Abstract
Linker for activation of T cells (LAT) is an adaptor protein whose tyrosine phosphorylation is critical for transduction of the T cell receptor (TCR) signal. LAT phosphorylation is accomplished by the protein tyrosine kinase ZAP-70, but it is not at all clear how LAT (which is not associated with the TCR) encounters ZAP-70 (which is bound to the TCR). Here we show that LAT associates with surface CD4 and CD8 coreceptors and that its association is promoted by the same coreceptor cysteine motif that mediates Lck binding. In fact, LAT competes with Lck for binding to individual coreceptor molecules but differs from Lck in its preferential association with CD8 rather than CD4 in CD4+CD8+ thymocytes. Importantly, as a consequence of LAT association with surface coreceptors, coengagement of the TCR with surface coreceptors induces LAT phosphorylation and the specific recruitment of downstream signaling mediators to coreceptor-associated LAT molecules. These results point to a new function for CD4 and CD8 coreceptors in TCR signal transduction, namely to promote LAT phosphorylation by ZAP-70 by recruiting LAT to major histocompatibility complex–engaged TCR complexes.
Keywords: T cell receptor, development, signaling, thymus, phosphorylation
Activation of T lymphocytes in response to antigenic stimulation is initiated by interaction of surface TCRs with antigenic peptides bound to MHC molecules. Inside the cell, TCR engagement of MHC–peptide complexes results in activation of src family protein tyrosine kinases (PTKs)1 p56lck (Lck) and p59fyn to phosphorylate specialized signaling motifs (referred to as immunoreceptor tyrosine-based activation motifs [ITAMs]) that are present in the cytosolic tails of the invariant components of surface TCR complexes 1 2 3 4. Phosphorylated ITAMs then recruit ZAP-70 to surface TCR complexes, whereupon the recruited ZAP-70 molecules are themselves tyrosine phosphorylated and activated 5 6 7 8 9 10. These proximal TCR signaling events are significantly enhanced by MHC-induced coengagement of TCR with surface CD4/CD8 coreceptor molecules, since such coengagement serves to juxtapose TCR complexes with coreceptor-associated Lck molecules and so promotes efficient phosphorylation of TCR ITAMs 11 12 and efficient activation of TCR-associated ZAP-70 PTK molecules 13. Further propagation of the TCR signal requires tyrosine phosphorylation by ZAP-70 of linker for activation of T cells (LAT), a 36/38-kd membrane-associated protein that, upon tyrosine phosphorylation, is subsequently bound by downstream signaling mediators 14 15 16. Thus, tyrosine phosphorylation of LAT marks the transition between proximal and downstream signaling events initiated by TCR engagement of MHC–peptide complexes.
Because of LAT's central role in TCR signal transduction, it is important to understand how TCR-associated ZAP-70 PTK molecules encounter LAT in order to tyrosine phosphorylate it. In this study, we examined the possibility that LAT, like Lck, might be associated with CD4 and CD8 coreceptor molecules and so might be colocalized with TCR-associated ZAP-70 molecules upon MHC-induced coengagement of the TCR with CD4/CD8 coreceptors. We report that LAT does associate with CD4 and CD8 coreceptors in T cells, and that coengagement of TCR with surface coreceptor molecules induces LAT phosphorylation and recruitment of downstream signaling mediators to coreceptor-associated LAT molecules.
Materials and Methods
Cell Preparation and Immunoprecipitations.
The care of experimental animals was in accordance with National Institutes of Health guidelines. Single cell suspensions of lymph node cells and thymocytes were prepared from C57BL/6 or CD8β− mice thymi. Purified populations of CD4+CD8+ thymocytes >96% pure were obtained by anti-CD8 panning of whole thymocyte populations and selecting the adherent cells, as described previously 17. Cells (0.5–3 × 108, as indicated) were lysed in ice-cold lysis buffer containing either 1% Triton X-100 or 60 mM octylglucoside. After clarification (10,000 g for 10 min), cell lysates were subjected to immunoprecipitation with the indicated antibodies. Immunoprecipitates were resolved on SDS-PAGE under reducing conditions. Antibodies used for immunoprecipitation were specific for: CD4 (GK1.5 or RM4.5; PharMingen), CD8α (53-67; PharMingen), or CD8β (53-5.8; PharMingen); and TCR-ζ (serum 551), Lck (serum 688), or LAT (serum 3023) 14. Antibodies used for immunoblotting were specific for: LAT (serum 3023); Lck (serum 688); or phosphotyrosine (4G10; Upstate Biotechnology). For immunoprecipitations, mAbs were directly coupled to CnBr-activated Sepharose beads (Amersham Pharmacia Biotech), except when indicated otherwise.
Thymocyte Stimulation.
Pervanadate treatment (final concentration of 0.01 mM Na3VO4 in the presence of 4.5 mM H2O2, extemporaneously prepared) was conducted for 10 min at 37°C. TCR cross-linking experiments were performed essentially as described 13. Antibodies used for cross-linking were as follows: anti–TCR-β (H57-597 18), anti-CD4 (GK 1.5; PharMingen), and anti-CD8α (2.43 19 or 53-6.7 [PharMingen]). Thymocytes were cultured for 18 h at 37°C, pelleted, and resuspended at 107/ml in ice-cold RPMI containing 1 mM Na3VO4 and biotinylated antibodies (10 μg/ml). After 10 min at 4°C, the cells were pelleted and resuspended at 108/ml in RPMI containing 20 μg/ml of streptavidin (Southern Biotechnology Associates) previously prewarmed at 37°C. After a 5-min incubation at 37°C, cells were pelleted and lysed in octylglucoside-containing buffer.
DNA Constructs and Transfections.
cDNAs encoding mouse CD4, CD8α, and CD8β were provided by Dr. Jane Parnes (Stanford University, Stanford, CA 20). Fragments encoding each coreceptor domain, or mutant derivatives thereof, were prepared by restriction enzyme digestion, PCR amplification, or as double stranded oligonucleotides, and ligated to generate the indicated constructs. The CD8α AAM mutation, converting cysteines 227 and 229 to alanines, was introduced using PCR-mediated site-directed mutagenesis 21. Amino acid sequences (single letter code) at the modified junctions were as follows: ABB, LDFACD/ITTLSL; AAT, LICYA/RSR; ABA, LDFACD/ITTLSL … VYFYCA/RSRKRVC; 4AA, GVNQTD/IYIWAPL; 4BB, GVNQTD/ITTLSL. The sequences of all PCR-amplified and oligonucleotide-encoded regions were verified by dideoxy sequencing for the presence of the desired modifications and the absence of additional mutations. Wild-type and mutant cDNAs were introduced in pcDNA3 (Invitrogen) for expression in 293T cells. Expression vectors for mouse and human LAT have been described 14 22. 293T cells were transfected using the calcium phosphate coprecipitation method 23. Total plasmid amount was kept constant among samples by adjusting the amount of empty expression vectors. Cells were harvested 36–40 h after transfection. Expression of the coreceptor derivatives in each sample was verified by cell surface staining with anti-CD4 (GK 1.5), anti-CD8α (53-6.7), and anti-CD8β (53-5.8) mAbs and cytofluorimetric analysis.
Results
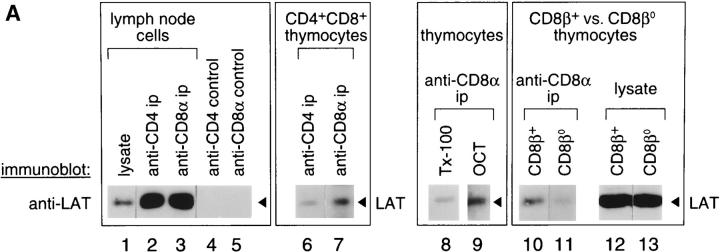
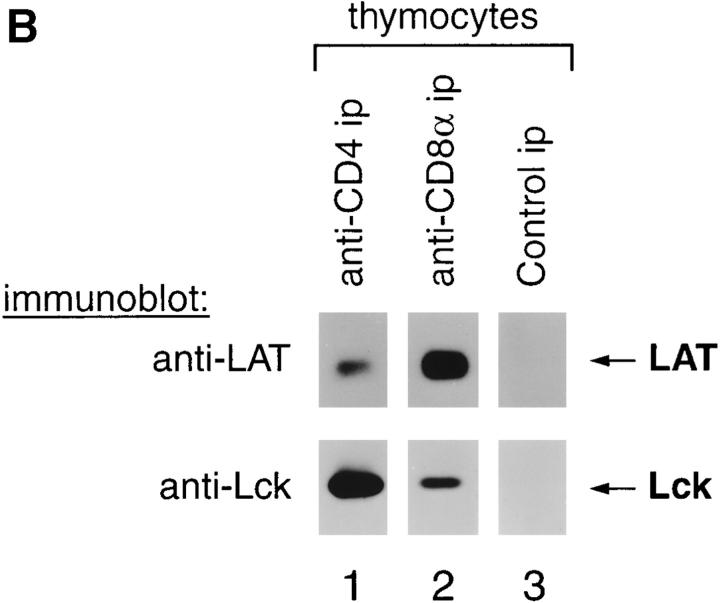
To determine if coreceptor–LAT complexes existed on unstimulated murine T cells and thymocytes, we immunoblotted anti-CD4 and anti-CD8 immunoprecipitates for LAT (Fig. 1 A, left). In lymph node T cells that express CD4 and CD8 coreceptors on separate cell populations, we found that LAT was associated with both CD4 and CD8 (Fig. 1 A, lanes 2 and 3). In immature CD4+CD8+ thymocytes, which express both CD4 and CD8 on individual cells, we found that LAT associated with CD8 in significantly greater amounts than with CD4 (Fig. 1 A, lanes 6 and 7). Interestingly, the hierarchy of LAT binding in immature CD4+CD8+ thymocytes (CD8 > CD4) is reciprocal to Lck, which binds CD4 > CD8 (24 25; Fig. 1 B).
Figure 1.
Association of LAT with CD4 and CD8 coreceptors in mature T cells and immature thymocytes. (A) Mouse lymph node cells (3 × 108 cell equivalents/lane) or thymocytes (0.5 × 108 cell equivalents/lane) were detergent solubilized by 1% Triton X-100 (lanes 1–8) or 60 mM octylglucoside (OCT, lanes 9–13), and the resulting lysates were immunoprecipitated with either anti-CD4 or anti-CD8α mAbs directly coupled to beads. Unfractionated cell lysates (3–6 × 106 cell equivalents/lane) and immunoprecipitates (ip) were resolved by SDS-PAGE, immunoblotted with a LAT-specific rabbit antiserum, and visualized by enhanced chemiluminescence. In contrast to anti-CD4 and anti-CD8α immunoprecipitates, which contained LAT, control anti-ζ immunoprecipitates did not contain LAT (data not shown). As additional negative specificity controls, antibody-coupled beads in the absence of lysate were also immunoblotted for LAT (lanes 4 and 5). In lanes 10–13, anti-CD8α immunoprecipitates and unfractionated cell lysates from CD8β− thymocytes were assessed for LAT and compared with wild-type CD8β+ thymocytes. (B) Mouse thymocytes were lysed in 60 mM octylglucoside, immunoprecipitated (ip) with anti-CD4 or anti-CD8 mAb directly coupled to beads, and immunoblotted with either anti-Lck or anti-LAT. LAT was preferentially immunoprecipitated by CD8, whereas Lck was preferentially immunoprecipitated by CD4 (lanes 1 and 2). As an additional specificity control, neither LAT nor Lck was immunoprecipitated by beads in the absence of anti-CD4 or anti-CD8 antibodies (lane 3).
We then analyzed CD8–LAT associations in thymocytes in greater detail. LAT molecules are largely localized to detergent-resistant areas of the plasma membrane referred to as glycolipid-enriched membrane microdomains (GEMs [22, 26]), which also contain subpopulations of both CD4 and CD8 molecules 27. Thus, it was conceivable that coimmunoprecipitation of LAT with CD4/CD8 coreceptors might simply reflect the presence of LAT and CD4/CD8 coreceptors in the same GEM. This was not the case, however, as LAT–CD8 associations were maintained in anti-CD8 immunoprecipitations of cells solubilized by the detergent octylglucoside, which solubilizes GEMs (28 29; Fig. 1 A, lanes 8 and 9). In fact, detergent solubilization with octylglucoside increased the amount of LAT detected in anti-CD8 immunoprecipitates (Fig. 1 A, lanes 8 and 9).
Next, we assessed whether LAT association with CD8 required both components of CD8 (α and β). In normal mice, CD8 is expressed on thymocytes and thymic-derived T cells as an αβ heterodimer, whereas in CD8β− mice, CD8 is expressed as an αα homodimer 30 31. Despite similar amounts of LAT in whole cell lysates from normal and CD8β− thymocytes (Fig. 1 A, lanes 12 and 13), the amount of CD8-associated LAT was markedly greater in CD8β+ thymocytes than in CD8β− thymocytes, although a small amount of LAT binding to CD8αα homodimers was evident in CD8β− thymocytes (Fig. 1 A, lanes 10 and 11). Thus, CD8β contributes to CD8–LAT association, as it does to CD8–Lck association 32 33. For Lck, CD8β is thought to increase accessibility to a binding site present in the cytosolic tail of CD8α, and this may be the case for LAT as well.
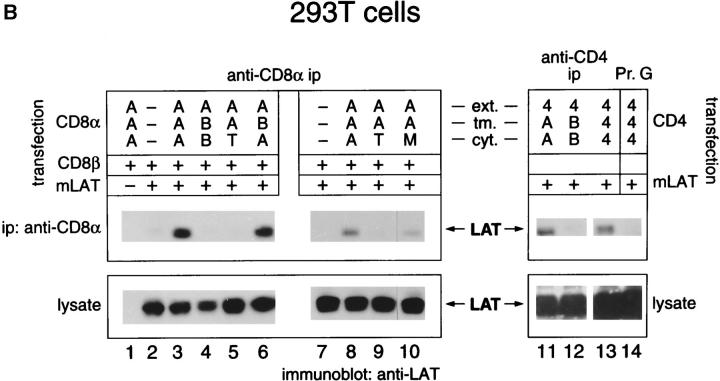
To determine the molecular basis for LAT–coreceptor associations, we cotransfected 293T cells, a human transformed kidney cell line expressing SV40 T antigen, with murine LAT (mLAT) and murine coreceptor molecules (CD8α and β or CD4). The transfected coreceptor molecules were either wild-type or variants containing altered transmembrane or cytosolic domains (Fig. 2 A). Surface expression of transfected coreceptor molecules was quantitated by immunofluorescence and flow cytometry and was found to be similar within all experimental groups (Table ). Lysates from transfected 293T cells were immunoprecipitated by anti-CD8α mAbs (Fig. 2 B, left) or anti-CD4 mAb (Fig. 2 B, right), and the immunoprecipitates were immunoblotted for LAT. Both CD8–LAT and CD4–LAT complexes were observed in 293T cells transfected with intact (AAA, 444) coreceptor molecules (Fig. 2 B, lanes 3 and 13), demonstrating that coreceptor–LAT complexes could form in nonlymphoid cells and did not require Lck, which is not expressed in 293T cells. We further documented that LAT did not associate with CD4 or CD8α variants expressing either the cytosolic tail of CD8β (lanes 4 and 12) or lacking a cytosolic tail altogether (lanes 5 and 9). Thus, the cytosolic tails of CD4 and CD8α are the main determinants for LAT association, suggesting that a sequence common to both cytosolic tails is involved in LAT binding.
Figure 2.
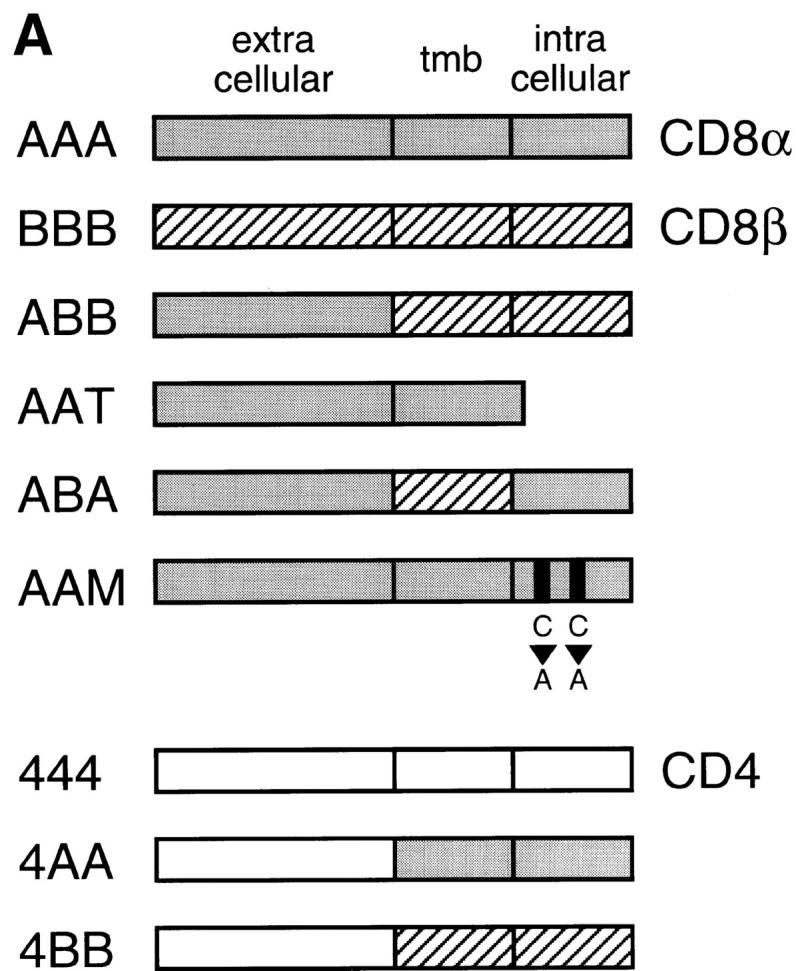
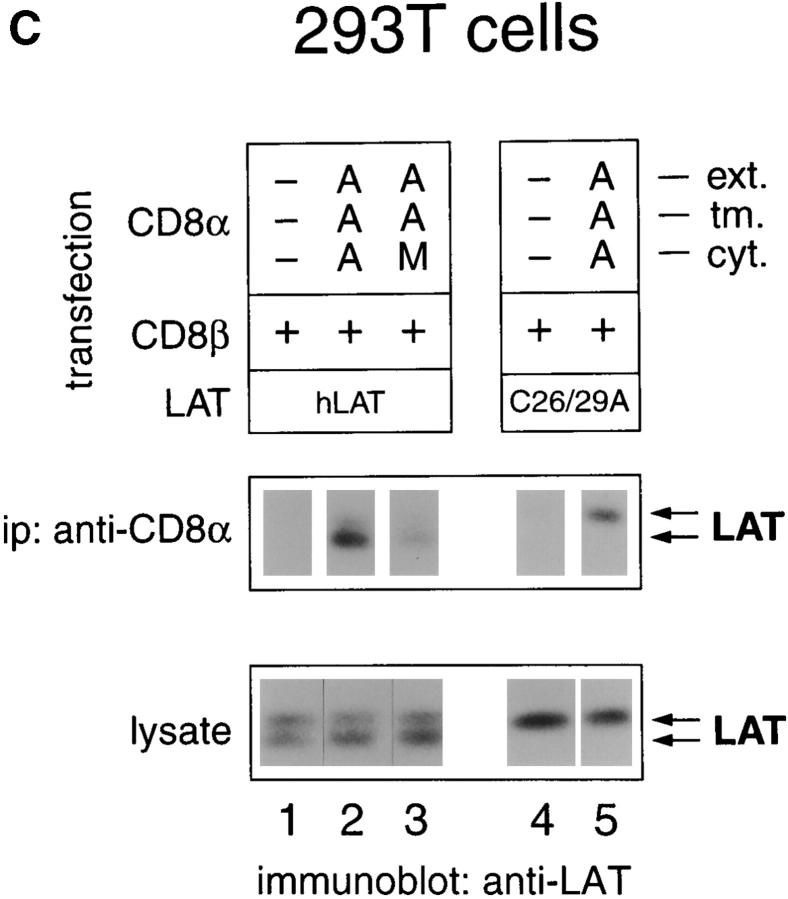
Mapping the coreceptor site for LAT association. (A) Schematic of the coreceptor derivatives used in this study. Mouse CD8α (AAA), CD8β (BBB), and CD4 (444) molecules (references 20 and 44) are schematically depicted by gray, hatched, or white bars and designated by a three-letter code indicating the origin of their extracellular, transmembrane (tmb), and intracellular domains. In addition to intact coreceptor molecules (AAA, BBB, 444), we constructed variant coreceptor molecules either lacking a cytosolic tail (AAT) or containing shuffled coreceptor domains. A mutant CD8α construct (AAM) was also generated by mutating to alanines the cytosolic cysteines at positions 227 and 229 of CD8α. (B) 293T kidney cells were cotransfected with the indicated expression vectors and analyzed 36–40 h after transfection for coreceptor–LAT associations. CD8 transfections involved cotransfection with both CD8α and CD8β vectors in addition to LAT, whereas CD4 transfections only involved cotransfection with CD4 and LAT. Cells were detergent solubilized with Triton X-100; identical results were obtained with cells detergent solubilized with octylglucoside. Anti-CD8α immunoprecipitations (ip, left) were performed with anti-CD8α mAb covalently coupled to Sepharose beads, and two independent experiments are shown (lanes 1–6 and lanes 7–10). Anti-CD4 immunoprecipitations (right) were performed using anti-CD4 mAb adsorbed onto protein G–Sepharose beads (Pr. G, lanes 11–13). As a negative control for anti-CD4 immunoprecipitations, cell lysates were immunoprecipitated with protein G–Sepharose beads alone without anti-CD4 mAb (lane 14). Assessment of unfractionated cell lysates revealed that transfected cells within each experiment expressed essentially equivalent amounts of LAT (lower panel), although they differed in the amount of LAT present in anti-coreceptor immunoprecipitations (upper panel). In addition, surface expression of coreceptor molecules in each cell transfection was quantitated by immunofluorescence and flow cytometry (see Table ). Densitometric analysis of the films indicated that the amount of AAM-bound LAT (lane 10) was 30% of the amount of AAA-bound LAT (lane 8). Essentially identical results were also obtained using 125I-labeled protein A instead of chemiluminescence to visualize LAT. (C) Association of hLAT with CD8. 293T cells were cotransfected with wild-type (AAA) or mutant (AAM) versions of CD8α, wild-type (hLAT) or mutant (C26/29A) versions of human LAT, and CD8β. Cells were solubilized in octylglucoside, immunoprecipitated, and resolved by SDS-PAGE. In lanes 1–3, wild-type LAT migrated as two bands of 36 and 38 kD. However, wild-type CD8α preferentially associated with the lower 36-kD band (lane 2); in contrast, the AAM mutant of CD8α bound neither band well. In lanes 4–5, it can be seen that the C26/29A mutant of human LAT (in which cysteines 26 and 29 were mutated to alanines) migrated as a single band of 38 kD that could still associate with CD8. Relative fluorescence intensities (as defined in Table ) for anti-CD8α immunostaining of transfected cells were <0.01 (cells in lanes 1 and 4), 1.0 (lane 2), 0.7 (lane 3), and 0.9 (lane 5).
Table 1.
Coreceptor Expression in Transfected 293T Cells
| Exp. | Sample | Coreceptor derivative | Mean fluorescence intensity | Relative fluorescence intensity | |
|---|---|---|---|---|---|
| CD8α | CD4 | ||||
| 1 | 1 | AAA | 195 | 1.0 | |
| 2 | – | 1 | <0.01 | ||
| 3 | AAA | 183 | 0.9 | ||
| 4 | ABB | 103 | 0.6 | ||
| 5 | AAT | 346 | 1.8 | ||
| 6 | ABA | 265 | 1.4 | ||
| 2 | 7 | – | 1 | <0.01 | |
| 8 | AAA | 702 | 1.0 | ||
| 9 | AAT | 528 | 0.8 | ||
| 10 | AAM | 536 | 0.8 | ||
| 3 | 11 | 4AA | 319 | 1.3 | |
| 12 | 4BB | 269 | 1.1 | ||
| 13 | 444 | 248 | 1.0 | ||
| 14 | 444 | 248 | 1.0 | ||
Surface expression of transfected coreceptor molecules on 293T kidney cells from Fig. 2 B was quantitated by surface staining with either anti-CD8α (experiment [Exp.] 1 and 2) or anti-CD4 (experiment 3) mAbs and flow cytometry. Surface staining of transfected cells was unimodal, and mean fluorescence intensities (expressed in fluorescence units) are indicated for each construct. Within each experiment, mean fluorescence intensity was normalized for each construct relative to that of the wild-type coreceptor construct (either AAA or 444), whose relative fluorescence intensity was set at 1.0.
Sequence homology between the cytosolic domains of CD4 and CD8α is restricted to a short region centered around a CxC cysteine motif that is involved in Lck binding 34 35. To examine the possible role of this cytosolic CxC cysteine motif in LAT–coreceptor associations, we constructed a CD8α variant (AAM) in which both cytosolic cysteines C227 and C229 were mutated to alanines (Fig. 2 A). We found that substitution with alanine of these two cysteines diminished CD8α associations with murine LAT by 70% (Fig. 2 B, compare lanes 8 and 10). Thus, the cytosolic CxC cysteine motif important for coreceptor associations with Lck is also important for coreceptor associations with LAT.
Because LAT molecules contain two molecular species that migrate at either 36 or 38 kD in SDS-PAGE 14 22, we wished to determine if coreceptors preferentially associated with one or the other form of LAT. To address this issue, we used human LAT (hLAT) because the 36- and 38-kD forms of hLAT are easily distinguishable (Fig. 2 C, lysate). We found that CD8 coreceptors were associated almost exclusively with the lower 36-kD form of LAT, and that that association was dependent on the cytosolic cysteines in the CD8α tail (Fig. 2 C, lanes 1–3). However, appearance of the lower 36-kD form of hLAT is dependent on two juxtamembrane cysteines (C26, C29) in the cytosolic tail of hLAT, which are palmitoylated and responsible for targeting LAT molecules to GEMs 22. Alanine substitution of both cysteines (C26/29A) removes the palmitoylation sites and results in migration of LAT as a single 38-kD (upper) band (Fig. 2 C, lanes 4 and 5). Interestingly, despite the fact that the C26/29A mutant LAT molecule cannot be palmitoylated and targeted to GEMs, we found that the mutant LAT can still associate with surface CD8 coreceptor molecules (Fig. 2 C, lanes 4 and 5). These results confirm that CD8–LAT associations are the result of specific protein–protein interactions and not simply of colocalization of both molecules in GEMs. However, we do not yet understand why CD8 normally preferentially associates with the lower band of LAT when it is also clearly capable of binding to the upper band.
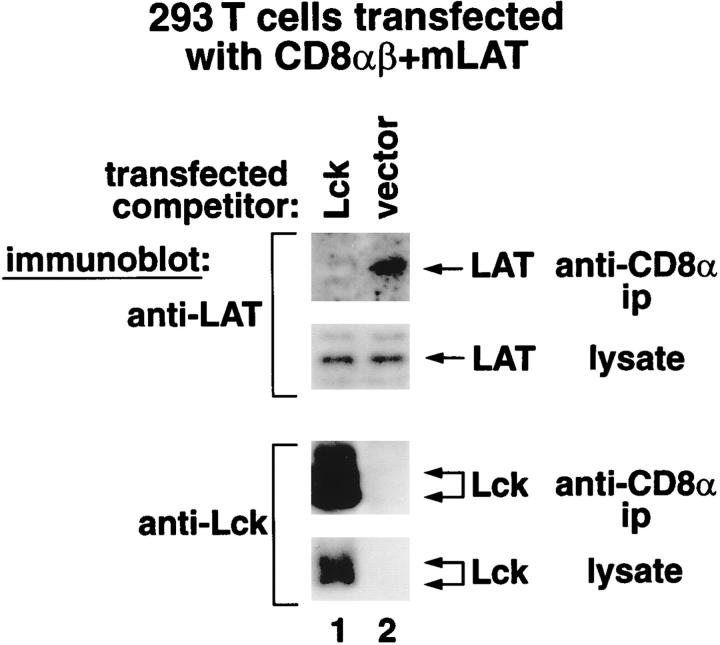
Since the cytosolic cysteine motif in CD8α promotes binding of LAT as well as Lck, we considered that LAT and Lck might compete for binding to individual coreceptor molecules. To address this issue, we transiently expressed CD8α, CD8β, and mLAT in 293T cells. In addition, we also transfected the cells with an excess of either Lck-containing vector or empty vector (Fig. 3). Even though Lck did not affect the amount of LAT present in the cell lysates, Lck abrogated the ability of LAT to be immunoprecipitated by anti-CD8α (Fig. 3). Thus, Lck protein, when present in excess, competes with LAT for binding to CD8.
Figure 3.
Overexpression of Lck inhibits CD8–LAT association in 293T cells. 293T cells were transfected with CD8α, CD8β, and mLAT, and either an Lck expression vector (10-fold excess over the mLAT vector, lane 1) or the same amount of empty control vector (lane 2). Expression of Lck did not detectably change either total LAT expression or surface CD8 expression (data not shown). However, overexpression of Lck resulted in CD8–Lck associations and in the inhibition of CD8–LAT associations. Relative fluorescence intensities (as defined in Table ) for anti-CD8α immunostaining of cells in lane 1 and 2 were identically 1.0.
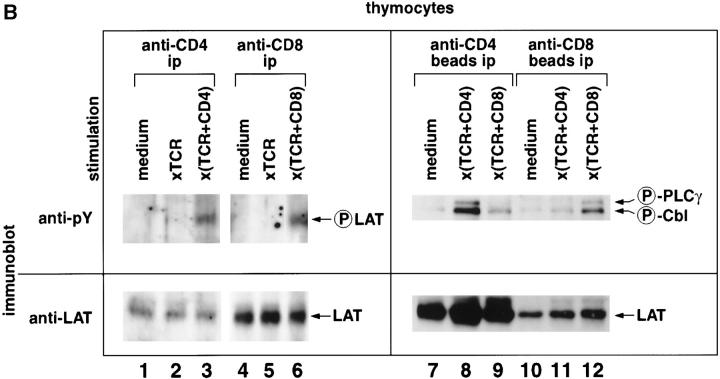
Since LAT function in TCR signal transduction depends on its tyrosine phosphorylation, we examined the ability of coreceptor-associated LAT molecules to be tyrosine phosphorylated. Treatment of thymocytes with pervanadate to induce activation of intracellular protein tyrosine kinases 36 resulted in tyrosine phosphorylation of CD8-associated LAT and recruitment of LAT-binding phosphoproteins (Fig. 4 A) previously identified to include phospholipase C (PLC)-γ1 and Cbl, among others 14. Tyrosine phosphorylation of coreceptor-associated LAT molecules was also induced in thymocytes by antibody-mediated coengagement of TCR with either CD4 or CD8, but was not induced by TCR engagement alone (Fig. 4 B, lanes 1–6). Coengagement of TCR with either CD4 or CD8 also resulted in the appearance of phosphoprotein bands indicative of PLC-γ1 and Cbl (Fig. 4 B, lanes 7–12). Importantly, formation of oligomeric signaling complexes on coreceptor-associated LAT molecules occurred with a remarkable degree of specificity, preferentially forming on coreceptor-associated LAT molecules that had been coengaged with TCR, compared with LAT molecules that had not been coengaged with TCR. That is, PLC-γ1 and Cbl were preferentially recruited to CD4-associated LAT molecules by coengagement of TCR with CD4 compared with coengagement of TCR with CD8 (Fig. 4 B, lanes 7–9); and, reciprocally, PLC-γ1 and Cbl were preferentially recruited to CD8-associated LAT molecules by coengagement of TCR with CD8 compared with coengagement of TCR with CD4 (Fig. 4 B, lanes 10–12). The small amount of phosphorylated Cbl that was immunoprecipitated by antibodies specific for the nonengaged coreceptor probably reflects the passive and inadvertent capture of some nonengaged coreceptor molecules within the TCR aggregate (Fig. 4 B, lanes 9 and 11). Thus, coreceptors can promote LAT phosphorylation by TCR-associated ZAP-70 molecules and the subsequent recruitment of downstream signaling mediators.
Figure 4.
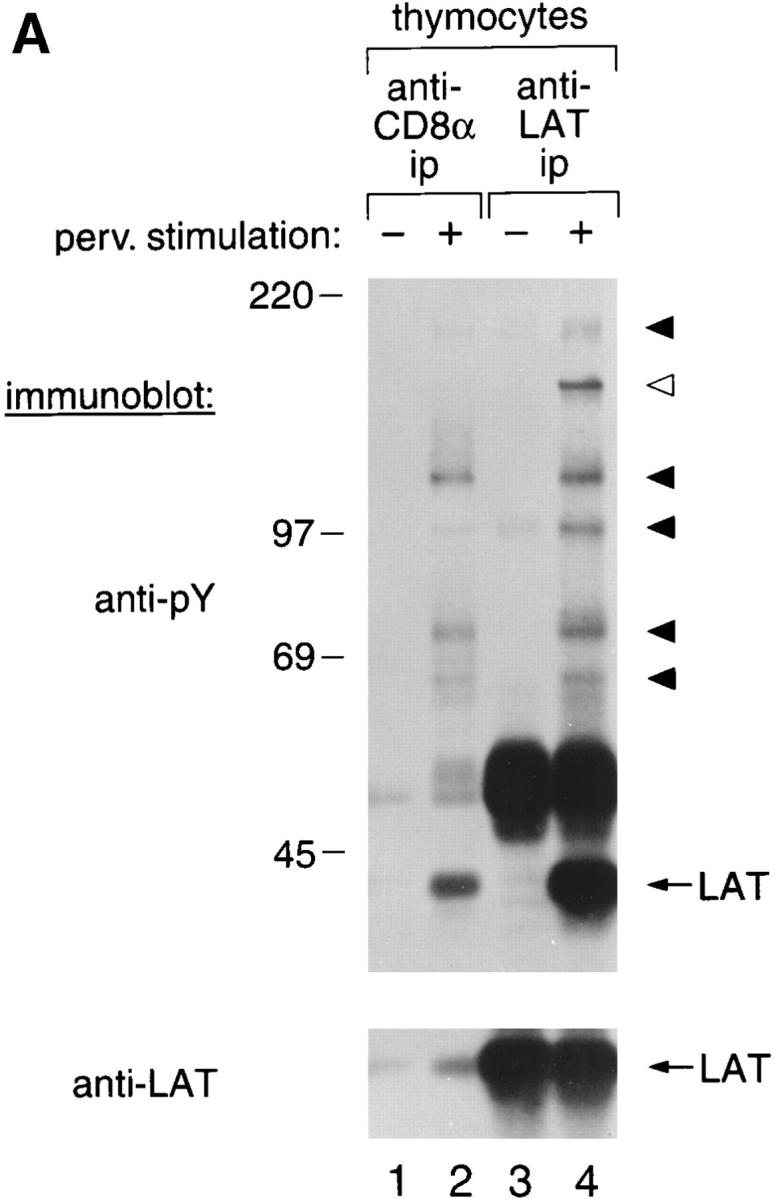
Tyrosine phosphorylation of coreceptor-associated LAT molecules and recruitment of downstream signaling intermediates. (A) Octylglucoside-solubilized lysates from thymocytes stimulated with medium or sodium pervanadate (perv.) were immunoprecipitated (ip), resolved on SDS-PAGE, and immunoblotted with antiphosphotyrosine (anti-pY, upper panel) or anti-LAT (lower panel) antibodies. Molecular weight markers are indicated. Black arrowheads point to phosphotyrosyl proteins found in both anti-LAT and anti-CD8α immunoprecipitates. White arrowhead indicates a phosphotyrosyl protein associated to total LAT, but not detectably present in this CD8α immunoprecipitate. None of these phosphotyrosyl bands were observed in immunoprecipitates prepared in the absence of specific antibody. The 50-kD band in lanes 3 and 4 is Ig H chain from the precipitating antibody. (B) Coengagement of TCR with surface coreceptor molecules results in tyrosine phosphorylation of coreceptor-associated LAT molecules and specific recruitment of downstream signaling intermediates. Cultured thymocytes were stimulated either by TCR cross-linking, or by TCR plus CD4 cocross-linking or TCR plus CD8 cocross-linking and detergent solubilized with octylglucoside. Lysates were immunoprecipitated with either anti-CD4 plus protein G–Sepharose (lanes 1–3) or anti-CD8 plus protein G–Sepharose (lanes 4–6). Because protein G–Sepharose may bind and precipitate the anti-TCR and anti-coreceptor mAbs used for signaling, we did not use protein G–Sepharose to assess the molecular specificity of TCR plus coreceptor cocross-linking. Rather, we used coreceptor-specific mAbs directly coupled to beads (lanes 7–12). Importantly, the immunoprecipitating coreceptor mAbs we coupled to beads were specific for different epitopes on CD4 and CD8 than those used for surface cross-linking so that the stimulating antibodies would not interfere with coreceptor immunoprecipitations. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with either antiphosphotyrosine (anti-pY) or anti-LAT antibodies. The positions of two phosphoproteins of 140 and 120 kD, corresponding by molecular mass to phosphorylated (circled P) PLC-γ1 and Cbl, are indicated (lanes 7–12). TCR plus CD4 cocross-linking resulted in preferential recruitment and phosphorylation of these proteins to CD4-associated LAT molecules (lanes 7–9), and, reciprocally, TCR plus CD8 cocross-linking resulted in preferential recruitment and phosphorylation of these proteins to CD8-associated LAT molecules (lanes 10–12).
Discussion
This study demonstrates that the LAT adaptor molecule associates with CD4 and CD8 surface coreceptors, and that such coreceptor associations are mutually exclusive with Lck. Indeed, the site on the cytosolic tail of CD4 and CD8α coreceptors to which LAT binds overlaps the site to which Lck binds, resulting in competition between Lck and LAT for coreceptor binding. Because of their association with coreceptor molecules, LAT molecules would be juxtaposed with TCR complexes upon coengagement of MHC–peptide complexes. In fact, we found that oligomeric complexes of downstream signaling mediators preferentially formed on those LAT molecules that were associated with coreceptors coengaged with the TCR. Thus, this study provides one solution to the problem of how TCR-associated ZAP-70 molecules can efficiently find their LAT substrate.
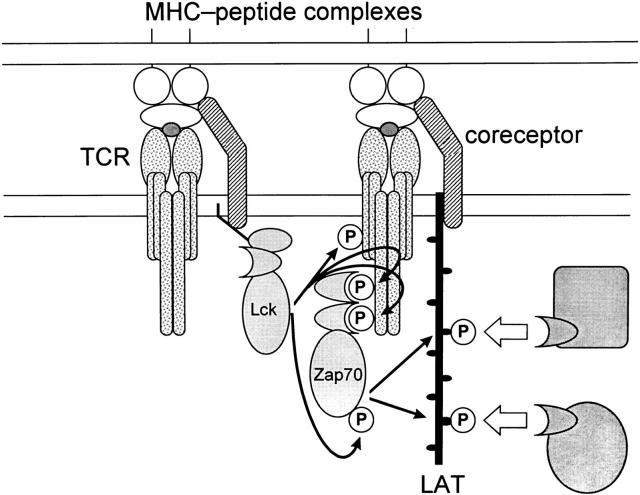
Colocalization of LAT with TCR would be a second function performed by CD4 and CD8 coreceptor molecules in TCR signal transduction, with colocalization of Lck and TCR being the only previously known function. In fact, we think that the two coreceptor functions are analogous to one another, in that coengagement of TCR with CD4 or CD8 coreceptor molecules by MHC–peptide complexes serves to physically juxtapose both Lck and LAT with TCR (schematized in Fig. 5). As a result, coreceptors promote both the initiation of TCR signaling and the activation of downstream signaling mediators. It is tempting to consider the implications of our present observations on the distinct but overlapping roles performed by CD4 and CD8 coreceptors in promoting TCR signal transduction in immature CD4+CD8+ thymocytes during T cell development in the thymus, as CD4 engagement by intrathymic MHC class II molecules would preferentially promote Lck activation 25, whereas CD8 engagement by intrathymic MHC class I molecules would preferentially promote LAT phosphorylation and activation of downstream mediators. It is conceivable that such coreceptor-induced differences in TCR signal transduction pathways influence lineage choices, but this possibility has not yet been examined. Unfortunately, LAT knockout mice have not been informative for this issue, as the absence of LAT arrests thymocyte development at an early CD4−CD8− stage of development that precedes expression of CD4 and CD8 coreceptors as well as the point at which CD4/CD8 lineage determination occurs 37.
Figure 5.
Model for the role of coreceptor-associated molecules in TCR signal transduction. Coengagement of TCR and CD4/CD8 coreceptor molecules by MHC–peptide complexes induces the physical approximation of coreceptor-associated Lck molecules within TCR aggregates, resulting in efficient tyrosine phosphorylation of TCR ITAMs, recruitment of ZAP-70, and efficient tyrosine phosphorylation of TCR-associated ZAP-70 molecule. In addition, coengagement of TCR and CD4/CD8 coreceptor molecules by MHC–peptide complexes also induces the physical juxtaposition of coreceptor-associated LAT molecules within TCR aggregates, promoting the efficient tyrosine phosphorylation of LAT by TCR-associated ZAP-70 molecules. Coreceptor-associated tyrosine-phosphorylated LAT molecules are then bound via SH2 domain interactions by downstream mediators of TCR signal transduction. In this model, CD4/CD8 coreceptors perform two important functions in TCR signal transduction: colocalization of TCR with Lck, and colocalization of TCR with LAT. We think that these two coreceptor functions are mediated by individual coreceptor molecules that are associated either with Lck or with LAT, but alternative stoichiometries are possible.
The association of LAT with surface CD4 and CD8 coreceptor molecules provides one solution to the problem of how TCR-associated ZAP-70 molecules manage to contact and phosphorylate LAT molecules. Indeed, we found that association of LAT with CD4 and CD8 coreceptors was of functional significance for TCR signal transduction, as: (a) coreceptor-associated LAT molecules were tyrosine phosphorylated upon TCR–coreceptor coengagement, and, more importantly, (b) the scaffold for oligomerization of downstream signaling mediators preferentially formed on coreceptor-associated LAT molecules that were coengaged with TCR. Thus, coreceptor-induced colocalization of LAT with TCR promotes downstream TCR signaling events.
We found that coreceptor–LAT associations are promoted by the same dicysteine motif in the coreceptor tails that promotes Lck binding. Nevertheless, we do not think that Lck and LAT bind to coreceptor molecules through identical mechanisms. Association of coreceptor molecules with Lck involves formation of a zinc-dependent complex between the dicysteine motif in the coreceptor tail and two cysteines in Lck 38, and strictly requires those cysteines to be present in both the coreceptor tail and the Lck molecule 34 35. In contrast, association of coreceptor molecules with LAT does not require cysteines in LAT, as CD8–LAT interactions were not abolished by mutation of LAT dicysteines to alanines. In fact, association of coreceptor molecules with LAT also does not strictly require the presence of the dicysteine motif in the coreceptor tail, as mutation of these coreceptor cysteines did not abrogate (but significantly reduced) association with LAT. Thus, we think that the molecular basis for LAT–coreceptor association is distinct from that of Lck–coreceptor association despite their overlapping binding sites. That Lck and LAT actually bind to overlapping sites in the cytosolic tail of CD8α is indicated by cotransfection experiments in which Lck was found to disrupt LAT binding to CD8. Such mutual exclusivity of binding may account for the opposite coreceptor binding preferences of LAT and Lck in CD4+CD8+ thymocytes, such that LAT may be primarily associated with CD8 because Lck is primarily associated with CD4. However, we favor the possibility that Lck and LAT have intrinsically different binding preferences for CD4 and CD8 coreceptor molecules such that Lck binds CD4 > CD8 and LAT binds CD8 > CD4.
LAT molecules are normally palmitoylated and, as a consequence, localized to GEMs 22. Importantly, however, LAT–coreceptor associations are mediated through protein–protein interactions and do not simply reflect their colocalization in GEMs, as LAT–coreceptor associations occur even in the absence of LAT palmitoylation. Indeed, a nonpalmitoylated version of LAT (C26/29A LAT) which does not localize in GEMs still associated with CD8. Nevertheless, it should be appreciated that subpopulations of CD4 and CD8 coreceptor molecules are normally present in GEMs 27, perhaps as a result of their association with palmitoylated LAT molecules. Consistent with such a possibility, we found that detection of CD8–LAT complexes was significantly increased by solubilization of cells with the detergent octylglucoside, which solubilizes GEMs far better than the detergent Triton X-100.
Surface CD4/CD8 coreceptors are not strictly required for TCR signal transduction, as CD4−CD8− T cells, in the absence of either CD4 or CD8 coreceptor molecules, competently transduce TCR signals. Consequently, there must exist coreceptor-independent mechanisms for promoting LAT phosphorylation by ZAP-70, although they may be less efficient than the physical colocalization resulting from LAT association with surface coreceptor molecules. It is conceivable that trapping of GEM-associated LAT molecules between aggregated TCR complexes can induce LAT phosphorylation. Such a possibility is suggested by the finding that TCR stimulation results in the migration of TCR components into GEMs 39 40. Importantly, however, it is not at all clear that ZAP-70 actually translocates to GEMs, a necessary event for ZAP-70 phosphorylation of GEM-associated LAT, as conflicting results have been reported 22 39 40. In any event, the inadvertent trapping of LAT between TCR aggregates would be expected to be significantly less efficient than the juxtaposition of LAT and TCR induced by coengagement of MHC–peptide complexes.
It has been suggested that LAT phosphorylation by ZAP-70 may be enhanced by proteins containing Src homology 2 (SH2) domains such as 3BP2, phosphatidylinositol-3 (PI-3) kinase p85 regulatory subunit, or PLC-γ1. 3BP2 is an SH2-containing adaptor protein that binds to tyrosine-phosphorylated forms of LAT and ZAP-70 41. Because 3BP2 contains only one SH2 domain, it is not evident how it could couple activated ZAP-70 to LAT. PI-3 kinase p85 subunit is a protein that contains two SH2 domains and has been shown in platelets to couple tyrosine-phosphorylated LAT with other tyrosine-phosphorylated molecules such as FcR γ chain 42. Similarly, PLC-γ also has two SH2 domains and could conceivably couple ZAP-70 to LAT 43. However, because SH2 domain–containing linker proteins only bind tyrosine-phosphorylated substrates, they would be expected to promote ZAP-70 associations with already phosphorylated LAT molecules; they would not be expected to promote LAT's initial phosphorylation by ZAP-70. Consequently, the molecular basis for the initial phosphorylation of LAT by ZAP-70 in the absence of CD4 and CD8 coreceptors remains uncertain.
In conclusion, this study documents the association of LAT with CD4 and CD8 coreceptor molecules in resting T cells and thymocytes, and documents that coengagement of coreceptor molecules with surface TCR results in tyrosine phosphorylation of LAT and recruitment of downstream signaling mediators.
Acknowledgments
We thank Jane Parnes (Stanford University) for the generous gifts of CD4 and CD8 cDNAs; A. Bhandoola for help with lymph node cell preparations; D. Winkler for antibody preparations, oligonucleotide synthesis, and DNA sequencing; and J. Ashwell, S. Bunnell, and D. Singer for critical reading of the manuscript.
Footnotes
1used in this paper: GEM, glycolipid-enriched membrane microdomain; ITAM, immunoreceptor tyrosine-activated motif; LAT, linker for activation of T cells; PI-3, phosphatidylinositol-3; PLC, phospholipase C; PTK, protein tyrosine kinase; SH2, src homology 2
References
- Marth J.D., Peet R., Krebs E.G., Perlmutter R.M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985;43:393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Cooke M.P., Perlmutter R.M. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol. 1989;1:66–74. [PubMed] [Google Scholar]
- Weiss A., Littman D.R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Wange R.L., Samelson L.E. Complex complexessignaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Romeo C., Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Wange R.L., Malek S.N., Desiderio S., Samelson L.E. Tandem SH2 domains of ZAP-70 bind to T cell antigen receptor zeta and CD3 epsilon from activated Jurkat T cells. J. Biol. Chem. 1993;268:19797–19801. [PubMed] [Google Scholar]
- Iwashima M., Irving B.A., van Oers N.S., Chan A.C., Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Watts J.D., Affolter M., Krebs D.L., Wange R.L., Samelson L.E., Aebersold R. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase ZAP-70. J. Biol. Chem. 1994;269:29520–29529. [PubMed] [Google Scholar]
- Chan A.C., Dalton M., Johnson R., Kong G.H., Wang T., Thoma R., Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wange R.L., Guitian R., Isakov N., Watts J.D., Aebersold R., Samelson L.E. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J. Biol. Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- Barber E.K., Dasgupta J.D., Schlossman S.F., Trevillyan J.M., Rudd C.E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc. Natl. Acad. Sci. USA. 1989;86:3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M.A., Horak E.M., Samelson L.E., Bolen J.B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Wiest D.L., Ashe J.M., Abe R., Bolen J.B., Singer A. TCR activation of ZAP70 is impaired in CD4+ CD8+ thymocytes as a consequence of intrathymic interactions that diminish available p56lck. Immunity. 1996;4:495–504. doi: 10.1016/s1074-7613(00)80415-x. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LATthe ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Weber J.R., Orstavik S., Torgersen K.M., Danbolt N.C., Berg S.F., Ryan J.C., Tasken K., Imboden J.B., Vaage J.T. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J. Exp. Med. 1998;187:1157–1161. doi: 10.1084/jem.187.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco T.S., Kadlecek T., Zhang W., Samelson L.E., Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Singer A., Hsi E.D., Samelson L.E. Intrathymic signalling in immature CD4+CD8+ thymocytes results in tyrosine phosphorylation of the T-cell receptor zeta chain. Nature. 1989;341:651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- Kubo R.T., Born W., Kappler J.W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J. Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A.L., Fitch F.W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J. Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- Zamoyska R., Derham P., Gorman S.D., von Hoegen P., Bolen J.B., Veillette A., Parnes J.R. Inability of CD8 alpha′ polypeptides to associate with p56lck correlates with impaired function in vitro and lack of expression in vivo. Nature. 1989;342:278–281. doi: 10.1038/342278a0. [DOI] [PubMed] [Google Scholar]
- Landt O., Grunert H.P., Hahn U. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene. 1990;96:125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylationits essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Graham F.L., van der Eb A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Veillette A., Zuniga-Pflucker J.C., Bolen J.B., Kruisbeek A.M. Engagement of CD4 and CD8 expressed on immature thymocytes induces activation of intracellular tyrosine phosphorylation pathways. J. Exp. Med. 1989;170:1671–1680. doi: 10.1084/jem.170.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest D.L., Yuan L., Jefferson J., Benveniste P., Tsokos M., Klausner R.D., Glimcher L.H., Samelson L.E., Singer A. Regulation of T cell receptor expression in immature CD4+CD8+ thymocytes by p56lck tyrosine kinasebasis for differential signaling by CD4 and CD8 in immature thymocytes expressing both coreceptor molecules. J. Exp. Med. 1993;178:1701–1712. doi: 10.1084/jem.178.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Cerny J., Stockinger H., Horejsi V. Noncovalent associations of T lymphocyte surface proteins. Eur. J. Immunol. 1996;26:2335–2343. doi: 10.1002/eji.1830261010. [DOI] [PubMed] [Google Scholar]
- Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Thomas P.M., Samelson L.E. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J. Biol. Chem. 1992;267:12317–12322. [PubMed] [Google Scholar]
- Crooks M.E., Littman D.R. Disruption of T lymphocyte positive and negative selection in mice lacking the CD8 beta chain. Immunity. 1994;1:277–285. doi: 10.1016/1074-7613(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Fung-Leung W.P., Kundig T.M., Ngo K., Panakos J., De Sousa-Hitzler J., Wang E., Ohashi P.S., Mak T.W., Lau C.Y. Reduced thymic maturation but normal effector function of CD8+ T cells in CD8β gene-targeted mice. J. Exp. Med. 1994;180:959–967. doi: 10.1084/jem.180.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie H.Y., Ravichandran K.S., Burakoff S.J. CD8 β chain influences CD8 α chain–associated Lck kinase activity. J. Exp. Med. 1995;181:1267–1273. doi: 10.1084/jem.181.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie H.Y., Mong M.S., Itano A., Crooks M.E., Littman D.R., Burakoff S.J., Robey E. The cytoplasmic domain of CD8 beta regulates Lck kinase activation and CD8 T cell development. J. Immunol. 1998;161:183–191. [PubMed] [Google Scholar]
- Shaw A.S., Chalupny J., Whitney J.A., Hammond C., Amrein K.E., Kavathas P., Sefton B.M., Rose J.K. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol. Cell. Biol. 1990;10:1853–1862. doi: 10.1128/mcb.10.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.M., Brodsky M.H., Irving B.A., Levin S.D., Perlmutter R.M., Littman D.R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Secrist J.P., Burns L.A., Karnitz L., Koretzky G.A., Abraham R.T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J. Biol. Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- Zhang W., Sommers C.L., Burshtyn D.N., Stebbins C.C., DeJarnette J.B., Trible R.P., Grinberg A., Tsay H.C., Jacobs H.M., Kessler C.M. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- Lin R.S., Rodriguez C., Veillette A., Lodish H.F. Zinc is essential for binding of p56(lck) to CD4 and CD8alpha. J. Biol. Chem. 1998;273:32878–32882. doi: 10.1074/jbc.273.49.32878. [DOI] [PubMed] [Google Scholar]
- Montixi C., Langlet C., Bernard A.M., Thimonier J., Dubois C., Wurbel M.A., Chauvin J.P., Pierres M., He H.T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. Embo (Eur. Mol. Biol. Organ.) J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Deckert M., Tartare-Deckert S., Hernandez J., Rottapel R., Altman A. Adaptor function for the Syk kinases-interacting protein 3BP2 in IL-2 gene activation. Immunity. 1998;9:595–605. doi: 10.1016/s1074-7613(00)80657-3. [DOI] [PubMed] [Google Scholar]
- Gibbins J.M., Briddon S., Shutes A., van Vugt M.J., van de Winkel J.G., Saito T., Watson S.P. The p85 subunit of phosphatidylinositol 3-kinase associates with the Fc receptor gamma-chain and linker for activator of T cells (LAT) in platelets stimulated by collagen and convulxin. J. Biol. Chem. 1998;273:34437–34443. doi: 10.1074/jbc.273.51.34437. [DOI] [PubMed] [Google Scholar]
- Williams B.L., Irvin B.J., Sutor S.L., Chini C.C., Yacyshyn E., Bubeck Wardenburg J., Dalton M., Chan A.C., Abraham R.T. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1832–1844. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourvieille B., Gorman S.D., Field E.H., Hunkapiller T., Parnes J.R. Isolation and sequence of L3T4 complementary DNA clonesexpression in T cells and brain. Science. 1986;234:610–614. doi: 10.1126/science.3094146. [DOI] [PubMed] [Google Scholar]