Abstract
The hexavalent meningococcal vaccine HexaMen, containing six PorAs on two vesicles, was tested in clinical studies. Although fourfold increases in serum bactericidal activity (SBA) titers against all of the PorAs were observed, there were significant differences between PorA-specific SBA titers. SBA titers were mainly directed against one PorA from each vesicle, P1.5-2,10 and P1.5-1,2-2, and were lower against the other PorAs, especially P1.7-2,4 and P1.19,15-1. We investigated whether these differences were due to immunological interference that resulted in competition between the three PorAs on the same vesicle or whether they were caused by a difference in the immunogenicities of the separate PorAs. Therefore, mice were immunized either with HexaMen, with six monovalent outer membrane vesicles (OMVs) representing the same six PorAs simultaneously (HexaMix), or with only one of the monovalent OMVs. The immunoglobulin G and SBA titers after HexaMen immunization in mice resembled the results obtained in clinical studies. Although immunization with HexaMix gave higher titers than immunization with HexaMen for some PorAs, the pattern of high and low titers was the same. Similar differences in immunogenicity between subtypes were seen after monovalent immunization when interference was eliminated as a cause of the differences. Monovalent immunization resulted in higher titers for P1.5-1,2-2 and P1.7,16 than immunization with HexaMen. However, no significant differences were found for the weakly immunogenic PorAs, P1.7-2,4 and P1.19,15-1. Since immunization with the six PorAs in the trivalent presentation form (HexaMen) and in the mixture of monovalent vesicles (HexaMix) resulted in the same pattern of high and low titers, we concluded that the differences between the PorA-specific responses are due to differences in the immunogenicities of the various PorAs and not due to interference that results in competition between different PorAs.
Meningococcal disease is one of the major health problems in children and adolescents in many countries. The clinical symptoms vary from self-limiting bacteremia to meningitis or fulminant sepsis, and the overall mortality is 7 to 10%. Neisseria meningitidis serogroup B still causes the majority of the infections in northern Europe (4), and an effective vaccine is needed to control the disease. The meningococcal serogroup B capsular polysaccharide is unsuitable as a vaccine candidate due to its structural similarity to human glycoproteins (8). Therefore, vaccine research has been focused on outer membrane proteins, mainly PorA, since this outer membrane protein is known to elicit strong bactericidal antibodies (15). This protein consists of 16 transmembrane regions with eight surface-exposed loops (22), is expressed on the membrane as a homotrimer (10), and functions as a cationic porin (20). Human and murine bactericidal antibodies are mainly directed against two hypervariable regions in loop 1 (VR1) and loop 4 (VR2) of PorA (15, 24).
Outer membrane vesicle (OMV) vaccines derived from clinical isolates, containing one PorA, have been developed in Cuba (serosubtype P1.19,15), Norway (serosubtype P1.7,16), and the United States (serosubtype P1.7-2,3). These vaccines were tested in several clinical studies (2, 17, 19). The induced serum bactericidal activity (SBA) was mainly serosubtype specific and was low for heterologous strains. Due to the occurrence of a considerable number of serosubtypes in clinical isolates, protection was limited. To improve protection, a hexavalent vaccine has been developed at the National Institute for Public Health and the Environment, Bilthoven, The Netherlands (5, 23). This vaccine (HexaMen) consists of OMVs of two trivalent strains, each expressing three serosubtypes (one strain expresses P1.7,16, P1.5-1,2-2, and P1.19,15-1, and the other expresses P1.5-2,10, P1.12-1,13, and P1.7-2,4), and covers at least one-half of the clinical serogroup B isolates in The Netherlands. HexaMen has been proven to be safe and immunogenic in clinical studies in The Netherlands and the United Kingdom (3, 7, 16), but there are significant differences between PorA-specific SBA titers. The SBA titers are highest against serosubtypes P1.5-2,10 and P1.5-1,2-2, moderate against P1.7,16 and P1.12-1,13, and relatively low against P1.7-2,4 and P1.19,15-1 (3, 7). The immunoglobulin G (IgG) isotype distributions appear to be similar for all six PorAs and cannot explain the difference in SBA (6, 14).
The aim of this study was to investigate whether the presentation form of the vaccine influences the PorA-specific IgG and SBA responses in mice against each of the six PorAs or, alternatively, whether the presence of multiple PorAs results in immunological competition. We compared the PorA-specific IgG responses and SBA titers in sera of mice immunized with HexaMen, mice immunized with a mixture of six monovalent OMVs expressing the same six PorAs (HexaMix), and mice immunized with each monovalent OMV separately. We found that the trivalent presentation form has only a limited effect on the PorA-specific response compared to the effect of the mixed monovalent presentation form. The PorAs differed in immunogenicity, independent of the presentation form and independent of simultaneous immunization with other PorAs.
MATERIALS AND METHODS
OMV vaccine preparations.
(i) Strains. The hexavalent meningococcal OMV vaccine was produced by using two different trivalent N. meningitidis strains, strains HP16215 and HP10124. Strains HP16215 and HP10124 are similar to the previously described and clinically tested strains PL16215 and PL10124 (7, 23), except that the third porA gene is inserted into the ClaI site ca. 80 bp downstream of the rmp gene instead of into the SnaBI site in an additional rmp gene. Each of the strains produces three different PorA proteins (HP16215 produces P1.7,16, P1.5-1,2-2, and P1.19,15-1, and HP10124 produces P1.5-2,10, P1.12-1,13, and P1.7-2,4), expresses GalE lipopolysaccharide (LPS), and is capsular polysaccharide negative.
Monovalent OMV vaccines were obtained from PorB/Rmp-negative variants of N. meningitidis strain H44/76 (P1.7,16) and five PorB/Rmp-negative isogenic PorA strains (TR52 [P1.5-1,2-2], TR10 [P1.5-2,10], TR1213 [P1.12-1,13], TR15 [P1.19,15-1], and TR4 [P1.7-2,4]), which differed only in the PorA gene, as described previously (16). OMVs of the PorA-deficient strain HI-5 were used as a control for enzyme-linked immunosorbent assay (ELISA) coating (20).
(ii) OMV isolation.
OMVs were obtained by extraction of bacteria with 0.5% deoxycholate in 0.1 M Tris-HCl-10 mM EDTA (pH 8.6) and were purified by differential centrifugation (9). Characterization and control of the hexavalent PorA OMV vaccine have been described previously (5). The protein concentration was determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.), and the percentage of PorA was determined by scanning a sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis gel, followed by Coomassie brilliant blue staining. The LPS content was determined by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (11), followed by silver staining (21), by using a standard curve. The functional activity of LPS was determined by a Limulus amebocyte lysate (LAL) assay as described by Claasen et al. (5) by using the official standard (BRP3; National Institute for Biological Standards Control, Potters Bar, United Kingdom).
To prepare HexaMix, the six PorB/Rmp-negative monovalent OMVs were mixed shortly before use so that the same amount of each PorA was present.
Immunizations.
Immunization experiments were performed with groups consisting of eight female BALB/c mice that were 6 to 8 weeks old. Group 1 mice received HexaMen (two trivalent vesicles of HP10124 and HP16215) having a total PorA content of 9 μg. The mice in group 2 were immunized with HexaMix, which had a total PorA content of 9 μg (1.5 μg of each PorA). The mice in groups 3 to 8 each received one of the six monovalent OMVs with a PorA content of 1.5 μg. The volume of each dose was 0.3 ml, and each dose contained 0.45 mg of AlPO4 as an adjuvant. Immunizations were given subcutaneously on days 0 and 28, and serum was collected on day 42. Experiments were carried out in duplicate.
Antibody ELISA.
PorA-specific IgG antibody titers were determined by an ELISA. Briefly, flat-bottom 96-well microtiter plates (Immulon 2; Nunc, Roskilde, Denmark) were coated overnight at room temperature with OMVs of each of the six monovalent PorB/Rmp-negative vaccine strains (3 μg of protein/ml). As a control for PorA specificity, we used OMVs of the PorA-deficient strain HI-5 for coating plates (3 μg of protein/ml). After overnight incubation, the plates were washed three times with a 0.03% Tween 80 solution in tap water. The plates were then incubated for 80 min at 37°C with threefold serial dilutions of the serum samples in phosphate-buffered saline containing 0.05% Tween 80. After incubation, the plates were washed three times with 0.03% Tween 80 in tap water. PorA-specific IgG levels were measured by using goat anti-mouse IgG-horseradish peroxidase conjugate (Southern Biotechnology Associates Inc., Birmingham, Ala.). The conjugate was diluted 1:5,000 in phosphate-buffered saline containing 0.05% Tween 80 and 0.5% skim milk powder (Protifar; Nutricia, Zoetermeer, The Netherlands), and 100 μl was added to the wells. The plates were sealed and incubated at 37°C for 80 min. The plates were then washed three times with 0.03% Tween 80 in tap water and once with tap water alone. A peroxidase substrate (100 μl of 3,3′,5,5′-tetramethylbenzidine with 0.01% H2O2 in 0.11 M sodium acetate buffer [pH 5.5]) was added to each well, and the plates were incubated for l0 min at room temperature. The reaction was stopped by adding 100 μl of 2 M H2SO4 to each well. The IgG antibody titers were expressed as the log10 of the serum dilution giving 50% of the maximum optical density at 450 nm.
Serum bactericidal assay.
The SBA of mouse sera against strain H44/76 (B:15:P1.7,16), the five PorB/Rmp-positive isogenic PorA strains (TR52, TR15, TR1213, TR4, and TR10), and HI-5 were determined after two immunizations as previously described (24). Briefly, sera were diluted 1:5 in Grey’s balanced salt solution containing 0.5% bovine serum albumin and complement inactivated (30 min, 56°C), and serial dilutions were added to 96-well plates. Bacteria were grown in Mueller-Hinton broth (approximately 80 min, 37°C) until the optical density at 620 nm was 0.220 to 0.240, diluted in GBSS containing 0.5% bovine serum albumin, and added to the wells (total concentration, 104 CFU/ml). Each preparation was incubated for 20 min at room temperature. Baby rabbit complement (80%) was added, zero-time samples were plated, and the 96-well plates were incubated at 37°C for 60 min. The SBA titer was calculated by determining the log2 reciprocal of the serum dilution that resulted in ≥90% killing based on the concentrations in the zero-time samples. When the SBA titers exceeded 1:640 (log2 >9.32), the serum was diluted 1:200 before complement inactivation, which enabled measurement of maximum titers of 1:25,600 (log2 14.64).
Statistics.
The IgG titer was expressed as the log10 value of the geometric mean titer (GMT) obtained for each group of mice plus the standard error of the mean. The SBA titer was expressed as the log2 average value obtained for each group of mice. Experiments were performed in duplicate. Differences between titers were considered significant at P values of ≤0.05, as determined by the Student t test.
RESULTS
OMV vaccines.
After deoxycholate extraction, the two trivalent OMV preparations contained 2.7 and 4.0 mg of protein per ml. The protein consisted of approximately 85 to 90% PorA. Minor amounts of Rmp, Opa, and Opc were also present, while PorB was absent. The amount of GalE LPS in the OMVs was 7.5 to 10% of the amount of protein, and the activity was found to be 370 to 1,000 endotoxin unit (EU)/mg of protein.
The monovalent OMV vaccines were diluted so that the protein content of each vaccine was 1.0 mg/ml. The relative PorA contents varied and were 85% for P.5-2,10 OMVs, 88% for P1.12-1,13, P1.7,16, and P1.19,15-1 OMVs, 90% for P1.7-2,4 OMVs, and 94% for P1.5-1,2-2 OMVs. PorB and Rmp were absent. The amount of wild-type (L3) LPS in monovalent OMVs was 4 to 7.5% of the amount of protein, which resulted in a functional activity of 8,000 EU/mg of protein in the combined HexaMix vaccine as determined by the LAL assay.
Comparison of PorA-specific responses.
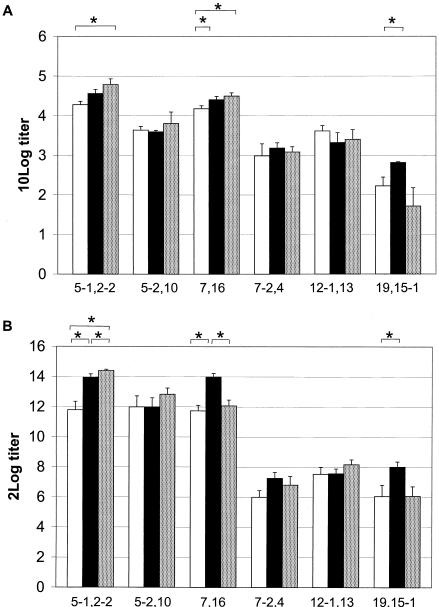
The IgG titers against OMVs of Rmp/PorB-negative isogenic strains and the functional SBA responses to six Rmp/PorB-positive isogenic strains were measured following HexaMen, HexaMix, and monovalent OMV immunizations (Fig. 1A). The SBA titers after the different immunizations were compared (Fig. 1B). Irrespective of the presentation form or the presence of other PorAs, P1.5-1,2-2 always raised the highest titers, followed by P1.7,16 and P1.5-2,10. P1.12-1,13 raised moderate titers, and P1.7-2,4 and P1.19,15-1 raised the lowest titers. The order of the SBA responses was the same for all three immunizations: P1.5-1,2-2 > P1.7,16 ≥ P1.5-2,10 > P1.12-1,13 > P1.7-2,4 > P1.19,15-1.
FIG. 1.
IgG GMT (A) and SBA titers (B) against six PorAs after immunization with HexaMen (open bars), HexaMix (solid bars), and homologous monovalent OMVs (dotted bars). ELISA plates were coated with PorB/Rmp-negative OMVs consisting of 85 to 94% PorA. The error bars indicate the standard errors of the means. Asterisks indicate significant differences in PorA-specific titers following the different types of immunization (P ≤ 0.05).
After HexaMen immunization, the IgG titers against P1.5-1,2-2 and P1.7,16 OMVs were significantly higher than the IgG titers against all other proteins (Fig. 1A). The titers against P1.5-2,10 and P1.12-1,13 OMVs were also significantly higher than the titers against P1.7-2,4, while the titers against P1.19,15-1 OMVs were the lowest titers observed. The SBA titers showed the same pattern, and the titers against the P1.5-1,2-2, P1.5-2,10, and P1.7,16 strains were at least 12-fold higher than titers against the P1.7-2,4, P1.12-1,13, and P1.19,15-1 strains (Fig. 1B).
HexaMix immunization resulted in the same pattern of PorA-specific IgG and SBA titers that was seen after HexaMen immunization: P1.5-1,2-2 ≥ P1.7,16 > P1.5-2,10 ≥ P1.12-1,13 ≥ P1.7-2,4 ≥ P1.19,15-1 (Fig. 1). Even though PorAs were present on monovalent OMVs and equal amounts of the PorAs were in the HexaMix vaccine, the IgG and SBA titers against P1.5-1,2-2 and P1.7,16 were significantly higher than the titers against the other PorAs.
Immunization with the six PorAs separately again resulted in the same pattern of PorA-specific IgG and SBA responses that was obtained after immunizations with HexaMen and HexaMix; the same three PorAs raised the highest titers, and the same three PorAs raised lower titers (Fig. 1).
When PorA-specific IgG and SBA titers were compared, the P1.5-1,2-2-, P1.7,16-, and P1.19,15-1-specific titers were lower after HexaMen immunization than after HexaMix or monovalent immunization. The P1.19,15-1 IgG and SBA titers were highest after HexaMix immunization, but there was not a significant difference between monovalent immunization and HexaMen immunization. The anti-P1.5-2,10, anti-P1.7-2,4, and anti-P1.12-1,13 IgG and SBA titers after HexaMen immunization were not lower than the SBA titers obtained after monovalent or HexaMix immunization. Overall, the pattern of SBA titers closely resembled the pattern of the IgG titers, although the IgG and SBA titers for individual serum samples were poorly correlated. The titers were PorA specific, since the IgG and SBA titers against the PorA-deficient strain HI-5 were generally low and absent, respectively (data not shown).
Although the values of some PorA-specific titers varied among the immunization protocols used, the patterns of immunogenicity of the PorAs were the same after all three types of immunization; P1.5-1,2-2 was the most immunogenic, followed by P1.7,16, P1.5-2,10, and P1.12-1,13. The titers against P1.7-2,4 and P1.19,15-1 were low, independent of the immunization. The immunogenicities of PorAs differed and appeared to be only partially dependent on the trivalent presentation form or the presence of other PorAs.
DISCUSSION
HexaMen has been shown to be a safe and immunogenic vaccine. However, considerable differences in the IgG and SBA titers against the different PorAs were found in clinical studies in The Netherlands and the United Kingdom (3, 7). Antibodies were mainly directed against one PorA of each type of vesicle: P1.5-1,2-2 for OMVs of strain HP16215 and P1.5-2,10 for OMVs of strain HP10124, followed by P1.7,16 and P1.12-1,13.
Here we showed that the pattern of PorA-specific IgG and SBA titers after HexaMen immunization matched the pattern of immunogenicity of PorAs in clinical studies, which indicates that the mouse model can function as a predictor of immunogenicity of PorA in humans. The four PorAs that raise the highest titers in humans did so in mice as well. The lowest titers were observed for PorA subtypes P1.19,15-1 and P1.7-2,4. P1.12-1,13 gave moderate titers in the mouse model, similar to what was observed in humans. Minor differences were observed between the human and mouse responses. P1.5-1,2-2 appeared to be the most immunogenic protein in mice, while P1.5-2,10 was the most immunogenic protein in humans and the response to P1.7,16 was greater in mice than in humans. The difference in the magnitudes of the PorA-specific SBA responses induced with HexaMen is not restricted to BALB/c mice and humans, since HexaMen immunization of NIH mice (5), Wistar rats (unpublished data), and infant cynomolgus monkeys (25) resulted in the same pattern.
In this study, the trivalent presentation form of PorA in HexaMen gave rise to an IgG and SBA response profile that was very similar to that obtained with a mixture of monovalent OMVs. PorA, which is present in homotrimers on monovalent OMVs (10), may form heterotrimers in HexaMen. If so, this had no effect on the profile of the IgG and SBA responses and only a limited effect on the sizes of the titers against individual PorAs.
Some differences between the responses to HexaMen and HexaMix were observed. HexaMix gave rise to significantly higher titers for the strong immunogenic PorAs P1.5-1,2-2 and P1.7,16. After HexaMen immunization the SBA titers of sera against these PorAs were still high (average titers, at least 2,400) and most likely offered good protection against these PorAs. HexaMix immunization resulted in higher P1.19,15-1 titers than HexaMen or monovalent immunization. There was, however, no difference in the P1.19,15-1 titers between monovalent immunization and HexaMen immunization. One possible explanation for these minor differences between HexaMix and HexaMen may be the endotoxin activity of the L3 LPS in HexaMix, which is 4.5-fold higher than the activity of GalE LPS in HexaMen, as determined by the LAL assay.
Finally, the differences in PorA-specific titers also appeared when mice were immunized with monovalent OMVs, which eliminated any form of immunological interference by either the putative trivalent presentation form in HexaMen or the simultaneous administration of six PorAs. This finding shows that the trivalent presentation form or the simultaneous administration of six PorAs has no effect on the pattern and only a limited effect on the sizes of the PorA-specific responses. Thus, the differences in the immune responses to the six PorAs in HexaMen are not due to immunologic interference but are due to differences in the immunogenicities of the PorAs.
The differences in immunogenicity between PorAs in this study are in line with the observations of other workers. First, both Tappero et al. (19) and Perkins et al. (17) showed that the P1.7,16 OMV vaccine (NIPH, Oslo, Norway) was more immunogenic than the P1.19,15-based OMV vaccine (Finlay Institute, Havana, Cuba) in clinical studies. In Icelandic teenagers (19), the proportions of responders (defined as individuals who exhibited at least a fourfold increase in the SBA) to the P1.19,15 OMV vaccine were not higher than the proportions of responders in the control group, whereas the P1.7,16 OMV vaccine resulted in a significant increase in the number of responders. A booster dose given 12 months later resulted in an increase in the number of SBA responders, and again immunization with P1.19,15 OMVs resulted in a lower proportion of responders (44%) than immunization with P1.7,16 OMVs resulted in (84%). When SBA titers induced by these vaccines were compared, the difference was even more pronounced; the GMT for the P1.19,15 group was 9.2, and the GMT for the P1.7,16 group was 64.6. A clinical study in Chile with these vaccines (19) indicated that after two doses of the P1.19,15 OMV vaccine, 38 to 56% of the individuals responded with a fourfold increase in the SBA titer, while the P1.7,16 OMV vaccine resulted in 75 to 96% responders. Both OMV vaccines are heterologous for antigens other than PorA. Even though other antigens, like Opc, induce some bactericidal antibodies (18), PorA is known to be the major antigen for bactericidal antibodies, and SBA titers are mainly PorA directed. In HexaMen, in which the effects of other antigens are the same for all PorAs, the difference between P1.7,16 and P1.19,15-1 was still striking, and the SBA GMT for P1.7,16 (5.86) was threefold higher than the GMT for P1.19,15-1 (1.95) in toddlers (7).
Second, similar results were obtained by using recombinant PorA with liposomes as the vaccine carrier (H. E. Humphries, K. Jolley, J. N. Williams, M. Christodoulides, and J. E. Heckels, Abstr. 13th Int. Pathogenic Neisseria Conf., p. 262, 2002). P1.7-2,4 and P1.19,15 also raised lower titers than P1.7,16 and P1.5-1,10-4 raised after immunization with separate monovalent, combined monovalent, and tetravalent liposomes (Humphries et al., Abstr. 13th Int. Pathogenic Neisseria Conf.), also suggesting that certain PorAs are immunodominant.
The cause of the immunodominance of one PorA over other PorAs is unknown, since the PorAs are approximately 90% homologous and were thought to behave similarly with respect to immunogenicity. The amino acid differences are mainly located in the two hypervariable regions, loop 1 and loop 4. The extracellular exposure of these loops suggests that the differences in immunogenicity should be due to weaker or less accessible B-cell epitopes in P1.7-2,4 and P1.19,15-1. This may be caused by physical and chemical properties, like hydrophobicity and the length of the loops. Loop 1 of P1.7-2,4 is three amino acids shorter than loop 1 of P1.7,16 but is three, four, and five amino acids longer than loop 1 of P1.19,15-1, P1.5-1,2-2, and P1.5-2,10, respectively. The accessibility of these loops may be influenced by downward folding, but the length of loop 1 gives no indication of a weaker epitope for P1.7-2,4 and P1.19,15-1. Loop 4, on the other hand, is two amino acids shorter in both weak immunogenic PorAs (P1.7-2,4 and P1.19,15-1), indicating that there may be reduced accessibility of this loop for antibodies and/or antigen-presenting cells in induction of the immune response. This hypothesis is supported by the observation that most antibodies after P1.7-2,4 OMV immunization are directed against loop 4 and only a few antibodies are directed against loop 1. Also, the combination of loops may play a role in the accessibility of the epitopes, since steric hindrance of loop 4 can prevent accessibility of loop 1 (1). The lower accessibility of the loops may play a role in the induction of the immune response, as well as in measurement of the induced immune response.
Apart from the differences in the hypervariable regions, there are also a number of amino acid differences located throughout the conserved regions of PorA, and this may result in differences in T-cell epitopes in the various PorAs. This can happen either directly, due to an amino acid variation in the T-cell epitope, or indirectly, due to altered antigen processing caused by variations in flanking amino acids. Little is known about the T-cell epitopes generated after bacterial antigen uptake and processing. The processing of a whole protein into T-cell epitopes may very well be affected by the flanking amino acids, since the cysteine protease asparagine endopeptidase, for example, is known to specifically cut after asparagine residues (12) and an asparagine endopeptidase processing site has a major impact on the presentation of a T-cell epitope (13). The small variations between PorAs can have a major impact on the T-cell epitopes, contributing to the differences in immunogenicity between various PorAs. The roles of PorA-specific B and T cells and their epitopes must be clarified further if an improved multiple-PorA-based OMV vaccine is to be developed. Until this is done, HexaMen, with its broad protection, relatively easy production, and pattern of immunogenicity of PorAs similar to that of HexaMix, is a good vaccine candidate for broad prevention of meningococcal disease caused by meningococci serogroup B.
Acknowledgments
We thank N. J. D. Nagelkerke for help with statistics, D. Borsboom for performing LAL assays, and J. Koch for performing PorA determination by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Editor: J. N. Weiser
REFERENCES
- 1.Bart, A., J. Dankert, and E. A. van der Ende. 1999. Antigenic variation of the class I outer membrane protein in hyperendemic Neisseria meningitidis strains in The Netherlands. Infect. Immun. 67:3842-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, and W. Silva. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Chilean National Committee for Meningococcal Disease. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright, K., R. Morris, H. Rumke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 17:2612-2619. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright, K., N. Noah, and H. Peltola. 2001. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Report of a European advisory board meeting, Vienna, Austria, 6-8 October, 2000. Vaccine 19:4347-4356. [DOI] [PubMed] [Google Scholar]
- 5.Claassen, I., J. Meylis, P. van der Ley, C. Peeters, H. Brons, J. Robert, D. Borsboom, A. van der Ark, I. van Straaten, P. Roholl, B. Kuipers, and J. Poolman. 1996. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 14:1001-1008. [DOI] [PubMed] [Google Scholar]
- 6.de Kleijn, E., L. van Eijndhoven, C. Vermont, B. Kuipers, H. van Dijken, H. Rumke, R. De Groot, L. van Alphen, and G. van den Dobbelsteen. 2001. Serum bactericidal activity and isotype distribution of antibodies in toddlers and schoolchildren after vaccination with RIVM hexavalent PorA vesicle vaccine. Vaccine 20:352-358. [DOI] [PubMed] [Google Scholar]
- 7.De Kleijn, E. D., R. De Groot, J. Labadie, A. B. Lafeber, D. G. van den Dobbelsteen, L. van Alphen, H. van Dijken, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. C. Rumke. 2000. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 18:1456-1466. [DOI] [PubMed] [Google Scholar]
- 8.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355-357. [DOI] [PubMed]
- 9.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, and U. Rye. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. [PubMed]
- 10.Jansen, C., A. Wiese, L. Reubsaet, N. Dekker, H. de Cock, U. Seydel, and J. Tommassen. 2000. Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochim. Biophys. Acta 1464:284-298. [DOI] [PubMed] [Google Scholar]
- 11.Lesse, A. J., A. A. Campagnari, W. E. Bittner, and M. A. Apicella. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 126:109-117. [DOI] [PubMed] [Google Scholar]
- 12.Manoury, B., E. W. Hewitt, N. Morrice, P. M. Dando, A. J. Barrett, and C. Watts. 1998. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature 396:695-699. [DOI] [PubMed] [Google Scholar]
- 13.Manoury, B., D. Mazzeo, L. Fugger, N. Viner, M. Ponsford, H. Streeter, G. Mazza, D. C. Wraith, and C. Watts. 2002. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat. Immunol. 3:169-174. [DOI] [PubMed] [Google Scholar]
- 14.Martin, S., F. Sadler, R. Borrow, M. Dawson, A. Fox, and K. Cartwright. 2001. IgG antibody subclass responses determined by immunoblot in infants' sera following vaccination with a meningococcal recombinant hexavalent PorA OMV vaccine. Vaccine 19:4404-4408. [DOI] [PubMed] [Google Scholar]
- 15.McGuinness, B. T., P. R. Lambden, and J. E. Heckels. 1993. Class 1 outer membrane protein of Neisseria meningitidis: epitope analysis of the antigenic diversity between strains, implications for subtype definition and molecular epidemiology. Mol. Microbiol. 7:505-514. [DOI] [PubMed] [Google Scholar]
- 16.Peeters, C. C., H. C. Rumke, L. C. Sundermann, E. M. Rouppe van der Voort, J. Meulenbelt, M. Schuller, A. J. Kuipers, P. van der Ley, and J. T. Poolman. 1996. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine 14:1009-1015. [DOI] [PubMed] [Google Scholar]
- 17.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 18.Rosenqvist, E., A. Musacchio, A. Aase, E. A. Hoiby, E. Namork, J. Kolberg, E. Wedege, A. Delvig, R. Dalseg, T. E. Michaelsen, and J. Tommassen. 1999. Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis. Infect. Immun. 67:1267-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 20.Tommassen, J., P. Vermeij, M. Struyve, R. Benz, and J. T. Poolman. 1990. Isolation of Neisseria meningitidis mutants deficient in class 1 (PorA) and class 3 (PorB) outer membrane proteins. Infect. Immun. 58:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 22.van der Ley, P., J. E. Heckels, M. Virji, P. Hoogerhout, and J. T. Poolman. 1991. Topology of outer membrane porins in pathogenic Neisseria spp. Infect. Immun. 59:2963-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Ley, P., J. van der Biezen, and J. T. Poolman. 1995. Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine 13:401-407. [DOI] [PubMed] [Google Scholar]
- 24.van der Voort, E. R., P. van der Ley, J. van der Biezen, S. George, O. Tunnela, H. van Dijken, B. Kuipers, and J. Poolman. 1996. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect. Immun. 64:2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Voort, E. R., M. Schuller, J. Holst, P. de Vries, L. P. van der Ley, D. G. van den Dobbelsteen, and J. Poolman. 2000. Immunogenicity studies with a genetically engineered hexavalent PorA and a wild-type meningococcal group B outer membrane vesicle vaccine in infant cynomolgus monkeys. Vaccine 18:1334-1343. [DOI] [PubMed] [Google Scholar]