Abstract
It has been proposed that CD2, which is highly expressed on T cells, serves to enhance T cell–antigen presenting cell (APC) adhesion and costimulate T cell activation. Here we analyzed the role of CD2 using CD2-deficient mice crossed with transgenic mice expressing a T cell receptor specific for lymphocytic choriomeningitis virus (LCMV)-derived peptide p33. We found that absence of CD2 on T cells shifted the p33-specific dose–response curve in vitro by a factor of 3–10. In comparison, stimulation of T cells in the absence of lymphocyte function–associated antigen (LFA)-1–intercellular adhesion molecule (ICAM)-1 interaction shifted the dose–response curve by a factor of 10, whereas absence of both CD2–CD48 and LFA-1–ICAM-1 interactions shifted the response by a factor of ∼100. This indicates that CD2 and LFA-1 facilitate T cell activation additively. T cell activation at low antigen density was blocked at its very first steps, as T cell APC conjugate formation, TCR triggering, and Ca2+ fluxes were affected by the absence of CD2. In vivo, LCMV-specific, CD2-deficient T cells proliferated normally upon infection with live virus but responded in a reduced fashion upon cross-priming. Thus, CD2 sets quantitative thresholds and fine-tunes T cell activation both in vitro and in vivo.
Keywords: adhesion, costimulation, virus, cross-priming, T cell
Tcell activation is a carefully orchestrated process that involves the TCR and a multitude of accessory molecules expressed on T cells and APCs. Operationally, two types of molecules that modulate TCR-mediated T cell activation can be distinguished: (a) accessory molecules such as LFA-1, which facilitate TCR triggering by promoting T cell–APC adhesion 1 2; and (b) costimulatory molecules such as CD28, which enhance T cell activation without affecting the rate of TCR triggering 2 3.
Until recently, it was not possible to easily distinguish between accessory and costimulatory molecules, as there was no means to experimentally separate TCR triggering from the outcome of T cell activation. However, the observation that functionally engaged TCRs are rapidly internalized 4 now allows us to quantitatively assess TCR triggering and dissect it from T cell activation. It has previously been shown that TCR internalization occurs normally in the absence of CD28 2. Although it is possible that some molecules may affect the ratio of TCR engagement/internalization, TCR downregulation is, at this time, the best assay to measure TCR triggering with minimal influence by costimulatory molecules. We have previously taken advantage of this possibility and used it to analyze the respective roles of LFA-1 and CD28 in T cell activation 2. In this study, we assessed the role of CD2 in a similar experimental setup.
CD2, a member of the Ig superfamily, is highly expressed on T cells and can bind to CD58/LFA-3 5, CD59 6, or CD48 7 8 expressed on APCs. In mice, CD48 appears to be the major, if not the only, ligand. The interaction of CD2 with its ligand(s) has been extensively studied at both the physicochemical and the structural level. The affinity of CD2 for CD58 or CD48 was found to be low (∼5 × 10−5 M for mouse CD48 and somewhat higher for human CD58) and to exhibit rapid on and off rates (for review see reference 9). However, the fact that both CD2 and its ligands are membrane bound is critical for their interaction, as it restricts the localization of receptors and ligands essentially to two dimensions. This increases the operational concentrations of the compounds and has been referred to as two-dimensional affinity 10 11. Similar rules apply to other membrane-bound receptor ligand pairs, as has been pointed out for the TCR MHC–peptide interaction 12 13.
Various roles for CD2 in T cell activation have been proposed, including function as an adhesion molecule 1 14 15 16, thereby reducing amounts of antigen required for T cell activation 14, as a costimulatory molecule 17 18 19, or as a direct promoter of T cell activation 20. Moreover, CD2 has been implicated in the induction of T cell anergy 21 22 and has been reported to modulate cytokine production by T cells 23 24 and regulate positive selection 25. Surprisingly, however, CD2-deficient mice did not show an obvious phenotype and could efficiently cope with viral infections 26 27, undermining the view that CD2 plays a major role in T cell activation. Only recently, CD2 again attracted attention, because CD2AP, a CD2 adapter protein, has been shown to help orchestrate receptor patterning and cytoskeletal rearrangement 28.
To quantitatively analyze the role of CD2 in T cell activation both in vitro and in vivo, we crossed transgenic mice expressing an MHC class I–restricted TCR specific for lymphocytic (L)CMV-derived peptide p33 29, with CD2-deficient mice 26 and analyzed the functional properties of the CD2-deficient T cells. We found that CD2 reduces the minimal amount of antigen required for T cell activation both in vitro and in vivo, a function shared with LFA-1 2. The results show that CD2 does not promote T cell activation by a costimulatory mechanism as described for CD28 2 3 30 but, rather, by simply facilitating T cell–APC interaction at low antigen concentrations. Such a mechanism is compatible with a critical role for CD2 in organizing the T cell–APC contact site.
Materials and Methods
Mice and Viruses.
Transgenic mice expressing a TCR specific for peptide p33 in association with H-2Db 29 and CD2- 26 and intercellular adhesion molecule (ICAM)1-1–deficient 31 mice have been described previously. LCMV-WE was grown on L cells at a low multiplicity of infection. Recombinant vaccinia virus expressing LCMV-GP (Vacc-LCMV-GP) 32 was originally obtained from Dr. D.H.L. Bishop (Oxford University, Oxford, UK) and was grown on BSC cells at a low multiplicity of infection. Vacc-LCMV-GP was inactivated with UV light using an XL-1500 UV cross-linker (Spectronics Corp.). To produce recombinant LCMV-GP for the cross-priming experiments, Vacc-LCMV-GP was inactivated by UV light and used to infect BSC cells at a multiplicity of infection of 10. 24 h later, cells were harvested and sonicated. Cell debris corresponding to 5 × 106 cells was injected per recipient.
Peptides.
Peptides p33 (KAVYNFATM) and A4Y (KAVANFATM) were generated at the Amgen Institute (Boulder, CO) by a solid phase method using the Fmoc/tBu-based protocol on an ABI-431 instrument. The crude product was purified by HPLC. p33 defines the major CTL epitope on LCMV-GP in the H-2b haplotype 33. To prevent disulphide bonds, the COOH-terminal cysteine (C) has been replaced by methionine (M) 34.
In Vitro T Cell Proliferation, Production of IFN-γ, Induction of Ca2+ Flux, and Conjugate Formation.
Spleen cells from TCR-transgenic control or CD2-deficient mice (105 cells/well) were stimulated with thioglycollate-elicited macrophages (5 × 104 cells/well) derived from control or ICAM-1–deficient mice pulsed with graded doses of peptide p33 or A4Y in flat-bottom 96-well plates. Macrophages were pulsed for 1 h at 37°C and subsequently washed two times. Proliferation was assessed 36 h later by pulsing cultures with [3H]thymidine for 12 h. Production of IFN-γ was assessed in supernatants as described 35. Induction of Ca2+ flux and presence of T cells forming specific conjugates was assessed as described using INDO-1–pulsed T cells (Cat. no. I-3261; Sigma Chemical Co.) 2.
Induction of TCR Downregulation.
Spleen cells from TCR-transgenic mice (105/well) were mixed with peptide pulsed macrophages (2 × 105/well) from control or ICAM-1–deficient mice, centrifuged, and incubated at 37°C (5% CO2) in IMDM supplemented with 10% FCS in round-bottom 96-well plates. After 4 h, cells were harvested and stained for CD8 (PE; PharMingen) and Vα2 (FITC; PharMingen); Vα2 expression is shown for CD8+ T cells (see Fig. 3).
Figure 3.
CD2–CD48 and LFA-1–ICAM-1 enhance T cell activation by altering signal 1. Thioglycollate-elicited macrophages derived from control or ICAM-1–deficient mice were pulsed with various doses of peptide p33, mixed with T cells derived from TCR-transgenic control or CD2-deficient mice, and centrifuged together. Expression of TCR (Vα2) was assessed 4 h later on CD8+ T cells. (A) TCR expression is shown for various combinations after stimulation with 10−10 M p33-pulsed macrophages. (B) Mean fluorescence of TCR expression is shown as a function of the peptide concentration for the various combinations. One representative experiment of three is shown. ▪, CD2+ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
In Vivo Activation of T Cells Using Peptide.
TCR-transgenic control or CD2-deficient mice were injected intravenously with various doses of peptide p33 in saline. 24 h later, spleen cells were harvested and stained for expression of CD8 (FITC; PharMingen), Vα2 (PE; PharMingen), and CD44 (biotin; PharMingen) followed by streptavidin–allophycocyanin (PharMingen) and analyzed by flow cytometry.
In Vivo Expansion and Effector Cell Induction.
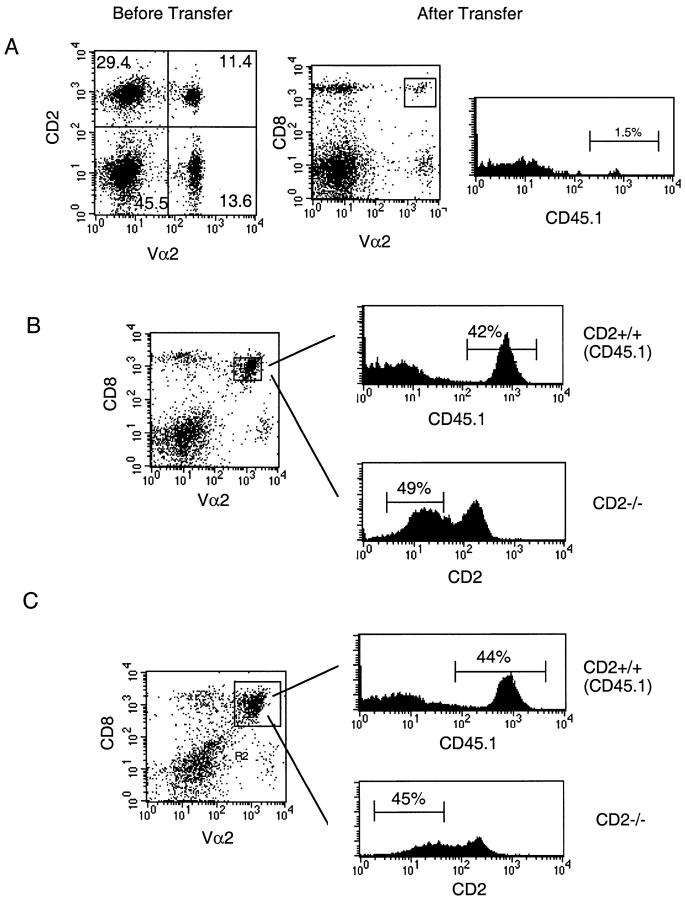
Spleen cells from TCR-transgenic CD2-deficient (CD45.2) or control (CD45.1) mice (106 cells) were adoptively transferred into normal C57BL/6 recipient mice. 1 h later, mice were challenged with live LCMV, Vacc-GP, UV light–inactivated Vacc-LCMV-GP, or recombinant LCMV-GP. 6 or 8 d later, spleen cells were harvested and stained with anti-CD2 antibodies (FITC), anti-CD8 (allophycocyanin), and anti-Vα2 (PE) or with anti-CD45.1 (FITC), anti-CD8 (allophycocyanin), and anti-Vα2 (PE).
Results
Shifted Dose Response in the Absence of CD2–CD48 and LFA-1–ICAM-1 Interaction.
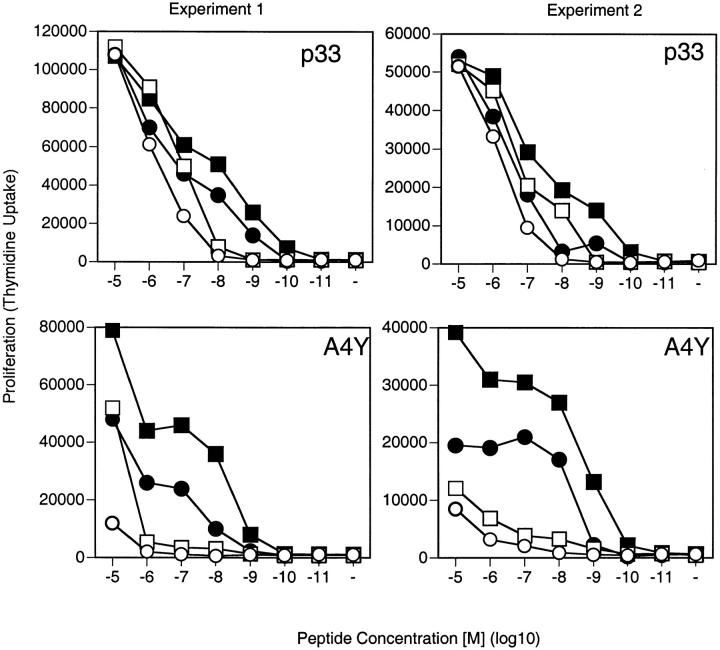
We crossed CD2-deficient mice with transgenic mice expressing a TCR specific for LCMV-derived peptide p33 to study activation of CD2-deficient T cells. To this end, splenocytes of CD2-deficient and control TCR-transgenic mice were stimulated with thioglycollate-elicited macrophages pulsed with various concentrations of peptide p33. As shown in Fig. 1, the absence of CD2 shifted the dose–response curve by a factor of 3–10. To additionally analyze the role of the LFA-1–ICAM-1 interaction in this context, we compared ICAM-1–deficient and control macrophages as APCs for CD2-deficient and CD2-competent transgenic T cells. As reported previously 2, the absence of LFA-1–ICAM-1 interaction shifted the dose–response curve of T cells upon stimulation with graded doses of peptide by a factor 10. Interestingly, the interference with both CD2 and LFA-1 pathways shifted the dose response by a factor of ∼100. Thus, CD2 and LFA-1 facilitated T cell activation in an additive manner (Fig. 1).
Figure 1.
CD2–CD48 and LFA-1–ICAM-1 interactions enhance T cell proliferation at low peptide densities. Thioglycollate-elicited macrophages derived from control (filled symbols) or ICAM-1–deficient (open symbols) mice were pulsed with various doses of peptide p33 or the low-affinity ligand A4Y and used to stimulate T cells derived from TCR-transgenic control (squares) or CD2-deficient (circles) mice. Proliferation was assessed 36 h later by means of [3H]thymidine incorporation. Two independent experiments are shown. ▪, CD2+ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
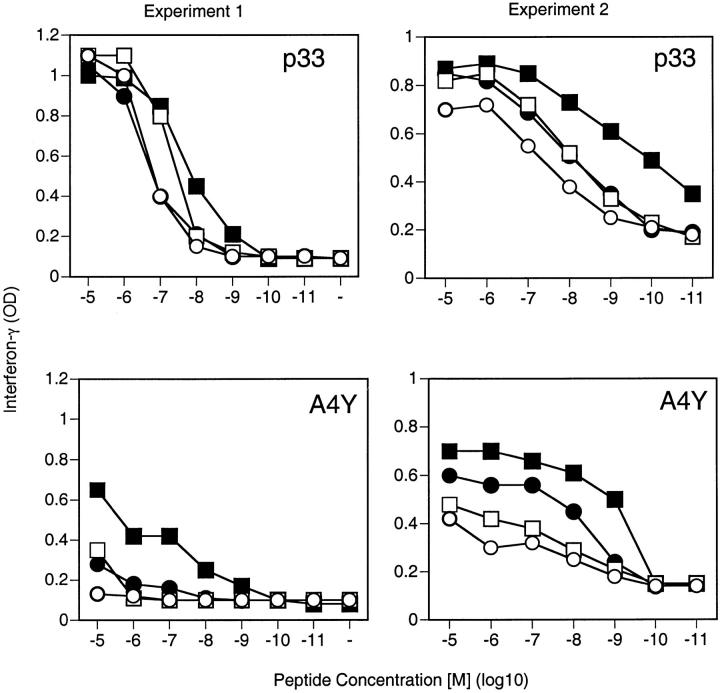
As expected, if the low-affinity ligand A4Y was used for the experiments, a similar albeit more pronounced shift in the dose–response curve could be observed (Fig. 1). Measurement of IFN-γ production showed results similar to the proliferative response. Both CD2 and LFA-1 enhanced IFN-γ production at low peptide concentrations, with the effects being more dramatic upon stimulation with the low-affinity ligand A4Y (Fig. 2). Thus, CD2–CD48 and LFA-1–ICAM-1 regulate T cell responses similarly by reducing the minimal amount of antigen required for activation and act in an additive manner.
Figure 2.
CD2–CD48 and LFA-1–ICAM-1 interaction enhances IFN-γ production at low peptide densities. Thioglycollate-elicited macrophages derived from control (filled symbols) or ICAM-1–deficient (open symbols) mice were pulsed with various doses of peptide p33 or the low-affinity ligand A4Y and used to stimulate T cells derived from TCR-transgenic control (squares) or CD2-deficient (circles) mice. Production of IFN-γ was assessed by ELISA 3 d later from culture supernatants. Two independent representative experiments are shown. ▪, CD2+ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
CD2 Facilitates Generation of Signal 1 by Enhancing TCR Triggering at Low Antigen Doses.
Functionally triggered TCRs are internalized shortly after stimulation 4 36. TCR downregulation can therefore be used to assess the number of functionally triggered TCRs and thus the amount of signal 1 3 37. To assess whether CD2 altered the intensity of signal 1, T cells from TCR-transgenic control or CD2-deficient mice were incubated with peptide-pulsed control or ICAM-1–deficient macrophages. Expression levels of TCRs were assessed 4 h later (Fig. 3a and Fig. b). TCR downregulation was reduced in the absence of either CD2 or ICAM-1 at low peptide densities (Fig. 3a and Fig. b). Moreover, as observed for the proliferative responses, ICAM-1 and CD2 acted in an additive fashion, and the dose–response curve of TCR downregulation was similar to the dose–response curve of the proliferative response (Fig. 3 B).
CD2 Promotes Ca2+ Flux and Conjugate Formation at Low Peptide Concentrations.
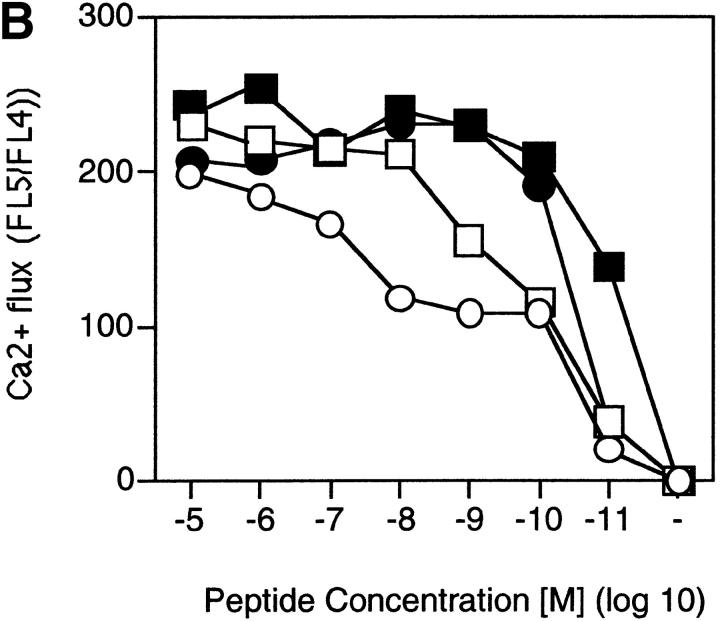
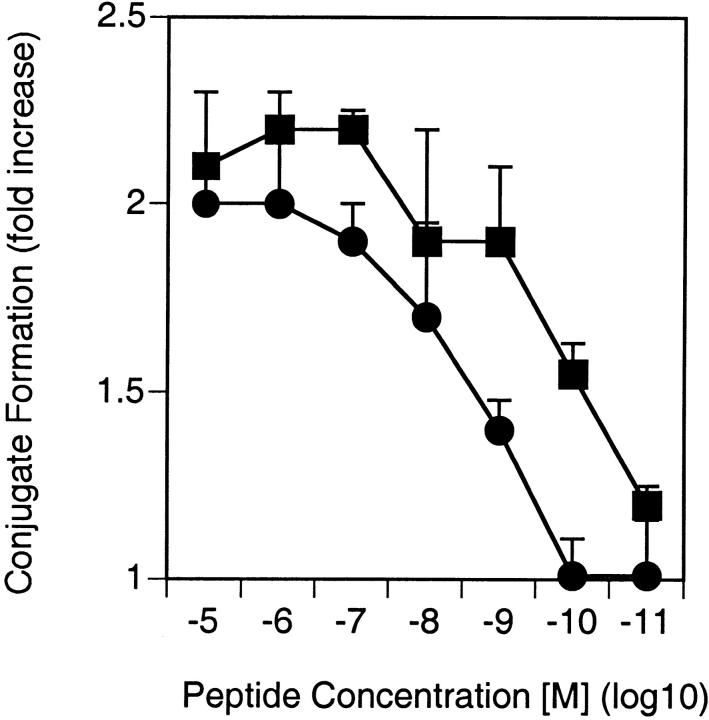
One of the earliest signals induced in T cells upon antigenic stimulation are increased intracellular free Ca2+ levels ([Ca2+]i). To test whether the enhanced TCR triggering at low peptide concentrations in the presence of LFA-1–ICAM-1 or CD2–CD48 interactions would translate into increased Ca2+ fluxes, CD2-deficient and control T cells were loaded with INDO-1 and stimulated with peptide-pulsed ICAM-1–deficient or control macrophages, and [Ca2+]i was assessed (Fig. 4a and Fig. b). As suggested by the data on TCR downregulation, both CD2 on T cells and ICAM-1 on APCs promoted increased [Ca2+]i at low antigen concentrations (Fig. 4a and Fig. b). Moreover, CD2–CD48 and LFA-1–ICAM-1 interactions had an additive effect. Importantly, as previously observed for T cell clones 14, CD2 facilitated T cell–APC conjugate formation at low antigen concentrations, indicating that CD2 primarily promotes adhesion of T cells to APCs (Fig. 5). Thus, the primary function of CD2 seems to be to enhance adhesion of T cells to APCs at low antigen concentrations, facilitating the generation of a T cell–APC contact site required for sustained signaling 38.
Figure 4.
CD2–CD48 and LFA-1–ICAM-1 enhance Ca2+ fluxes at low antigen concentration. Thioglycollate-elicited macrophages derived from control or ICAM-1–deficient mice were pulsed with various doses of peptide p33, mixed with INDO-1–pulsed, purified CD8+ T cells derived from TCR-transgenic control or CD2-deficient mice, and centrifuged together. Elevation of [Ca2+]i was assessed by measuring the FL5/FL4 ratio. (A) FL5/FL4 ratio is shown after stimulation with 10−11 M p33-pulsed macrophages. (B) Mean FL5/FL4 ratios are shown as a function of the peptide concentration for the various combinations. Baseline FL5/FL4 values were subtracted for the calculation. One representative experiment of two is shown. ▪, CD2+ ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
Figure 5.
CD2–CD48 interaction enhances T cell–APC conjugate formation. Thioglycollate-elicited macrophages were pulsed with various doses of peptide p33, mixed with INDO-1–pulsed, purified CD8+ T cells derived from TCR-transgenic control (▪) or CD2-deficient (•) mice, and centrifuged together, and conjugate formation was assessed. Data from two independent experiments were pooled, and the average and SD is shown.
CD2 Sets Quantitative Thresholds in T Cell Activation In Vivo.
To assess the role of CD2 in T cell activation in vivo, TCR-transgenic CD2-deficient and control mice were injected with various doses of peptide p33 in saline, and expression of the activation marker CD44 was assessed 1 (Fig. 6) and 3 d (not shown) later. As expected from the in vitro experiments, presence of CD2 decreased the minimal amount of peptide required for the upregulation of CD44.
Figure 6.
CD2 reduces the amount of peptide required for in vivo induction of CD44 expression. TCR-transgenic control (▪) and CD2-deficient (•) mice were injected with various amounts of peptide p33, and expression of CD44 was assessed 24 h later on CD8+Vα2+ T cells. The average of two mice is shown per dose. One representative experiment of three is shown.
To assess the role of CD2 in viral infections, an adoptive transfer system was employed 2 39. Spleen cells (106) from TCR-transgenic CD2-deficient and control mice were mixed at a 1:1 ratio and adoptively transferred into nonirradiated C57BL/6 mice and immunized with LCMV (200 PFU) or a recombinant vaccinia virus expressing LCMV-GP (Vacc-GP; 2 × 106 PFU). To enable selective identification of the control versus CD2-deficient TCR-transgenic T cells, TCR-transgenic control mice on a CD45.1 background were used. Expansion of transferred TCR-transgenic control and CD2-deficient T cells was subsequently assessed 6 (Fig. 7) or 8 d (not shown) later. No significant difference between CD2-deficient and control T cells was observed. These results are in agreement with an earlier report, in which CD2-deficient mice were found to mount normal LCMV-specific CD8+ T cell responses 27. Surprisingly, even the absence of both functional CD2 and LFA-1 together also failed to interfere with the response, as CD2-deficient T cells transferred into ICAM-1–deficient mice expanded normally upon infection with LCMV or Vacc-GP (not shown).
Figure 7.
Normal in vivo expansion of LCMV-GP–specific, CD2-deficient, TCR-transgenic T cells upon infection with live virus. CD45.1+ TCR-transgenic control spleen cells were mixed 1:1 with CD45.1+ CD2-deficient spleen cells and adoptively transferred into C57BL/6 recipient mice. (A) Left panel: 1:1 distribution of CD2+ and CD2− TCR+ T cells was confirmed before transfer. Center and right panels: splenocytes from mice that received 106 cells of the mixture shown in the left panel 6 d earlier in the absence of an infection were stained for Vα2, CD8, and CD45.1 expression; <2% of CD8+Vα2+ T cells were derived from the adoptively transferred T cells. (B and C) Recipient mice were infected with LCMV (200 PFU; B) or recombinant vaccinia virus expressing LCMV-GP (2 × 106 PFU; C). CD45.1 expression was assessed for CD8+Vα2+ T cells, revealing expansion of CD2+ control T cells (upper right panels). CD2 expression was assessed similarly for CD8+Vα2+ T cells, revealing expansion of CD2-deficient T cells (lower right panels). Similar results were obtained 8 d after infection. One representative experiment of three is shown.
The experiments performed so far suggested that CD2 plays a major role in T cell activation at low antigen densities. However, viral antigens are usually expressed at high densities, and it may therefore not be surprising that CD2-deficient T cells are able to respond normally to viral infections. To assess the role of CD2 in a situation where antigen is less abundant, we used a recombinant vaccinia virus expressing LCMV-GP (Vacc-GP), which was inactivated with UV light before infection. This treatment prevents the virus from undergoing full replication cycles, and endogenously produced antigens will therefore only reach low densities. For the experiment, a 1:1 mixture of CD2-deficient TCR-transgenic T cells obtained from CD45.2 mice and control CD2-competent transgenic T cells obtained from CD45.1 mice (in a total of 106 spleen cells) was transferred into C57BL/6 mice, which were subsequently immunized with UV light–inactivated Vacc-GP (2 × 106 PFU before inactivation). Control and CD2-deficient T cells could be conveniently distinguished by assessing CD45.1 versus CD45.2 and CD2 expression. As expected, the expansion of the transferred T cells was dramatically reduced compared with a challenge infection with live virus (Fig. 8 A). Moreover, CD2-deficient T cells were clearly less efficiently proliferating than the control T cells. Note that CD45.1+ and CD2-deficient Vα2+CD8+ T cells only account for ∼60% of the cells. This is due to the presence of endogenous CD8+Vα2+ T cells. Thus, CD2 expression on T cells becomes critical in vivo in a situation where virus derived antigens are limiting.
Figure 8.
Impaired in vivo expansion of LCMV-GP–specific, TCR-transgenic, CD2-deficient T cells upon cross-priming. CD45.1+ TCR-transgenic control spleen cells were mixed 1:1 with CD45.1+ CD2-deficient spleen cells and adoptively transferred into C57BL/6 recipient mice. (A) Recipient mice were immunized with UV light–inactivated recombinant vaccinia virus expressing LCMV-GP, and spleen cells were analyzed 6 d later. CD45.1 expression was assessed for CD8+Vα2+ T cells, revealing expansion of CD2+ control T cells (upper right panels). CD2 expression was assessed similarly for CD8+ Vα2+ T cells, revealing expansion of CD2-deficient T cells (lower right panels). (B) Recipient mice were immunized with LCMV-GP associated with cellular debris, and spleen cells were analyzed 6 d later. CD45.1 expression was assessed for CD8+Vα2+ T cells, revealing expansion of CD2+ control T cells (upper right panels). CD2 expression was assessed similarly for CD8+Vα2+ T cells, revealing expansion of CD2-deficient T cells (lower right panels). Percentage of CD45.1+ control T cells versus CD2-deficient T cells was <3% in the absence of immunization. One representative experiment of two is shown.
CTLs are usually primed by endogenously produced antigens reaching the class I pathway. However, MHC class I molecules may under some conditions also be loaded by exogenous antigens, leading to activation of specific T cells in a process called cross-priming. We have previously shown that exogenous LCMV-GP is able to reach the class I pathway if associated with cellular debris 40. To test whether CD2 may be required for optimal CTL induction upon cross-priming, a 1:1 mixture of CD2-deficient (CD45.2) and control (CD45.1) TCR-transgenic T cells (total of 106 spleen cells) was transferred into C57BL/6 mice, which were subsequently immunized with recombinant LCMV-GP in association with cellular debris. 6 d later, the presence of CD45.1+ control and CD2-deficient TCR-transgenic T cells was assessed in the spleen (Fig. 8 B). Although the CD2-deficient T cells were activated and proliferated upon cross-priming, the expansion was less dramatic than that observed for the control cells. This indicates that CD2 participates in regulation of T cell expansion upon cross-priming.
Discussion
This study demonstrates that CD2–CD48 and LFA-1–ICAM-1 interactions enhance T cell activation in an additive fashion by similar mechanisms. Both interactions facilitate TCR triggering by increasing T cell–APC interactions at low antigen densities, fine-tuning T cell responses in vitro and in vivo.
CD2 as an Accessory Molecule: Adhesion Versus Costimulation.
T cell activation may be described in terms of the two-signal model, where signal 1 describes TCR-mediated signals and signal 2 refers to signals delivered by costimulatory molecules, which facilitate full T cell activation and prevent the induction of T cell tolerance 2 41 42 43; we have operationally discriminated these as signal 2c and 2t, respectively 2. As TCRs productively triggered by MHC–peptide complexes are rapidly internalized 4 36, the rate of TCR internalization may serve as a quantitative measure for the amount of signal 1 a T cell is receiving at a given time point 2. Thus, a true costimulatory molecule would enhance T cell activation without changing signal 1, i.e., TCR internalization (unless it modulates the ratio of TCR engagement versus internalization) 2. This is the case for CD28, which does not affect TCR internalization but nevertheless enhances T cell activation, apparently by increasing TCR-mediated signals intracellularly 2 3 30. It has recently been suggested that rearrangement of membrane rafts rich in glycosphingolipids may be critical in this process 44 45. In contrast, CD2 does not seem to affect T cell activation other than by increasing signal 1 (i.e., the number of triggered TCRs) at low antigen densities. In fact, the dose–response curves of T cell–APC conjugation, TCR internalization, Ca2+ flux, and T cell proliferation are similarly shifted toward higher antigen concentrations in the absence of CD2, indicating that CD2 enhances T activation by facilitating T cell–APC interactions at low antigen densities. Thus, CD2 may be viewed as an adhesion molecule rather than a costimulatory molecule. This view is compatible with the recent observation that CD2 recruits an adapter molecule (CD2AP) to the T cell–APC contact site, helping to rearrange the cytoskeleton. Such a rearrangement is presumably required for a firm and stable T cell–APC interaction 28. Our observations also fit the hypothesis that CD2 may bring T cells and APCs into close proximity, helping to exclude large molecules such as CD45 from the contact site 16. In particular, the finding that CD2 is dispensable at high antigen concentrations may be explained by the notion that (a) the sizes of the TCR and CD2 are similar and (b) the CD2–CD48 interaction exhibits an affinity that is on the order of the TCR MHC–peptide interaction. Thus, large numbers of TCR MHC–peptide interactions may be able to substitute for CD2.
We have previously argued that LFA-1 effects activation of CD8+ T cells primarily by promoting T cell–APC adhesion 2. This may be different for CD4+ T cells, as it has been reported that LFA-1 specifically promotes Th1 development 46. Thus, it remains possible that CD2 may affect activation of CD4+ T cells in a similarly qualitative fashion. However, we recently found that LFA-1 shifts the Th1/Th2 cytokine balance by shifting the dose response of CD4+ Th cells. In fact, absence of LFA-1 increased the minimal antigen concentration required for activation of TCR-transgenic Th cells by a factor of ∼100 (Ruedl, C., M.F. Bachmann, and M. Kopf, manuscript submitted for publication). Because induction of Th1 cells was also shifted by a factor of 100, this indicated that absence of LFA-1 shifted the response from Th1 to Th2 by globally shifting the dose response of CD4+ Th cells. Thus, although we cannot exclude the possibility that CD2 affects CD4+ T cell responses distinctly from CD8+ T cell responses, there is no data supporting such an assumption at this point.
The In Vivo Role of CD2.
CD2-deficient mice have been found to mount largely normal T cell responses upon infection with LCMV 27. In the light of our observation that CD2 is dispensable for T cell activation at high antigen concentrations, this earlier finding may not be surprising, as viral infection usually leads to high local antigen density. Using CD2-deficient T cells from TCR-transgenic mice specific for a peptide derived from LCMV, we could confirm the CD2 independence of the anti-LCMV CTL response 27. Moreover, using a recombinant vaccinia virus expressing LCMV-GP, we could demonstrate that the CTL response elicited by vaccinia virus was also CD2 independent. More surprisingly, T cell responses were still unaffected in the absence of both LFA-1–ICAM-1 and CD2–CD48 interactions (not shown). Thus, antiviral immune responses may often be generated in the absence of CD2 and/or LFA-1. However, CD8+ T cell activation was clearly impaired in vivo in the absence of CD2 if limited amounts of antigen were used for immunization, as, for example, with immunization regimens involving low amounts of peptide or UV light–inactivated recombinant vaccinia virus. The latter results may be particularly interesting because they indicate that viruses do not target a particular APC in vivo that can prime CTLs in the absence of CD2 but rather suggest that CD2 dependence is dictated by antigen quantity. Thus, viruses that replicate intracellularly to high titers can prime T cells in the absence of CD2, whereas abortive viral infections that do not reach high levels of intracellular protein require CD2 for full T cell activation. This latter class of immunization may therefore be representative for infections with attenuated viruses that induce only abortive infections. Moreover, antigens introduced to the immune system by cross-presentation also did not reach high densities of class I molecules and therefore required the presence of CD2 for optimal T cell responses. Thus, T cell responses against abundant antigens occur in the absence of CD2, whereas T cell responses against rare and cross-presented antigens require the presence of CD2 for optimal responses.
Acknowledgments
We thank Christiane Ruedl and Marco Colonna for critical reading of the manuscript, Barbara Ecabert for excellent technical assistance, Marc Dessing for the [Ca2+]i measurements, Nigel Killeen for CD2-deficient mice, and Jose Carlos Gutierrez-Ramos for KAM-1–deficient mice.
The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche, Basel, Switzerland.
Footnotes
1used in this paper: ICAM, intercellular adhesion molecule
References
- Springer T.A., Dustin M.L., Kishimoto T.K., Marlin S.D. The lymphocyte function-associated LFA-1, CD2 and LFA-3 moleculescell adhesion receptors for the immune system. Annu. Rev. Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., McKall-Faienza K., Schmits R., Bouchard D., Beach J., Speiser D.E., Mak T.W., Ohashi P.S. Distinct roles for LFA-1 and CD28 during activation of naive T cellsadhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- Viola A., Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- Valitutti S., Müller S., Cella M., Padovan E., Lanzavecchia A. Serial triggering of many T cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Dustin M.L., Sanders M.E., Shaw S., Springer T.A. Purified lymphocyte function–associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J. Exp. Med. 1987;165:677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn W.C., Menu E., Bothwell A.L., Sims P.J., Bierer B.E. Overlapping but nonidentical binding sites on CD2 for CD58 and a second ligand CD59. Science. 1992;256:1805–1807. doi: 10.1126/science.1377404. [DOI] [PubMed] [Google Scholar]
- Kato K., Koyanagi M., Okada H., Takanashi T., Wong Y.W., Williams A.F., Okumura K., Yagita H. CD48 is a counter-receptor for mouse CD2 and is involved in T cell activation. J. Exp. Med. 1992;176:1241–1249. doi: 10.1084/jem.176.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrin M.S., Mouhtouris E., Vaughan H.A., Warren H.S., Parish C.R. CD48 is a low affinity ligand for human CD2. J. Immunol. 1993;151:4606–4613. [PubMed] [Google Scholar]
- Davis S.J., Ikemizu S., Wild M.K., van der Merwe P.A. CD2 and the nature of protein interactions mediating cell-cell recognition. Immunol. Rev. 1998;163:217–236. doi: 10.1111/j.1600-065x.1998.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Dustin M.L., Ferguson L.M., Chan P.Y., Springer T.A., Golan D. Visualization of CD2 interaction with LFA-3 and determination of the two-dimensional dissociation constant for adhesion receptors in a contact area. J. Cell Biol. 1996;132:465–474. doi: 10.1083/jcb.132.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A.S., Dustin M.L. Making the T cell receptor go the distancea topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- Karjalainen K. High sensitivity, low affinity—paradox of T-cell receptor recognition. Curr. Opin. Immunol. 1994;6:9–12. doi: 10.1016/0952-7915(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Salzmann M., Bachmann M.F. Estimation of maximal affinities between T cell receptors and MHC/peptide complexes. Mol. Immunol. 1998;35:65–71. doi: 10.1016/s0161-5890(98)00020-0. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Lawton T., Novick D., Recny M.A., Siliciano R.F., Wallner B.P., Reinherz E.L. Role of interaction of CD2 molecules with lymphocyte function-associated antigen 3 in T-cell recognition of nominal antigen. Proc. Natl. Acad. Sci. USA. 1990;87:2603–2607. doi: 10.1073/pnas.87.7.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y., Reiser H. Enhanced murine CD4+ T cell responses induced by the CD2 ligand CD48. Eur. J. Immunol. 1998;28:4325–4331. doi: 10.1002/(SICI)1521-4141(199812)28:12<4325::AID-IMMU4325>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Davis S.J., van der Merwe A.P. The structure and ligand interactions of CD2implications for T cell function. Immunol. Today. 1996;17:177–187. doi: 10.1016/0167-5699(96)80617-7. [DOI] [PubMed] [Google Scholar]
- Bierer B.E., Peterson A., Gorga J.C., Herrmann S.H., Burakoff S.J. Synergistic T cell activation via the physiological ligands for CD2 and the T cell receptor. J. Exp. Med. 1988;168:1145–1156. doi: 10.1084/jem.168.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guiner S., Le Drean E., Labarriere N., Fonteneau J.F., Viret C., Diez E., Jotereau F. LFA-3 co-stimulates cytokine secretion by cytotoxic T lymphocytes by providing a TCR-independent activation signal. Eur. J. Immunol. 1998;28:1322–1331. doi: 10.1002/(SICI)1521-4141(199804)28:04<1322::AID-IMMU1322>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Peng X., Kasran A., Bullens D., Ceuppens J.L. Ligation of CD2 provides a strong helper signal for the production of the type 2 cytokines interleukin-4 and -5 by memory T cells. Cell. Immunol. 1997;181:76–85. doi: 10.1006/cimm.1997.1197. [DOI] [PubMed] [Google Scholar]
- Ohno H., Ushiyama C., Taniguchi M., Germain R.N., Saito T. CD2 can mediate TCR/CD3-independent T cell activation. J. Immunol. 1991;146:3742–3746. [PubMed] [Google Scholar]
- Guckel B., Berek C., Lutz M., Altevogt P., Schirrmacher V., Kyewski B.A. Anti-CD2 antibodies induce T cell unresponsiveness in vivo. J. Exp. Med. 1991;174:957–967. doi: 10.1084/jem.174.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G.M., Imboden J.B. CD2 and the regulation of T cell anergy. J. Immunol. 1995;155:2805–2807. [PubMed] [Google Scholar]
- Gollob J.A., Li J., Reinherz E.L., Ritz J. CD2 regulates responsiveness of activated T cells to interleukin 12. J. Exp. Med. 1995;182:721–731. doi: 10.1084/jem.182.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter W., Schwarz M., Cerwenka A., Knapp W. The role of CD2 as a regulator of human T-cell cytokine production. Immunol. Rev. 1996;153:107–122. doi: 10.1111/j.1600-065x.1996.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Teh S.J., Killeen N., Tarakhovsky A., Littman D.R., Teh H.S. CD2 regulates the positive selection and function of antigen-specific CD4− CD8+ T cells. Blood. 1997;89:1308–1318. [PubMed] [Google Scholar]
- Killeen N., Stuart S.G., Littman D.R. Development and function of T cells in mice with a disrupted CD2 gene. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:4329–4336. doi: 10.1002/j.1460-2075.1992.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.F., Rall G.F., Killeen N., Littman D., Oldstone M.B. CD2-deficient mice generate virus-specific cytotoxic T lymphocytes upon infection with lymphocytic choriomeningitis virus. J. Immunol. 1993;151:6259–6264. [PubMed] [Google Scholar]
- Dustin M.L., Olszowy M.W., Holdorf A.D., Li J., Bromley S., Desai N., Widder P., Rosenberger F., van der Merwe P.A., Allen P.M. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- Pircher H.P., Bürki K., Lang R., Hengartner H., Zinkernagel R.M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Tuosto L., Acuto O. CD28 affects the earliest signaling events generated by TCR engagement. Eur. J. Immunol. 1998;28:2131–2142. doi: 10.1002/(SICI)1521-4141(199807)28:07<2131::AID-IMMU2131>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Xu H., Gonzalo J.A., St Pierre Y., Williams I.R., Kupper T.S., Cotran R.S., Springer T.A., Gutierrez-Ramos J.C. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1–deficient mice. J. Exp. Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany M., Oehen S., Schulz M., Hengartner H., Mackett M., Bishop D., Zinkernagel R.M. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunisation with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur. J. Immunol. 1989;19:417–424. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- Pircher H.P., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R.M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Pircher H.P., Brduscha K., Steinhoff U., Kasai M., Mizuochi T., Zinkernagel R.M., Hengartner H., Kyewski B., Müller K.P. Tolerance induction by clonal deletion of CD4+8+ thymocytes in vitro does not require dedicated antigen presenting cells. Eur. J. Immunol. 1993;23:669–674. doi: 10.1002/eji.1830230315. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Barner M., Viola A., Kopf M.F. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 1999;29:291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Meuer S.C., Hussey R.E., Cantrell D.A., Hodgdon J.C., Schlossman S.F., Smith K.A., Reinherz E.L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc. Natl. Acad. Sci. USA. 1984;81:1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Oxenius A., Speiser D.E., Mariathasan S., Hengartner H., Zinkernagel R.M., Ohashi P.S. Peptide-induced T cell receptor down-regulation on naive T cells predicts agonist/partial agonist properties and strictly correlates with T cell activation. Eur. J. Immunol. 1997;27:2195–2203. doi: 10.1002/eji.1830270912. [DOI] [PubMed] [Google Scholar]
- Penninger J.M., Crabtree G.R. The actin cytoskeleton and lymphocyte activation. Cell. 1999;96:9–12. doi: 10.1016/s0092-8674(00)80954-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann C., Brduscha-Riem K., Blaser C., Zinkernagel R.M., Pircher H. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J. Exp. Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Kündig T.M., Freer G., Li Y., Bishop D.H., Hengartner H., Zinkernagel R.M. Induction of protective cytotoxic T cells with viral proteins. Eur. J. Immunol. 1994;24:2228–2236. doi: 10.1002/eji.1830240944. [DOI] [PubMed] [Google Scholar]
- Cohn M., Langman R.E. The protectonthe unit of humoral immunity selected by evolution. Immunol. Rev. 1990;115:11–147. doi: 10.1111/j.1600-065x.1990.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R.H. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Bluestone J.A. New perspectives of CD28-B7 mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- Wulfing C., Davis M.M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Salomon B., Bluestone J.A. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J. Immunol. 1998;161:5138–5142. [PubMed] [Google Scholar]