Abstract
The 4-1BB receptor (CDw137), a member of the tumor necrosis factor receptor superfamily, has been shown to costimulate the activation of T cells. Here we show that anti–mouse 4-1BB monoclonal antibodies (mAbs) inhibit thymus-dependent antibody production by B cells. Injection of anti–4-1BB mAbs into mice being immunized with cellular or soluble protein antigens induced long-term anergy of antigen-specific T cells. The immune response to the type II T cell–independent antigen trinintrophenol-conjugated Ficoll, however, was not suppressed. Inhibition of humoral immunity occurred only when anti–4-1BB mAb was given within 1 wk after immunization. Anti–4-1BB inhibition was observed in mice lacking functional CD8+ T cells, indicating that CD8+ T cells were not required for the induction of anergy. Analysis of the requirements for the anti–4-1BB–mediated inhibition of humoral immunity revealed that suppression could not be adoptively transferred with T cells from anti–4-1BB–treated mice. Transfer of BALB/c splenic T cells from sheep red blood cell (SRBC)-immunized and anti–4-1BB–treated mice together with normal BALB/c B cells into C.B-17 severe combined immunodeficient mice failed to generate an anti-SRBC response. However, B cells from the SRBC-immunized, anti–4-1BB–treated BALB/c mice, together with normal naive T cells, exhibited a normal humoral immune response against SRBC after transfer, demonstrating that SRBC-specific B cells were left unaffected by anti–4-1BB mAbs.
Keywords: 4-1BB receptor, costimulation, humoral immunity, anergy
The 4-1BB receptor, CDw137, is a member of the TNFR superfamily 1, which is reportedly expressed on activated T and NK cells in mice 1 2. Several studies have demonstrated that the 4-1BB receptor serves as a potent costimulatory molecule for T cells 3 4 5 6 and, in vivo, for NK cells (our unpublished observations). The natural ligand for the 4-1BB receptor, a molecule known as 4-1BB ligand, is constitutively expressed on resting B cells and macrophages and is costimulatory for anti-μ–mediated B cell activation 7. We have previously demonstrated through a combination of in vitro and in vivo studies in the mouse that anti–4-1BB mAbs preferentially activate CD8+ T cells 8 and protect them from superantigen (SAg)-induced apoptotic death 9. Anti–4-1BB mAb–costimulated CD8+ T cells secreted large quantities of IFN-γ 8 and TNF-α (our unpublished observations) and developed into antigen-specific CTLs 8. In tumor-bearing mice, we found that anti–4-1BB–induced CTLs eradicated large established tumors even when the tumors were poorly immunogenic and refractive to CD28/CD80-mediated costimulation 10. Given the fact that B cells express the 4-1BB ligand and that CD8+ T cells are known to function as suppressor cells, we examined in vivo the effect of anti–4-1BB mAbs on the generation of humoral immunity to thymus-dependent and thymus-independent (TI) antigens. We made use of three model antigens commonly employed for the study of humoral immunity in mice. Sheep (S)RBCs and human (hu)IgG are thymus-dependent antigens. Trinintrophenol (TNP)–Ficoll is a type II TI antigen. The studies described here demonstrate that injection of anti–4-1BB mAbs into mice undergoing immunization to T cell–dependent antigens blocked the development of humoral immunity. In contrast, injection of anti–4-1BB mAbs in mice immunized with TNP–Ficoll was without effect, and the mice generated a normal humoral anti-TNP response. Anti–4-1BB–induced immune suppression is long lasting and independent of circulating anti–4-1BB mAbs.
Materials and Methods
Animals.
8–12-wk-old female BALB/c, C57BL/6, and C57BL/6 β2-microglobulin–deficient mice were purchased from The Jackson Laboratory. Animals were maintained under a standard protocol with free access to food and water.
Antibodies and Fusion Proteins.
The generation and characterization of 1D8 and 3E1 anti–mouse 4-1BB mAbs and murine 4-1BB–huIg soluble fusion protein has been previously described 8, and both antibodies are rat IgG2A molecules having identical functional properties. 6E9 is a rat IgG2A anti–human CD40 ligand mAb that does not react with mouse CD40 ligand and was provided by Dr. Tony Siadak (Bristol-Myers Squibb).
Experimental Design.
Female BALB/c mice (The Jackson Laboratory) were immunized intravenously with 108 SRBCs (Colorado Serum Co.) on day 0 and challenged 7 wk later in the same manner. In some experiments, mice received multiple challenges at varying time points following the same procedure. huIgG (Calbiochem Corp.) was administered in two doses of 50 μg each on days 0 and 6 and then challenged at varying time points depending on the nature of the experiment with 10 μg of huIgG injected intravenously. Mice were bled at indicated intervals, and total antibody response to solubilized SRBC membrane proteins was measured 11. Humoral immunity to TNP–Ficoll (TNP-Ficoll-TNP[20]-AGG-AECM-Ficoll), purchased from Biosearch Technologies, was established by injection of 50 μg of TNP–Ficoll intravenously on day 0 and again on day 14. Antibody responses to TNP were measured by ELISA using TNP-conjugated OVA as the substrate.
ELISA.
4-1BB Ig was bound to 96-well plates (Immunolon-2; Dynatech Labs, Inc.) at 0.1 μg/ml in PBS overnight at 4°C. Wells were washed and blocked by incubation for 1 h with specimen diluent (Genetic Systems, Inc.). Antibodies or antisera were diluted or solubilized in specimen diluent for 1 h at 22°C. Wells were washed and incubated with several different reagents, depending on the assay. For routine binding assays and hybridoma supernatant screening, wells were incubated with peroxidase-conjugated goat anti–rat IgG (Calbiochem Corp.). For mAb isotyping, wells were incubated with peroxidase-conjugated isotype-specific mouse anti–rat mAbs (Zymed Labs., Inc.). For pharmacokinetic assay, wells were incubated with biotinylated RG7 (mouse anti–rat κ chain), washed, and then incubated with streptavidin–HRPO (horseradish peroxidase; Amersham). After final washing, all assays were developed with TMB substrate (3,3′5,5′-tetramethylbenzidine; Kirkegaard & Perry Labs., Inc.). Reactions were stopped with the addition of 1 N H2SO4, and optical density was measured at 450–725. ELISAs for monitoring ligand blocking of 4-1BB–Ig were performed as described previously 8. For anti-SRBC responses, mice were bled at indicated intervals, and total antibody response to SRBC membranes was measured in ELISA with HRPO-conjugated antibodies (Amersham). Solubilized SRBC membrane proteins were prepared as previously described and coated overnight onto Immunolon II plates (Dynatech Labs, Inc.) at 2.5 μg/ml PBS and washed five times before the mouse serum to be assayed was added. Serial dilutions beginning at 1:5 of each sera sample were performed in triplicate as described earlier. After a 30-min incubation at 4°C, the plates were washed five times with PBS before the addition of HRPO-conjugated anti-rat isotype-specific antibody (Amersham; 1:2000 final dilution). After a second 30-min incubation at 4°C, the plates were washed five times and then developed with TMB.
Results
Pharmacokinetics of Rat (IgG2A) Anti–Mouse 4-1BB mAbs.
We analyzed the serum half-life of all of our anti–4-1BB mAbs by pharmacokinetic analysis. 200 μg of the rat (IgG2A) mAb 1D8 was injected intravenously into the tail vein on days 0, 2, 4, and 6 into five mice. The mice were periodically bled, and serum levels of rat IgG2A were determined in triplicate by ELISA for each mouse and expressed as the mean ± SD. The serum half-life of anti–4-1BB mAb 1D8 was found to be 7.5 d (Fig. 1). Identical data were obtained for 12 other rat IgG2A isotype anti–4-1BB mAbs tested.
Figure 1.
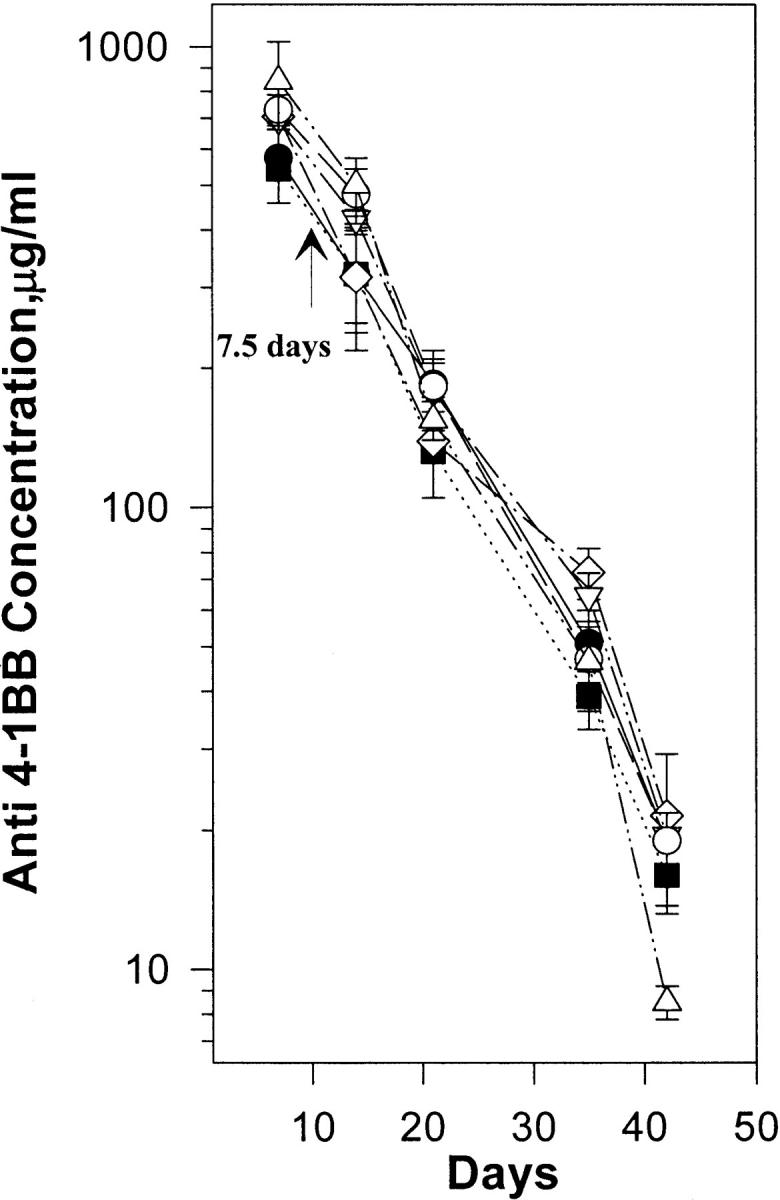
Pharmacokinetics of 1D8 mAbs. Serum levels of anti–4-1BB mAbs at varying time points were determined by ELISA. Five 8–12-wk-old female BALB/c mice were given four doses of 200 mg of 1D8 mAb intravenously on days 0, 2, 4, and 6. At the indicated times, sera were collected from each mouse, and the concentration of serum 1D8 was determined by measuring its binding to 4-1BB–huIgG1 fusion protein immobilized on ELISA plates and comparing the results to those obtained from a standard curve generated using purified 1D8 mAb. The figure shows the clearance rates of five mice. Each assay was carried out in triplicate. Serum concentrations at various time points were determined as described. The results are shown as the mean ± SD of three mice.
Inhibition of Humoral Immunity to SRBC and huIgG.
When injected into mice, cellular antigens such as SRBC or soluble proteins such as huIgG are strong, T cell–dependent antigens. To determine the effect of anti–4-1BB mAbs on the generation of humoral immunity to these antigens, we injected two groups of 15 female BALB/c mice with either SRBC (group I) or huIgG (group II). Each group was divided into three sets of five mice. One set was injected intravenously with 200 μg of 1D8 mAb in 200 μl of PBS beginning on the day of immunization and continuing every other day for four days (800 μg total). A second set received the isotype-matched negative control mAb 6E9, and the third set received PBS. In later experiments, as little as 50 μg of anti–4-1BB was injected with similar results. The mice were bled weekly, and their anti-SRBC or -huIgG serum titers were determined in triplicate by ELISA. At week 5, the mice were challenged with antigen. The results, repeated three times with similar data, are shown in Fig. 2. The mice produced a primary and a vigorous secondary humoral response to SRBC (Fig. 2 A) and huIgG (Fig. 2 B). However, when coinjected with anti–4-1BB mAb 1D8, the animals generated neither a primary nor secondary humoral response. By week 12, a small but significant anti-SRBC response occurred in the suppressed mice, and the response could be boosted with subsequent antigenic challenge. The isotype control mAb had no effect on the development of a primary or secondary humoral response to either antigen.
Figure 2.


Inhibition of anti-SRBC and anti-huIgG responses by 1D8 mAb but not by 4-1BB–huIg. Groups of five BALB/c mice were immunized with SRBC or huIgG in 0.5 ml of PBS by tail vein injection. On days 0, 2, 4, and 6, mice were injected with 200 mg of 1D8 (•), 6E9 (♦) in PBS, PBS alone (▪), or the 4-1BB–huIg soluble fusion protein (▴). Serum samples were collected as indicated and assayed in triplicate by ELISA for anti-SRBC–reactive antibodies of all IgG isotypes (A). The results shown are the mean ± SD for each group of five mice. Similarly, mice immunized with huIgG (B) and injected with 1D8 (•), 6E9 (♦) or PBS (▪) were likewise assayed for an anti-huIgG response. At week 7, the mice were challenged with a second dose of SRBC or huIgG, and their anti-SRBC/huIgG titers were followed through week 11.
Anti–4-1BB mAbs Block Humoral Immunity Only if Given Shortly after Immunization.
To determine how long after antigen injection anti–4-1BB mAbs would block humoral immunity to SRBC, we injected two groups of mice with SRBC. One group of mice received a single injection of 200 μg of anti–4-1BB mAb 1D8 on day 0. The second group of mice was similarly treated, except that anti–4-1BB administration did not occur until day 10 after immunization. Fig. 3 demonstrates that antibody no longer inhibited the development of humoral immunity when the injection of anti–4-1BB mAbs was delayed by 10 d. In subsequent experiments, it was found that full suppressive activity of anti–4-1BB mAbs could be obtained 72–96 h after immunization (data not shown).
Figure 3.

Anti–4-1BB mAbs block humoral immunity only if given during the first week of immunization. Two groups (five mice per group) of BALB/c mice were immunized on day 0 with SRBC as described above. One group of mice received anti–4-1BB mAb 1D8 as described in the Fig. 1 legend on day 0. Injection of anti–4-1BB in the second group was carried out as for the first group, but injection was delayed until day 10. When anti–4-1BB mAbs were injected at the time of immunization, the mice failed to generate either a primary or secondary humoral response to SRBC (♦). However, if the injection of anti–4-1BB mAbs was delayed by 10 d (•), there was no inhibition of either a primary or secondary response to SRBC antigens.
Anti–4-1BB mAbs Do Not Block Humoral Immunity to TNP–Ficoll.
TNP–Ficoll induces a type II TI humoral immune response. Type II TI responses do not require T–B cell cognate interactions but are greatly enhanced by T cell help 12. Anti–4-1BB mAbs were injected into mice immunized with TNP–Ficoll, and the antibody response to TNP was measured by assaying serum samples for their reactivity with TNP–OVA. Fig. 4 demonstrates that injection of anti–4-1BB mAbs did not inhibit the ability of the mice to generate a humoral anti-TNP response. These results suggest that anti–4-1BB mAbs do not affect B cell function, an observation consistent with the fact that murine B cells do not express the 4-1BB receptor.
Figure 4.
Anti–4-1BB mAbs fail to block type II TI humoral responses. Three groups (five mice per group) of 8–12-wk-old female BALB/c mice were injected intravenously with 50 mg of TNP–Ficoll on days 0 and 14. In addition, one group was injected intravenously with 200 mg of 3E1 (♦) on day 0; a second group was injected intravenously with 200 mg of 6E9 (▴), and the last group was injected with PBS (•).
Cellular Requirements for 4-1BB mAb–mediated Inhibition of Humoral Immunity.
We have previously shown that anti–4-1BB mAb is a potent costimulatory reagent for CD8+ T cells and, to a lesser degree, for CD4+ T cells 8. To determine if CD8+ T cells are required to mediate anti–4-1BB inhibition of T cell–dependent humoral immunity, we immunized C57BL/6 β2-microglobulin–deficient mice with SRBC. These mice fail to develop normal CD8+ T cells during thymic selection due to their failure to express functional MHC class I molecules. Simultaneous injection of anti–4-1BB mAb completely blocked both primary and a secondary anti-SRBC response despite the absence of CD8+ T cells (not shown).
Furthermore, T cell adoptive transfer into naive recipients could not transfer 4-1BB mAb–induced suppression. Splenic T cells (4 × 107) from SRBC-immune mice that were immunized and injected with either anti–4-1BB mAb or an isotype control mAb was injected intravenously into naive BALB/c mice together with SRBC. All of the recipient mice produced normal primary and secondary humoral responses to SRBC, showing no sign of adoptive suppression (not shown).
We also wished to know if helper T cell function was affected by anti–4-1BB mAbs. C.B-17 SCID mice were reconstituted with BALB/c T and B cells in the following manner. Three groups of mice, five mice per group, were reconstituted with (a) T and B cells from naive untreated mice, (b) T cells from SRBC-immunized and anti–4-1BB–injected mice and B cells from untreated mice, or (c) T cells from untreated mice and B cells from SRBC-immunized, anti–4-1BB–injected mice. All recipient groups were injected with SRBC and challenged 5 wk later. In the total procedure, the donor mice received a single injection of 200 μg of antibody and two injections of SRBC. The recipient mice received two injections of SRBC only. The results shown in Fig. 5 demonstrate that adoptive transfer of T cells from SRBC-immunized and anti–4-1BB–injected mice along with B cells from untreated mice failed to generate an antiSRBC humoral response. This procedure was carried out under conditions in which T and B cells from untreated mice injected into C.B-17 SCID mice generated a primary and secondary response to SRBC. Likewise, adoptive transfer of T cells from untreated mice together with B cells from SRBC-immunized and anti–4-1BB–injected mice into C.B-17 SCID mice produced normal primary and secondary humoral responses to SRBC.
Figure 5.
Adoptively transferred T cells from 3E1-treated, SRBC-immunized mice fail to support normal anti-SRBC responses in C.B-17 SCID mice. BALB/c mice were immunized with SRBC and injected intravenously with 200 mg of 3E1. 2 wk later, the mice were challenged with SRBC, and 3 wk thereafter, the mice were killed and 2–4 × 107 splenic T or B cells were injected intravenously into recipient C.B-17 SCID mice. SCID mice receiving T cells from anti–4-1BB–treated mice were injected with an equal number of B cells from naive BALB/c mice (•). Mice that received B cells from anti–4-1BB–injected mice were injected with an equal number of T cells from naive mice (▪). A third group of SCID mice received T and B cells from naive mice (♦). At weeks 5 and 9 after transfer, SCID mice were challenged with SRBC. At week 9, mice were immunized with an unrelated antigen, keyhole limpet hemocyanin (KLH). Serum samples were collected weekly and assayed for anti-SRBC titer. Results are expressed as the mean ± SD from each treatment group. KLH responses were normal (▴).
Discussion
Anti–4-1BB mAb is a potent costimulatory agent for T cells, especially CD8+ T cells 8. We report here that anti–4-1BB mAbs effectively block the humoral immune response of B cells against T cell–dependent antigens. A series of experiments designed to explore the mechanism of 4-1BB–mediated suppression of humoral immunity is described in this paper.
Antibody against the 4-1BB molecule, which is not expressed on B cells, does not inhibit the function of B cells directly, as demonstrated by several independent pieces of evidence. First, anti–4-1BB mAbs do not inhibit the T cell–independent antibody production by B cells. Second, B cells from suppressed animals are fully active in generating humoral immunity when T cell help from nonsuppressed animals is provided. Lastly, a 4-1BB–huIg fusion protein that binds to the 4-1BB ligand expressed by B cells cannot inhibit antigen-specific T cell–dependent humoral immunity to SRBC (data not shown). These observations point to suppression of T cell help by anti–4-1BB mAbs, the induction of helper T cell anergy by anti–4-1BB mAbs, or deletion.
T cell help is provided by CD4+ T cells. As these cells express 4-1BB, it is conceivable that the antibody directly induces anergy of these cells. This possibility is consistent with our previously published findings demonstrating that although anti–4-1BB mAbs profoundly costimulated anti-CD3–activated CD8+ T cells to proliferate, they marginally activated CD4+ T cells 8. In the same report, we demonstrated that there were marked qualitative and quantitative differences in protein tyrosine phosphorylation between CD8+ and CD4+ T cells. Most notably, phosphorylation patterns obtained with CD4+ T cells after stimulation with anti-CD3 and anti–4-1BB were similar to the pattern observed when T cells are stimulated with TCR/MHC-restricted, partially agonistic peptides 13. Indirect induction of helper T cell anergy through suppressor cells is an alternative explanation for which our experiments did not provide convincing evidence. An exclusive involvement of CD8+ suppressor T cells can be ruled out by our observation that suppression was observed in mice that lacked CD8+ T cells. However, these animals may have developed alternative methods of helper T cell regulation, e.g., using CD4+ T cells or CD4/CD8 double-negative T cells for this regulatory purpose 14.
It may be informative to compare CD4+ T cell anergy induced with anti–4-1BB mAbs with CD4+ T cell anergy induced by SAg 15 16. SAg has been shown to activate a majority of responding CD4+ T cells, with subsequent apoptotic death of most of them; a minority of responding T cells survive a primary SAg stimulus with long-term anergy 15 16 17 18. Anergy is controlled by regulatory (suppressor) T cells, the removal of which restores the proliferative response of “anergic” CD4+ T cells to SAg stimulation 19. The regulatory T cells appear to be idiotype specific, and they regulate the activity of preactivated (memory) T cells but not that of naive CD4+ T cells. In BALB/c mice, the regulatory T cells are found in the CD8+ T cell population. Such regulatory CD8+ T cells may function in two ways to inhibit CD4+ T cell activity: they may destroy their targets 20 or induce proliferative anergy 19 21. It has been shown in mice that express the transgene for a SAg-reactive Vβ chain in T cells and therefore lack TCR diversity that anergy of preactivated CD4+ T cells is mediated by CD4/CD8 double-negative CD3+ T cells 14.
The assumption of a role for regulatory cells in mediating 4-1BB mAb–mediated suppression of helper T cell function in the humoral response to T cell–dependent antigens is compatible with the findings presented in this report. Regulatory T cells may express the CD8+ phenotype, but this is not an absolute requirement. Therefore, anti–4-1BB mAb–mediated suppression of humoral immunity in CD8 T cell–deficient mice is not contradictory to the concept. The fact that regulatory T cells affect preactivated (memory) CD4+ T cells but not naive T cells in the SAg system is consistent with the notion in this study that a 4-1BB mAb–suppressed T cell population is not conferring helper T cell anergy to CD4+ T cells.
Taking all these considerations into account, we may conclude that 4-1BB mAb may induce helper T cell anergy in the humoral immune response by directly blocking helper T cells or by inducing regulatory T cells that block the activation as well as the reactivation of antigen-specific helper T cells on a long-term basis.
This study introduces a unique agent to suppress the humoral immune response to T cell–dependent antigens in a lasting and exhaustive fashion. The antibody does not induce humoral immunity against itself and can therefore be repeatedly employed without concern for loss of efficacy due to antibody production against therapeutic mAbs and against other immunogenic biologics. The antibody may be considered for active suppression of antibody-mediated autoimmune reactions.
References
- Pollok K.E., Kim Y.-J., Zhou Z., Hurtado J., Kim K.K., Pickard R.T., Kwon B.S. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- Mellero I., Johnston J.V., Shufford W.W., Mittler R.S., Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell. Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- Hurtado J.C., Kim S.H., Pollack K.E., Lee Z.H., Kwon B.S. Potential role of 4-1BB in T cell activation. Comparison with the costimulatory molecule CD28. J. Immunol. 1995;155:3360–3367. [PubMed] [Google Scholar]
- DeBenedette M.A., Chu N.R., Pollack K.E., Hurtado J., Wade W.F., Kwon B.S., Watts T.H. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphomas by cAMP. J. Exp. Med. 1995;181:985–992. doi: 10.1084/jem.181.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado J., Kim Y.-J., Kwon B.S. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- DeBenedette M.A., Shahinian A., Mak T.W., Watts T.H. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J. Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- Pollok K.E., Kim Y.-J., Hurtado J., Zhou Z., Kim K.K., Kwon B.S. 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-μ-primed splenic B cells. Eur. J. Immunol. 1994;24:367–374. doi: 10.1002/eji.1830240215. [DOI] [PubMed] [Google Scholar]
- Shuford W.W., Klussman K., Tritchler D.D., Loo D.T., Chalupny J., Siadak A.W., Brown T.J., Emswiler J., Raecho H., Larsen C.P. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification of cytotoxic T cell responses. J. Exp. Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi C., Mittler R.S., Vella A.T. Cutting edge4-1BB is a bona fide CD8 T cell survival signal. J. Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- Melero I., Shufford W.W., Newby S.A., Aruffo A., Ledbetter J.A., Hellstrom K.E., Mittler R.S., Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- Temple L., Kawabata T.T., Munson A.E., White K.L. Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 1993;21:412–419. doi: 10.1006/faat.1993.1116. [DOI] [PubMed] [Google Scholar]
- Van den Eertwegh A., Noelle R., Roy M., Sheperd D., Aruffo A., Ledbetter J., Boersma W., Claassen E. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J. Exp. Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrenas J., Chau L.A., Smith J., Bluestone J.A., Germain R.N. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide–MHC molecule ligands. J. Exp. Med. 1997;185:219–229. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley L.S., Cauley K.A., Shub F., Huston G., Swain S.L. Transferable anergysuperantigen treatment induces CD4+ T cell tolerance that is reversible and requires CD4−CD8− cells and interferon γ. J. Exp. Med. 1997;186:71–81. doi: 10.1084/jem.186.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannecker G., Mecheri S., Staiano Coico L., Hoffmann M.K. A characteristic Mls-1a response precedes Mls-1a anergy in vivo. J. Immunol. 1991;146:2083–2087. [PubMed] [Google Scholar]
- Rammensee H.G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature Vβ6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989;339:541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cellsclonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Wang Z.-Q., Orlikowsky T., Dudhane A., Trejo V., Dannecker G.E., Pernis B., Hoffmann M.K. Staphylococcal enterotoxin B-induced T cell anergy is mediated by regulatory T cells. Immunology. 1998;94:331–339. doi: 10.1046/j.1365-2567.1998.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ware R., Stall L., Flaherty L., Chess L., Pernis B. Murine CD8+ T cells that specifically delete autologous CD4+ T cells expressing Vβ8+ TCRa role of the Qa-1 molecule. Immunity. 1995;2:185–194. doi: 10.1016/s1074-7613(95)80079-4. [DOI] [PubMed] [Google Scholar]
- Schultz H., Geiselmart A., Sappler S., Neithammer D., Hoffmann M.K., Dannecker G. The superantigen Staphylococcus enterotoxin B induces a strong and accelerated secondary T-cell response rather than anergy. Immunology. 1996;87:49–54. [PMC free article] [PubMed] [Google Scholar]




