Abstract
The Trypanosoma cruzi trans-sialidase can sensitize mice to become highly susceptible to T. cruzi invasion, through mechanisms that remain unknown. In pursuing this observation, we found that purified trans-sialidase induces the selective release of biologically active interleukin (IL)-6 in naive human intestinal microvascular endothelial cells (HIMECs), peripheral blood mononuclear cells (PBMCs), and bladder carcinoma cells. The trans-sialidase action was independent of its catalytic activity, as demonstrated with a genetically engineered trans-sialidase mutant, an enzymatically active polypeptide, and cocultures of PBMCs with epimastigotes and trypomastigotes. Instead, the trans-sialidase action was reproduced with a recombinant COOH-terminal tandem repeat and with synthetic peptides modeled on the tandem repeat. Most interesting, HIMECs infected with a trypomastigote population expressing trans-sialidase effectively released IL-6, but did not upon infection with the counterpart trypomastigote population expressing low trans-sialidase levels. IL-6 is a key factor in the regulation and symptom formation of infection caused by several types of viruses, such as HIV and influenza A virus. However, the function of IL-6 in protozoan and other parasitic diseases remains unclear. The unique findings presented here suggest that trans-sialidase is a major inducer of IL-6 secretion in T. cruzi infection, independently of immune cell activation. Such IL-6 secretion might underlie some features of Chagas's disease, such as pyrexia, neuroprotection, and fibrosis, and might result in the undermining of normal acquired immunity against T. cruzi.
Keywords: interleukin 6, trans-sialidase, cytokines, Trypanosoma cruzi, endothelial cells
The protozoan Trypanosoma cruzi causes Chagas's disease, a chronic debilitating condition afflicting millions of people in this Hemisphere. T. cruzi invasion results in the production of several cytokines, some of which, like IL-6, can be released independently of antigenic stimulation of B and T lymphocytes. This is the case in the in vitro infection of human umbilical vein endothelial cells (HUVECs), where T. cruzi upregulates the gene for IL-6 and other cytokines 1. Likewise, coculture of normal human PBMCs with T. cruzi results in the proliferation of lymphocytes and the production of cytokines including IL-6 2. In vivo, IL-6 is found to be consistently elevated in the sera of animals experimentally infected with T. cruzi 3 and in humans with acute Chagas's disease 1. Inflamed heart, aorta, and spleen of chagasic animals, as well as the chronic chagasic heart of humans, are enriched in IL-6–producing cells, particularly endothelial cells and mononuclear cells 4 5 6 7. These results suggest that IL-6 is upregulated in Chagas's disease not only by immune but also by nonimmune mechanisms. However, the molecular basis for the IL-6 upregulation in T. cruzi infection is unknown.
IL-6 is a multifunctional cytokine that regulates the infection of several types of viruses. For example, normal PBMCs exposed to HIV enhance production of IL-6 8. In addition, the majority of HIV-infected individuals exhibit elevated serum levels of IL-6 9. IL-6 seems to have an autocrine effect on HIV replication, because blocking IL-6 production dramatically reduced viral titers 10. HIV upregulates expression of IL-6 through the viral transactivating Tat, which interacts with IL-6 promoter to increase IL-6 transcription 11. These findings suggest an important role for IL-6 in maintaining HIV infection. Influenza provides another example of a direct relationship between viral infection and IL-6 production. As with HIV, exposure of normal human PBMCs to human influenza A virus results in the production of IL-6 and other cytokines 12. Furthermore, IL-6 levels in nasal lavage fluids and in the circulation of human volunteers infected with influenza A virus correlated directly with viral titers, temperature, mucus production, and symptom score 13. These results implicate IL-6 as an important mechanism both in symptom formation and in host defense in influenza. IL-6 is also implicated in rhinovirus infection 14.
However, not much is known about the function of IL-6 in infections produced by parasites, including T. cruzi 15 16. Ascertaining the role IL-6 plays in Chagas's disease would be facilitated if it was known what in T. cruzi induces IL-6 production in normal cells before the development of acquired immunity, as this might give clues to how the parasite attempts to subvert immune regulation or how the host seeks to eliminate parasitism. The results presented here identify for the first time a T. cruzi glycoprotein with trans-sialidase (TS) activity capable of selectively upregulating IL-6 secretion in naive human cells independently of antigenic stimulation.
TS was originally discovered by its ability to catalyze the release of sialic acid from glycoconjugates in solution or on cell surfaces (i.e., neuraminidase or sialidase activity [17]). Subsequently, it was demonstrated that TS could transfer sialic acid to β-galactosyl acceptors (sialyl transferase activity [18 19 20; for a review, see reference 21]). TS is attached to the trypomastigote outer membrane through a glycosylphosphatidylinositol anchor 22 23 and is present as a soluble factor in the extracellular milieu in the bloodstream of T. cruzi–infected mice 17 and humans 24. Therefore, the T. cruzi neuraminidase is strategically located for a role in mediating trypanosome–host interactions, such as by altering immune functions.
A few years ago, we found that mice sensitized with TS in their footpads became highly susceptible to trypanosomal infection compared with mice sensitized with control medium, bacterial neuraminidase, or LPS 25. The dosage (low nanograms per mouse) and kinetics (1–2 h before parasite inoculation) of TS sensitization suggested that the enzyme enhanced virulence by altering the dynamics of innate and/or acquired immune responses to T. cruzi, independent of antigenic stimulation of B and T cell receptors. This could be accomplished if TS were to activate cytokine receptor signaling pathways by binding either directly to cytokine receptors or to other surface receptors whose activation would lead to cytokine secretion. If so, the TS-dependent cytokines could, in turn, subvert normal anti–T. cruzi immune responses.
To begin to test this hypothesis, we screened for cytokines in media conditioned by TS stimulation of various human cells relevant to the pathogenesis of Chagas's disease. We found that intact TS and recombinant and synthetic fragments of TS selectively stimulated secretion of biologically active IL-6 in primary cultures of human intestinal microvascular endothelial cells (HIMECs) and in normal PBMCs. Surprisingly, the secretagogue action of TS relied on its COOH-terminal tandem repeat (TR) and not on its catalytic domain (CD). These findings raise the possibility that TS plays a role in T. cruzi invasion and in the pathology of Chagas's disease, at least in part, by upregulating IL-6 secretion in naive cells.
Materials and Methods
Cell Culture.
Dr. Claudio Fiocchi (Case Western Reserve University School of Medicine, Cleveland, OH) provided primary cultures of HIMECs isolated from normal jejunal mucosa/submucosal tissue 26. They were cultured in fibronectin-coated plasticware in MCDB medium (Sigma Chemical Co.) supplemented with 20% fetal bovine serum, 90 μg/ml heparin, and 50 μg/ml of endothelial cell growth factor (Sigma Chemical Co.). T-24 cells (CRL-1998; American Type Culture Collection) were cultured in M199 medium supplemented with 10% fetal bovine serum. PBMCs were purified by Ficoll-Paque gradient as described 27. Vero cells were grown in RPMI medium with 5% Nu serum and infected with the Silvio X-10/4 strain of T. cruzi as described previously 25. Epimastigotes were grown as described previously 17 23.
Purification of Native TS and Related Proteins.
TS was purified by immunoaffinity chromatography as described previously 20. In brief, supernatants from T. cruzi–infected Vero cells were passed on a protein G–Sepharose (Amersham Pharmacia Biotech) column adsorbed with mAb TCN-2 specific for the LTR domain of TS. Bound TS was eluted with TR1 peptide (DSSAHGTPSTPA) in the presence of 0.1% octyl glucopyranoside. TS was concentrated by ultrafiltration in Amicon-10 and washed extensively with PBS, pH 7.8, to remove the TR peptide. The neuraminidase from Vibrio cholera (VCNA) was purchased from Calbiochem. Penetrin (PN-1) was isolated by heparin affinity chromatography as described previously 28. All reagents, glassware, and plasticware used in the isolation of the various proteins were LPS free. To eliminate residual LPS, the purified materials were passed through two distinct resins that remove endotoxin by different mechanisms, END-X B15 (Associates of Cape Cod, Woods Hole, MA) and AffinityPack™ Detoxi-Gel™ (Pierce Chemical Co.), following the recommendations of the manufacturers.
Cloning and Expression of Catalytic and LTR Domains of TS.
The DNA fragment corresponding to the CD was amplified by PCR on DNA template from TS clone 19Y of the Silvio strain of T. cruzi trypomastigotes 22. The primers for CD were 5′-GGAATTCCATATGGCACCCGGATCGAGCCGAGTT-3′ and 5′-CCGCTCGAGGCTCAAGAACAAGGTCCTGATCG-3′. The amplified fragment was cloned into pET-23b (Novagen) with a stretch of six His residues at the COOH terminus of the expressed proteins. For protein production, plasmids were used to transform the BL21 DE3 bacterial strain (Novagen) containing a chromosomal copy of the T7 RNA polymerase gene. Expression was induced by isopropyl-β-d-thiogalactopyranoside (IPTG). To isolate the recombinant CD fragment, bacterial lysates were prepared by osmotic shock in 40 mM phosphate buffer, pH 7.5, 0.3 M NaCl, 1% Triton X-100, 1 mM PMSF, followed by brief sonication. The CD peptide was purified on a Ni2+-nitrilotriacetic acid (NTA)/agarose column as recommended by the manufacturer (Novagen). CD was further purified by FPLC on the anion-exchange column Mono-Q HR (Amersham Pharmacia Biotech), as described previously 20 25. All reagents were LPS free and passed through the END-X B15 resin to remove residual LPS.
The full-length COOH-terminal LTR (LTR fragment) of Silvio TS, clone 7F 22, was isolated as follows: LTR, subcloned from a pMelBac plasmid (Invitrogen) containing the TS gene, was digested with PvuII-SalI and ligated into EcoRV-SalI sites of pET-20b (Novagen). The LTR DNA was introduced into the NcoI-HindIII sites of pFASTBAC HTb vector (GIBCO BRL). The Bac-to-Bac system (GIBCO BRL) was used to generate recombinant baculovirus, which in turn was used to infect Sf 9 cells. Recombinant LTR protein was purified by Ni2+-NTA column (Novagen) affinity chromatography. The LTR fragment thus generated contains the full-length TR of clone 7F with 44 repeats plus TS sequences of 26 and 40 amino acids upstream and downstream of the repeat, respectively. Purified LTR was passed through AffinityPack™ Detoxi-Gel™ to remove residual LPS.
Preparation of TS-154 and TS-H32 Constructs.
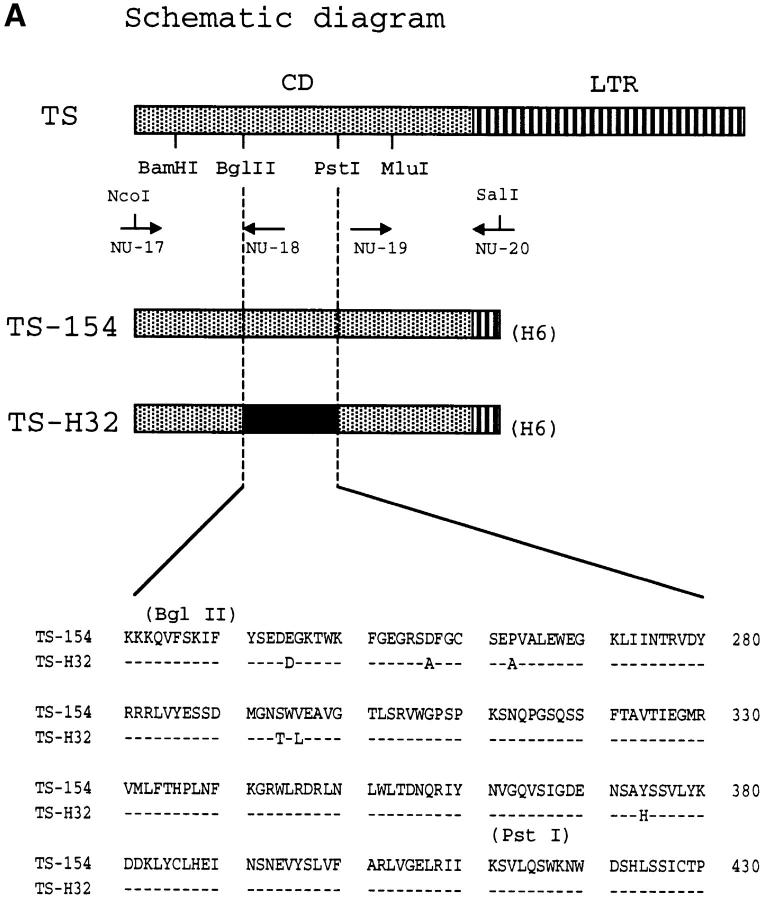
Construct TS-154 is derived from enzymatically active trans-sialidase clone 154 of the Y strain of T. cruzi 29. The NH2 terminus of clone 154 was amplified by PCR using primers NU-17 (27 mer, 5′-GCCCATGGCACCCGATCGAGCCGAGTT-3′) and NU-18 (20 mer, 5′-CGGAATTTTCATCACCAATG-3′) with restriction sites shown in Fig. 4 A. NU-17 was designed to introduce starting ATG codon just before the NH2 terminus of mature TS protein, and a NcoI site for subcloning. The PCR product of NU-17 and NU-18 was treated with NcoI and BglII, and subcloned into the NcoI and BamHI sites of pET-21d (Novagen). Most of the 12 amino acid repeats and hydrophobic region at the COOH terminus were removed by PCR using primers NU-19 (24 mer, 5′-GTTCCGAACGGTTTGAAGTTTGCG-3′) and NU-20 (25 mer, 5′-CTGTCGACGGGAGTTGAGGGCGTAC-3′). NU-20 corresponds to the partial sequence of the TR and, in addition, contains a SalI site. The PCR product with the minimum numbers of repeats (i.e., five repeats) was selected and used to replace the original COOH terminus of clone 154 at unique MluI site (see Fig. 4 A). The DNA fragment with unique BamHI-SalI sites was ligated to the pET-21d plasmid containing the NH2-terminal region of TS using the BamHI and XhoI sites of the vector, to yield construct TS-154 (see Fig. 4 A). To generate construct TS-H32, the BglII-PstI DNA fragment of pTS-154 was replaced with the corresponding fragment from the gene 121 29. The trans-sialidase gene 121 is catalytically inactive due to a single amino acid difference in the BglII-PstI fragment, with histidine (H374) replacing tyrosine in the catalytically active TS gene 154 29. Thus, construct TS-H32 should be enzymatically inactive, as determined experimentally (see Fig. 4 B). All constructs were verified by automated sequencing (ABI Perkin Elmer) using BigDye terminator. Production and purification of TS-154 and TS-H32 proteins were identical to the method described above for the CD construct.
Figure 4.
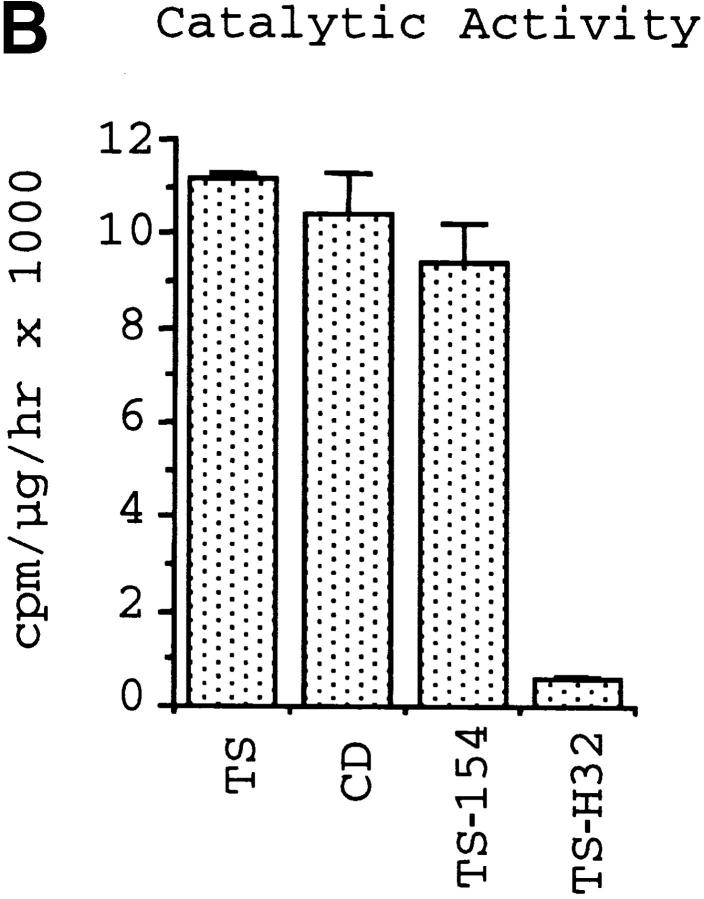
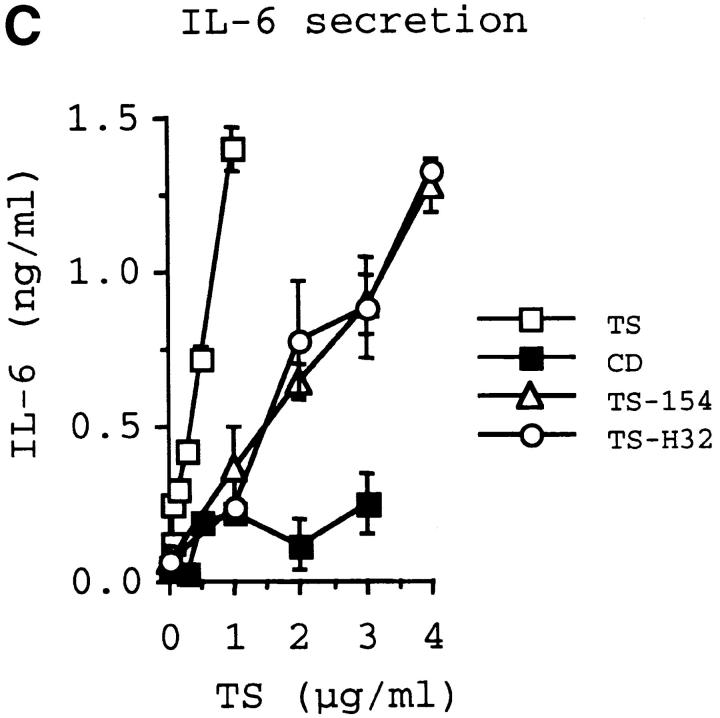
The catalytic activity of TS does not mediate IL-6 release in PBMCs and T-24 cells. Diagram of TS (Silvio strain, clone 7F; see reference 22) and of TS-154 and TS-H32 constructs (derived from Y strain, clones 154 and 121, respectively; see reference 29). NU-17, -18, -19, and -20 refer to primers used to make the constructs (see Materials and Methods). H6 refers to the 6xHis tag. Insert shows the sequence comparison between TS-154 and TS-H32 in the BglII-PstI fragment of TS. The sequence of TS-H32 is identical to the sequence of TS-154 except where indicated with the amino acid letter code. (B) Catalytic activity for each TS construct. (C) IL-6 secretory activity of TS and its respective derivatives for PBMCs. Similar results were obtained with T-24 cells.
Immunoassays for Cytokines.
Endothelial cells and T-24 cells were plated on 24-well plates at a density of 105 cells/well, while PBMCs were plated in the similar wells at 106/well. Cells were incubated with the various test reagents at the concentrations and for the time indicated in the figures in triplicate points. Polymyxin B was used at 10 μg/ml in all cell cultures, as it did not affect any parameter tested. Cytokines and chemokines released in the culture supernatants were measured by ELISA assay following the instructions of the manufacturer (Endogen). The cytokines tested were IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-γ, and TNF-α, and the chemokines were RANTES (regulated upon activation, normal T cell expressed and secreted) and monocyte chemotactic protein 1 (MCP-1). Negative control were cells incubated in medium containing polymyxin B at 10 μg/ml, and positive control always included cells incubated with bacterial LPS at the low nanogram per milliliter range (for PBMCs or HIMECs) or in the microgram per milliliter range for carcinoma T-24 cells. In some experiments, IL-1β or TNF-α was used as positive control for cytokine release. Bioassay for IL-6 was performed using IL-6–dependent human DS-1 cells 30. In brief, 104 cells/well were plated in 96-well plates and incubated for 24 h in IL-6–free medium (10% FCS in RPMI) containing several dilutions of TS-conditioned medium (TS/CM) or exogenous rIL-6, pulsed for 4 h with 0.5 μCi [3H]thymidine, and harvested to determine radioactivity incorporation in a microplate scintillation instrument (Packard). In some experiments, a neutralizing IL-6 rabbit IgG or normal rabbit IgG (Endogen) was added to the dilutions of TS/CM before assaying for growth stimulation of DS-1 cells. Conditioned media were prepared by incubating PBMCs or T-24 cells for 24 h in 10% FCS/RPMI without (CM) or with (TS/CM) TS at 1 μg/ml. Supernatants were centrifuged at 1,000 g to remove cell debris and were kept frozen at –20°C until use.
PCR of Reverse-transcribed mRNA.
Semiquantitative analysis of cytokine mRNA was performed by the primer-dropping method 31. RNA of endothelial cells (105) or PBMCs (106) that had been or not stimulated with TS at 1 μg/ml for 24 h, was purified by acid guanidinium isothiocyanate-phenol-chloroform-TRI reagent (Molecular Research Center). 2 μg of total RNA was converted to cDNA in a volume of 20 μl using random hexamer primers according to the manufacturer's instructions (GIBCO BRL). Primers for human IL-6 were 5′-ATGAACTCCTTCTCCACAAGCGC and 5′-GAAGAGCCCTCAGGCTGGACTG, and for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-CGGAGTCAACGGATTTGGTCGTAT and 5′-AGCCTTCTCCATGGTGGTGAAGAC. The PCR mixture contained 20 mM Tris-HCl, pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 1 mM dNTP, 200 pM primers, and 1 U of Taq polymerase in 50 μl. Thermal cycling conditions were: denaturation step, 95°C for 5 min; 35 cycles of 95°C for 30 s, 60°C for 30 s, and 75°C for 1 min, and a final step of 75°C for 5 min. At cycle 9, GAPDH primers were added. 5 μl of the PCR product was analyzed through 2% agarose gel electrophoresis in the presence of 1 μg/ml ethidium bromide.
Cocultures of Epimastigotes and Trypomastigotes with PBMCs.
Normal human PBMCs (106/well) were coincubated in 10% FCS/RPMI with either epimastigotes or trypomastigotes (105/well for each stage) for 48 h at 37°C. IL-6 was measured by ELISA in cell-free supernatants (obtained by centrifugation of the cultures at 2,500 g for 5 min) at various times after the start of the cocultures. TS activity in the parasites was obtained before the start of the cultures.
Kinetics of IL-6 Production by HIMECs Incubated with TS+ and TS− Populations and with Leishmania major.
T. cruzi trypomastigotes expressing TS on their surface (TS+ parasites) or expressing low or no TS (TS− parasites) were purified by magnetic beads coated with the mAb TCN-2, as described previously 32. HIMECs, plated (5 × 104/well) on 24-well plates for 24 h, were infected with 106 trypomastigotes (unfractionated, TS+, or TS−) or with L. major promastigotes, strain V1 (gift from Dr. Stephen Beverley, Washington University, St. Louis, MO). At various times after infection, aliquots of the supernatants were collected, centrifuged at 1,000 g, filtered in 0.22 μm nitrocellulose, and assayed for IL-6 by ELISA.
LTR Depletion.
200 μl of protein G–Sepharose (Amersham Pharmacia Biotech) was adsorbed with culture supernatants of the mAb TCN (IgG1 isotype) or of a control mAb IgG1 specific for p-azo-phenylarsonate (provided by Dr. Thereza Imanishi-Kari, Tufts University School of Medicine), and washed with 20 vol of LPS-free PBS, pH 7.2. 3 μg of LTR was loaded to either protein G/TCN-2 or protein G/control IgG1 column; the effluent was reapplied five times to the respective column. The last flow-through of each column was collected in 200 μl in a separate LPS-free tube, and the columns were washed with five column volumes of PBS. Elution of bound LTR was by mixing the resins with a spatula, followed by centrifugation at 250 g for 5 min at 4°C. Effluents and eluates were constituted to the original volume and added to T-24 cells. After 24 h, IL-6 in the T-24 cell supernatants was determined by ELISA.
IL-6 Production by PBMCs Stimulated with TR Peptides.
TR peptides (see Table ) were synthesized at the Tufts University Synthesis Facility. Each peptide was purified by HPLC, and the number of amino acids and molecular weight of each peptide were verified by mass spectrography. Peptides were dissolved in growth medium (10% FCS in RPMI) and added at various concentrations to 106 PBMCs/well in 24-well plates. After 24 h, IL-6 was assayed by ELISA in the conditioned supernatants.
Table 1.
Structure of Synthetic TR Peptides Modeled on the Proximal Sequence of the LTR from Silvio TS
| Peptides | Amino acid sequence |
|---|---|
| TR1 | DSSAHGAPSTPA |
| TR2 | DSSAHGAPSTPADSSAHGTPSTPV |
| TR3 | DSSAHGAPSTPADSSAHGTPSTPVDSSAHGTPSTPA |
| TR4 | DSSAHGAPSTPADSSAHGTPSTPVDSSAHGTPSTPADSSAHSTPSTPA |
| TR5 | DSSAHGAPSTPADSSAHGTPSTPVDSSAHGTPSTPADSSAHSTPSTPADSSAHSTPSTPA |
Note that the TR1 unit is faithfully reproduced in the other peptides except as indicated by underlining.
Results
TS Promotes IL-6 Secretion in Normal HIMECs, PBMCs, and in Cancer Cells.
Endothelial cells mediate tissue inflammation, in part, through the secretion of proinflammatory cytokines and the selective expression of adhesion molecules in the vascular bed 33. Most studies with endothelial cells rely on HUVECs, as they are readily available and easy to isolate. However, the majority of physiological and pathological events mediated by endothelial cells, in particular inflammation, wound healing, and leukocyte homing through postcapillary venules, occur at the level of microcirculation 34. Furthermore, endothelial cell populations vary from organ to organ and in different vessels within an organ 35. Most important, the pattern of cytokine secretion and proliferation of a microvascular endothelial cell, HIMEC, differed from that of HUVECs in response to various stimuli 36 37. Thus, due to the relevance of the intestinal microvascular endothelium to the pathogenesis of the megacolon in Chagas's disease, and the possibility that TS subverts host immune responses by directly reacting with nonimmune cells, we attempted to determine whether HIMECs isolated from normal human mucosa would secrete cytokines in response to TS stimulation.
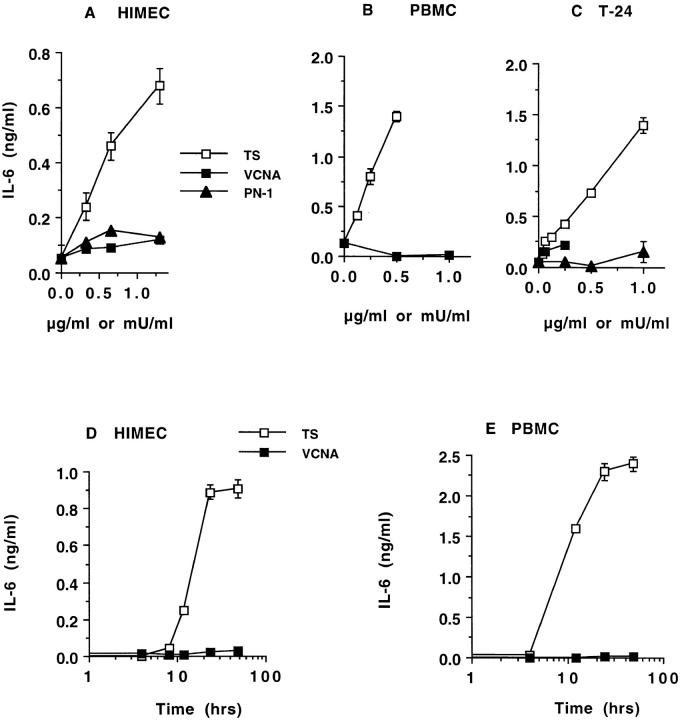
For this purpose, we incubated HIMEC monolayers with various concentrations of purified TS for 24 h and measured the concentration of eight cytokines (IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-γ, and TNF-α) and two chemokines (RANTES and monocyte chemotactic protein 1 [MCP-1]) in the conditioned HIMEC supernatants. We found the concentration of IL-6 to be elevated in the conditioned supernatants in a manner dependent on the TS input (Fig. 1 A). In contrast, the concentration of the other cytokines did not increase in response to TS stimulation under conditions in which bacterial LPS at 50 ng/ml released IL-1β, IL-8, and TNF-α (data not shown). Similar IL-6 upregulation was observed with two other primary cultures of HIMECs (data not shown). The IL-6 concentration produced by HIMECs in response to 1 μg/ml of TS corresponded to that induced by 50 ng/ml of a bacterial LPS (data not shown). Thus, HIMECs appear to be much more sensitive to produce IL-6 in response to bacterial LPS than to TS. A control VCNA corresponding to the neuraminidase activity of TS did not stimulate detectable IL-6 secretion in HIMECs (Fig. 1 A), suggesting that the TS action did not depend on its intrinsic glycosidase activity. Another control protein, the T. cruzi heparin-binding penetrin (PN-1) thought to promote parasite invasion 28, was not effective in stimulating IL-6 secretion (Fig. 1 A), further emphasizing the selectivity of the TS action.
Figure 1.
Dose–response (A–C) and time course (D and E) of IL-6 release by normal human cells and cancer cells. HIMECs (A), PBMCs (B), and T-24 carcinoma cells (C) were incubated with the indicated concentrations of TS, VCNA, and PN-1, and after 24 h, the conditioned supernatants were analyzed for their content of IL-6 and other cytokines by ELISA. HIMECs (D) and PBMCs (E) were incubated with 1 μg/ml TS or 1 mU/ml VCNA for the times indicated. The VCNA units correspond to the neuraminidase activity of TS. Results similar to those in A, B, and C were repeated 10, 9, and 10 times, respectively.
TS also stimulated IL-6 release in normal PBMCs (Fig. 1 B), another class of human cells relevant to the immunity against T. cruzi. The result in Fig. 1 B is for one blood donor, but a similar result was obtained with three other blood donors (data not shown). In addition, TS stimulated IL-6 secretion in the human bladder carcinoma T-24 cell line (Fig. 1 C). Previous work has shown that T-24 cells express IL-6 constitutively and in response to cytokine stimuli 38 39. TS-induced IL-6 secretion in the T-24 bladder carcinoma cells further underscores the neuraminidase action for nonimmune cells. Interestingly, the IL-6 output resulting from the 24-h stimulation of T-24 carcinoma cells with 1 μg/ml of TS corresponded to that induced by 0.75 μg/ml of bacterial LPS under the same conditions (data not shown). Thus, in this cancer cell line, TS was nearly as good as bacterial LPS in stimulating IL-6 secretion.
IL-6 is produced by a large variety of cells, including endothelial cells, monocytes, fibroblasts, keratinocytes, T cells, mast cells, neutrophils, tumor cell lines, and cells of neural origin 40. Although we found that TS stimulates IL-6 production in endothelial cells and PBMCs, it is likely that the neuraminidase will also stimulate IL-6 release in other cell types. However, normal human neutrophils, which are capable of secreting IL-6 in response to various stimuli such as TNF-α and GM-CSF 41, did not produce detectable IL-6 when stimulated with TS under conditions in which TNF-α did (data not shown). Thus, not every human cell type may secrete IL-6 upon stimulation with TS.
Kinetics of TS-dependent IL-6 Secretion.
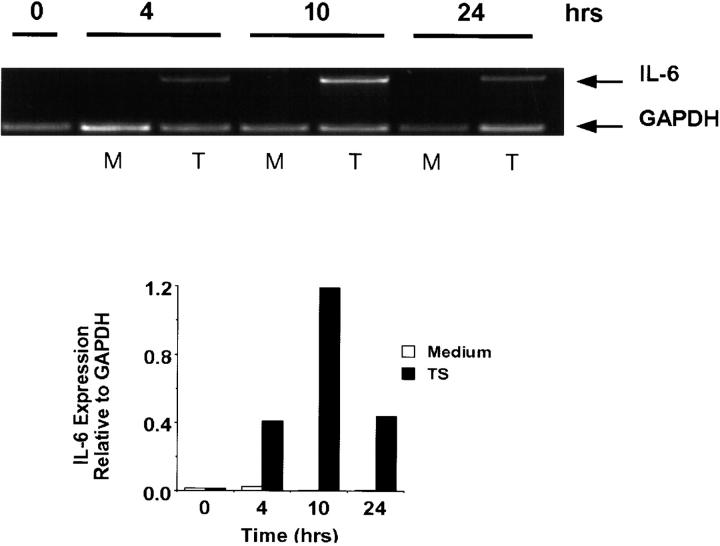
The kinetics of TS-induced IL-6 secretion revealed maximum effect after 24 h stimulation in both HIMECs and PBMCs (Fig. 1D and Fig. E). This response followed the upregulation of IL-6 transcripts, which was maximal 4–10 h after TS stimulation (Fig. 2). These results suggest that TS triggers synthesis and then secretion of IL-6, consistent with the effect of conventional cytokine agonists in the same cell types 37 38.
Figure 2.
Semiquantitative determination of PCR-amplified IL-6 mRNA in resting and TS-activated PBMCs. Representation of amplified IL-6 and GAPDH mRNA after stimulation with TS at 1 μg/ml for the times indicated. Top, agarose gel; bottom, densitometric measurement. Experiments repeated three times with similar results.
TS-conditioned Cell Supernatants Restore Growth of an IL-6–dependent B Lymphoma Cell Line.
The DS-1 B lymphoma cells have an intact IL-6 receptor signaling pathway, but because they cannot produce IL-6, the cells will die unless exogenous human IL-6 is added to the culture medium 30. Therefore, this cell line is a useful probe for the biological assay of human IL-6.
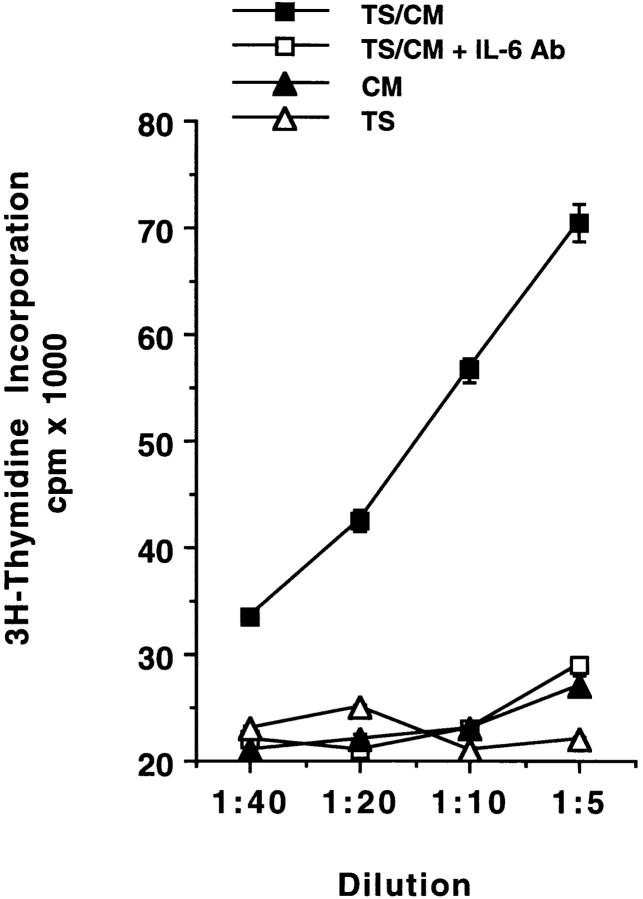
To determine whether TS induces secretion of biologically active IL-6, we measured the ability of conditioned media to promote growth of DS-1 cells. We prepared conditioned media by incubating PBMCs or T-24 cells for 24 h in 10% FCS/RPMI without (CM) or with (TS/CM) TS at 1 μg/ml. We found that TS/CM, but not CM, restored growth of the DS-1 lymphoma cells in a dose-dependent manner, and that a neutralizing IL-6 antibody suppressed the growth-promoting activity of TS/CM (Fig. 3). These results indicate that TS-dependent IL-6 released by PBMCs and T-24 cells is biologically active. In addition, we also conclude that the T. cruzi neuraminidase is not an IL-6 agonist because it did not restore growth of the DS-1 cells when the enzyme was added to normal growth medium lacking exogenous IL-6 (Fig. 3).
Figure 3.
TS/CM restores growth of IL-6–dependent DS-1 B lymphoma cells. PBMCs and T-24 cells were incubated without (CM) or with (TS/CM) TS for 24 h, and the resulting conditioned media were used at the indicated dilutions to supplement IL-6–free medium for DS-1 cells. A neutralizing IL-6 rabbit IgG at 1 μg/ml was added to each dilution of the TS/CM (TS/CM + IL-6 Ab). Normal rabbit IgG had no effect on the growth-promoting action of TS/CM (data not shown). TS was added directly to the IL-6–free growth medium at 1 μg/ml, which corresponds to 1:5 dilution shown on the x-axis. The plot above is for TS-CM from PBMC cultures, but similar results were obtained with T-24 cultures. Experiment repeated twice with similar results.
TS Mediates IL-6 Release in Nonimmune Cells through Its COOH-terminal TR, but Not Through Its CD.
To begin to dissect structural features of TS that elicit IL-6 release in normal human cells, we first determined whether the catalytic activity of TS mediates IL-6 secretion in immunologically naive human cells. For this, we compared the action of the catalytically inactive TS mutant TS-H32 with that of catalytically active TS-154 protein. Both TS-154 and TS-H32 constructs derive from the Y strain of T. cruzi 29, whereas the TS used in the experiments displayed in Fig. 1 Fig. 2 Fig. 3 Fig. 4 was derived from the Silvio strain 22. Both TS-H32 and TS-154 constructs contain five TR units (Fig. 4 A). Although TS-H32 differs from TS-154 in six amino acid substitutions in the CD (Fig. 4 A), the difference in enzymatic activity between the two constructs is attributed to a single amino acid, with tyrosine (Y374) in the active gene (TS-154) changed to histidine in the inactive gene (TS-H32) (Fig. 4a and Fig. b). To our surprise, enzymatically active TS-154 was as powerful as enzymatically inactive TS-H32 in stimulating IL-6 secretion in PBMCs (Fig. 4 C) and T-24 cells (data not shown).
The results with the TS-154 and TS-H32 constructs suggest that the catalytic activity of TS does not mediate IL-6 release in naive cells. This was confirmed with the use of recombinant CD of TS. The NH2-terminal domain of TS is 663 amino acids long and contains the enzymatically active CD (Fig. 4 A). In contrast, the enzymatically inactive COOH-terminal domain is made up of a 12 amino acid unit repeated 44 times in the 7F clone of TS derived from the Silvio strain of T. cruzi (LTR) (22; and data not shown). Recombinant His-tagged CD was purified from Escherichia coli lysates by binding to and elution from Ni2+-NTA/agarose column and by ion-exchange chromatography on Mono-Q columns (FPLC; see Materials and Methods). Recombinant His-tagged LTR was isolated from insect cell lysates by immunoaffinity to the mAb TCN-2 and to Ni2+-agarose columns (see Materials and Methods).
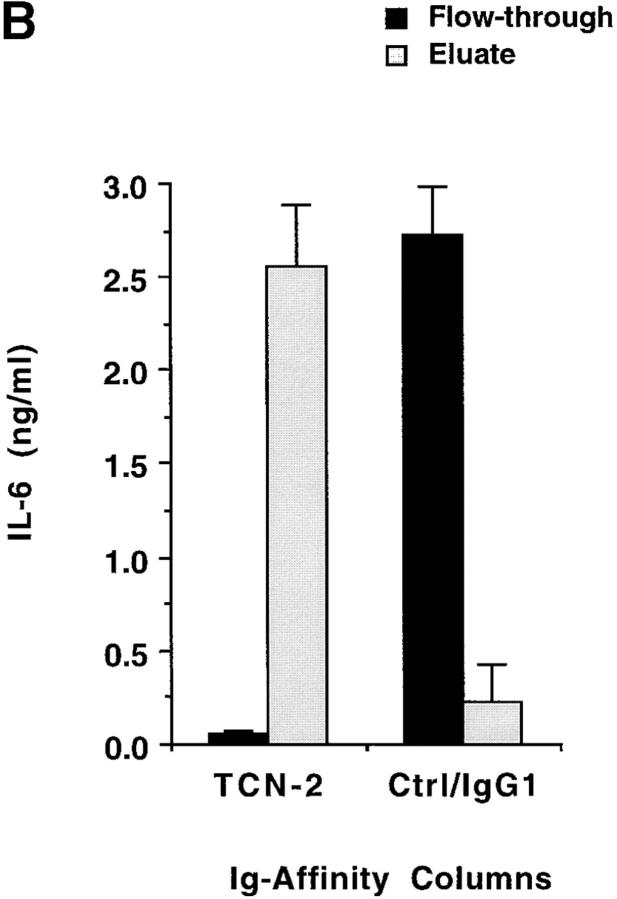
Addition of CD to T-24 cells did not promote IL-6 release, whereas addition of LTR effectively released IL-6 in a dose-dependent manner (Fig. 5 A). Similar results were obtained in the IL-6 release by PBMCs, in which LTR, but not CD, upregulated IL-6 release (data not shown). To confirm that LTR is a mediator of IL-6 release, we depleted LTR from solution in a protein G–Sepharose column adsorbed with TCN-2. TCN-2 is an IgG1 mAb specific for LTR routinely used in our lab to purify TS 20. The flow-through of such TCN-2 affinity column, which was devoid of LTR polypeptide, as determined by immunoblot analysis (data not shown), did not stimulate IL-6 release in T-24 cells (Fig. 5 B). In contrast, the flow-through of a control IgG1 column contained LTR polypeptide and stimulated IL-6 secretion (Fig. 5 B). Furthermore, elution of LTR from the TCN-2/protein G column restored the IL-6–secretory power of the original preparation, whereas similar elution from the IgG1 control column did not (Fig. 5 B). Therefore, these results establish LTR as mediator of IL-6 secretion in naive human cells.
Figure 5.
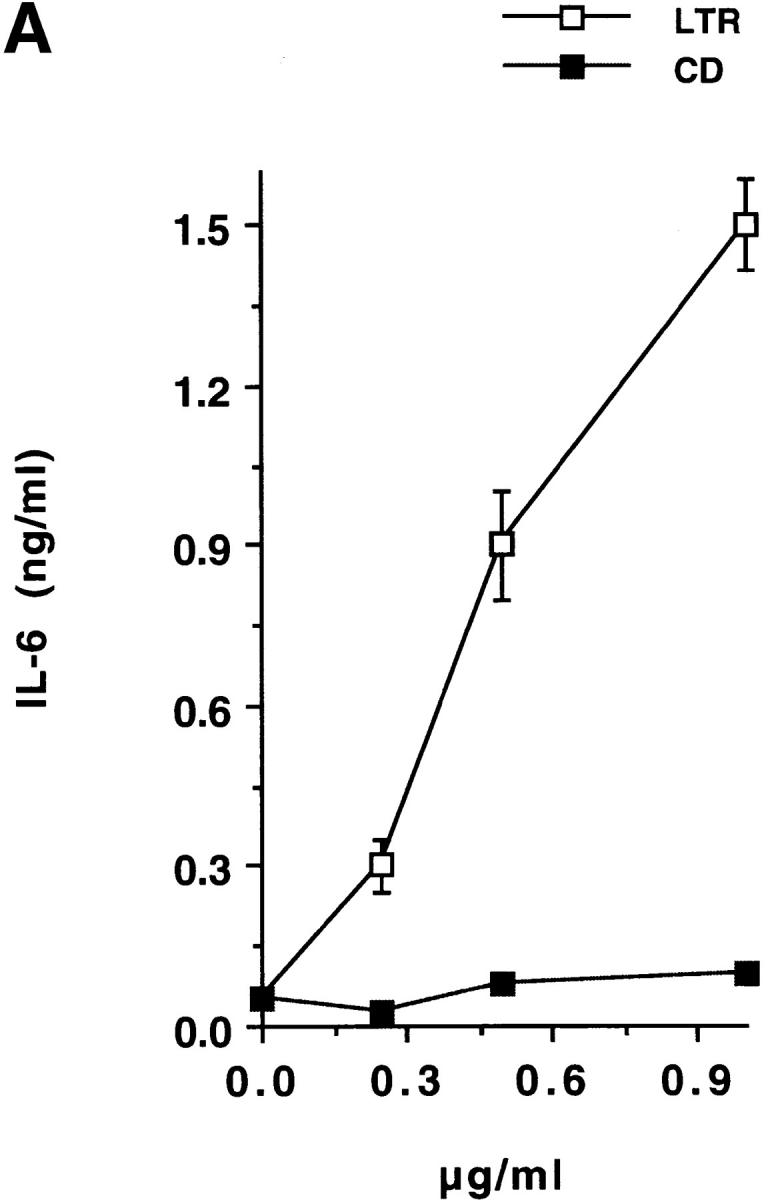
TS stimulates IL-6 release in T-24 cells via its TR. (A) Dose–response. T-24 cells were stimulated for 24 h with the indicated concentrations of recombinant LTR or CD domains of TS. IL-6 in the conditioned supernatants was assayed by ELISA. Similar results were obtained with PBMCs. (B) Immunodepletion of LTR. An LTR solution was passed through a protein G–Sepharose column adsorbed with either anti-LTR mAb TCN-2 (TCN-2) or control anti–p-azo-phenylarsonate IgG1 (Ctrl/IgG1). The flow-through of both columns was added to T-24 cells for 24 h, and the resulting conditioned media were assayed for IL-6 by ELISA. Eluate for each column was obtained by mechanical stirring of the agarose followed by centrifugation. Eluates were tested for IL-6–secretory activity in the same way as the flow-through.
Epimastigotes, Whose TS Lacks LTR, Do Not Efficiently Induce IL-6 Secretion in Normal PBMCs.
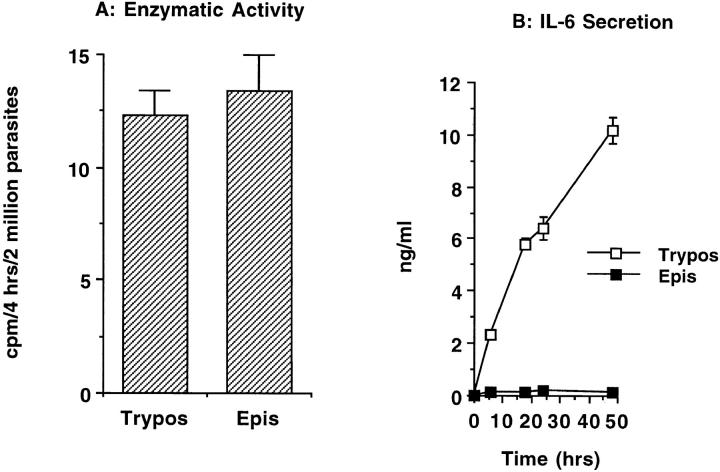
Epimastigote, a noninvasive stage of T. cruzi, expresses TS with the same carbohydrate specificity as the trypomastigote enzyme (17 42; Fig. 6 A). Yet, coculture of normal PBMCs with live epimastigotes did not result in IL-6 secretion under conditions in which coculture with trypomastigotes did (Fig. 6 B). This is not a paradox, because the TS of epimastigote, with a CD 84% identical to the counterpart TS of trypomastigotes 42, lacks LTR, as determined by immunoblot analysis with LTR-specific mAb 23 42 and by gene cloning 43. Therefore, the failure of live epimastigotes to release IL-6 from PBMCs supports the conclusion that LTR, and not CD, is the active TS domain upregulating IL-6 in normal human cells. In addition, it emphasizes the selectivity of trypomastigote TS in stimulating cytokine secretion in normal cells.
Figure 6.
Live trypomastigotes, but not epimastigotes, induce IL-6 secretion in normal PBMCs. (A) The trans-sialidase activity of trypomastigotes (Trypos) and epimastigotes (Epis). Live parasites (2 × 106 per reaction) were added to the TS reaction mixture containing 1% nonionic detergent to lyse the cells. Newly formed sialylated [14C]lactosamine was determined after 4 h incubation at room temperature. (B) IL-6 secretion after coculture of trypomastigotes with normal PBMCs. 106 PBMCs were cocultured with 105 trypomastigotes or epimastigotes for 48 h, and IL-6 was determined in the cell-free supernatants at the indicated times.
Synthetic Peptides Based on Silvio LTR Reproduce the Secretagogue Action of Intact TS.
Given that LTR is the domain that mediates for IL-6 secretion, and that the LTR of TS-154 and TS-H32 contains only five COOH-terminal repeats (Fig. 4 A), one would expect that LTR-based synthetic peptides up to five units should stimulate IL-6 secretion in human cells.
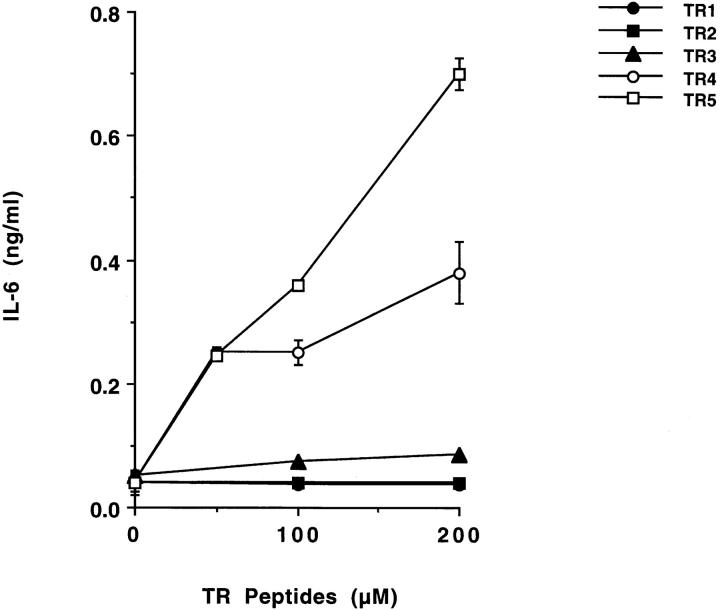
To test the above hypothesis, we prepared synthetic peptides TR1, TR2, TR3, TR4, and TR5, which correspond to the 12, 24, 36, 48, and 60 amino acid sequence proximal to the COOH terminus of the catalytic domain of Silvio TS (22; Table ). We then tested whether these synthetic peptides could stimulate IL-6 release in PBMCs. We found that TR4 and TR5 peptides were active in promoting IL-6 release, whereas TR1, TR2, and TR3 were less active (Fig. 7). The results with TR peptides confirm that the TR is the TS moiety that mediates IL-6 release in naive human cells.
Figure 7.
LTR-based synthetic peptides stimulate IL-6 release in PBMCs. Synthetic peptides, at the indicated concentrations, were added to PBMCs for 24 h, and the conditioned supernatants were assayed for IL-6 by ELISA. TR1 refers to 1 repeat (12 amino acids), TR2 to 2 repeats, and so on. For the sequence of the repeats, see Table .
HIMECs Strongly Induce IL-6 Secretion When Infected with a Trypomastigote Population Bearing a TS+, but Not a TS− Phenotype.
Extracellular trypomastigotes can be subdivided into two populations based on the relative abundance of TS (32; Fig. 8 A). These two populations can be readily separated from one another by differential affinity to magnetic beads coated with LTR-specific mAb TCN-2 32. The subset that produces TS, named TS+, represents 25% of the total trypomastigote population, whereas the subset that does not produce or produces very little TS, termed TS−, constitutes the majority of the trypomastigotes 32. Interestingly, TS+ parasites of the Silvio strain are short and stumpy (length = 9.3 ± 2.8 μm) and morphologically distinct from the TS− parasites, which are slender (length = 18.2 ± 4.3 μm) (Fig. 8 C). Live TS+ trypomastigotes moved slowly and sluggishly in liquid medium (10% FCS/RPMI) at room temperature, contrary to the TS− trypomastigotes, which migrated swiftly with a whipping movement (data not shown). However, the dimorphism and contrasting movements of TS+ and TS− trypomastigotes were not characteristic features of two other T. cruzi strains, Tulahuen and MV-13 (data not shown). Thus, there does not appear to be a relation between the expression of TS and a particular morphological type of T. cruzi. It remains to be determined why the TS+ and TS− trypomastigotes of the Silvio strain, but not of the other strains, are so morphologically distinguishable from one another.
Figure 8.
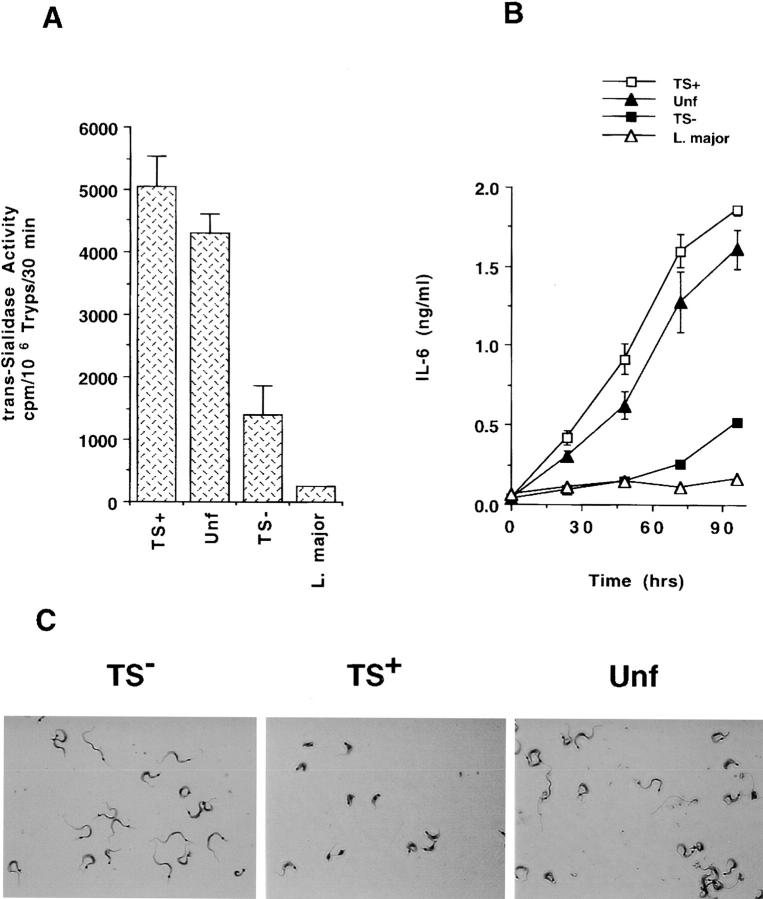
Differential release of IL-6 by HIMECs after infection with TS+ and TS− trypomastigotes. (A) The trans-sialidase activity of unfractionated trypomastigotes (Unf ), trypomastigote populations with phenotypes TS+ and TS−, and L. major promastigotes. (B) IL-6 released by HIMECs after infection with the indicated trypomastigote populations and L. major promastigotes. (C) The morphology of TS−, TS+, and unfractionated trypomastigotes of the Silvio X-10/4 of T. cruzi stained with Diff-Quik. Original magnification: ×1,200.
Nevertheless, the availability of purified TS+ and TS− populations offered a unique opportunity to test the power of live parasites with variable TS abundance to upregulate IL-6 in normal human cells. Therefore, we challenged HIMEC monolayers with live TS+ and TS− parasites and followed the time course of IL-6 released in the trypanosome-containing culture supernatants. It was remarkable to find that TS+ trypomastigotes were much more effective than TS−, and somewhat better than unfractionated trypomastigotes, in inducing intestinal endothelial cells to release IL-6 (Fig. 8 B). The result in Fig. 8 B is for the subpopulations of the Silvio strain, but similar results were obtained with the TS+ and TS− subsets of the Tulahuen strain (data not shown). Because the TS+ parasites of the Tulahuen strain are a mixture of stumpy and slender forms, the power of T. cruzi to induce IL-6 secretion in naive human cells depends on the TS content, rather than on the morphology of the parasites. Furthermore, the inability of L. major promastigotes (which do not have TS activity [see Fig. 8 A]) to release IL-6 in HIMECs (Fig. 8 B) suggests that parasite burden per se does not suffice to induce IL-6 secretion in naive human cells.
Discussion
Infection of mammals by parasites and other microbes results in the release of cytokines and other mediators of the inflammatory response. The composition of the cytokines, which depends on the nature of the infecting organism and on the host genotype, may be critical for the resistance or susceptibility to microbial invasion, as best exemplified by the infection of mice with the protozoan L. major 44 and of humans with the bacterium Mycobacterium leprae 45.
It is generally accepted that cytokine networks result from antigenic stimulation of lymphocytes and macrophages. However, these antigen-driven host responses can be subverted by a group of viral and bacterial proteins, termed virokines and bacteriokines, respectively, which are capable of changing the dynamics of the cytokine networks without directly activating B and T cell receptors 46. Virokines tend to suppress host immune responses by neutralizing inflammatory cytokines, as is the case of the protein B15R of vaccinia virus, which binds IL-1β 47. Alternatively, virokines may downmodulate immune responses by mimicking antiinflammatory cytokines, like the protein BCRF1 of Epstein-Barr virus, which is 70% identical to IL-10 48. On the other hand, bacteriokines, including LPS and exotoxins, are more likely to stimulate proinflammatory cytokines, thereby enhancing pathogenesis 46. Whether protozoan parasites can alter host immune responses through molecules functionally equivalent to virokines and bacteriokines (i.e., “protokines”) remains to be determined.
The findings reported in this paper identify TS as a protein that could alter the dynamics of the cytokine network by upregulating IL-6 secretion in normal human cells. As shown in Fig. 1, Fig. 5, Fig. 7, and Fig. 8, TS and its TR were active against naive microvascular endothelial cells and PBMCs. Furthermore, given that IL-6 may be produced by many other cell types, such as fibroblasts and epithelial cells, the range of cell target for IL-6 release by TS could be broader than the vascular endothelium and blood mononuclear cells. This was indicated by the ability of TS to induce IL-6 release in the T-24 bladder carcinoma cells (Fig. 1) and by parallel experiments with mouse cells, which revealed TS to be an upregulator of IL-6 secretion in naive splenocytes, bone marrow cells, and peritoneal cells from BALB/c and other strains of mice (Gao, W., and M.A. Pereira, manuscript in preparation). Therefore, TS upregulates IL-6 secretion in various cell types and animal species.
The kinetics of TS-dependent IL-6 release in vitro (Fig. 1D and Fig. E, and Fig. 2) suggests that, in vivo, TS should stimulate IL-6 release before the full development of acquired immune response against T. cruzi. This could be accomplished when T. cruzi first encounters the mammalian host, such as after an insect bite when the parasites enter the host through the mucosa, usually around the eye; or when T. cruzi enters the host during blood transfusion or congenitally, in which case the TS+ parasites gain access into the circulation where they should react with endothelial cells and PBMCs to trigger IL-6 release. In addition, TS may also promote IL-6 secretion in vivo as a soluble mediator. Indeed, soluble neuraminidase was detected, before the parasites, in the blood of a T. cruzi–infected individual 24. Copious amounts of the enzyme are released in monolayers of cells infected with T. cruzi 20. Our findings reported here show that soluble enzyme and TS-expressing parasites are capable of inducing IL-6 secretion in normal cells.
There are several ways in which TS-dependent IL-6 could alter T. cruzi invasion. IL-6 promotes polyclonal activation of B and T lymphocytes 49 50 and is an important cofactor for Th2 T cell activation, necessary for the induction of humoral immune responses 51. Thus, the TS/IL-6 pathway could be directly relevant to the polyclonal lymphocyte responses that characterize acute Chagas's disease 52. In addition, IL-6 is a potent inducer of collagen secretion in human fibroblasts 53, and as such appears to underlie the pathogenesis of systemic sclerosis, a connective tissue disease characterized by fibrosis in the skin and internal organs 54. The fibrogenic action of IL-6 could also be relevant to T. cruzi infection, as fibrosis is a prominent feature of acute and chronic Chagas's disease 55 56. Because chronic chagasic heart contains many IL-6–containing mononuclear cells and endothelial cells 4 5 6 7, IL-6 could in theory contribute to the fibrosis in the chronic heart, regardless of the mechanism stimulating production of the cytokine.
IL-6, in addition to mediating inflammatory and immune responses, may play an important role in a variety of activities of the central and peripheral nervous systems, such as cell-to-cell signaling, protection of neurons from insult, and neuronal growth and survival 57. Thus, TS-dependent IL-6 could be a factor in the neuroregeneration that characterizes the indeterminate phase of Chagas's disease in humans 55 and animals 58. In support of this hypothesis, we recently found TS to be extremely potent in protecting several types of neuronal cells from undergoing apoptosis induced by growth factor deprivation (Chuenkova, M., and M.A. Pereira, manuscript submitted for publication). What's more, the TS-induced neuroprotection was synergistic with two members of the IL-6 family, ciliary neurotrophic factor and leukemia inhibitory factor (Chuenkova, M., and M.A. Pereira, manuscript submitted for publication). Thus, the neurotrophic effect of TS-dependent IL-6 could be boosted by the action of TS and by the TS synergy with ciliary neurotrophic factor or leukemia inhibitory factor on the neurons.
TS itself has been implicated as a key factor in T. cruzi invasion. Experiments with cells in culture suggest that TS promotes parasite attachment to host cells through the recognition of sialyl-containing surface receptors 59 60. Experiments in vivo are also consistent with TS being a promoter of T. cruzi invasion, as suggested by the enhancement of infection in experimental models of Chagas's disease by LTR-specific polyclonal 61 and monoclonal 62 24 antibodies, and by the inhibition of infection by CD-specific antibodies 63 64. In addition, antibodies against TS-generated sialyl epitopes present on the trypomastigote surface block infection as well 65. Finally, the enhancement of T. cruzi virulence in BALB/c mice sensitized with low doses of the enzyme further supports the conclusion that TS is a major factor in promoting T. cruzi invasion of mammalian hosts 25. The novel findings reported here open up strategies to investigate an unsuspected role for TS in T. cruzi invasion and provide a new tool to study the function of IL-6 in Chagas's disease.
Acknowledgments
We thank Drs. Claudio Fiocchi and Gail West for the generous gift of HIMECs and for advice, Marina Chuenkova for the CD domain and for discussions, Edouard Vannier and Charles Dinarello for initial radioimmunoassays to detect IL-1β and TNF-α in TS-conditioned supernatants of PBMCs, Thereza Imanishi-Kari for the anti–p-azo-phenylarsonate mAb IgG1, and Daniel Eichinger for suggesting the use of the Y strain constructs.
This work was supported by National Institutes of Health grant AI18102.
Footnotes
Abbreviations used in this paper: CD, catalytic domain of TS; CM, conditioned medium; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HIMEC, human intestinal microvascular endothelial cell; HUVEC, human umbilical vein endothelial cell; NTA, nitrilotriacetic acid; PN-1, penetrin; RANTES, regulated upon activation, T cell expressed and secreted; TS, trans-sialidase; TR, tandem repeat; TS/CM, TS-stimulated CM; VCNA, Vibrio cholera neuraminidase.
References
- Tanowitz H.B., Gumprecht J.P., Spurr D., Calderon T.M., Ventura M.C., Raventos-Suarez C., Kellie S., Factor S.M., Hatcher V.B., Wittner M., Berman J.W. Cytokine gene expression of endothelial cells infected with Trypanosoma cruzi . J. Infect. Dis. 1992;166:598–603. doi: 10.1093/infdis/166.3.598. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W.C. Coculture of human peripheral blood mononuclear cells with Trypanosoma cruzi leads to proliferation of lymphocytes and cytokine production. J. Immunol. 1992;148:239–248. [PubMed] [Google Scholar]
- Truyens C., Angelo-Barrios A., Torrico F., van Damme J., Heremans H., Carlier Y. Interleukin-6 (IL-6) production in mice infected with Trypanosoma cruzieffect of its paradoxical increase by anti-IL-6 monoclonal antibody treatment on infection and acute-phase and humoral immune responses. Infect. Immun. 1994;62:692–696. doi: 10.1128/iai.62.2.692-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B., Melby P.C., Troyer D.A., Freeman G.L. Induction of proinflammatory cytokine expression in experimental acute chagasic cardiomyopathy. Biochem. Biophys. Res. Commun. 1996;223:365–371. doi: 10.1006/bbrc.1996.0900. [DOI] [PubMed] [Google Scholar]
- Sunnemark D., Ulfgren A.-K., Orn A., Harris R.A. Cytokine production in hearts of Trypanosoma cruzi-infected CBA micedo cytokine patterns in chronic stage reflect the establishment of myocardial pathology? Scand. J. Immunol. 1996;44:421–429. doi: 10.1046/j.1365-3083.1996.d01-328.x. [DOI] [PubMed] [Google Scholar]
- Sunnemark D., Frostegard J., Orn A., Harris R.A. Cellular and cytokine characterization of vascular inflammation in CBA/J mice infected with Trypanosoma cruzi . Scand. J. Immunol. 1998;48:480–484. doi: 10.1046/j.1365-3083.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Tarleton R. Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infections. Exp. Parasitol. 1996;84:203–213. doi: 10.1006/expr.1996.0106. [DOI] [PubMed] [Google Scholar]
- Glienke W., Esser R., von Briessen H., Schuster K., Muller S., Unger R., Andreesen R., Stutte H.J., Rubsamen-Waigman H. Cytokine expression of HIV-infected monocytes/macrophages at the single-cell level. Res. Virol. 1994;145:193–197. doi: 10.1016/s0923-2516(07)80022-7. [DOI] [PubMed] [Google Scholar]
- Breen E.C., Rezai A.R., Nakajima K., Beall G.N., Mitsuyasu R.T., Hirano T., Kishimoto T., Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J. Immunol. 1990;144:480–484. [PubMed] [Google Scholar]
- Rieckmann P., Poli G., Kehlr J.H., Fauci A.S. Activated B lymphocytes from human immunodeficiency virus–infected individuals induce virus expression in infected T cells and a promonocytic cell line, U1. J. Exp. Med. 1991;173:1–5. doi: 10.1084/jem.173.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino C., Ruocco M.R., Chen X., Mallardo M., Baudi F., Trematerra S., Quinto I., Venuta S., Scala G. HIV-1 Tat induces the expression of the interleukin-6 (IL-6) gene by binding to the IL-6 leader RNA and by interacting with CAAT enhancer-binding protein beta (NF-IL6) transcription factors. J. Biol. Chem. 1997;272:14883–14892. doi: 10.1074/jbc.272.23.14883. [DOI] [PubMed] [Google Scholar]
- Gong J.-H., Sprenger H., Hinder F., Bender A., Schmidt A., Horch S., Nain M., Gemsa D. Influenza A virus infection of macrophages. Enhanced tumor necrosis factor-α (TNF-α) gene expression and lipopolysaccharide-triggered TNF-α release. J. Immunol. 1991;147:3507–3513. [PubMed] [Google Scholar]
- Hayden F.G., Fritz R.S., Lobo M.C., Alvord W.G., Strober W., Straus S.E. Local and systemic cytokine responses during experimental human influenza A virus infection. J. Clin. Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Tang W., Ray A., Wu Y., Einarsson O., Landry M.L., Gwaltney J., Jr., Elias J.A. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. J. Clin. Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Coffman R.L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- DosReis G.A. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol. Today. 1997;13:335–342. doi: 10.1016/s0169-4758(97)01073-9. [DOI] [PubMed] [Google Scholar]
- Pereira M.E.A. A developmentally regulated neuraminidase activity in Trypanosoma cruzi . Science. 1983;219:1444–1447. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Schenkman S.L., de Carvalho P., Nussenzweig V. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzyme. J. Exp. Med. 1992;175:567–575. doi: 10.1084/jem.175.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A.J., Pollevick G.D., Mautner M., Buschiazzo A., Sanchez D.O., Frasch A.C.C. Identification of the gene(s) coding for the trans-sialidase of Trypanosoma cruzi . EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:1705–1710. doi: 10.1002/j.1460-2075.1992.tb05221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P., Doom J.P., Chuenkova M., Manger I.D., Pereira M.E.A. Enzymatic characterization of the β-d-galactoside α2,3-trans-sialidase from Trypanosoma cruzi . J. Biol. Chem. 1993;268:9886–9891. [PubMed] [Google Scholar]
- Schenkman S., Eichinger D., Pereira M.E.A., Nussenzweig V. Structural and functional properties of trypanosoma trans-sialidase. Annu. Rev. Microbiol. 1994;48:499–532. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- Pereira M.E.A., Mejia J.S., Ortega-Barria E., Matzilevich D., Prioli R.P. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low-density lipoprotein receptor, and type III modules of fibronectin. J. Exp. Med. 1991;174:179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg I., Prioli R.P., Ortega-Barria E., Pereira M.E.A. Stage-specific phospholipase C mediated release of Trypanosoma cruzi neuraminidase. Mol. Biochem. Parasitol. 1991;46:303–306. doi: 10.1016/0166-6851(91)90054-a. [DOI] [PubMed] [Google Scholar]
- De Titto E., Araujo F.G. Serum neuraminidase activity and hematological alterations in acute human Chagas' disease. Clin. Immunol. Immunopathol. 1988;46:157–161. doi: 10.1016/0090-1229(88)90016-5. [DOI] [PubMed] [Google Scholar]
- Chuenkova M., Pereira M.E.A. Trypanosoma cruzi trans-sialidaseenhancement of virulence in a murine model of Chagas' disease. J. Exp. Med. 1995;181:1693–1703. doi: 10.1084/jem.181.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong S.A., Pizarro T.T., Klein J.S., Cominelli F., Fiocchi C. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology. 1998;114:1244–1256. doi: 10.1016/s0016-5085(98)70431-7. [DOI] [PubMed] [Google Scholar]
- Coligan J.E., Kruisbeek A.M., Margulies D.H., Shevach E.M., Strober W. Current Protocols in Immunology 1995. John Wiley & Sons, Inc; New York: pp. 7.1.1–7.1.2 [Google Scholar]
- Ortega-Barria E., Pereira M.E.A. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991;67:411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- Uemura H., Schenkman S., Nussenzweig V., Eichinger D. Only some members of a gene family of Trypanosoma cruzi encode proteins that express both trans-sialidase and neuraminidase activities. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:3837–3844. doi: 10.1002/j.1460-2075.1992.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock G.H., Long C.A., Riley M.L., White J.D., Kurman C.C., Fleischer T.A., Tsokos M., Brown M., Serbousek D., Schwietermann W.D. Characterization of a new IL-6 dependent human B-lymphoma cell line in long term culture. Cytokine. 1993;5:480–489. doi: 10.1016/1043-4666(93)90039-8. [DOI] [PubMed] [Google Scholar]
- Wong H., Anderson W.D., Cheng T., Riabowo K.T. Monitoring mRNA expression by polymerase chain reactionthe “primer-dropping” method. Anal. Biochem. 1994;223:251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- Pereira M.E.A., Zhang K., Gong Y., Herrera E.M., Ming M. Invasive phenotype of Trypanosoma cruzi restricted to a population expressing trans-sialidase. Infect. Immun. 1996;64:3884–3892. doi: 10.1128/iai.64.9.3884-3892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerlick R.A., Lawley T.J. Role of microvascular endothelial cells in inflammation. J. Invest. Dermatol. 1993;100:111S–115S. doi: 10.1111/1523-1747.ep12356595. [DOI] [PubMed] [Google Scholar]
- Granger D.N., Kubes P. The microcirculation and inflammationmodulation of leukocyte-endothelial cell adhesion. J. Leukoc. Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- Augustin H.G., Kozian D.T., Johnson R.C. Differentiation of endothelial cellsanalysis of the constitutive and activated endothelial cell phenotypes. Bioessays. 1994;16:901–906. doi: 10.1002/bies.950161208. [DOI] [PubMed] [Google Scholar]
- Binion D.G., West G.A., Ina K., Ziats N.P., Emancipator S.N., Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895–1907. doi: 10.1053/gast.1997.v112.pm9178682. [DOI] [PubMed] [Google Scholar]
- Nilsen E.M., Johansen F.-E., Jahnsen F.L., Lundin K.E.A., Scholz T., Brandtzaeg P., Haraldsen G. Cytokine profiles of cultured microvascular endothelial cells from human intestine. Gut. 1998;42:635–642. doi: 10.1136/gut.42.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik J., Baresova M., Viklicky V., Jakoubkova J., Sainerova H., Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumor-specific antigen. Int. J. Cancer. 1973;11:765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO (Eur. Mol. Biol. Organ.) J. 1987;6:2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Sutherland M.S.K. IL-6 as a drug discovery target. Drug Discovery Today. 1998;3:202–213. [Google Scholar]
- Cicco N.A., Lindemann A., Content J., Vandenbussche P., Lubbert M., Gauss J., Mertelsmann R., Herrmann F. Inducible production of interleukin-6 by human polymorphonuclear neutrophilsrole of granulocyte-macrophage colony stimulating factor and tumor necrosis factor-alpha. Blood. 1990;75:2049–2052. [PubMed] [Google Scholar]
- Chaves L.B., Briones M.R., Schenkman S. Trans-sialidase from Trypanosoma cruzi epimastigotes is expressed at the stationary phase and is different from the enzyme expressed in trypomastigotes. Mol. Biochem. Parasitol. 1993;61:97–106. doi: 10.1016/0166-6851(93)90162-q. [DOI] [PubMed] [Google Scholar]
- Briones M.R., Egima C.M., Schenkman S. Trypanosoma cruzi trans-sialidase gene lacking C-terminal repeats and expressed in epimastigote forms. Mol. Biochem. Parasitol. 1995;70:9–17. doi: 10.1016/0166-6851(95)00004-k. [DOI] [PubMed] [Google Scholar]
- Reiner S.L., Locksley R.M. The regulation of immunity to Leishmania major . Annu. Rev. Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Wang X.H., Ohmen J.D., Uyemura K., Rea T.H., Bloom B.R., Modlin R.L. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 1992;149:1470–1475. [PubMed] [Google Scholar]
- Wilson M., Seymour R., Henderson B. Bacterial perturbation of cytokine networks. Infect. Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcami A., Smith G.L. A soluble receptor for interleukin–1 beta encoded by vaccinia virusa novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Hsu D.H., de Waal-Malfyt R., Fiorentino D.F., Dang M.-N., Vieira P., deVries J., Spits H., Mosmann T.R., Moore K.W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- Uyttenhove C., Coulie P.G., Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J. Exp. Med. 1988;167:1417–1427. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M., Anguita J., Nakamura T., Fikrig E., Flavell R.A. Interleukin (IL)-6 directs the differentiation of IL-4–producing CD4+ T cells. J. Exp. Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoprio P.M., Eisen H., Forni L., D'Imperio Lima M.R., Joskowicz M., Coutinho A. Polyclonal lymphocyte responses to murine Trypanosoma cruzi infection. I. Quantitation of both T- and B-cell responses. Scand. J. Immunol. 1986;24:661–668. doi: 10.1111/j.1365-3083.1986.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Duncan M.R., Berman B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J. Invest. Dermatol. 1991;97:686–692. doi: 10.1111/1523-1747.ep12483971. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Hara M., Wright T.M. Endogenous IL-1α from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J. Clin. Invest. 1999;103:1253–1260. doi: 10.1172/JCI4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kÿberle F. Pathogenesis of Chagas' disease. Ciba Found. Symp. 1974;20:137–147. [Google Scholar]
- Andrade Z.A. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found. Symp. 1983;99:214–233. doi: 10.1002/9780470720806.ch12. [DOI] [PubMed] [Google Scholar]
- Gruol D.L., Nelson T.E. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol. Neurobiol. 1997;15:307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- Tafuri W.L. Pathogenesis of lesions of the autonomic nervous system of the mouse in experimental acute Chagas' disease. Am. J. Trop. Med. Hyg. 1970;19:405–417. doi: 10.4269/ajtmh.1970.19.405. [DOI] [PubMed] [Google Scholar]
- Ming M., Chuenkova M., Ortega-Barria E., Pereira M.E.A. Mediation of Trypanosoma cruzi invasion by sialic acid on the host cell and trans-sialidase on the trypanosomes. Mol. Biochem. Parasitol. 1993;59:243–252. doi: 10.1016/0166-6851(93)90222-j. [DOI] [PubMed] [Google Scholar]
- Schenkman R.P., Vandekerckhove F., Schenkman S. Mammalian cell sialic acid enhances Trypanosoma cruzi invasion. Infect. Immun. 1993;61:898–902. doi: 10.1128/iai.61.3.898-902.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalesco R., Pereira M.E.A. Antibody to Trypanosoma cruzi neuraminidase enhances infection in vitro and identifies a subpopulation of trypomastigotes. J. Immunol. 1988;140:617–625. [PubMed] [Google Scholar]
- Prioli R.P., Mejia J.S., Pereira M.E.A. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J. Immunol. 1990;144:4384–4389. [PubMed] [Google Scholar]
- Costa F., Franchin G., Pereira-Chioccola V.L., Riberao M., Schenkman S., Rodrigues M.M. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine. 1998;16:768–774. doi: 10.1016/s0264-410x(97)00277-6. [DOI] [PubMed] [Google Scholar]
- Leguizamon M.S., Campetella O.E., Gonzalez Cappa S.M., Frasch A.C.C. Mice infected with Trypanosoma cruzi produce antibodies against the enzymatic domain of trans-sialidase that inhibit its activity. Infect. Immun. 1994;62:3441–3446. doi: 10.1128/iai.62.8.3441-3446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchin G., Pereira-Chioccola V.L., Schenkman S., Rodrigues M.M. Passive transfer of a monoclonal antibody specific for a sialic acid-dependent epitope on the surface of Trypanosoma cruzi trypomastigotes reduces infection in mice. Infect. Immun. 1997;65:2548–2554. doi: 10.1128/iai.65.7.2548-2554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]