Abstract
Triggering of Fas (CD95) by its ligand (FasL) rapidly induces cell death via recruitment of the adaptor protein Fas-associated death domain (FADD), resulting in activation of a caspase cascade. It was thus surprising that T lymphocytes deficient in FADD were reported recently to be not only resistant to FasL-mediated apoptosis, but also defective in their proliferative capacity. This finding suggested potentially dual roles of cell growth and death for Fas and possibly other death receptors. We report here that CD3-induced proliferation and interleukin 2 production by human T cells are blocked by inhibitors of caspase activity. This is paralleled by rapid cleavage of caspase-8 after CD3 stimulation, but no detectable processing of caspase-3 during the same interval. The caspase contribution to T cell activation may occur via TCR-mediated upregulation of FasL, as Fas-Fc blocked T cell proliferation, whereas soluble FasL augmented CD3-induced proliferation. These findings extend the role of death receptors to the promotion of T cell growth in a caspase-dependent manner.
Keywords: caspase, T cell activation, Fas, costimulation, apoptosis
Death receptors typified by TNF receptor 1 (TNFR1) and Fas mediate apoptosis in a wide array of cell types through the ligand-induced association of adaptor proteins that in turn recruit a series of aspartic acid–specific proteases known as caspases 1. In the case of Fas, oligomerization of FasL promotes the binding of Fas-associated death domain protein (FADD) to the death domain of Fas 2. This allows the association of caspase-8 and its activation through cleavage of a precursor to an active form. The resulting protease cascade activates caspase-3, leading to eventual apoptosis 3.
Although activation-induced cell death (AICD) of T lymphocytes is well described as a Fas-dependent process for previously activated cycling T cells, resting T cells are resistant to Fas-mediated apoptosis 4 5. This information, coupled with the surprising observation that murine T cells either deficient in FADD or expressing a dominant negative form of FADD do not proliferate to TCR signals 6 7 8 9, further implicates a required contribution by the death receptor pathway in T cell growth. In these studies, we observe that CD3 stimulation of resting human T cells leads to processing of caspase-8, but not of caspase-3, within 4 h of activation. In addition, inhibitors of caspase activation block T cell proliferation. Fas-Fc is also capable of blocking T cell growth, suggesting that TCR-induced FasL upregulation may be at least partly responsible for initiating caspase activation.
Materials and Methods
Cell Preparation, Proliferation, and IL-2 Assay.
Purified human T cells were prepared by Ficoll-Hypaque centrifugation followed by rosetting with sheep erythrocytes. Positively rosetted lymphocytes were at least 98% CD3+ by flow cytometry. Purified T cells were cultured in 96-well plates at 5 × 104 cells per well and preincubated for 30 min with the indicated concentrations of caspase peptide blockers Ile-Glu-Thr-Asp fluoromethyl ketone (IETD-fmk), benzyloxycarbonyl-Val-Ala-Asp (zVAD)-fmk, Asp-Glu-Val-Asp (DEVD)-fmk, and Tyr-Val-Ala-Asp (YVAD)-fmk (Enzyme Systems Products), or a similar dilution of the stock solvent DMSO. Cells were then stimulated with the indicated concentrations of immobilized anti-CD3 antibody TR66 at either an optimal concentration of 3 μg/ml or suboptimally at 0.5 μg/ml. To some cultures containing suboptimal anti-CD3 was added either soluble recombinant fluoresceinated antigen (FLAG)-tagged FasL at the concentrations shown (Alexis Corp.), with or without cross-linking by 1 μg/ml of anti-FLAG antibody (M2; Sigma Chemical Co.); with soluble IgM anti-CD28 antibody 28/34 at 5 μg/ml; or with immobilized Fas-Fc (Alexis Corp.); or human IgG at the concentrations shown. Proliferation was measured by tritiated thymidine ([3H]TdR) incorporation during the final 18 h of a 4-d culture. Supernatants for IL-2 production were taken from PBLs (106/ml) that were stimulated for 24 h with immobilized anti-CD3 (3 μg/ml), with or without each caspase blocker (50 μM), or with cross-linked FasL (50 ng/ml). IL-2 levels were assayed using the CTLL bioassay.
Western Blots.
Cells were washed once with PBS, and lysed in lysis buffer (50 mM Tris-HCl, pH 7.5), 1% Triton X-100, 2 mM dithiothreitol, 2 mM sodium vanadate, and protease inhibitor cocktail (Complete™; Boehringer Mannheim), followed by centrifugation. Postnuclear lysates from 2 × 106 cells per lane were separated by SDS-PAGE, and analyzed by Western blotting using antibodies to caspase-3 (Transduction Laboratories) or caspase-8 (PharMingen).
Cell Cycle Analysis.
Cells were stimulated by immobilized anti-CD3 (0.5 μg/ml), anti-CD3/FasL (50 ng/ml plus anti-FLAG, 1 μg/ml), anti-CD3/anti-CD28 (28/34, IgM soluble at 10 μg/ml), or medium control. Samples were taken on each day for 5 d, washed in PBS, and then stained in 250 μl using 50 μg/ml propidium iodide (PI) in 0.1% Triton X-100, 4 mM sodium citrate, and 360 U/ml RNase, pH 7.2. Cells were incubated for 30 min at 37°C, and then 250 μl of salt solution was added (50 μg/ml PI, 0.1% Triton X-100, 0.4 M NaCl, pH 7.2). Samples were stored in the dark at 4°C for at least 1 h, and then analyzed within 24 h by flow cytometry.
Results and Discussion
T Cell Proliferation Is Caspase Dependent.
Stimulation of purified resting human T lymphocytes by anti-CD3 antibody was extensively blocked by the caspase inhibitors IETD-fmk and zVAD-fmk over a dose range of 12.5–50 μM (Fig. 1 A). By contrast, two other caspase blockers, YVAD-fmk and DEVD-fmk, showed no inhibition of T cell growth at similar concentrations. Although differences in the specificity of these caspase blockers has been suggested, they are actually irreversible blockers, with the ability to titrate all accessible caspases. Thus, the different degrees of inhibition by the various blockers should not be viewed as implicating a particular caspase. An alternative explanation for the differences would be varying degrees of permeability by the caspase blockers in primary T cells.
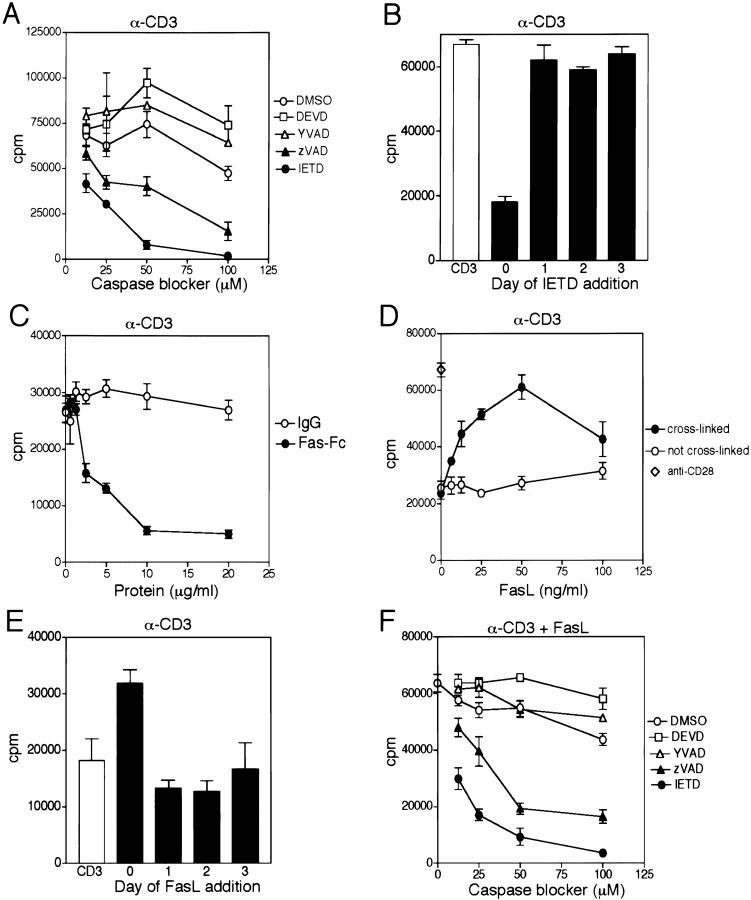
Figure 1.
CD3-induced T cell proliferation is blocked by inhibitors of caspases and Fas-Fc, and augmented by FasL. (A) Resting purified human T cells were stimulated with immobilized anti-CD3 (3 μg/ml) in the presence of the indicated concentrations of the caspase inhibitors IETD-fmk, zVAD-fmk, DEVD-fmk, and YVAD-fmk, or control DMSO at the same dilution. Proliferation was monitored by [3H]TdR incorporation after 3 d. (B) T cells were stimulated with immobilized anti-CD3 (3 μg/ml) in the absence or presence of IETD (50 μM), added on the days indicated. Proliferation was determined after 3 d. (C) T cells were activated with immobilized anti-CD3 (0.5 μg/ml) in the presence of either immobilized Fas-Fc or human IgG at the indicated concentrations. (D) Resting T cells were stimulated with suboptimal immobilized anti-CD3 (0.5 μg/ml) in the presence of the indicated concentrations of soluble FLAG-tagged FasL, with or without cross-linking by anti-FLAG. A comparison for costimulation is shown using the same concentrations of bound anti-CD3 and soluble IgM anti-CD28 (⋄). (E) Proliferation of T cells by anti-CD3 (0.5 μg/ml) in the absence or presence of cross-linked FasL (50 ng/ml), added on the days indicated. (F) T cells were cultured with the indicated caspase inhibitors, and stimulated with anti-CD3 (0.5 μg/ml) and cross-linked FasL (50 ng/ml). Similar results were observed in three experiments.
We also considered the possibility of nonspecific toxicity selectively by IETD-fmk and zVAD-fmk. However, the addition of exogenous IL-2 to cultures containing IETD-fmk or zVAD-fmk could largely overcome the block in proliferation (data not shown). Furthermore, to block proliferation, the addition of IETD-fmk had to occur within the first 24 h of CD3 stimulation, after which it conferred no inhibition even at relatively high concentrations of 50 μM (Fig. 1 B). This observation also underscores the view that a caspase is involved in early T cell activation events, and that evidence of its processing should therefore be examined early, before secondary activation-induced cell death (AICD) complicates the picture, as cycling T cells become sensitive to FasL-induced apoptosis.
A possible sequence by which TCR stimulation could activate the caspase cascade would be via the known TCR-induced upregulation of FasL 10 that would in turn engage surface Fas. Consistent with this view, immobilized Fas-Fc, but not control human IgG, largely blocked suboptimal (0.5 μg/ml) anti-CD3 activation of T cell growth in a dose-dependent manner (Fig. 1 C). This finding does not exclude the additional possibility that Fas-Fc might also confer a negative signal directly retrograde through FasL 11. However, in agreement with the former model, the addition of soluble FasL to suboptimal doses of anti-CD3 promoted a dose-dependent increase of T cell growth by as much as threefold (Fig. 1 D). At higher concentrations of anti-CD3 (3.0 μg/ml), there was less block of proliferation by Fas-Fc, and less augmentation of proliferation by FasL (data not shown). This is in agreement with similar findings using anti-Fas antibodies (12; our unpublished observations). The magnitude of this costimulation approached that seen with the same concentration of anti-CD3 and an optimal dose of anti-CD28. FasL costimulation was observed only when FasL was oligomerized through cross-linking of its FLAG tag using anti-FLAG, which mimics active membrane-bound FasL 13. As with the caspase blockers, costimulation with FasL needed to be delivered within the first 24 h of CD3 activation, otherwise no effect was observed (Fig. 1 E). Similar to the findings with CD3 stimulation, the costimulation of T cell growth conferred by FasL was also blocked by the same two caspase inhibitors (Fig. 1 F).
CD3 Activation and CD3/FasL Costimulation Cleave Caspase-8.
Evidence for activation of caspase-8 and caspase-3 was investigated by Western blot within 2–4 h of T cell activation by anti-CD3, with or without FasL (Fig. 2 A, top). Purified resting T cells were treated with immobilized suboptimal anti-CD3 (0.5 μg/ml) antibody in the absence or presence of cross-linked FasL or with cross-linked FasL alone, and caspase processing was analyzed during the first 4 h. Given the need to block caspase activity within the first 24 h, we elected to examine early time intervals after activation. It was also reasoned that evidence of caspase processing after this time might reflect FasL-induced apoptosis of actively dividing T cells.
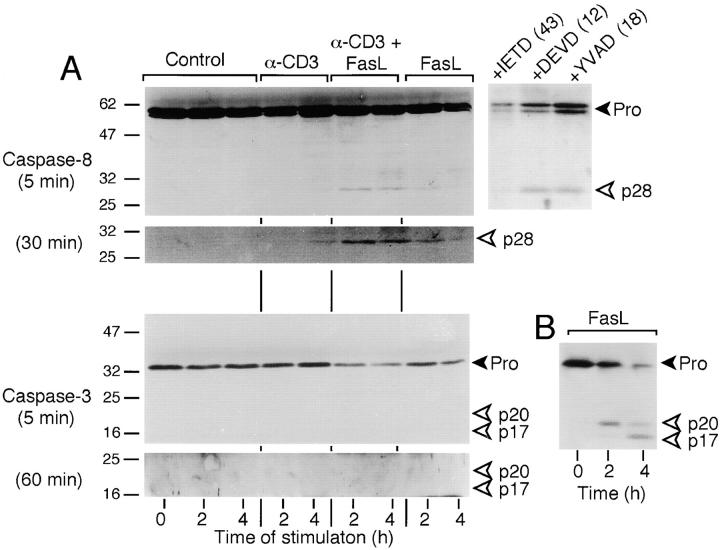
Figure 2.
CD3/Fas costimulation results in cleavage of caspase-8, but not of caspase-3. (A) Purified human T cells (98%) were cultured with either no stimulation (Control), immobilized anti-CD3 alone (0.5 μg/ml), anti-CD3 plus soluble FasL (50 ng/ml) cross-linked by its FLAG tag, or cross-linked FasL alone. Cell lysates were made after the indicated times of culture, and were analyzed sequentially by Western Blot for expression of cleaved forms of caspase-8 and caspase-3. The full-length forms (Pro) of the proteins are indicated by the black arrowheads, whereas the cleaved forms are indicated with open arrowheads. The larger top panels for each caspase show exposure times of 5 min, whereas the smaller bottom panels represent 30-min (caspase-8) or 60-min (caspase-3) exposures. An additional three samples of T cells were stimulated with anti-CD3/FasL for 6 h in the presence of the caspase-8 blocker IETD-fmk, caspase-3 blocker DEVD-fmk, or caspase-1 blocker YVAD-fmk, each at 50 μM. The ratio of procaspase to cleaved caspase-8, assessed by densitometry, is indicated in parentheses. (B) Positive control for cleavage of caspase-3 in Jurkat T cells treated with cross-linked FasL (50 ng/ml) for the times indicated. The levels of cell death by PI staining were consistently <1% in all cultures except the sample with IETD, which in one experiment was 4.2%. These findings were consistent in two additional experiments.
Caspase-8 processing in primary T cells is detectable by cleavage of its full-length 55-kD form between the procaspase and caspase domains to release a cleaved 28-kD fragment. As shown in Fig. 2 A, no evidence of caspase-8 cleavage was detectable in unstimulated T cells for up to 4 h, or even after overnight culture (data not shown). However, cleavage of caspase-8 became apparent by 4 h after suboptimal anti-CD3 stimulation (0.5 μg/ml) alone, or within 2 h using anti-CD3 plus FasL (Fig. 2 A). This was particularly visible by longer exposure of the blot (Fig. 2 A, bottom). Similar to the proliferation findings, the amount of the cleaved p28 fragment was more intense at high doses of anti-CD3 (3.0 μg/ml), but less augmentation by FasL was observed (data not shown). Thus, FasL costimulation was best observed for both proliferation and caspase-8 processing at suboptimal doses of anti-CD3. The cleavage of caspase-8 was not secondary to apoptosis, as <1% cell death was detectable by PI staining in either the unstimulated controls or stimulated cultures up to 24 h later (data not shown). Furthermore, IETD-fmk, but not DEVD-fmk or YVAD-fmk, at the same dose (50 μM) that inhibited T cell growth also blocked cleavage of caspase-8 during anti-CD3/FasL costimulation over 6 h (Fig. 2 A).
Although caspase-3 cleavage was readily detectable in FasL-treated Jurkat T cells undergoing apoptosis (Fig. 2 B), no indication of caspase-3 cleavage products could be found in primary T cells costimulated with anti-CD3/FasL over 4 h, even with longer exposure of the blot (Fig. 2 A, bottom), or with costimulation for as long as 22 h (data not shown). Consequently, cleavage of poly(ADP-ribose) polymerase (PARP), a substrate for caspase-3, was also not observed (data not shown). This indicated that processed caspase-8 did not lead to detectable processing of caspase-3 under these conditions of CD3/Fas costimulation.
FasL Costimulation Increases IL-2 Production and Cell Cycling.
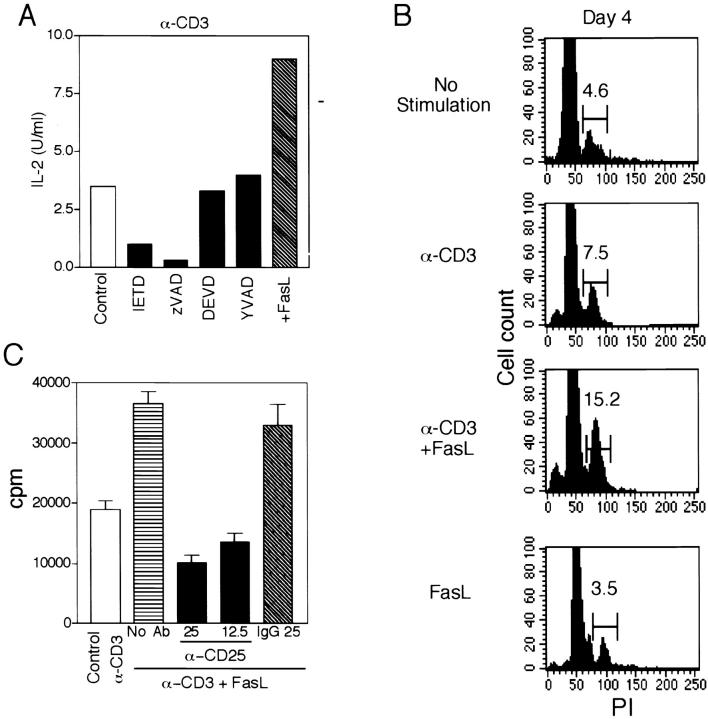
The augmented proliferation conferred by anti-CD3/FasL was paralleled by increased production of IL-2, which was also blocked by IETD-fmk and zVAD-fmk (Fig. 3 A). As IL-2 promotes entry of T cells into the S phase of the cell cycle, the effect of FasL costimulation on cell cycling was examined. As shown for day 4 in Fig. 3 B, and summarized for all days in Table , FasL costimulation with CD3 increased the percentage of cells in S+G2+M over CD3 alone, to levels at least as high as observed with CD3 plus CD28 activation. Furthermore, the increased proliferation with FasL could be at least partly blocked by addition of anti-CD25 (Fig. 3 C), suggesting that FasL costimulation may occur partly through upregulation of cytokines necessary for T cell proliferation.
Figure 3.
Caspase blocking inhibits IL-2 production, which is augmented by FasL along with cell cycling. (A) T cells were cultured at 106/ml with immobilized anti-CD3 and cross-linked FasL in the absence or presence of the indicated caspase inhibitors (50 μM). Supernatants were removed after 24 h and assayed for IL-2 using the CTLL bioassay. (B) Cell cycle analysis using PI was performed daily for 5 d on T cells after stimulation with the indicated agents. Shown are the results on day 4 from one experiment. All time points of two separate experiments are summarized in Table . (C) T cells were activated with anti-CD3 in the absence or presence of FasL. To cultures stimulated by anti-CD3 plus FasL was added either no additional antibody, anti-CD25 (25 μg/ml, 12.5 μg/ml), or control IgG (25 μg/ml). Proliferation was measured after 3 d.
Table 1.
Increased Cell Cycling with CD3/FasL Costimulation
| Stimulus | Percentage of cells in S+G2+M | ||||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| Experiment 1 | |||||
| Medium | 2.6 | 2.6 | 3.6 | 3.4 | 3.9 |
| α-CD3 | 6.8 | 5.0 | 8.8 | 5.8 | 7.5 |
| α-CD3/FasL | 11.9 | 9.2 | 14.7 | 9.5 | 10.1 |
| FasL | 3.4 | 4.5 | 4.2 | 5.2 | 4.6 |
| α-CD3/α-CD28 | 6.2 | 10.5 | 10.3 | 9.7 | 7.9 |
| Experiment 2 | |||||
| Medium | 5.1 | 4.6 | 2.1 | ||
| α-CD3 | 6.5 | 7.5 | 4.2 | ||
| α-CD3/FasL | 9.6 | 15.2 | 12.2 | ||
| FasL | 3.6 | 3.5 | 1.7 | ||
T cells were activated by the indicated stimulus, and samples were taken on the days shown, permeabilized, and stained with PI. Cell cycle analysis was performed by flow cytometry, and gates were set for the S+G2+M phases of the cell cycle.
Combined with the reported T cell activation defect in FADD-deficient mice and FADD-dominant negative mice 6 7 8 9, the current findings suggest a model whereby T cell activation via TCR upregulates surface FasL, which engages Fas and leads to recruitment of FADD and caspase activation. However, in resting T cells, this does not result in apoptosis, but rather augments IL-2 gene activation and cell proliferation. The notion that FasL can augment proliferation of resting T cells may be more broadly applicable to other death receptors such as TNF-related apoptosis-inducing ligand receptor (TRAIL-R) and TNFR-related apoptosis-mediated receptor/death receptor 3 (TRAMP/DR3) 1, as well as to other cell types. These other death receptors may be particularly important in initiating proliferation of primary T cells lacking Fas. In addition to T cells, fibroblasts have also been observed to manifest increased proliferation with anti-Fas antibodies 12 14, as well as with FasL (our unpublished observations).
The exact caspase(s) required for proliferation by primary T cells and its substrate cannot be concluded at present, given the broad specificity of the caspase blockers. However, one might speculate that a proximal caspase in the Fas signal cascade is required for activation of primary T cells, given our findings of cleavage of caspase-8, but not of caspase-3, under conditions of FasL costimulation. A likely substrate for caspase-8 by which a death signal could be diverted to a proliferative response would be cellular FADD-like IL-1β–converting enzyme (FLICE) inhibitory protein (c-FLIP), the natural inhibitor of Fas-induced cell death. c-FLIP is homologous to caspase-8, but bears a nonfunctional caspase domain. c-FLIP contains two potential caspase cleavage sites, and as such functions as a substrate trap for caspase-8, while also competing for binding to FADD 15. In further studies we have observed that c-FLIP is cleaved during T cell activation, and its overexpression in Jurkat T cells or in transgenic mouse T cells increases the activities of the mitogen-activated protein (MAP) kinase, extracellular signal–regulated kinase (ERK), and nuclear factor (NF)-κB after CD3 stimulation, leading to augmented IL-2 production. In addition, Fas costimulation of normal T cells with CD3 also augments the activator protein 1 (AP-1) and NF-κB pathways (our manuscript in preparation). It is apparent from these findings that inhibitors of caspases could prove valuable in therapeutic maneuvers to diminish T cell activation.
Acknowledgments
We thank Dr. Salvatore Valitutti for the anti-CD3 antibody and Dr. Pedro Romero for the anti-CD28 antibody.
This work was supported by grants of the Swiss National Science Foundation (to J. Tschopp), and the National Institutes of Health (AI36333 and F06 TW02294, to R.C. Budd).
Footnotes
N.J. Kennedy and T. Kataoka contributed equally to this work.
References
- Ashkenazi A., Dixit V.M. Death receptorssignaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Kischkel F.C., Hellbardt S., Behrmann I., Germer M., Pawlita M., Krammer P.H., Peter M.E. Cytotoxicity-dependent Apo-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Singer G.G., Abbas A.K. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Klas C., Debatin K.M., Jonker R.R., Krammer P.H. Activation interferes with the APO-1 pathway in mature human T cells. Int. Immunol. 1993;5:625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- Newton K., Harris A.W., Bath M.L., Smith K.G.C., Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.M., Wen B.G., Chinnaiyan A.M., O'Rourke K., Dixit V.M., Hedrick S.M. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cado D., Chen A., Kabra N.H., Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- Yeh W.C., Pompa J.L., McCurrach M.E., Shu H.B., Elia A.J., Shahinian A., Ng M., Wakeham A., Khoo W., Mitchell K. FADDessential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Anel A., Buferne M., Boyer C., Schmitt-Verhulst V.A., Golstein P. T cell receptor-induced Fas ligand expression in cytotoxic T lymphocyte clones is blocked by protein tyrosine kinase inhibitors and cyclosporin A. Eur. J. Immunol. 1994;24:2469–2476. doi: 10.1002/eji.1830241032. [DOI] [PubMed] [Google Scholar]
- Desbarats J., Duke R.C., Newell M.K. Newly discovered role for Fas ligand in the cell-cycle arrest of CD4+ T cells. Nat. Med. 1998;4:1377–1382. doi: 10.1038/3965. [DOI] [PubMed] [Google Scholar]
- Alderson M.R., Armitage R.J., Maraskovsky E., Tough T.W., Roux E., Schooley K., Ramsdell F., Lynch D.H. Fas transduces activation signals in normal human T lymphocytes. J. Exp. Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P., Holler N., Bodmer J.L., Hahne M., Frei K., Fontana A., Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal B.B., Singh S., LaPushin R., Totpal K. Fas antigen signals proliferation of normal human diploid fibroblast and its mechanism is different from tumor necrosis factor receptor. FEBS Lett. 1995;364:5–8. doi: 10.1016/0014-5793(95)00339-b. [DOI] [PubMed] [Google Scholar]
- Irmler M., Thome M., Hahne M., Schneider P., Hofmann K., Steiner V., Bodmer J.L., Schroter M., Burns K., Mattmann C. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]