Abstract
T1/ST2, an orphan receptor with homology with the interleukin (IL)-1 receptor family, is expressed constitutively and stably on the surface of T helper type 2 (Th2) cells, but not on Th1 cells. T1/ST2 is also expressed on mast cells, which are critical for Th2-mediated effector responses. To evaluate whether T1/ST2 is required for Th2 responses and mast cell function, we have generated T1/ST2-deficient (T1/ST2−/−) mice and examined the roles of T1/ST2. Naive CD4+ T cells isolated from T1/ST2−/− mice developed to Th2 cells in response to IL-4 in vitro. T1/ST2−/− mice showed normal Th2 responses after infection with the helminthic parasite Nippostrongylus brasiliensis as well as in the mouse model of allergen-induced airway inflammation. In addition, differentiation and function of bone marrow–derived cultured mast cells were unaffected. These findings demonstrate that T1/ST2 does not play an essential role in development and function of Th2 cells and mast cells.
Keywords: gene targeting, interleukin 1 receptor family, mast cell, asthma, T helper cells types 1 and 2
The orphan receptor T1/ST2 belongs to the IL-1R family. The T1/ST2 gene was originally identified as a late response gene that was induced by either serum or overexpression of v-mos and Ha-ras oncogenes in murine fibroblasts 1 2 3. Alternative 3′ processing of the primary transcript generates two messengers of 2.7 and 5 kb long. The short mRNA encodes a 38.5-kD peptide with similarity to the extracellular portion of the IL-1Rs 4, and is secreted from the cell as a heavily glycosylated protein of 50–60 kD. The short mRNA is found in embryonic tissues and mammary tumors, and is induced in fibroblasts upon stimulation with proliferation-inducing agents such as serum, platelet-derived growth factor, fibroblast growth factor, 12-O-tetradecanoyl-phorbol-13-acetate, and lysophosphatidic acid 2 5 6. On the other hand, the long mRNA encodes a single transmembrane-spanning protein with significant homology to the type 1 IL-1R. Expression of the long mRNA was restricted to hematopoietic organs and the lung 5 7. The gene for ST2 was mapped to mouse chromosome 1 closely linked to the il-1r locus containing both type I and type II receptor genes, in support of their common ancestry 8. Despite their similarity to the IL-1R, the T1/ST2 proteins do not bind either IL-1α or IL-1β 9 10. Two different groups have reported the identification of putative T1/ST2 ligands, but it is still unclear whether these proteins indeed represent the physiological T1/ST2 ligands 9 11.
Th cells are categorized into two subsets, Th1 and Th2 cells, based on their cytokine production. Th1 cells secrete IL-2 and IFN-γ, which promote mainly cellular immunity. Th2 cells produce IL-4, IL-5, IL-10, and IL-13, and promote mainly humoral immunity 12 13. The Th1 to Th2 balance determines the outcome of a wide variety of immune responses involving infectious, autoimmune, and allergic diseases. Acquired resistance against intracellular bacteria is associated with the Th1 response, whereas resistance against helminths is achieved by the Th2 response 14. Autoimmune diseases such as multiple sclerosis and Crohn's disease are associated with an abnormally strong Th1 response, whereas allergic diseases seem to involve an abnormally strong Th2 response 15 16 17 18. Both Th1 and Th2 cells are derived from a common precursor cell named naive Th cells: IL-12 stimulates the differentiation of naive Th to Th1 cells 19, whereas IL-4 regulates Th2 differentiation 20 21. These cytokines are produced in the early phase of infection: IL-12 derives from infected macrophages 22, and IL-4 from basophils, mast cells, and CD4+NK1.1+ T cells 23 24 25.
Recently it has been demonstrated that T1/ST2 is preferentially expressed on murine Th2 cells and is important for Th2 effector functions. Using differential display PCR comparing a panel of Th1 and Th2 clones, Xu et al. 26 and Löhning et al. 27 independently identified T1/ST2 as a gene expressed on Th2 but not Th1 cells. T1/ST2 is constitutively and stably expressed on the surface of murine Th2 cells. Furthermore, Th1/Th2 cytokine coexpression is observed in the T1/ST2-negative population but not in the T1/ST2-positive population, indicating that surface expression of T1/ST2 is an advanced commitment to the Th2 phenotype. In vivo administration of anti-T1/ST2 Ab enhanced Th1 responses by increasing IFN-γ production and decreasing IL-4 and IL-5 synthesis, and thereby induced resistance to Leishmania major infection in BALB/c mice and exacerbated collagen-induced arthritis in DBA/1 mice. In addition, pretreatment with anti-T1/ST2 Ab or T1/ST2 fusion protein abrogated both the accumulation of eosinophils in the airways and the secretion of Th2 cytokines in a murine model of Th2-dependent allergic airway inflammation. Mast cells are critical effectors in Th2 cell–dependent immune responses and in the pathogenesis of Th2 cell–dependent disorders. Mast cells are also found to express T1/ST2 at high levels 28 29.
In this study, we have generated mice deficient in the T1/ST2 gene, and using the mutant mice we examined the roles of T1/ST2 in helminth infection and a mouse model of asthma, as well as in mast cell differentiation and function.
Materials and Methods
Reagents.
Recombinant murine IL-12 was provided by Hayashibara Biochemical Laboratories, Inc. (Okayama, Japan). FITC-conjugated anti–mouse T1/ST2 Ab (clone DJ8) was purchased from Morwell Diagnostics GmbH. Levels of IL-4, IL-5, IL-10, and IFN-γ were determined by ELISA kit (Genzyme).
Cell Culture.
Lymphoid cell growth medium consisted of RPMI 1640 medium (Life Technologies) supplemented with 10% (vol/vol) fetal bovine serum, 100 μM 2-ME, 5 U/ml penicillin, and 50 μg/ml streptomycin. Bone marrow cell culture medium consisted of αMEM (Life Technologies) supplemented with 10% (vol/vol) fetal bovine serum, 5 U/ml penicillin, and 50 μg/ml streptomycin.
Generation of T1/ST2-deficient Mice.
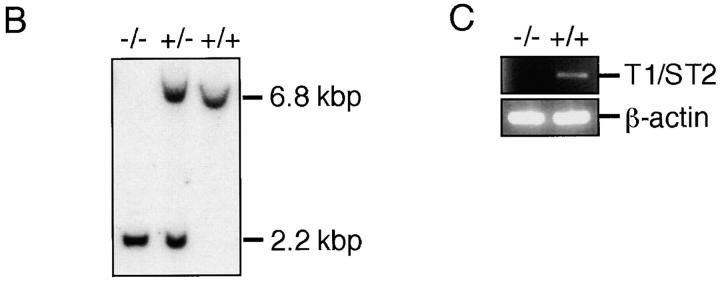
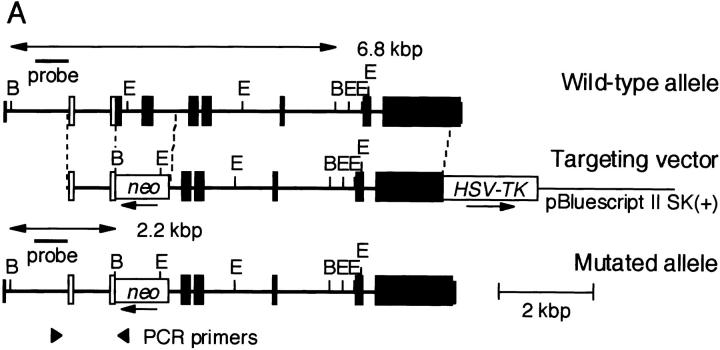
The T1/ST2 locus on the mouse genome has been submitted to the EMBL/GenBank/DDBJ database (available under accession no. X60184). A targeting vector was designed to replace a 1,280-bp genomic fragment encoding amino acids 1–96 of the mature T1/ST2 with neomycin resistance gene (neo) from pMC1-neo (Stratagene). The targeting vector was flanked by the 5.8-kbp fragment at the 3′ end and the 1.0-kbp fragment at the 5′ end. A herpes simplex virus–thymidine kinase gene (HSV-tk) was inserted into the 3′ end of the vector. The targeting vector was linearized with SalI and electroporated into E14.1 embryonic stem (ES) cells. The clones resistant to G418 and ganciclovir were screened by PCR and confirmed by Southern blot analysis with the probe shown in Fig. 1 A for homologous recombination. An external primer (5′-AGGTGGATTATGACGTTGTGCTCATGG-3′) specific for the T1/ST2 gene upstream of the targeting construct and a neo primer (5′-ATCGCCTTCTATCGCCTTCTTGACGAG-3′) specific for neo were used in the PCR screening. Generation of chimeric mice and mutant mice was essentially as described previously 30.
Figure 1.
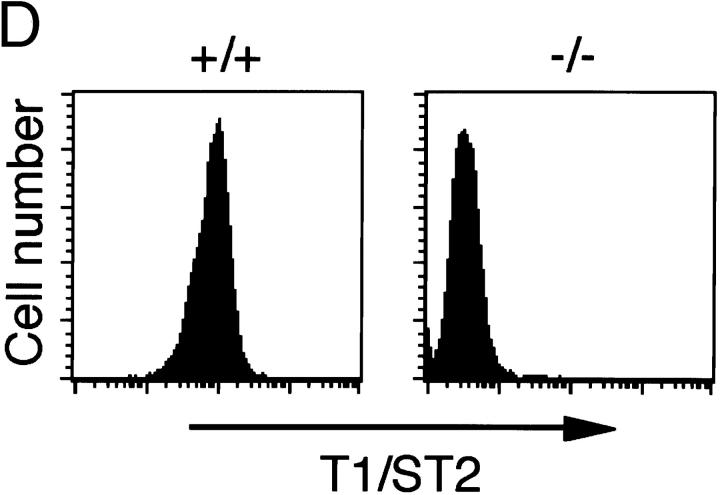
Targeted disruption of the mouse T1/ST2 gene. (A) Targeting vector and restriction map of the T1/ST2 locus. The restriction map of the wild-type allele, targeting vector, and mutated allele is shown. Open and filled boxes denote the noncoding and coding exons, respectively. The orientation of the neomycin resistance gene (neo) and the herpes simplex virus–thymidine kinase gene (HSV-TK) are indicated by the arrow. E, EcoRI; B, BamHI. (B) Southern blot analysis of offspring from the heterozygote intercrosses. Genomic DNA was extracted from mouse tails, digested with BamHI, electrophoresed, and hybridized with the probe as shown in A. The approximate size of the wild-type band is 6.8 kbp, and the mutated band is 2.2 kbp. +/+, wild-type; +/−, heterozygous mutant; −/−, homozygous mutant. (C) RT-PCR analysis of T1/ST2 mRNA. Total RNA was isolated from the bone marrow cells. PCR analysis was performed using specific primers for T1/ST2 or for β-actin (reference 38). (D) Expression of T1/ST2 on BMCMCs. BMCMCs were triple stained with FITC–anti-T1/ST2 Ab, PE–anti–c-Kit Ab, IgE/biotin–anti-IgE Ab/Streptavidin–Cy-Chrome. T1/ST2 expression was analyzed in histogram mode by gating on the IgE+c-Kit+ double positive population which was >99% of the cells analyzed.
In Vitro Induction of Th1/Th2 Cell Differentiation.
Splenocytes were incubated with biotinylated anti–I-Ab Ab (clone 25-9-17; PharMingen), biotinylated anti-CD8 Ab (clone 53-6.7; PharMingen), biotinylated anti–pan-NK cell Ab (clone DX5; PharMingen), biotinylated anti-CD45R Ab (clone RA3-6B2; PharMingen), and streptavidin microbeads (MACS; Miltenyi Biotec) followed by magnetic field separation to remove MHC class II, CD8, and B220-expressing cells and NK cells. Cell purity was analyzed by flow cytometry using FITC-conjugated anti-CD3 Ab (clone 145-2C11; PharMingen) and PE-conjugated anti-CD4 Ab (clone L3T4; PharMingen). Purified cells were cultured on 10 μg/ml anti-CD3 Ab–coated plates (clone 145-2C11; PharMingen) in the presence of exogenous cytokines and anticytokine Ab as indicated. Th2 differentiation was promoted in the presence of 1,000 U/ml IL-4 (PharMingen) and 10 μg/ml anti–IFN-γ Ab (clone XMG1.2; Endogen), whereas Th1 differentiation was promoted in the presence of 2 ng/ml IL-12. After the 5-d culture, cells were harvested and washed with HBSS. Cells (106/ml) were stimulated on an anti-CD3 Ab–coated 96-well plate for 24 h. Supernatants were analyzed by cytokine ELISA.
After 8 d of culture, cells were also analyzed for intracellular cytokines. Staining was performed by Cytofix/CytoPerm Plus kit (PharMingen). The following Abs were used: Cy-Chrome–conjugated anti-CD4 Ab (clone H129.19; PharMingen), PE-conjugated anti–IL-4 Ab (clone BVD4-1D11; PharMingen), and FITC-conjugated anti–IFN-γ Ab (clone XMG1.2; PharMingen). Samples were analyzed by three-color flow cytometry, and gates were set on CD4+ lymphocytes.
Nippostrongylus Infection.
800 third-stage Nippostrongylus brasiliensis larvae were injected subcutaneously into 10-wk-old T1/ST2−/− and wild-type control mice. Mice were killed 8 d after infection, and CD4+ T cells were enriched from the spleen by positive selection on LS+ columns (MACS), according to the manufacturer's instructions (Miltenyi Biotec). CD4+ T cells were cultured at 106/ml in 96-well plates coated with 10 μg/ml anti-CD3 Ab for 24 h. Supernatants were analyzed by cytokine ELISA. In a separate experiment, serum Ig levels were measured by ELISA.
Bone Marrow–derived Mast Cell Preparation and Stimulation.
Bone marrow was aseptically flushed from femora of 8-wk-old mice. The cells were cultured at 106/ml in bone marrow cell culture medium supplemented with 10% (vol/vol) PWM-stimulated spleen cell conditioned medium 31 for 3 wk. The bone marrow cultures were enriched for mast cells by repetitively transferring the suspension cell fraction into fresh culture flasks. After 3 wk of culture, culture medium was changed to bone marrow cell culture medium containing 10 ng/ml recombinant mouse IL-3 (Genzyme/Techne). After 1 wk of culture, mast cells were analyzed for T1/ST2 expression by incubating with IgE,κ (clone IgE-3; PharMingen), PE-conjugated anti–c-Kit (clone 3C1; Immunotech), and 0.8 μg/ml FITC-conjugated anti-T1/ST2 Ab, followed by biotinylated anti–mouse IgE (clone R35-92; PharMingen) and Cy-Chrome–conjugated streptavidin (PharMingen).
After washing, cells were resuspended at 106/ml and stimulated for 24 h with the polyclonal activator PMA at 50 ng/ml and 1 μM ionomycin. Supernatants were analyzed by cytokine ELISA. IgE cross-linking was performed by first incubating the cells for 30 min at 37°C in the presence of 3 μg/ml of monoclonal mouse IgE anti-dinitrophenyl group (DNP) (clone SPE-7; Sigma Chemical Co.). The mast cells were washed three times and then incubated with 10 ng/ml DNP30–40 HSA (Sigma Chemical Co.) for 30 min at 37°C. After the treatment with antigens, supernatants were measured for histamine concentration by RIA (SRL, Inc.).
Sensitization Procedure and Allergen Exposure.
Immunization and exposure of mice were carried out according to the method of Foster et al. 32. In brief, 8- or 10-wk-old mice were sensitized by intraperitoneal injection with 50 μg OVA/1 mg aluminum hydroxide in 0.9% saline on days 0 and 12. Nonsensitized mice received 1 mg of aluminum hydroxide in 0.9% saline. On day 24, mice were placed individually in a chamber and inhaled aerosolized OVA (10 mg/ml) in 0.9% saline (sensitized, OVA-challenged group) for 30 min three times at 1-h intervals, and then every second day thereafter, for 8 d. Nonsensitized mice received saline only (nonsensitized, nonchallenged group). The aerosols were generated by ultrasonic nebulization. 24 h after the last aeroallergen challenge, the mice were anesthetized by intraperitoneal injection of pentobarbital (80 mg/kg body wt) and used for the following experiments. The blood was collected, and serum Ig levels were measured by ELISA.
Bronchoalveolar Lavage.
The trachea was cannulated, and the airway lumina were washed twice with 0.5 ml of PBS (Ca2+, Mg2+ free) supplemented with 50 μM EDTA and 0.1% BSA. The bronchoalveolar lavage (BAL) fluid was immediately centrifuged (10 min at 4°C, 700 g). The BAL cells were resuspended in 0.2 ml of rat serum and counted using a hemocytometer. For differential cell counts, 2–4 × 103 cells were spun onto glass slides, air dried, fixed with ethanol, and stained with Giemsa solution. The numbers of eosinophils, neutrophils, lymphocytes, and macrophages in 200 cells were counted based on morphology and staining characteristics.
ELISA for Measurement of Serum Ig Level.
Serum IgG1 level was measured as described previously 33, except that the following reagents were used: goat anti–mouse IgG1, mouse IgG1, biotinylated goat anti–mouse IgG1, and streptavidin-conjugated alkaline phosphatase (Southern Biotechnology Associates). Serum IgE level was measured according to protocol supplied by PharMingen.
Results and Discussion
Generation of T1/ST2-deficient Mice.
The mouse T1/ST2 gene was disrupted by homologous recombination in E14.1 ES cells. A targeting vector was designed to delete the first and second ATG codons of T1/ST2 with the neomycin resistance gene (Fig. 1 A). 2 out of 733 clones screened were positive for homologous recombination. Two targeted ES clones were microinjected into C57BL/6 blastocysts, and successfully transmitted the disrupted T1/ST2 gene through the germline (Fig. 1 B). T1/ST2−/− mice were born at the expected Mendelian ratios (+/+: +/−: −/− = 55: 96: 49). The mice grew healthy and showed no obvious abnormalities until 20 wk old. Homozygous mutants of both sexes were fertile, and there was no significant difference in the birth rate between T1/ST2−/− and wild-type mice. Thus, T1/ST2 was not required for mouse development or fertility. Reverse transcription (RT)-PCR analysis using total RNA from bone marrow cells of T1/ST2−/− mice confirmed the absence of expression of T1/ST2 mRNA (Fig. 1 C). T1/ST2 has been shown to be abundantly expressed on the cell surface of mast cells and Th2 cells 26 27 28 29. When bone marrow cells were cultured in the presence of IL-3, these cells differentiated into mast cells expressing both high-affinity IgE receptor, Fc∈RI, and c-Kit receptor. Analysis by two-color dot plot revealed that >99% of the cells were IgE+c-Kit+ double positive (data not shown). In wild-type mice, these bone marrow–derived mast cells expressed T1/ST2 on their cell surface (Fig. 1 D). In contrast, T1/ST2 was not expressed on T1/ST2−/− mast cells. When splenic CD4+ T cells were skewed for Th2 cell differentiation, several cells expressed T1/ST2 on their cell surface (data not shown). However, Th2-skewed CD4+ T cells from T1/ST2−/− mice did not express T1/ST2 at all. Thus, targeted disruption of the T1/ST2 gene resulted in no expression of T1/ST2 protein in mice. FACS® analysis of the expression of CD3, B220, CD4, CD8, I-Ab, and IgM in thymocytes and splenocytes showed that lymphocyte composition was not altered in 6-wk-old T1/ST2−/− mice compared with wild-type mice of the same age (data not shown).
Normal Th2-mediated Functions in T1/ST2−/− Mice.
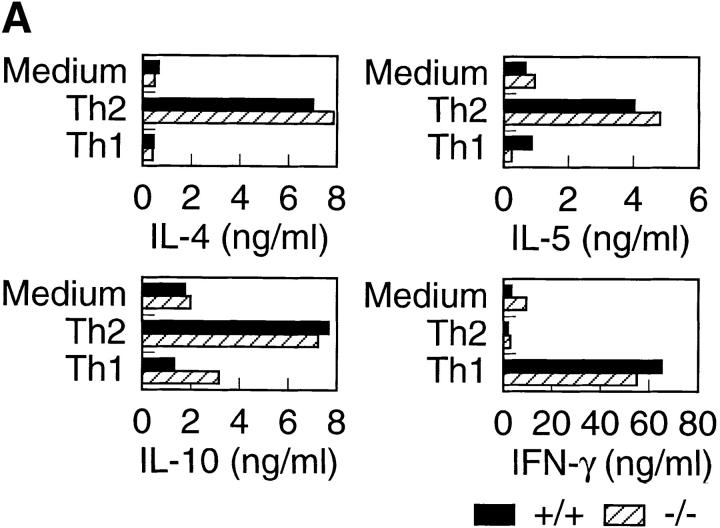
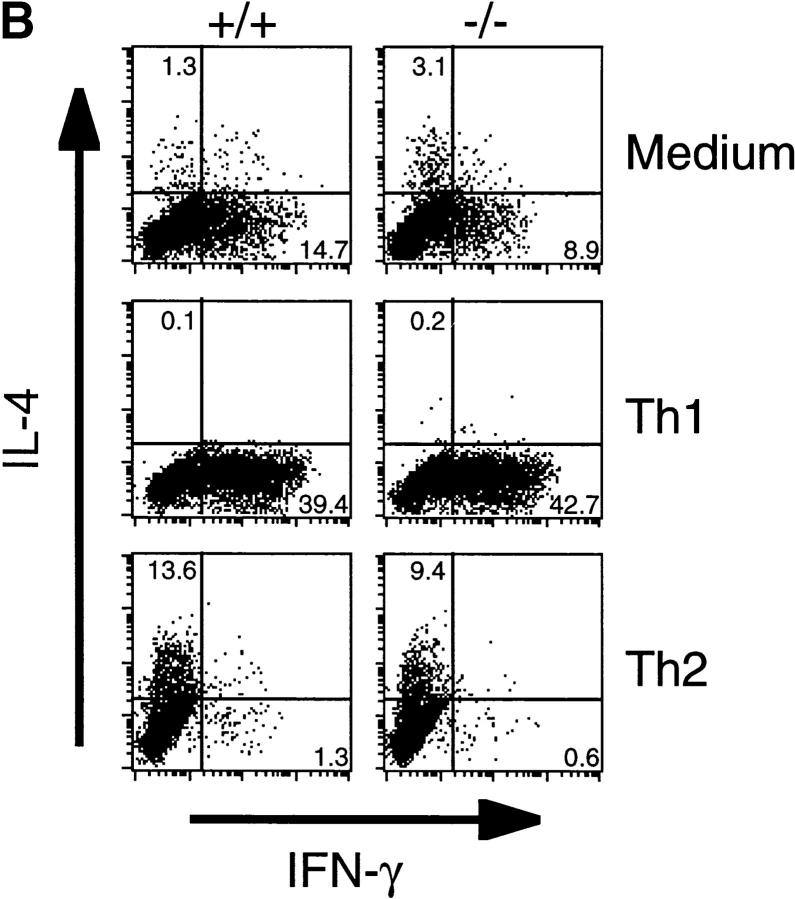
It has recently been shown that treatment of mice with anti-T1/ST2 Ab resulted in decreased Th2 cell–mediated functions, indicating that T1/ST2 might be functionally associated with Th2 cells 26 27. Therefore, we analyzed in vitro and in vivo Th2 responses of T1/ST2−/− mice. First, we explored in vitro differentiation assays to assess the effect of T1/ST2 deficiency on cytokine production from Th cells. CD4+ T cells were cultured for 5 d in the presence of IL-12 or IL-4 plus anti–IFN-γ Ab to develop into Th1 or Th2 cells, respectively. Th1-developing cells from both wild-type and T1/ST2−/− mice produced almost equal amounts of IFN-γ (Fig. 2 A). Th2-developing cells from wild-type mice produced increased levels of Th2 cytokines such as IL-4, IL-5, and IL-10. The cells from T1/ST2−/− mice also produced the equivalent levels of Th2 cytokines. Th cells from wild-type mice developed into IL-4 producers in response to IL-4 or into IFN-γ producers in response to IL-12 (Fig. 2 B). The cells from T1/ST2−/− mice developed into IL-4 or IFN-γ producer cells as did the cells from wild-type mice. These results indicate that IL-4–induced in vitro Th2 cell differentiation was not affected in T1/ST2−/− mice.
Figure 2.
Cytokine production after in vitro Th cell differentiation. (A) CD4+ T cells purified from spleen from wild-type and T1/ST2−/− mice were stimulated under Th1 or Th2 culture conditions for 5 d, washed, and restimulated on anti-CD3 Ab–coated plates for 24 h. Supernatants were analyzed for production of IL-4, IL-5, IL-10 and IFN-γ by ELISA. The experiment shown is representative of three independent experiments. (B) FACS® analysis of intracellular cytokine. CD4+ T cells were cultured under Th1 or Th2 condition for 8 d, then stimulated for 4 h with PMA/ionomycin, fixed, and stained for CD4, IL-4, and IFN-γ. For analysis, gate was set on CD4+ lymphocytes. The frequency of cytokine-producing CD4+ cells is indicated in the quadrants as a percent.
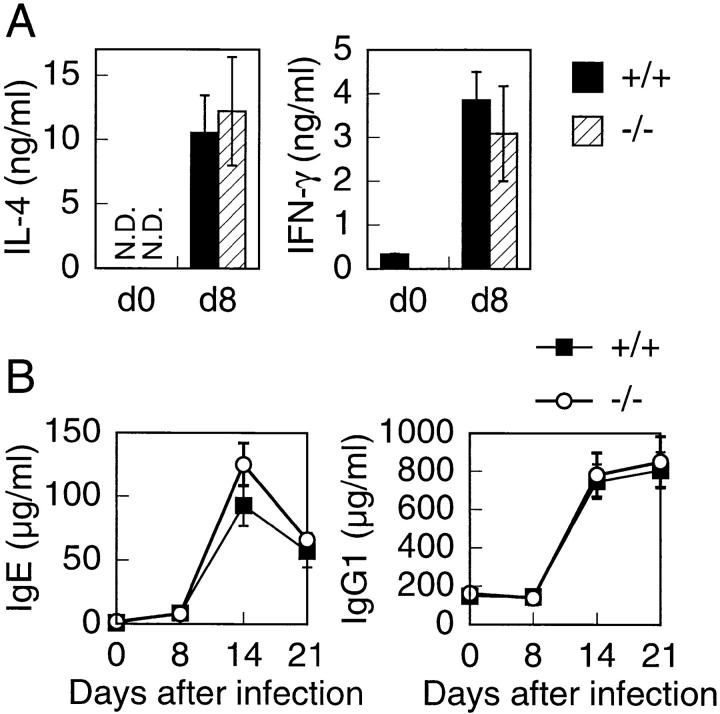
Next, we examined in vivo Th2 response by infecting with the intestinal parasitic nematode N. brasiliensis. CD4+ splenic T cells were prepared 8 d after infection and stimulated with immobilized anti-CD3 Ab for 24 h. The supernatants were assayed for cytokine production by ELISA. Both wild-type and T1/ST2−/− CD4+ T cells produced the same amounts of IL-4 (Fig. 3 A). IFN-γ production from both CD4+ T cells was almost the same. IL-4 is shown to be required for Ig class switching of B cells to IgE and IgG1 producers 20 21 34 35 36. Therefore, we measured serum Ig levels before and after N. brasiliensis infection. Total serum IgE and IgG1 levels after infection were almost equivalent in both wild-type and T1/ST2−/− mice (Fig. 3 B). These data indicate that T1/ST2 is not functionally involved in Th2 response in vivo.
Figure 3.
Cytokine production from splenic T cells and serum Ig levels in response to parasite infection. (A) Splenic CD4+ T cells were prepared from uninfected animals (d0) or animals 8 d after infection (d8) with N. brasiliensis. Cells were stimulated with immobilized anti-CD3 Ab for 24 h. Supernatants were analyzed by ELISA. Data are representative of three experiments consisting of cohorts of two mice at each time point. (B) T1/ST2−/− mice (eight mice) and wild-type (six mice) were infected with N. brasiliensis. Serum IgE and IgG1 levels were measured at days 0, 8, 14, and 21 after infection. Serum samples were assayed by ELISA. Data are presented as mean ± SEM. N.D., not detectable.
Normal Functions of Mast Cells in T1/ST2−/− Mice.
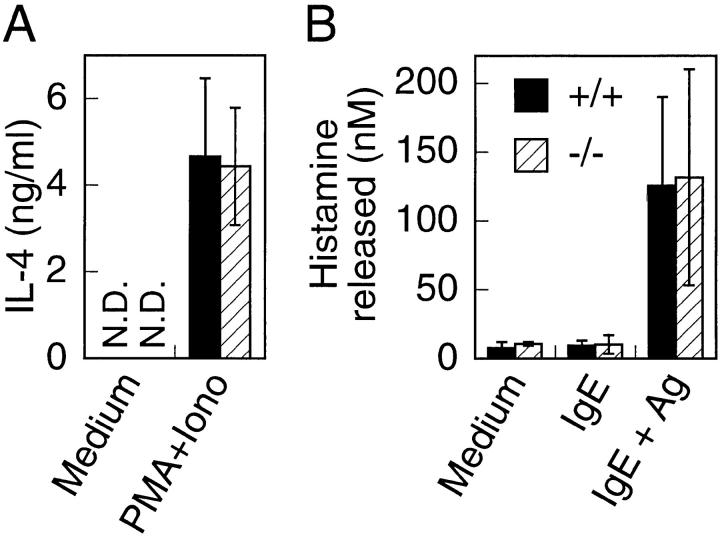
T1/ST2 has been shown to be expressed on bone marrow–derived cultured mast cells (BMCMCs) and peritoneal mast cells and to be regarded as a cell surface marker for the mast cell lineage 28 29. Mast cells have been shown to secrete IL-4 and histamine when stimulated. We analyzed production of these mediators from BMCMCs. Both wild-type and T1/ST2−/− BMCMCs secreted almost the same levels of IL-4 upon stimulation with PMA and ionomycin (Fig. 4 A). Further, both wild-type and T1/ST2−/− cells released almost equal levels of histamine in response to IgE and Ag (Fig. 4 B). Mast cells were classified into two subtypes: the connective tissue–type mast cell, which is found in the skin, musculature, perivascular tissues, and peritoneal cavity, and the mucosal mast cell, which is widely distributed in mucosal tissues of the respiratory tract and the intestinal lamina propria 37. The number of connective tissue–type mast cells in the back skin of T1/ST2−/− mice was almost the same as that of wild-type mice. The number of mucosal mast cells in the lamina propria of the stomach was also equivalent between T1/ST2−/− and wild-type mice, indicating that mast cell development is not affected in T1/ST2−/− mice (data not shown). Thus, our data suggest that T1/ST2 expression does not contribute to development and function of mast cells.
Figure 4.
Mast cell analysis. (A) BMCMCs from T1/ST2−/− and wild-type mice were stimulated with PMA and ionomycin for 24 h. Supernatants were analyzed by ELISA. (B) Histamine release from BMCMCs stimulated with IgE and specific antigen. The data shown are representative of the three experiments. Data are presented as mean ± SEM. N.D., not detectable.
Development of Allergen-induced Airway Inflammation in T1/ST2−/− Mice.
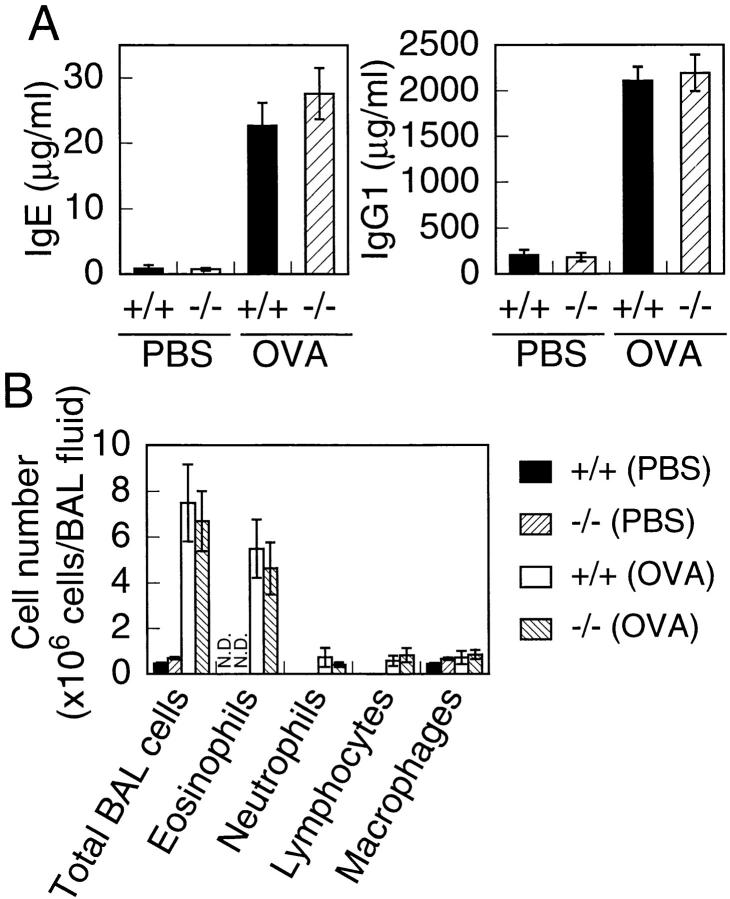
It has been shown that Th2-mediated eosinophilic lung inflammation of airways is attenuated by pretreatment with anti-T1/ST2 mAb 27. Therefore, we examined whether T1/ST2 deficiency influences inflammatory responses in the murine model of Th2-dependent allergic airway inflammation. Wild-type and T1/ST2−/− mice immunized with OVA were exposed to an aerosol of OVA. 24 h after OVA challenge, mice were bled, and serum concentrations of IgE and IgG1 were measured by ELISA. Serum concentrations of IgE and IgG1 were equally increased after OVA treatment in both wild-type and T1/ST2−/− mice (Fig. 5 A). We also analyzed the cells in BAL fluid 24 h after the exposure to OVA aerosol. The total cell number of BAL cells was dramatically increased after OVA exposure in both wild-type and T1/ST2−/− mice (Fig. 5 B). Furthermore, a dramatic increase in eosinophils was observed in both wild-type and T1/ST2−/− mice. Histological analysis of lung taken from both wild-type and T1/ST2−/− mice exposed to OVA displayed severe widespread inflammatory infiltration (data not shown). Thus, T1/ST2−/− mice exhibited normal eosinophilic response in the mouse model of allergic airway inflammation.
Figure 5.
Analysis of Th2 effector function of T1/ST2−/− mice on a mouse model of asthma. (A) Changes in serum concentrations of IgE and IgG1 induced by OVA treatment. Data are presented as mean ± SEM for six mice. (B) BAL cell number and cellular distribution of BAL fluid. BAL fluid was obtained from T1/ST2−/− and wild-type mice after treatment with or without OVA. The columns represent the absolute numbers of total BAL cells, eosinophils, neutrophils, lymphocytes and macrophages per animal. Data are presented as mean ± SEM for five mice.
In this study, we generated T1/ST2−/− mice and analyzed their phenotype. T1/ST2 has been shown to be expressed on the cell surface of Th2 cells, but not Th1 cells. Furthermore, administration of anti-T1/ST2 Ab resulted in reduced Th2-mediated functions in mice 26 27. Thus, T1/ST2 has been implicated in Th2-mediated functions. However, T1/ST2−/− mice displayed almost normal Th2 responses in both nematode infection and allergic airway inflammation. These results clearly demonstrate that T1/ST2 is not essential to Th2 cell functions. Since it has been shown that treatment of Th2 cells with anti-T1/ST2 Ab enhances complement-mediated lysis in vitro 26, the reduced Th2-mediated functions observed in the studies with anti-T1/ST2 Ab administration might be a result of complement-mediated elimination of the T1/ST2-positive cell population. In addition to Th2 cells, mast cells express high levels of T1/ST2. However, development and activity of mast cells were unaffected in T1/ST2−/− mice. Thus, our knockout study reveals that T1/ST2 does not play a critical role in development and function of Th2 cells and mast cells, although we cannot rule out the possibility that T1/ST2 may play some role in Th2 response. Alternatively, T1/ST2 may have an unknown function in these or other cell populations. At present, a ligand for T1/ST2 has not been identified. The identification of the ligand will reveal a novel function of T1/ST2 as well as its role in Th2 response.
Acknowledgments
We thank Dr. Tsuneyasu Kaisho for helpful discussion, Ms. Tomoko Aoki, Nana Iwami, and Etsuko Nakatani for technical assistance, and all members of our laboratory for their helpful advice in the preparation of this manuscript.
This work was supported by grants from the Ministry of Education, Science, and Culture of Japan.
References
- Werenskiold A.K., Hoffmann S., Klemenz R. Induction of a mitogen-responsive gene after expression of the Ha-ras oncogene in NIH 3T3 fibroblasts. Mol. Cell. Biol. 1989;9:5207–5214. doi: 10.1128/mcb.9.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler U., Andres A.C., Reichmann E., Schmahl W., Werenskiold A.K. T1, an immunoglobulin superfamily member, is expressed in H-ras-dependent epithelial tumours of mammary cells. Oncogene. 1993;8:609–617. [PubMed] [Google Scholar]
- Klemenz R., Hoffmann S., Werenskiold A.K. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA. 1989;86:5708–5712. doi: 10.1073/pnas.86.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- Rossler U., Thomassen E., Hultner L., Baier S., Danescu J., Werenskiold A.K. Secreted and membrane-bound isoforms of T1, an orphan receptor related to IL-1-binding proteins, are differently expressed in vivo. Dev. Biol. 1995;168:86–97. doi: 10.1006/dbio.1995.1063. [DOI] [PubMed] [Google Scholar]
- Kalousek M.B., Trub T., Schuermann M., Klemenz R. T1 is a c-Fos- and FosB-responsive gene which is induced by growth factors through multiple signal transduction pathways. J. Biol. Chem. 1994;269:6866–6873. [PubMed] [Google Scholar]
- Bergers G., Reikerstorfer A., Braselmann S., Graninger P., Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga S., Jenkins N.A., Gilbert D.J., Copeland N.G., Tetsuka T. Molecular cloning of the murine ST2 gene. Characterization and chromosomal mapping. Biochim. Biophys. Acta. 1991;1090:1–8. doi: 10.1016/0167-4781(91)90029-l. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K., Naito Y., Kuroiwa K., Arai T., Furukawa Y., Tomizuka H., Miura Y., Kasahara T., Tetsuka T., Tominaga S. The expression of ST2 gene in helper T cells and the binding of ST2 protein to myeloma-derived RPMI8226 cells. J. Biochem. (Tokyo). 1997;121:95–103. doi: 10.1093/oxfordjournals.jbchem.a021577. [DOI] [PubMed] [Google Scholar]
- Reikerstorfer A., Holz H., Stunnenberg H.G., Busslinger M. Low affinity binding of interleukin-1β and intracellular signaling via NF-κB identify Fit-1 as a distant member of the interleukin-1 receptor family. J. Biol. Chem. 1995;270:17645–17648. doi: 10.1074/jbc.270.30.17645. [DOI] [PubMed] [Google Scholar]
- Gayle M.A., Slack J.L., Bonnert T.P., Renshaw B.R., Sonoda G., Taguchi T., Testa J.R., Dower S.K., Sims J.E. Cloning of a putative ligand for the T1/ST2 receptor. J. Biol. Chem. 1996;271:5784–5789. doi: 10.1074/jbc.271.10.5784. [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., Cherwinski H., Bond M.W., Giedlin M.A., Coffman R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Mosmann T.R., Coffman R.L. TH1 and TH2 cellsdifferent patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- Laman J.D., Thompson E.J., Kappos L. Balancing the Th1/Th2 concept in multiple sclerosis. Immunol. Today. 1998;19:489–490. doi: 10.1016/s0167-5699(98)01320-6. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Trapasso F., Parrello T., Biancone L., Stella A., Iuliano R., Luzza F., Fusco A., Pallone F. Bioactive IL-18 expression is up-regulated in Crohn's disease. J. Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- Romagnani S. Th1 and Th2 in human diseases. Clin. Immunol. Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Magram J., Connaughton S.E., Warrier R.R., Carvajal D.M., Wu C.Y., Ferrante J., Stewart C., Sarmiento U., Faherty D.A., Gately M.K. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M.C., Bluethmann H., Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Rajewsky K., Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Hsieh C.S., Macatonia S.E., Tripp C.S., Wolf S.F., O'Garra A., Murphy K.M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Seder R.A., Paul W.E., Dvorak A.M., Sharkis S.J., Kagey-Sobotka A., Niv Y., Finkelman F.D., Barbieri S.A., Galli S.J., Plaut M. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc. Natl. Acad. Sci. USA. 1991;88:2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Pierce J.H., Watson C.J., Hanley-Hyde J., Nordan R.P., Paul W.E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Watson C., Hu-Li J., Paul W.E. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- Xu D., Chan W.L., Leung B.P., Huang F., Wheeler R., Piedrafita D., Robinson J.H., Liew F.Y. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhning M., Stroehmann A., Coyle A.J., Grogan J.L., Lin S., Gutierrez-Ramos J.C., Levinson D., Radbruch A., Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz D.R., Gheyselinck J., Klemenz R. Expression analysis of the soluble and membrane-associated forms of the interleukin-1 receptor-related T1 protein in primary mast cells and fibroblasts. Hybridoma. 1998;17:107–116. doi: 10.1089/hyb.1998.17.107. [DOI] [PubMed] [Google Scholar]
- Moritz D.R., Rodewald H.R., Gheyselinck J., Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J. Immunol. 1998;161:4866–4874. [PubMed] [Google Scholar]
- Takeda K., Noguchi K., Shi W., Tanaka T., Matsumoto M., Yoshida N., Kishimoto T., Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriu A., Sonoda S., Kanakura Y., Jozaki K., Yamatodani A., Kitamura Y. Proliferative potential of degranulated murine peritoneal mast cells. Blood. 1989;74:925–929. [PubMed] [Google Scholar]
- Foster P.S., Hogan S.P., Ramsay A.J., Matthaei K.I., Young I.G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho T., Schwenk F., Rajewsky K. The roles of gamma 1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses. Science. 1997;276:412–415. doi: 10.1126/science.276.5311.412. [DOI] [PubMed] [Google Scholar]
- Finkelman F.D., Katona I.M., Urban J.F., Jr., Snapper C.M., Ohara J., Paul W.E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc. Natl. Acad. Sci. USA. 1986;83:9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F.D., Holmes J., Katona I.M., Urban J.F., Jr., Beckmann M.P., Park L.S., Schooley K.A., Coffman R.L., Mosmann T.R., Paul W.E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Rothman P. Interleukin 4 targeting of immunoglobulin heavy chain class-switch recombination. Res. Immunol. 1993;144:579–583. doi: 10.1016/s0923-2494(05)80006-9. [DOI] [PubMed] [Google Scholar]
- Galli S.J. New concepts about the mast cell. N. Engl. J. Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Takeda K., Kamanaka M., Tanaka T., Kishimoto T., Akira S. Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J. Immunol. 1996;157:3220–3222. [PubMed] [Google Scholar]