to MBP. An additional and unexpected finding was that sensitization to ovalbumin caused a marked (80%) reduction in the viral content of the lungs. This was reversed by the antibody to IL-5, implicating a role for eosinophils in viral immunity.
Keywords: eosinophils, muscarinic receptors, parainfluenza virus, antigen challenge, viral immunity
Viruses are a significant cause of asthma exacerbations in adults and children 1. Although a variety of mechanisms may contribute to virus-induced asthma exacerbation, it is known that the neural control of the airways is markedly abnormal during viral infection. In normal subjects, viral infection causes airway hyperresponsiveness that is vagally mediated and is blocked by anticholinergics 2.
In the lungs, parasympathetic nerves provide the dominant autonomic control of airway smooth muscle 3. Release of acetylcholine onto M3 muscarinic receptors located on the airway smooth muscle causes bronchoconstriction 4. Under normal physiological conditions, release of acetylcholine from parasympathetic nerves is inhibited by prejunctional M2 muscarinic receptors (M2Rs)1 on the nerves 5. Stimulation of these receptors with acetylcholine or with muscarinic agonists such as pilocarpine decreases vagally induced bronchoconstriction via inhibition of acetylcholine release 5. Conversely, loss of M2R function or blockade of these neuronal receptors with antagonists leads to increased release of acetylcholine and increased vagally induced bronchoconstriction 6.
In antigen-challenged guinea pigs, hyperresponsiveness is due to loss of neuronal M2R function. Loss of M2R function is mediated by eosinophils, since depletion of eosinophils 7 or inhibition of eosinophil migration into the lungs 6 prevents loss of M2R function. Eosinophils release major basic protein (MBP), which is an endogenous allosteric antagonist for M2Rs 8. Heparin, which binds MBP, acutely restores M2R function and rapidly reverses airway hyperreactivity in antigen-challenged animals 9. In addition, an antibody to guinea pig eosinophil MBP (AbMBP) prevents M2R dysfunction and associated hyperreactivity, demonstrating that it is eosinophil MBP that is blocking neuronal M2Rs and causing hyperreactivity in antigen-challenged guinea pigs 10.
In contrast, virus-induced loss of M2R function is not mediated by eosinophils. Eosinophils are not typically increased in the bronchoalveolar lavage of virus-infected animals. Furthermore, heparin, which restores M2R function in antigen-challenged guinea pigs, has no effect in virus-infected guinea pigs 11.
In animals sensitized to a nonviral protein before viral infection, the inflammatory response to viral infection is switched to involve eosinophils. Coyle et al. have shown that, in mice, virus-specific CD8+ T cells respond to viral protein by generating IL-2 and INF-γ, leading to an influx of neutrophils and mononuclear cells into the airways 12. In contrast, virus-specific CD8+ T cells from animals sensitized to a nonviral protein, OVA, release large amounts of IL-4 and IL-5 in response to the same viral stimulus. In vivo, sensitization of mice leads to an eosinophilic response to viral antigen in the lung.
Since many asthmatics are atopic, their inflammatory response after viral infection might be altered in a similar manner. The experiments described in this paper were designed to test whether eosinophils and eosinophil MBP are responsible for virus-induced hyperreactivity and loss of M2R function in animals sensitized to OVA, but not subsequently challenged with OVA inhalation.
Materials and Methods
Animals.
Specific pathogen-free female Dunkin-Hartley guinea pigs (180–300 g; Hilltop Lab Animals) were used. All animals were shipped in filtered crates and kept in high-efficiency particulate-filtered air. Guinea pigs were fed a normal diet (Prolab; Agway) and were handled in accordance with the standards established by the United States Animal Welfare Acts set forth in National Institutes of Health guidelines and the “Policy and Procedures Manual” published by the Johns Hopkins University School of Hygiene and Public Health Animal Care and Use Committee.
Study Protocol.
Animals were sensitized to OVA by intraperitoneal injection on days 1, 3, and 5 (see sensitization below). 3 wk later, animals were exposed to either parainfluenza virus or control medium via nasal instillation (see viral infection below). Some animals were treated with AbMBPs on the day of infection and 2 d later. Another group of animals was treated with antibody to IL-5 (AbIL5), 5 and 3 d before infection. All studies of airway function, inflammatory responses, and lung viral content were done 4 d after infection.
Sensitization.
Pathogen-free guinea pigs were sensitized to OVA. Animals were injected intraperitoneally with 10 mg·kg−1 OVA (0.3 ml) every other day for a total of three injections. Nonsensitized, pathogen-free guinea pigs were used as controls. Sensitization to OVA was confirmed 21 d later by intravenously injecting 1.0 ml of 2.5% OVA randomly into a few animals from each group. This dose caused acute, lethal anaphylaxis in all groups of sensitized guinea pigs, but had no effect in nonsensitized guinea pigs.
Viral Infection.
Parainfluenza type 1 (Sendai virus, VR-105; American Type Culture Collection) was grown in rhesus monkey kidney cell monolayers in L-15 medium for 1 wk at 34°C. Cells and medium were frozen and thawed, cleared by low-speed centrifugation, and stored in aliquots at −70°C. Animals were anesthetized intramuscularly with ketamine (30 mg·kg−1) and xylazine (5 mg·kg−1), and were inoculated intranasally with 0.5 ml of virus stock diluted in Hank's PBS to produce a solution containing a 105 tissue culture ID50 (TCID50) ml−1 (105 times the concentration required to produce infection in 50% of rhesus monkey kidney monolayers). Infected and noninfected animals were housed in separate laminar flow rooms.
Pretreatments.
Some animals from both the sensitized virus-infected and nonsensitized virus-infected groups were given 240 μl·kg−1 of AbIL5 intraperitoneally 3 and 5 d before infection. Some of the sensitized virus-infected guinea pigs were pretreated intraperitoneally with AbMBP 13 1.5 ml immediately before infection, and again 2 d after infection.
Measurements of Pulmonary Inflation Pressure.
Experiments were conducted 4 d after viral infection. The guinea pigs were anesthetized intraperitoneally with urethane (1.5 g·kg−1). This dose produces a deep anesthesia lasting 8–10 h, although none of these experiments lasted longer than 4 h. Both jugular veins were cannulated for the administration of drugs. One internal carotid artery was cannulated for measurement of blood pressure using a DTX™ pressure transducer (Viggo-Spectramed), and the heart rate was derived from the blood pressure tracing using a tachograph. The trachea was cannulated, and the animals were ventilated with a positive pressure, constant volume rodent respirator (Harvard Apparatus, Inc.) at a tidal volume of 10 ml·kg−1 and a respiratory rate of 100 breaths·min−1. The animals were paralyzed by intravenously infusing succinylcholine (10 μg·kg−1·min−1). All animals were pretreated intraperitoneally with guanethidine (10 mg·kg−1) to deplete norepinephrine. Pulmonary inflation pressure (P pi) was measured at the trachea using a DTX™ pressure transducer. All signals were recorded on a polygraph (Grass Instrument Co.). Bronchoconstriction was measured as the increase in P pi above the basal inflation pressure produced by the ventilator. The sensitivity of the method was increased by taking the output P pi signal from the driver of one channel to the input of the preamplifier of a different channel on the polygraph. Thus, baseline P pi was recorded on one channel and increases in P pi above the baseline were recorded on a separate channel. With this method, increases in P pi as small as 2–3 mm H2O can be accurately recorded.
Studies of Vagal Hyperresponsiveness.
Both vagus nerves were cut, and the distal ends were placed on shielded electrodes immersed in liquid paraffin. Electrical stimulation of both vagus nerves produced bronchoconstriction and bradycardia. The vagus nerves were stimulated at frequencies ranging 2.0–25.0 Hz for 5 s at 120-s intervals, keeping both pulse duration (0.1 ms) and voltage (10.0 V) constant between groups. At the end of each experiment, heparin (2,000 U) was given intravenously to both sensitized virus-infected and nonsensitized virus-infected animals. After 15–20 min, the response to vagal stimulation was remeasured as above. After the completion of each experiment, atropine (1 mg·kg−1) was administered intravenously to confirm that bronchoconstrictions were due to cholinergic nerve stimulation. Changes in P pi were recorded on a Grass polygraph as described above.
Studies of Neuronal M2R Function.
30 min after administering guanethidine, baseline responses to electrical stimulation of the vagus nerves were obtained. Both vagus nerves were simultaneously stimulated at 1-min intervals (2 Hz, 0.2 ms, 2.5–30 V, 44 pulses per train). The function of autoreceptors is frequency dependent. Stimulation of M2Rs by endogenous acetylcholine is greatest at high frequencies of stimulation. Therefore, the ability of exogenous agonists to inhibit vagally induced bronchoconstriction via stimulation of M2Rs is more readily apparent at low frequencies of stimulation 5. Thus, the studies with the agonist pilocarpine were carried out at 2 Hz. The voltage was chosen at the beginning of each experiment (within a range of 2.5–30.0 V; mean 9.11 ± 0.92 V) to give an increase in P pi of ∼20 mmH2O (21.4 ± 0.75 mmH2O). Cumulative doses of pilocarpine (1–100 μg·kg−1) were administered intravenously, and the effect on vagally induced bronchoconstriction was measured. 30–100 μg·kg−1 of pilocarpine produced a small, transient bronchoconstriction. Therefore, the effect of these doses of pilocarpine on vagally induced bronchoconstriction was measured after the P pi had returned to baseline. At the end of each experiment, heparin (2,000 U) was given intravenously in both the sensitized virus-infected and nonsensitized virus-infected guinea pigs. After 15–20 min, the response to vagal stimulation was remeasured as above. After the completion of each experiment, atropine (1 mg·kg−1) was given intravenously to confirm that bronchoconstrictions were due to vagal stimulation. The results are expressed as a ratio of bronchoconstriction in the presence of pilocarpine to the bronchoconstriction in the absence of pilocarpine. Thus, a ratio <1 would indicate that pilocarpine was inhibiting vagally induced bronchoconstriction.
Studies of Postjunctional Muscarinic Receptor Function.
The function of muscarinic receptors on airway smooth muscle was tested in vagotomized guinea pigs by measuring bronchoconstriction in response to increasing intravenous doses of acetylcholine (1–10 μg·kg−1).
Bronchoalveolar Lavage.
At the end of the experiment, bronchoalveolar lavage was performed in situ via the tracheal cannula. The lungs were lavaged with five aliquots of 10.0 ml PBS. The recovered lavage fluid (40–45 ml) was centrifuged (350 g for 7 min). The cells were resuspended in 10 ml of deionized water to remove any erythrocytes before an additional 40 ml of PBS was added. Cells were centrifuged again, the supernatant was poured off, and the cells were resuspended in 10 ml of PBS. Cells were counted using a Neubauer Hemocytometer (Hausser Scientific Co.). Aliquots of the cell suspension were cytospun onto glass slides, stained with Diff-Quik® (Baxter Healthcare Corp.), and counted to obtain differential cell counts.
Virus Isolation and Titration.
Viral infection was confirmed in all guinea pigs that were exposed to parainfluenza virus by infection of rhesus monkey kidney cells with aliquots of lung homogenate from each animal (see Fig. 12). After physiological studies were completed, the guinea pig lungs were removed and stored at −70°C. Frozen samples were thawed, weighed, and homogenized in 2 ml PBS (Polytron™; Brinkmann). Virus was eluted from the tissue homogenate by incubating at 34°C for 1 h. The suspensions were centrifuged at 450 g for 30 min, and the supernatants were inoculated in serial 10-fold dilutions into fresh rhesus monkey kidney cell monolayers. After 1 wk of incubation at 34°C, the monolayers were washed and the medium replaced with a 0.5% suspension of guinea pig erythrocytes in Hank's PBS. After 1 h, the erythrocytes were washed off and the monolayers were examined under an inverted phase–contrast microscope (Olympus Corp.) for evidence of hemadsorption (sticking of erythrocytes to the surface of cells because of expression of viral hemagglutinin on these surfaces). Viral content was determined as the amount of lung homogenate required to produce infection in 50% of rhesus monkey kidney monolayers (the TCID50), and is expressed as TCID50/g lung wet wt. Only data from virus-exposed guinea pigs with confirmed parainfluenza infection are reported.
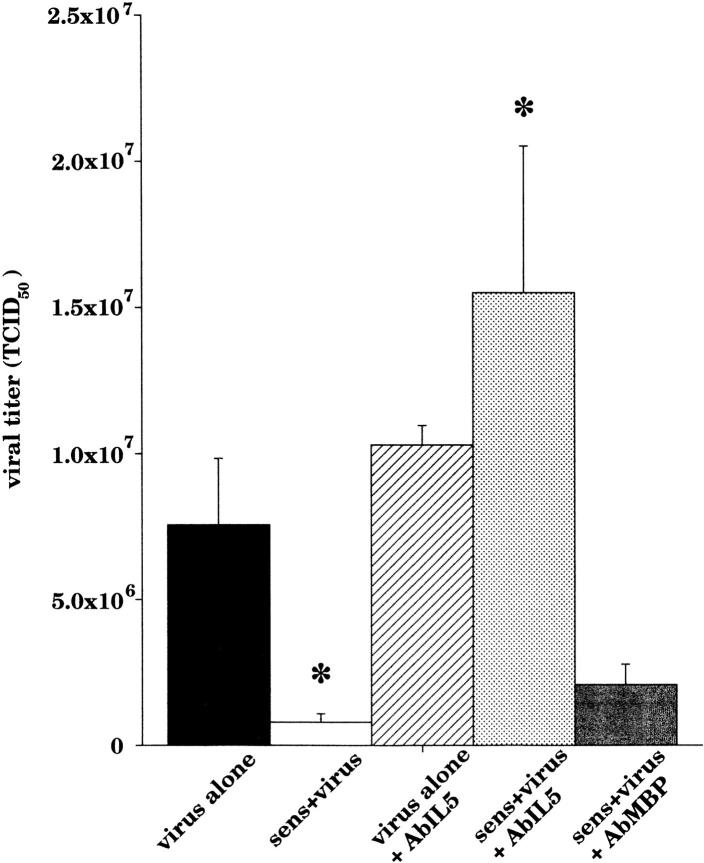
Figure 12.
Viral titers from the lungs of all virus-exposed guinea pigs were quantified. Sensitized virus-infected guinea pigs (white bar, n = 19) had a significant decrease in viral titer compared with nonsensitized virus-infected guinea pigs (black bar, n = 11, P = 0.04). Pretreatment with AbIL5 had no effect on nonsensitized virus-infected guinea pigs (hatched bar, n = 3), but caused a significant increase in recovered viral titers in sensitized virus-infected animals (light gray bar, n = 11, P < 0.0001). Sensitized virus-infected guinea pigs treated with AbMBP (dark gray bar, n = 7) continued to have decreased viral titers compared with untreated, sensitized virus-infected animals.
Drugs and Reagents.
Acetylcholine, atropine, guanethidine, heparin, OVA, pilocarpine, succinylcholine, and urethane were purchased from Sigma Chemical Co. Purified rabbit AbMBP was produced as described previously 13. Purified rat anti–mouse/human AbIL5 (TRFK-5) was purchased from PharMingen. All drugs were dissolved and diluted in 0.9% NaCl or PBS.
Statistics.
All data are expressed as mean ± SEM. Acetylcholine, frequency, and pilocarpine responses were analyzed using two-way analyses of variance for repeated measures. Baseline heart rates, blood pressures, P pi, and changes in P pi (before pilocarpine administration), histological measurements, and bronchoalveolar lavage were analyzed using analysis of variance (Statview 4.5; Abacus Concepts, Inc.). A P value of 0.05 was considered significant.
Results
Baseline Responses.
There were no significant differences among the groups for baseline heart rate ([beats per min] control, 261 ± 3.7; virus-infected, 255 ± 6.8; sensitized, 261 ± 5.7; sensitized virus-infected, 260 ± 4.2; virus-infected with AbIL5, 252.5 ± 11.0; sensitized virus-infected with AbIL5, 268 ± 6.5; and sensitized virus- infected with AbMBP, 270 ± 6.6), systolic blood pressure ([mmHg) control, 51.2 ± 1.7; virus-infected, 47.9 ± 2.3; sensitized, 51.3 ± 2.2; sensitized virus-infected, 49.0 ± 2.3; virus-infected with AbIL5, 42.5 ± 2.5; sensitized virus-infected with AbIL5, 45.6 ± 2.1; and sensitized virus-infected with AbMBP, 47.1 ± 1.5), diastolic blood pressure ([mmHg] control, 29.4 ± 1.5; virus-infected, 28.6 ± 2.1; sensitized, 30.8 ± 1.0; sensitized virus-infected, 29.0 ± 1.3; virus-infected with AbIL5, 28.8 ± 3.1; sensitized virus-infected with AbIL5, 27.8 ± 2.2; and sensitized virus-infected with AbMBP, 33.6 ± 1.8), or animal weight ([kg] control, 0.449 ± 0.02; virus-infected, 0.516 ± 0.03; sensitized, 0.476 ± 0.03; sensitized virus-infected, 0.434 ± 0.02; virus-infected with AbIL5, 0.424 ± 0.02; sensitized virus-infected with AbIL5, 0.461 ± 0.04; and sensitized virus-infected with AbMBP, 0.406 ± 0.02).
A positive pressure of 70–220 mmH2O (mean 128 ± 4.43 mmH2O) was needed to ventilate the animals. Sensitization of pathogen-free guinea pigs did not alter baseline P pi (90.7 ± 4.1, n = 14) compared with nonsensitized controls (92.4 ± 3.6, n = 17). Regardless of sensitization, viral infection increased baseline P pi (sensitized 156.2 ± 8.6, n = 21; nonsensitized 151.0 ± 8.5, n = 10; P < 0.0001). The increased baseline was not prevented by pretreatment with AbIL5 (sensitized 143.3 ± 9.6, n = 9; nonsensitized 143.3 ± 3.3, n = 3), but was attenuated by treatment with AbMBP in the sensitized virus-infected guinea pigs (112 ± 9.6, n = 7; P = 0.0004).
Simultaneous electrical stimulation of both cut vagus nerves at 2 Hz produced bronchoconstriction that was rapidly reversible upon cessation of stimulation. There were no significant differences among the groups for the voltages used (control, 5.8 ± 1.4; virus-infected, 6.8 ± 1.6; sensitized, 10.7 ± 3.4; sensitized virus-infected, 8.9 ± 1.6; virus-infected with AbIL5, 8.9 ± 2.0; sensitized virus-infected with AbIL5, 5.8 ± 1.4; and sensitized virus-infected with AbMBP, 11.6 ± 1.7) or the degree of bronchoconstriction produced ([mmH2O] control, 20.9 ± 0.5; virus-infected, 19.8 ± 1.1; sensitized, 25.0 ± 2.0; sensitized virus-infected, 20.6 ± 1.4; virus-infected with AbIL5, 19.2 ± 2.9; sensitized virus-infected with AbIL5, 23.2 ± 2.0; and sensitized virus-infected with AbMBP, 19.2 ± 2.6).
M2R Function.
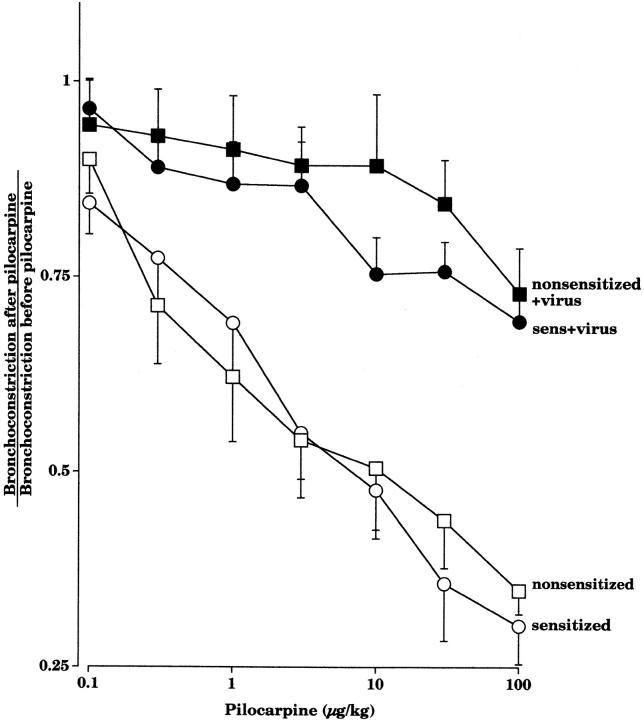
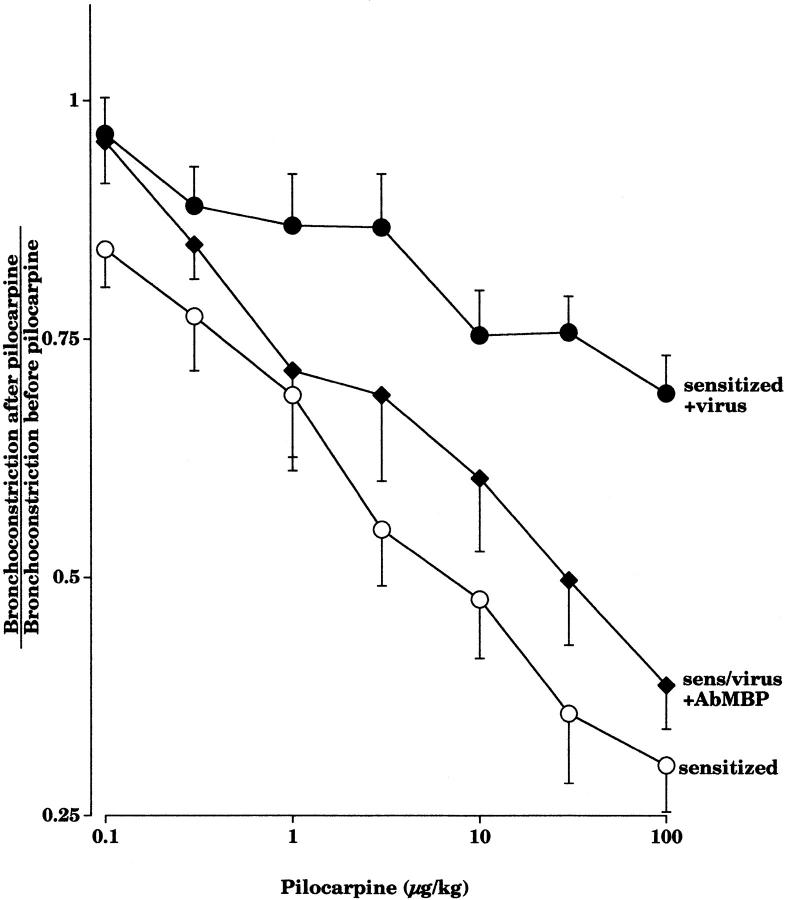
Pilocarpine inhibited vagally induced bronchoconstriction in a dose-dependent manner in pathogen-free guinea pigs, demonstrating that there are functional M2Rs on the parasympathetic nerves (Fig. 1). Sensitization to OVA did not affect the ability of pilocarpine to inhibit vagally induced bronchoconstriction. In virus-infected animals, irrespective of sensitization, pilocarpine no longer inhibited vagally induced bronchoconstriction (Fig. 1). This demonstrates that the neuronal M2Rs were dysfunctional in virus-infected guinea pigs.
Figure 1.
Pilocarpine (1–100 μg·kg−1 intravenous) inhibits vagally induced bronchoconstriction in pathogen-free guinea pigs, whether they are sensitized (open circles, n = 7) or not (open squares, n = 8). In contrast, pilocarpine does not inhibit vagally induced bronchoconstriction in virus-infected animals, whether they are sensitized (filled circles, n = 10) or not (filled squares, n = 7). Results are expressed as the ratio of vagally induced bronchoconstriction in the presence of pilocarpine to the response of vagal stimulation in the absence of pilocarpine. There was a significant difference between the pilocarpine dose–response curves in noninfected versus virus-infected guinea pigs (P < 0.0001). Each point is the mean, with SEM shown by vertical bars.
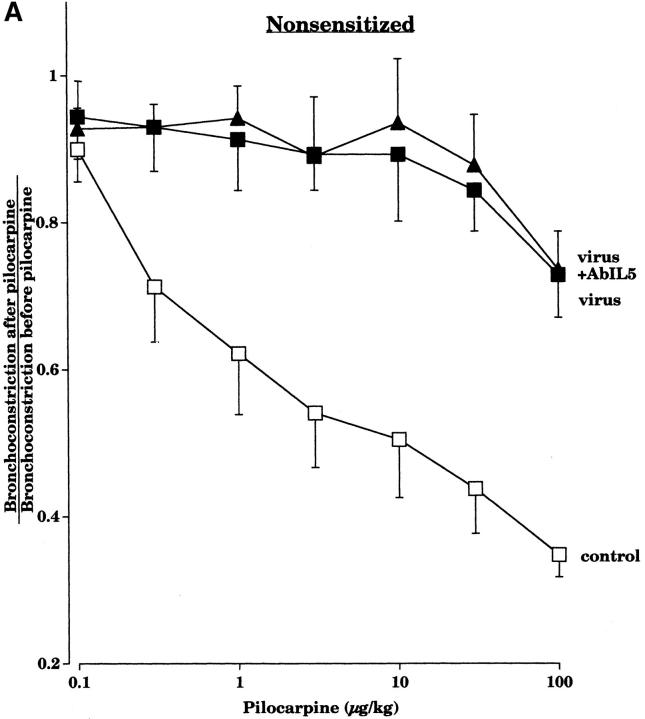
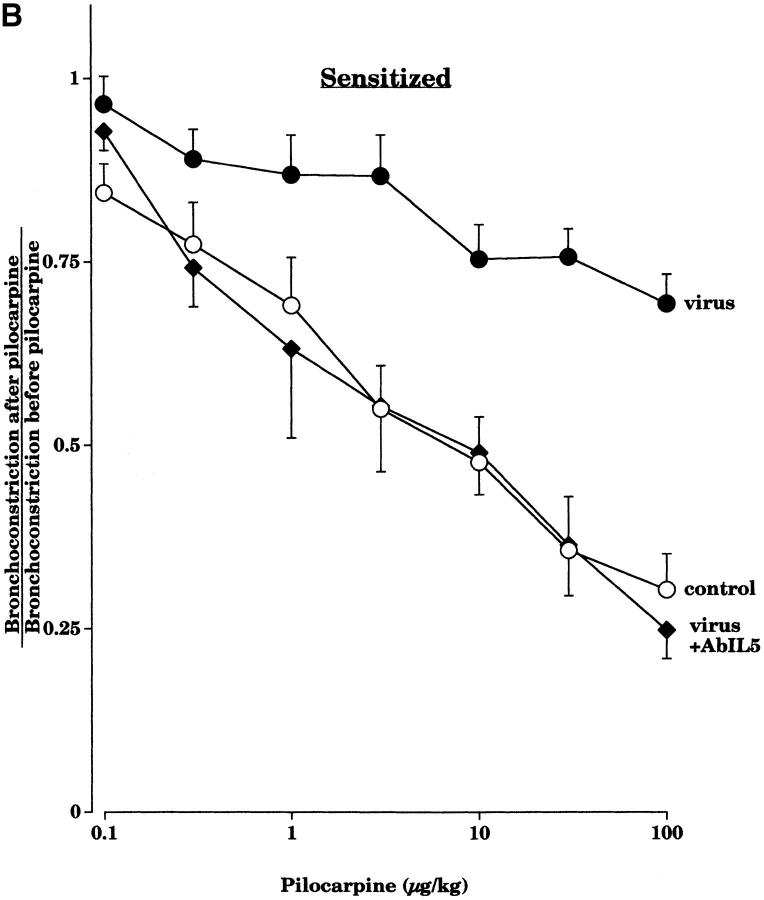
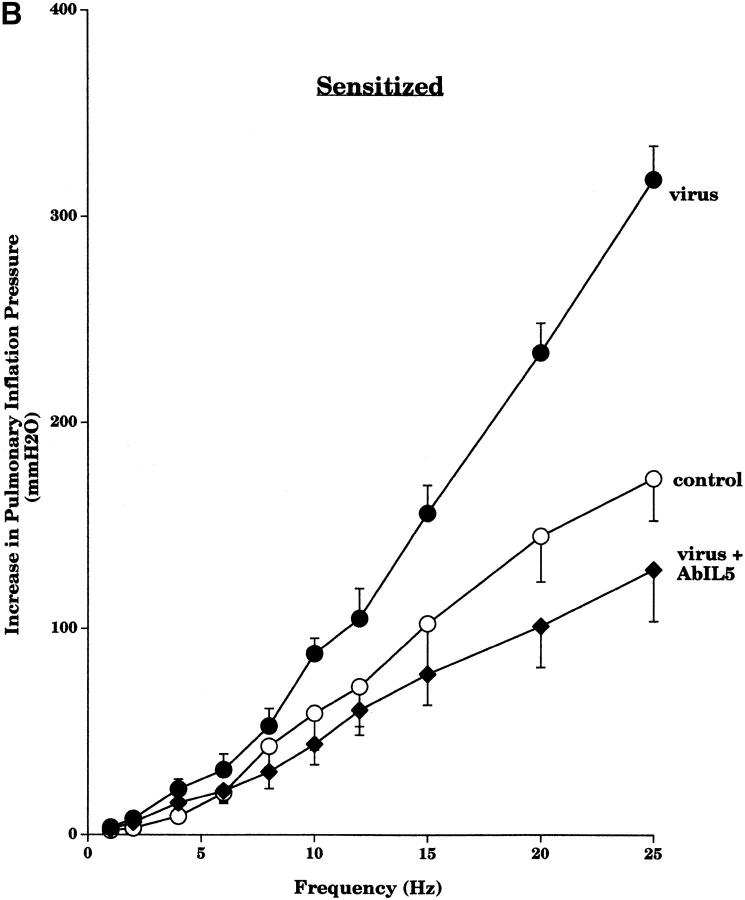
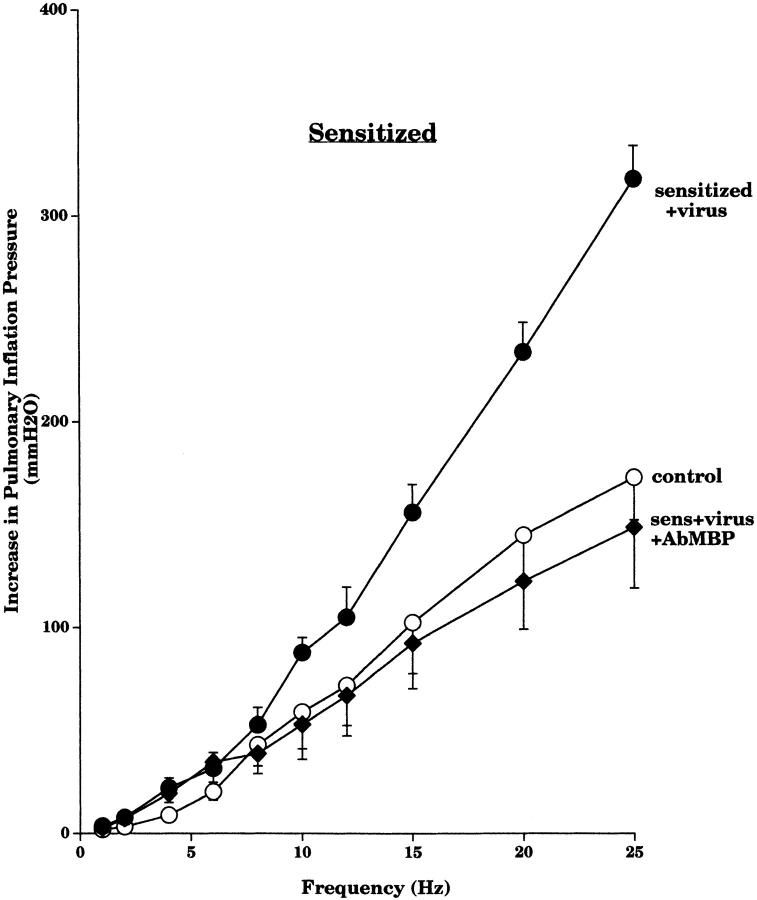
Pretreatment with an AbIL5 did not prevent virus-induced M2R dysfunction in nonsensitized guinea pigs (Fig. 2 A). In contrast, pretreatment with AbIL5 did prevent virus-induced M2R dysfunction in virus-infected guinea pigs sensitized to OVA (Fig. 2 B). Likewise, pretreatment of sensitized guinea pigs with AbMBP before viral infection also protected the function of the neuronal M2Rs (Fig. 3).
Figure 2.
Pretreatment with AbIL5 before viral infection did not protect the ability of pilocarpine to inhibit vagally induced bronchoconstriction in nonsensitized guinea pigs (A). Pilocarpine (1–100 μg·kg−1 intravenous) inhibited vagally induced bronchoconstriction in control animals (open squares, n = 8), but not in virus-infected animals (filled squares, n = 7) or in virus-infected animals pretreated with AbIL5 before infection (filled triangles, n = 5). In contrast, in sensitized guinea pigs, AbIL5 given before viral infection did protect the ability of pilocarpine to inhibit vagally induced bronchoconstriction (B). Pilocarpine (1–100 μg·kg−1 intravenous) inhibited vagally induced bronchoconstriction in sensitized control animals (open circles, n = 7), but not in sensitized virus-infected animals (filled circles, n = 10). However, pilocarpine did inhibit vagally induced bronchoconstriction in sensitized virus-infected animals pretreated with AbIL5 (filled diamonds, n = 5, P = 0.0006). The control and virus-infected data of Figs. A and B are the same as shown in Fig. 1. Each point is the mean, with SEM shown by vertical bars.
Figure 3.
Administration of AbMBP before viral infection of sensitized animals protected the ability of pilocarpine to inhibit vagally induced bronchoconstriction. Pilocarpine (1–100 μg·kg−1 intravenous) inhibited vagally induced bronchoconstriction in sensitized control animals (open circles, n = 7), but not in sensitized virus-infected animals (filled circles, n = 10), unless they were pretreated with AbMBP (filled diamonds, n = 5; P = 0.005). Each point is the mean, with SEM shown by vertical bars.
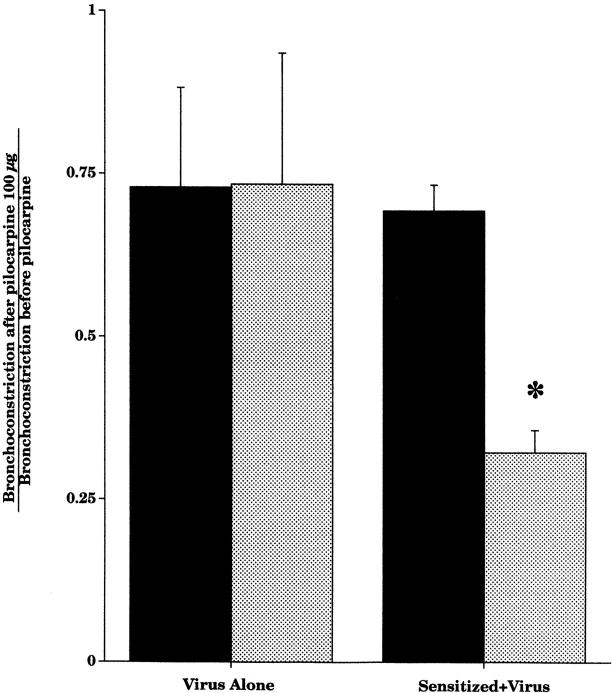
Heparin was administered after the maximal dose of pilocarpine had been given. Heparin did not inhibit vagally induced bronchoconstriction in the nonsensitized virus-infected guinea pigs (Fig. 4). However, in sensitized virus-infected guinea pigs, heparin did significantly inhibit vagally induced bronchoconstriction.
Figure 4.
Pilocarpine (100 μg·kg−1 intravenous) did not inhibit vagally induced bronchoconstriction in virus-infected animals, whether they were sensitized or not (black bars). Heparin (2,000 IU, intravenous) restored pilocarpine's ability to inhibit vagally induced bronchoconstriction in virus-infected animals with prior sensitization (n = 7, P = 0.0001), but not in nonsensitized virus-infected animals (n = 5, gray bars). Each point is the mean, with SEM shown by vertical bars.
Vagal Hyperreactivity in Sensitized and Nonsensitized Virus-infected Animals.
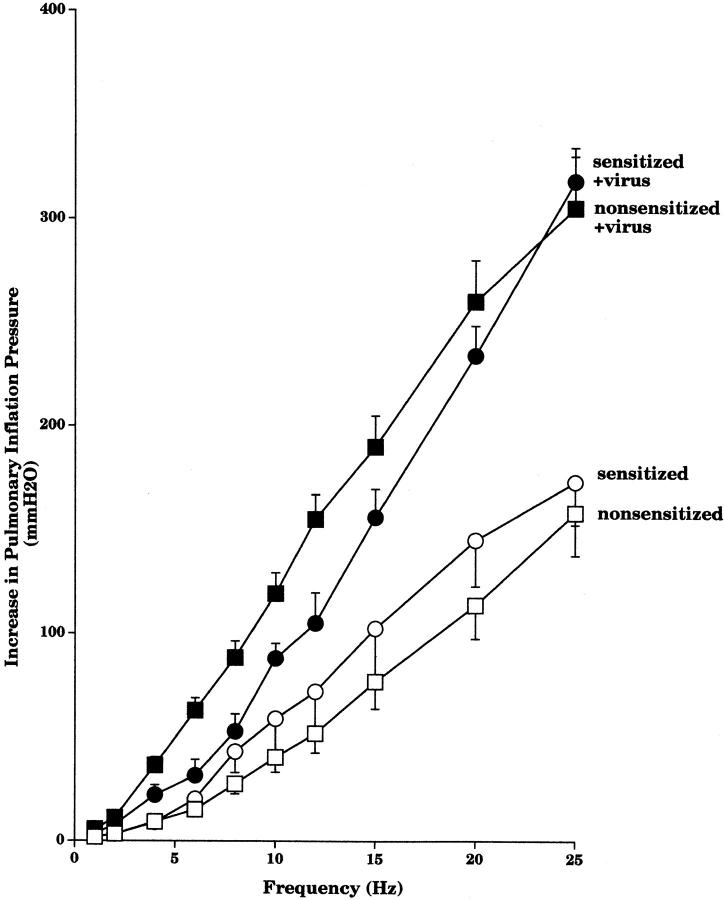
Simultaneous electrical stimulation of both cut vagus nerves (1–25 Hz) caused frequency-dependent bronchoconstriction. There was no difference between vagally induced bronchoconstriction in the uninfected sensitized and nonsensitized guinea pigs (Fig. 5). In contrast, after viral infection, vagally induced bronchoconstriction in both sensitized and nonsensitized animals was significantly greater than in their respective controls. This difference was not apparent at lower frequencies of stimulation, but became greater with increasing frequencies. Vagally induced bronchoconstriction was not significantly different between the sensitized and nonsensitized virus-infected groups.
Figure 5.
Simultaneous electrical stimulation of both cut vagus nerves (2.0–25 Hz, 10.0 V, 0.1 ms, 5 s) produced frequency-dependent bronchoconstriction measured as an increase in P pi. Viral infection significantly potentiated vagally induced bronchoconstriction in both sensitized (filled circles, n = 5) and nonsensitized (filled squares, n = 6) animals compared with their respective controls (open circles, n = 6; and open squares, n = 8; P < 0.0001). Results are expressed as the mean increase in P pi (mmH2O). Each point is the mean, with SEM shown by vertical bars.
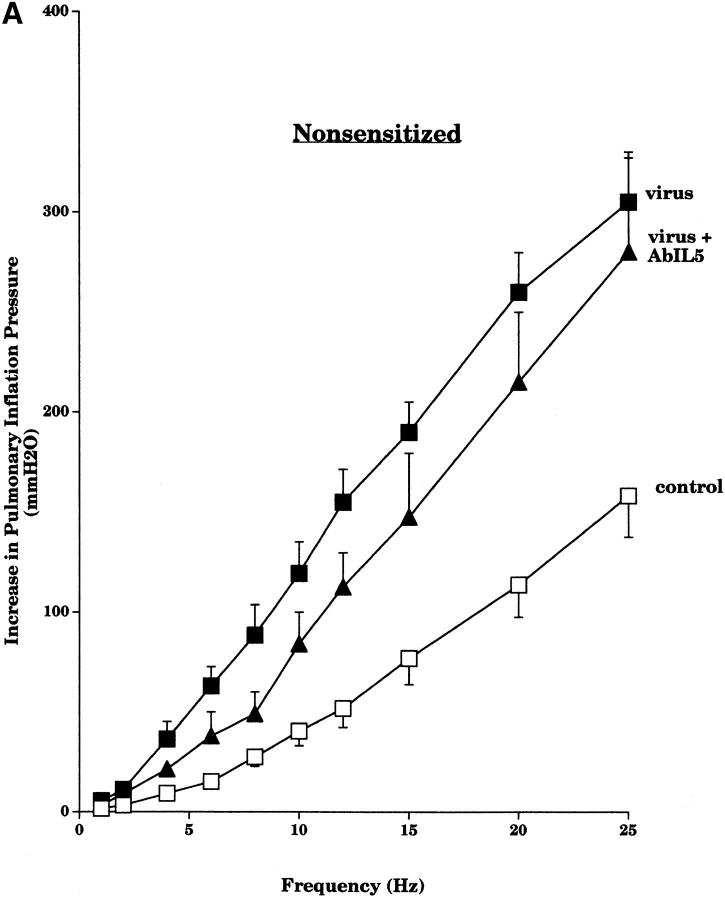
Pretreatment with AbIL5 did not prevent virus-induced vagal hyperreactivity in the nonsensitized guinea pigs (Fig. 6 A). In contrast, pretreatment with AbIL5 did prevent virus-induced vagal hyperreactivity in guinea pigs sensitized to OVA (Fig. 6 B).
Figure 6.
Pretreatment of nonsensitized animals with AbIL5 did not prevent virus-induced vagal hyperreactivity (A). Vagally induced bronchoconstriction in both nonsensitized virus-infected animals (filled squares, n = 6) and nonsensitized virus-infected animals pretreated with AbIL5 (filled triangles, n = 5) remained greater than in nonsensitized control animals (open squares, n = 8). In contrast, pretreatment of sensitized animals with AbIL5 did prevent virus-induced vagal hyperreactivity in sensitized animals (B). Vagally induced bronchoconstriction in sensitized virus-infected animals (filled circles, n = 5) was greater than in sensitized control animals (open circles, n = 6). Vagally induced bronchoconstriction in sensitized virus-infected animals pretreated with AbIL5 (filled diamonds, n = 5) was significantly decreased (P = 0.004) to become similar to sensitized controls. Results are expressed as the mean increase in P pi (mmH2O). Virus-infected and control data are the same as shown in Fig. 5. Each point is the mean, with SEM shown by vertical bars.
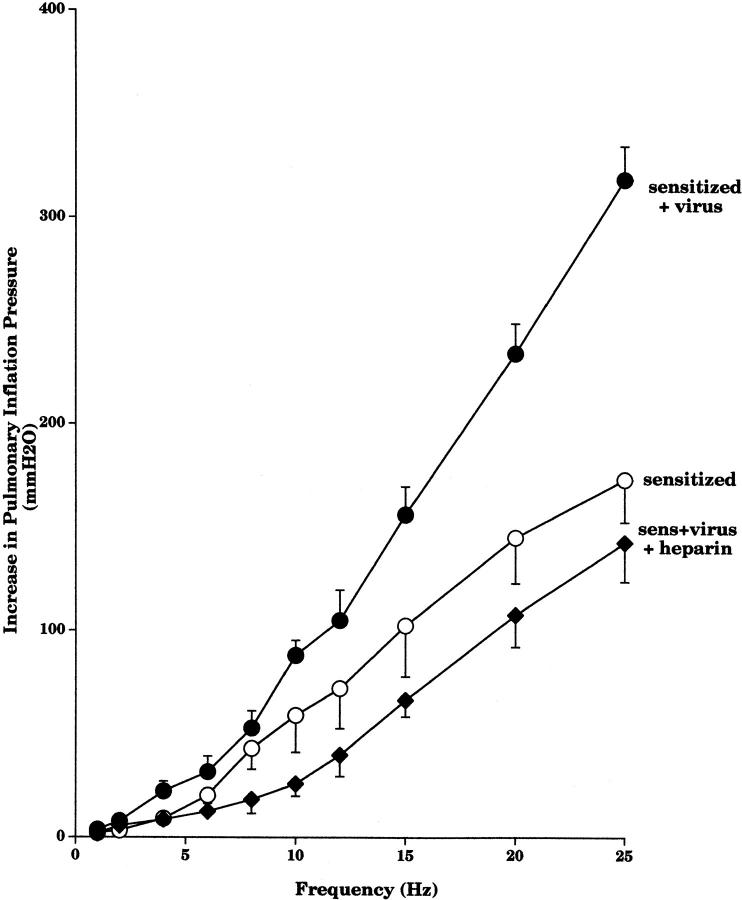
Pretreatment of the sensitized guinea pigs with AbMBP before viral infection prevented virus-induced vagal hyperreactivity in the sensitized guinea pigs (Fig. 7). Likewise, administration of heparin 30 min before testing vagal responses reversed virus-induced hyperreactivity to stimulation of the vagus nerves in sensitized animals (Fig. 8).
Figure 7.
Pretreatment with AbMBP prevented virus-induced vagal hyperreactivity in sensitized animals. Vagally induced bronchoconstriction in sensitized virus-infected animals (filled circles, n = 5) was greater than in sensitized control animals (open circles, n = 6). Vagally induced bronchoconstriction in sensitized virus-infected animals pretreated with AbMBP (filled diamonds, n = 5) was significantly decreased (P = 0.02) to become similar to sensitized control animals. Results are expressed as the mean increase in P pi (mmH2O). Each point is the mean, with SEM shown by vertical bars.
Figure 8.
Heparin reversed virus-induced vagal hyperreactivity in sensitized animals. Vagally induced bronchoconstriction in sensitized virus-infected animals (filled circles, n = 5) was significantly higher (P < 0.0001) than in sensitized control animals (open circles, n = 6). Vagally induced bronchoconstriction in sensitized virus-infected animals given heparin (filled diamonds, n = 5) was significantly decreased (P = 0.0004) to become similar to sensitized control animals. Virus-infected and control data are the same as shown in Fig. 5. Results are expressed as the mean increase in P pi (mmH2O). Each point is the mean, with SEM shown by vertical bars.
Smooth Muscle Responsiveness in Sensitized and Nonsensitized Virus-infected Animals.
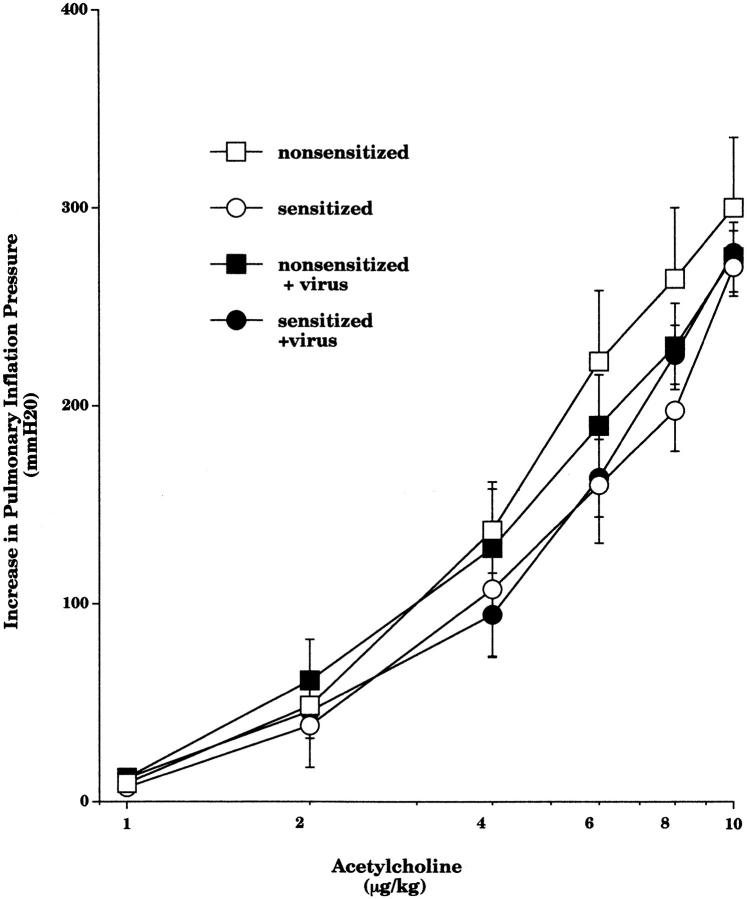
In vagotomized guinea pigs, intravenous acetylcholine (2–10 μg·kg−1) caused dose-dependent bronchoconstriction. Acetylcholine-induced bronchoconstriction was not affected by sensitization, viral infection (Fig. 9), or by any of the treatments (data not shown).
Figure 9.
Intravenous injection of acetylcholine (1–10 μg/kg) produced dose-dependent bronchoconstriction, measured as an increase in P pi. Acetylcholine dose–response measurements in nonsensitized controls (open squares, n = 7), sensitized controls (open circles, n = 5), nonsensitized virus-infected (filled squares, n = 7), and sensitized virus-infected guinea pigs (filled circles, n = 8) were not significantly different. Each point is the mean, with SEM shown by vertical bars.
Effect of Sensitization and Virus Infection on Inflammatory Cells Recovered in Lung Lavage.
The total number of cells recovered by lung lavage at the end of each experiment was increased by viral infection regardless of whether the animals were sensitized (Fig. 10). This increase was comprised of macrophages and neutrophils. Lymphocyte numbers were not significantly affected by either sensitization or viral infection.
Figure 10.
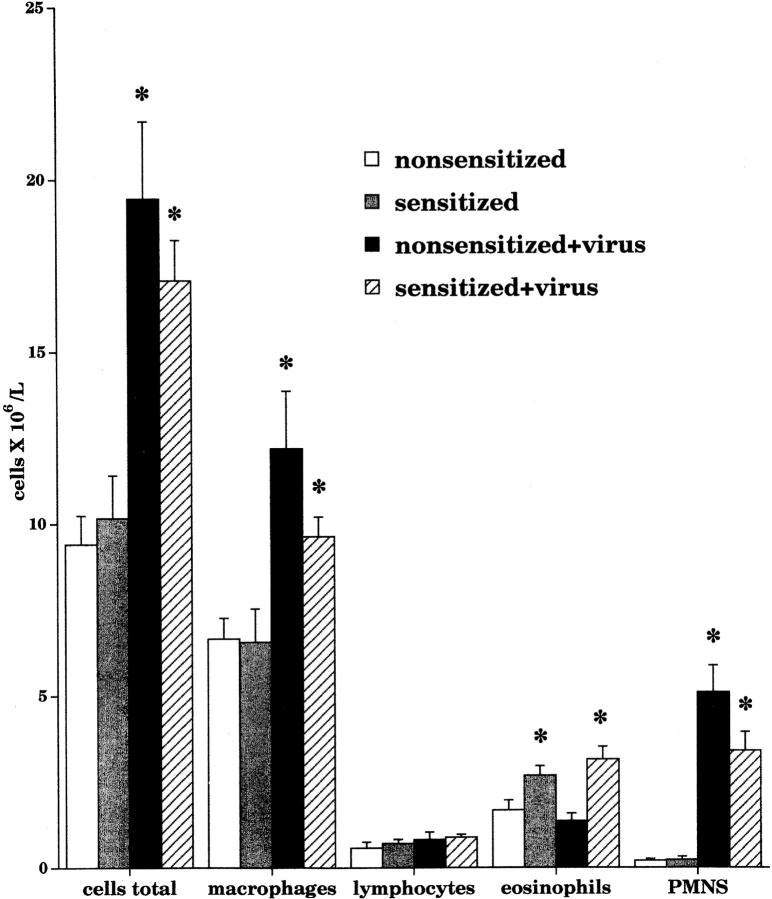
Airway leukocyte populations were measured in bronchoalveolar lavage. There was a significant increase (P = 0.0001) in the total inflammatory cell number after viral infection of both nonsensitized (black bar, n = 10) and sensitized (hatched bar, n = 16) animals compared with their respective controls (white and gray bars, n = 9–11). This increase consisted of macrophages and neutrophils. Regardless of viral infection, there was a significant increase in the number of eosinophils in sensitized animals (gray bar, P = 0.0295; and hatched bar, P = 0.0004). Data are expressed as the means of total cells recovered by lavage. Each point is the mean, with SEM shown by vertical bars.
Sensitization significantly increased the number of eosinophils recovered in the lavage fluid (white and gray bars; Fig. 10). This increase with sensitization was not further potentiated by viral infection (compare gray with hatched bars). Viral infection did not increase the number of eosinophils recovered in the lavage fluid in nonsensitized guinea pigs (compare white with black bars).
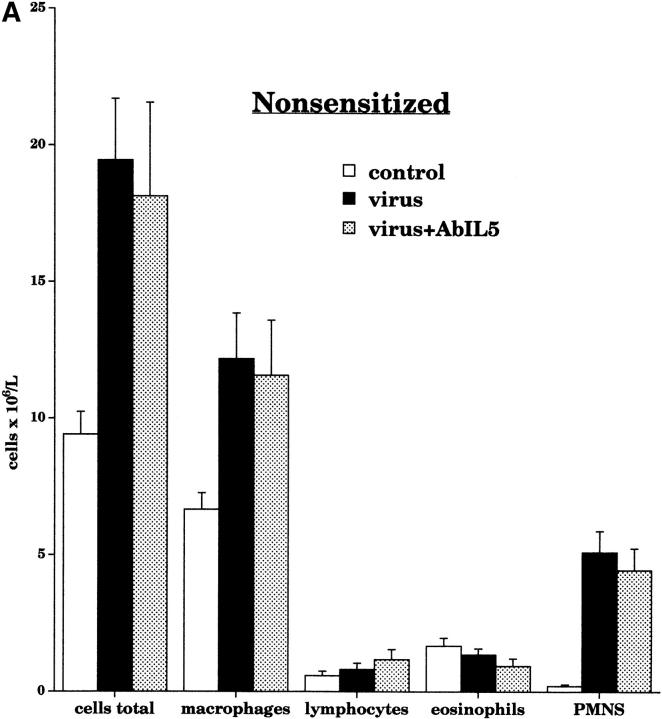
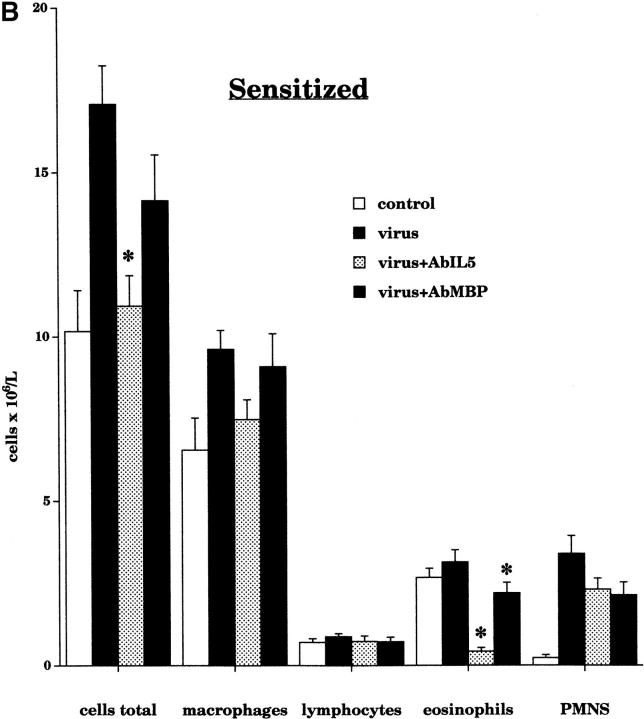
Treatment with AbIL5 not did not change leukocyte numbers in the lavage fluid of nonsensitized virus-infected guinea pigs (Fig. 11 A.) In contrast, the total number of inflammatory cells in the sensitized virus-infected animals was decreased by AbIL5 (Fig. 11 B). This decrease was due to a significant decrease in eosinophils. Pretreatment with AbMBP caused a small but statistically significant decrease in the number of eosinophils recovered from the airways (Fig. 11 B). Neither AbIL5 nor AbMBP significantly altered the numbers of lymphocytes recovered in the lavage fluid.
Figure 11.
Airway leukocyte populations were measured in bronchoalveolar lavage after pretreatment with AbIL5 and AbMBP. In nonsensitized virus-infected animals (black bars, n = 10), pretreatment with AbIL5 (gray bars, n = 5) did not change any of the leukocyte numbers (A). In contrast, pretreatment of sensitized virus-infected animals with AbIL5 (gray bars, n = 8) significantly decreased the number of eosinophils compared with sensitized virus-infected alone (black bars; n = 16, P < 0.0001) (B). Pretreatment of sensitized virus-infected animals with AbMBP (dark gray bars, n = 7) caused a slight but statistically significantly decrease in eosinophil numbers compared with sensitized virus-infected animals alone (black bars; n = 16, P = 0.04). In both AbIL5- and AbMBP-pretreated animals, neutrophil cell numbers were unaltered. Total leukocyte numbers were significantly decreased in sensitized virus-infected animals treated with AbIL5 (light gray bar) compared with sensitized virus-infected animals alone (black bar, P = 0.0025). Data are expressed as the means of total cells recovered by lavage. Each point is the mean, with SEM shown by vertical bars.
Effect of Sensitization on Viral Immunity.
Infectious virus was quantified in all guinea pigs that were exposed to parainfluenza virus (Fig. 12). Viral content of the lungs was significantly decreased in sensitized guinea pigs compared with nonsensitized virus-infected guinea pigs. Pretreatment with AbIL5 did not alter viral content of nonsensitized virus-infected guinea pigs. In contrast, there was a significant increase in the viral content of AbIL5-treated, sensitized virus-infected animals. Sensitized virus-infected animals treated with AbMBP did not have any significant change in the viral content. It is important to note that these results were collected from all of the infected animals, but the actual titer measurements were performed on separate days over a 4-mo period.
Discussion
The experiments described in this paper were designed to test whether the mechanism of M2R dysfunction and associated airway hyperreactivity in virus-infected guinea pigs differs between sensitized and nonsensitized animals. In control guinea pigs, the muscarinic agonist pilocarpine inhibited vagally induced bronchoconstriction in a dose-dependent manner, confirming that neuronal M2Rs were functioning to inhibit release of acetylcholine 5. Similarly, pilocarpine also inhibited vagally induced bronchoconstriction in the guinea pigs sensitized to OVA, confirming that sensitization alone does not inhibit neuronal M2R function 14. Likewise, sensitization does not induce vagal hyperreactivity, since vagally induced bronchoconstriction was also similar between sensitized and nonsensitized guinea pigs.
In contrast, viral infection of both the sensitized and nonsensitized guinea pigs caused loss of M2R function, since pilocarpine no longer inhibited vagally induced bronchoconstriction (Fig. 1). Viral infection also induced hyperreactivity in both the sensitized and nonsensitized guinea pigs, since vagal nerve stimulation was potentiated in both compared with their respective noninfected controls (Fig. 5). The degree of vagal hyperreactivity was similar in the sensitized and nonsensitized virus-infected animals. This virus-induced hyperreactivity was mediated by the parasympathetic nerves and not by a change in the responsiveness of airway smooth muscle, since bronchoconstriction induced by intravenous acetylcholine was not different among the groups (Fig. 9). Thus, viral infection causes loss of M2R function and vagal hyperreactivity, regardless of sensitization status.
In antigen-challenged guinea pigs, hyperreactivity to electrical stimulation of the vagus nerves as well as to intravenous histamine is mediated entirely by loss of neuronal M2R function 15. We have demonstrated that bronchoconstriction in response to stimulation of the vagus nerves is potentiated by viral infection in both sensitized and nonsensitized guinea pigs. Since the responsiveness of airway smooth muscle is not changed by viral infection or by sensitization, virus-induced hyperreactivity to vagal nerve stimulation results from increased release of acetylcholine due to loss of neuronal M2R function.
Eosinophils, through release of MBP, mediate loss of M2R function in antigen-challenged guinea pigs 10. In contrast, we have shown that virus-induced M2R dysfunction and vagal hyperreactivity in nonsensitized guinea pigs are not mediated by eosinophils. Depletion of eosinophils with AbIL5 did not prevent virus-induced M2R dysfunction or vagal hyperreactivity (Fig. 2 A and 6 A). Furthermore, heparin, which binds and neutralizes eosinophil MBP, did not reverse virus-induced M2R dysfunction or vagal hyperreactivity (Fig. 4). Thus, virus-induced loss of M2R function and hyperreactivity in nonsensitized guinea pigs is not mediated by eosinophils.
In contrast, virus-induced loss of M2R dysfunction and vagal hyperreactivity in sensitized guinea pigs is mediated by eosinophils, and specifically via eosinophil MBP. Depletion of eosinophils in sensitized, virus-infected guinea pigs (Fig. 10 B) prevented M2R dysfunction, and also prevented vagal hyperreactivity (Fig. 2 B and 6 B). Removing positively charged proteins with heparin both acutely restored M2R function and reversed vagal hyperreactivity in the sensitized virus-infected guinea pigs (Fig. 4 and Fig. 8). The role of MBP was confirmed, since treatment with AbMBP prevented both M2R dysfunction and vagal hyperreactivity in the sensitized virus-infected guinea pigs (Fig. 3 and Fig. 7). Thus, by sensitizing guinea pigs before viral infection, the mechanism of virus-induced M2R dysfunction and vagal hyperreactivity was switched to be clearly dependent on eosinophils and eosinophil MBP.
Traditionally, eosinophils are not prominent in the response of nonasthmatics to viruses. Airway viral infections cause a neutrophil and mononuclear cell influx into the airways 16. Typically, viral clearance has been attributed to T cell release of IFN-γ and TNF-α, and to direct killing by CD8+ cells and NK cells 17. A CD4+ T lymphocyte response may also contribute to viral clearance, and here again it is production of INF-γ by T cells that appears to be beneficial 18.
Under some circumstances, viral infections do cause airway eosinophilia. Children who wheeze with respiratory syncytial virus (RSV) infection have both increased eosinophils and eosinophil cationic protein (ECP) in their airways 19. With viral infections other than RSV, eosinophilia is seen in atopic individuals. During naturally acquired viral infection, children with asthma have large increases in eosinophil MBP regulated on activation, normal T cell expressed and secreted (RANTES), and macrophage-inhibitory protein 1α in their nasal secretions 20. After intranasal infection with rhinovirus, biopsies of the lower airways of individuals with asthma contain increased eosinophils that persist even into convalescence 21. In patients with asthma, the presence of eosinophils and MBP in their airways during periods of exacerbations has been well established 22. However, the pathogenesis and role of this eosinophilia in hyperresponsiveness have been unclear, considering that many of these exacerbations are triggered by viral infection 1 23.
In mice, viral infection can induce airway eosinophilia by production of IL-5 by T lymphocytes 24 25. It has been shown that, although under normal circumstances CD8+ T lymphocytes produce IFN-γ in response to viral infections, in an allergic milieu these CD8+ cells respond to viral infection by producing IL-5 12. Studies by Coyle et al. 12 used a transgenic mouse model in which the CD8+ T lymphocytes expressed the receptor for a particular viral glycoprotein. When these mice inhaled this glycoprotein, there was increased IFN-γ and an influx of neutrophils and mononuclear cells into the lung lavage. However, if the mice were first sensitized to a nonviral antigen, OVA, the response to inhaled viral glycoprotein was production of IL-5 and an influx of eosinophils. These investigators demonstrated that exposure of the transgenic CD8+ T lymphocytes to IL-4 changed their in vitro response to the viral glycoprotein from IFN-γ to IL-5. Thus, it was postulated that in an “atopic” animal (i.e., one that had been sensitized), the production of IL-4 conditions the CD8+ T lymphocytes so that their response to viral infection is to produce IL-5 and to promote pulmonary eosinophilia.
In vivo and in vitro experiments have investigated the specific interactions between eosinophils and viruses. Viral infection of epithelial cells causes release of the chemokines for eosinophil migration such as RANTES, monocyte chemotactic protein 1, and macrophage inhibitory protein 1α 26 27. Eosinophils also respond directly to RSV infection 28. After infection with rhinovirus, eosinophils have been shown to activate T cells by acting as APCs 29. This demonstrates that eosinophils are being recruited and activated by viral infections. The possible mechanism of virus-induced eosinophil activation and granulation has been studied by Olszewska-Pazdrak et al. 30. Incubation of eosinophils with RSV-infected epithelial cells increases expression of the adhesion molecule CD18 on the eosinophils. Upregulation of Mac-1 (CD11b/CD18) is critical to eosinophil activation 31, allowing the eosinophils to interact with infected respiratory epithelium and to release ECP. The increased expression of CD18 also allows virus-specific T cells to bind and activate eosinophils.
An unexpected finding was an IL-5– and eosinophil-dependent antiviral effect in sensitized animals. Sensitization before viral infection decreased the viral content of the lungs by >80% (Fig. 11). AbIL5 pretreatment reversed the effect of sensitization, increasing viral titers in the lungs of sensitized virus-infected guinea pigs. In contrast, pretreatment with AbIL5 in the nonsensitized virus-infected guinea pigs had no effect on viral titers. This suggests that in addition to changing the mechanism of M2R dysfunction, sensitization before viral infection has also changed the guinea pig's ability to clear virus. The increased eosinophils in sensitized virus-infected animals were associated with decreased viral titer, whereas depleting eosinophils with AbIL5 was associated with increased viral titers. Although treatment with AbMBP did cause a small but significant decrease in eosinophil number, this decrease was much less than the decrease associated with AbIL5 treatment (Fig. 10 B). Treatment with AbMBP did not alter the viral content in the lungs of sensitized virus-infected guinea pigs. Therefore, the mechanism of the eosinophil's apparent ability to enhance viral clearance does not involve eosinophil MBP, as AbMBP treatment did not interfere with the ability of sensitization to lower viral titers.
Although the activation of eosinophils by viral infection has been shown, the possible direct antiviral role of eosinophils is less clear. ECP and eosinophil-derived neurotoxin are both RNases 32 that can inhibit replication of RNA viruses such as parainfluenza 33. Domachowske et al. have demonstrated, in vitro, that eosinophils can directly inhibit both RSV and parainfluenza virus infectivity 34. They found that the mechanism of eosinophil antiviral activity is through the RNase activity of eosinophil-derived neurotoxin and ECP 35.
Another possible antiviral mechanism for eosinophils may be through release of nitric oxide (NO), which is an effective inhibitor of viral replication 36. While the virucidal effects of eosinophils through production of NO have not been investigated, eosinophils do use NO production to kill parasites 37. NO production by eosinophils is increased in sensitized guinea pigs 38. Exhaled NO levels are increased in patients with asthma 39, and specifically during virus-induced asthma attacks 40.
In addition to the possible antiviral effects of eosinophils, other effectors in the Th2 pathway may also play a role in viral clearance. In mice, IL-5 plays an important role in mucosal immunity through the induction of plasma cell release of IgA 41. IL-5 may also play a role in systemic immunity through B cell growth and differentiation 42 and induction of receptors for IL-2 43, although studies in human B cells have yielded conflicting results 44 45. IL-5 may also act to stimulate differentiation of cytotoxic T lymphocytes 46. Although viral clearance is primarily dependent on cytotoxic T cell responses, antibody responses can also affect viral clearance 47. Therefore, it is possible that the effect of AbIL5 on the viral titers recovered in our sensitized virus-infected guinea pigs could also have occurred through disruption of a noneosinophil inflammatory pathway.
We have demonstrated that virus-induced loss of M2R function in nonsensitized virus-infected guinea pigs is not mediated by eosinophils. In contrast, by sensitizing guinea pigs to a nonviral protein before viral infection, we have switched the response to subsequent viral infection to depend on eosinophils. We have demonstrated that in sensitized animals, virus-induced hyperreactivity results from activation of eosinophils, release of MBP, and inhibition of neuronal M2R function.
M2Rs are present in humans 48, and loss of M2R function is associated with airway hyperreactivity in patients with asthma 49 50. Although the neuronal M2Rs are functional in humans with stable asthma, their function is lost during naturally acquired viral infection 51.
Asthma attacks are often precipitated by viral infection and are characterized by an eosinophilic airway inflammatory response, yet viral infection is not traditionally associated with eosinophilia. We have shown that sensitizing guinea pigs to a nonviral protein before viral infection switches the inflammatory response to viral infection to include eosinophils. Many asthmatics are atopic and have increased eosinophils in their airways even during periods of quiescence 52. Our data suggest that eosinophils play an important role in the responses of patients with atopic asthma to viral infection. This altered mechanism may be important in understanding the role viruses play in triggering asthma exacerbations. In addition, under these circumstances, eosinophils may play an additional role in viral clearance.
Acknowledgments
This work was funded by grants from the Maryland Thoracic Society and the Royal College of Physicians and Surgeons of Canada (to D.J. Adamko), by National Institutes of Health grants HL-44727 (to A.D. Fryer), HL-55543 and HL-61013 (to D.B. Jacoby), AI-09728 and AI-34577 (to G.J. Gleich), and by the Center for Indoor Air Research and the American Heart Association (to D.B. Jacoby and A.D. Fryer).
Footnotes
1used in this paper: AbIL5, antibody to IL-5; AbMBP, antibody to eosinophil MBP; ECP, eosinophil cationic protein; MBP, major basic protein; M2R, M2 muscarinic receptor; NO, nitric oxide; Ppi, pulmonary inflation pressure; RSV, respiratory syncytial virus; TCID50, tissue culture ID50
Some of the data in this manuscript were published previously in abstract form (Adamko, D.J., B.L. Yost, A.D. Fryer, and D.B. Jacoby. 1999. Am. J. Respir. Crit. Care Med. 159:229).
References
- Johnston S., Pattermore P., Sanderson G., Smith S., Lampe F., Josephs L., Symington P., O'Toole S., Myint S., Tyrell D., Holgate S. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empey D.W., Laitinen L.A., Jacobs L., Gold W.M., Nadel J.A. Mechanisms of bronchial hyperreactivity in normal subjects following upper respiratory tract infection. Am. Rev. Respir. Dis. 1976;113:523–527. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- Nadel J.A., Barnes P.J. Autonomic regulation of the airways. Annu. Rev. Med. 1984;35:451–467. doi: 10.1146/annurev.me.35.020184.002315. [DOI] [PubMed] [Google Scholar]
- Roffel A.F., Elzinga C.R., Zaagsma J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm. Pharmacol. 1990;3:47–51. doi: 10.1016/0952-0600(90)90009-8. [DOI] [PubMed] [Google Scholar]
- Fryer A.D., Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br. J. Pharmacol. 1984;83:973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A.D., Costello R.W., Yost B.L., Lobb R.R., Tedder T.F., Steeber A.S., Bochner B.S. Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J. Clin. Invest. 1997;99:2036–2044. doi: 10.1172/JCI119372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbon C.L., Jacoby D.B., Fryer A.D. Pretreatment with an antibody to IL-5 preserves the function of pulmonary M2 muscarinic receptors in antigen challenged guinea-pigs. Am. J. Respir. Cell Mol. Biol. 1995;12:320–328. doi: 10.1165/ajrcmb.12.3.7873198. [DOI] [PubMed] [Google Scholar]
- Jacoby D.B., Gleich G.J., Fryer A.D. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J. Clin. Invest. 1993;91:1314–1318. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A.D., Jacoby D.B. Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-l-glutamate. J. Clin. Invest. 1992;90:2292–2298. doi: 10.1172/JCI116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.M., Jacoby D.B., Gleich G.J., Fryer A.D., Costello R.W. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 receptor function in antigen-challenged guinea pigs. J. Clin. Invest. 1997;100:2254–2262. doi: 10.1172/JCI119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A.D., Yarkony K.A., Jacoby D.B. The effect of leukocyte depletion on pulmonary M2 muscarinic receptor function in parainfluenza virus-infected guinea-pigs. Br. J. Pharmacol. 1994;112:588–594. doi: 10.1111/j.1476-5381.1994.tb13115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle A.J., Erard F., Bertrand C., Walti S., Pircher H., Le Gros G. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J. Exp. Med. 1995;181:1229–1233. doi: 10.1084/jem.181.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.M., Loegering D.A., Gleich G.J. Antiserum to the major basic protein of guinea pig eosinophil granules. Immunochemistry. 1976;13:743–746. doi: 10.1016/0019-2791(76)90194-4. [DOI] [PubMed] [Google Scholar]
- Fryer A.D., Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J. Appl. Physiol. 1991;71:2255–2261. doi: 10.1152/jappl.1991.71.6.2255. [DOI] [PubMed] [Google Scholar]
- Costello R.W., Evans C.M., Yost B.L., Belmonte K.E., Gleich G.J., Jacoby D.B., Fryer A.D. Antigen-induced hyperreactivity to histaminerole of the vagus nerves and eosinophils. Am. J. Physiol. 1999;20:L709–L714. doi: 10.1152/ajplung.1999.276.5.L709. [DOI] [PubMed] [Google Scholar]
- Walsh J.J., Dietlein L.F., Low F.N., Burch G.E., Mogabgab W.J. Bronchotracheal response in human influenza. Arch. Int. Med. 1960;108:376–388. doi: 10.1001/archinte.1961.03620090048006. [DOI] [PubMed] [Google Scholar]
- Ramshaw I., Ramsay A., Karupiah G., Rolph M., Mahalingam S., Ruby J. Cytokines and immunity to viral infection. Immunol. Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Graham M.B., Braciale V.L., Braciale T.J. Influenza virus–specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R., Kimpen J.L., Welliver R.C., Ogra P.L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J. Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- Teran L.M., Seminario M.C., Shute J.K., Papi A., Compton S.J., Low J.L., Gleich G.J., Johnston S.L. RANTES, macrophage-inhibitory protein 1α, and the eosinophil product major basic protein are released into upper respiratory secretions during virus-induced asthma exacerbations in children. J. Infect. Dis. 1999;179:677–681. doi: 10.1086/314618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin P.G., Fraenkel D.J., Sanderson G., Lampe F., Holgate S.T. Lower airways inflammatory response during rhinovirus colds. Int. Arch. Allergy Immunol. 1995;107:127–129. doi: 10.1159/000236951. [DOI] [PubMed] [Google Scholar]
- Frigas E., Loegering D.A., Solley G.O., Farrow G.M., Gleich G.J. Elevated levels of the eosinophil granule MBP in the sputum of patients with bronchial asthma. Mayo Clin. Proc. 1981;56:345–353. [PubMed] [Google Scholar]
- Rakes G.P., Arruda E., Ingram J.M., Hoover G.E., Zambrano J.C., Hayden F.G., Platts-Mills T.A.E., Heymann P.W. Rhinovirus and respiratory syncytial virus in wheezing children requires emergency careIgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- Li L., Xia Y., Nguyen A., Feng L., Lo D. Th2-induced eotaxin expression and eosinophilia coexist with Th1 responses at the effector stage of lung inflammation. J. Immunol. 1998;161:3128–3135. [PubMed] [Google Scholar]
- Spender L., Hussell T., Openshaw P. Abundant IFN-γ production by local T cells in respiratory syncytial virus induced eosinophil lung disease. J. Gen. Virol. 1998;79:1751–1758. doi: 10.1099/0022-1317-79-7-1751. [DOI] [PubMed] [Google Scholar]
- Becker S., Reed W., Henderson F., Noah T. RSV infection of human airway epithelial cells causes production of the β-chemokine RANTES. Am. J. Physiol. 1997;272:L512–L520. doi: 10.1152/ajplung.1997.272.3.L512. [DOI] [PubMed] [Google Scholar]
- Olszewska-Pazdrak B., Casola A., Saito T., Alam R., Crowe S., Mei F., Ogra P., Garofalo R. Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J. Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpen J.L.L., Garofalo R., Welliver R.C., Ogra P.L. Activation of human eosinophils in vitro by respiratory syncytial virus. Pediatr. Res. 1992;32:160–164. doi: 10.1203/00006450-199208000-00007. [DOI] [PubMed] [Google Scholar]
- Handzel Z.T., Busse W.W., Sedgwick J.B., Vrtis R., Lee W.M., Kelly E.A., Gern J.E. Eosinophils bind rhinovirus and activate virus-specific T cells. J. Immunol. 1998;160:1279–1284. [PubMed] [Google Scholar]
- Olszewska-Pazdrak B., Pazdrak K., Ogra P., Garofalo R.P. Respiratory syncytial virus-infected pulmonary epithelial cells induce eosinophil degranulation by a CD18-mediated mechanism. J. Immunol. 1998;160:4889–4895. [PubMed] [Google Scholar]
- Kato M., Abraham R.T., Okada S., Kita H. Ligation of the beta2 integrin triggers activation and degranulation of human eosinophils. Am. J. Respir. Cell Mol. Biol. 1998;18:675–686. doi: 10.1165/ajrcmb.18.5.2885. [DOI] [PubMed] [Google Scholar]
- Gleich G.J., Loegering D.A., Bell M.P., Checkel J.L., Ackerman S.J., McKean D.J. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic proteinhomology with ribonuclease. Proc. Natl. Acad. Sci. USA. 1986;83:3146–3150. doi: 10.1073/pnas.83.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B.P., Galinski M.S., Banerjee A.K. Characterization of an in vitro system for the synthesis of mRNA from human parainfluenza virus type 3. J. Virol. 1990;64:1135–1142. doi: 10.1128/jvi.64.3.1135-1142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domachowske J.B., Dyer K.D., Bonville C.A., Rosenberg H.F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J. Infect. Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- Domachowske J.B., Dyer K.D., Adams A.G., Leto T.L., Rosenberg H.F. Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 1998;26:3358–3363. doi: 10.1093/nar/26.14.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S.P., Siekierski E.S., Porter J.D., Richards S.M., Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J. Virol. 1998;72:934–942. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira S.H., Fonseca S.G., Romao P.R., Figueiredo F., Ferreira S.H., Cunha F.Q. Microbicidal activity of eosinophils is associated with activation of the arginine-NO pathway. Parasite Immunol. 1998;20:405–412. doi: 10.1046/j.1365-3024.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- Liu S.F., Haddad E.B., Adcock I., Salmon M., Koto H., Gilbey T., Barnes P.J., Chung K.F. Inducible nitric oxide synthase after sensitization and allergen challenge of Brown Norway rat lung. Br. J. Pharmacol. 1997;121:1241–1246. doi: 10.1038/sj.bjp.0701242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonov S.A., Yates D., Robbins R.A., Logan-Sinclair R., Shinebourne E.A., Barnes P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- de Gouw H.W., Grunberg K., Schot R., Kroes A.C., Dick E.C., Sterk P.J. Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur. Respir. J. 1998;11:126–132. doi: 10.1183/09031936.98.11010126. [DOI] [PubMed] [Google Scholar]
- Hiroi T., Yanagita M., Iijima H., Iwatani K., Yoshida T., Yakatshu K., Kiyono H. Deficiency of IL-5 receptor alpha-chain selectively influences the development of the common mucosal immune system independent IgA-producing B-1 cell in mucosal-associated tissues. J. Immunol. 1999;162:821–828. [PubMed] [Google Scholar]
- Kopf M., Gros G., Coyle A., Kosco-Vilbois M., Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol. Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Loughnan M.S., Takatsu K., Harada N., Nossal G.J.V. T cell replacing factor (interleukin 5) induces expression of interleukin 2 receptors on murine splenic B cells. Proc. Natl. Acad. Sci. USA. 1987;84:5399–5403. doi: 10.1073/pnas.84.15.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck E.J., Hirst E.M., Sanderson C.J. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow culturescomparison and interaction with IL-1, IL-3, IL-6, and GM-CSF. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- Bertolini J.N., Sanderson C.J., Benson E.M. Human interleukin-5 induces staphylococcal A Cowan 1 strain-activated human B cells to secrete IgM. Eur. J. Immunol. 1993;23:398–402. doi: 10.1002/eji.1830230215. [DOI] [PubMed] [Google Scholar]
- Takatsu K., Kikuchi Y., Takahashi T., Honjo T., Matsumoto M., Harada N., Yamaguchi N., Tominaga A. Interleukin 5, a T-cell derived B-cell differentiating factor also induces T lymphocytes. Proc. Natl. Acad. Sci. USA. 1987;84:4234–4238. doi: 10.1073/pnas.84.12.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P.A., Palladino G., Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class 1-restricted cytotoxic T cells. J. Immunol. 1991;148:212–217. [PubMed] [Google Scholar]
- Minette P.A., Barnes P.J. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea pig airways. J. Appl. Physiol. 1988;64:2532–2537. doi: 10.1152/jappl.1988.64.6.2532. [DOI] [PubMed] [Google Scholar]
- Minette P.A., Lammers J.W., Dixon C.M., McCusker M.T., Barnes P.J. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J. Appl. Physiol. 1989;67:2461–2465. doi: 10.1152/jappl.1989.67.6.2461. [DOI] [PubMed] [Google Scholar]
- Ayala L.E., Ahmed T. Is there loss of a protective muscarinic receptor in asthma? Chest. 1989;96:1285–1291. doi: 10.1378/chest.96.6.1285. [DOI] [PubMed] [Google Scholar]
- Keen H., Hurst V., Jack S., Pearson M.G., Warburton C., Calverley P.M.A., Costello R.W. Loss of function of pulmonary neuronal M2 receptors in subjects with mild bronchial hyperreactivity during a respiratory tract viral infection Eur. Respir. J. 12 1998. 149S(Abstr.) [Google Scholar]
- Kirby J.G., Hargreave F.E., Gleich G.J., O'Byrne P.M. Bronchoalveolar cell profiles of asthmatic and nonasthmatic subjects. Am. Rev. Respir. Dis. 1987;136:379–383. doi: 10.1164/ajrccm/136.2.379. [DOI] [PubMed] [Google Scholar]