Abstract
Larvae and adults of the parasitic blood fluke Schistosoma mansoni are resistant to killing by human complement. An earlier search by Parizade et al. for a schistosome complement inhibitor identified a 94-kDa surface protein which was named SCIP-1 (M. Parizade, R. Arnon, P. J. Lachmann, and Z. Fishelson, J. Exp. Med. 179:1625-1636, 1994). Following partial purification and analysis by mass spectrometry, we have determined SCIP-1 to be a surface-exposed form of the muscle protein paramyosin. As shown by immunofluorescence, anti-paramyosin antibodies label the surface of live schistosomula and adult worms. Like SCIP-1, purified native paramyosin reacts with a polyclonal rabbit anti-human CD59 antiserum, as shown by Western blot analysis. Also, the human complement components C8 and C9 bind to recombinant and native paramyosin. Analysis of paramyosin binding to fragments of C9 generated by thrombin or trypsin has demonstrated that paramyosin binds to C9 at a position located between Gly245 and Arg391. Paramyosin inhibited Zn2+-induced C9 polymerization and poly-C9 deposition onto rabbit erythrocytes (ER). In addition, paramyosin inhibited lysis of ER and of sensitized sheep erythrocytes by human complement. Finally, anti-paramyosin antibodies enhanced in vitro killing of schistosomula by normal and C4-depleted human complement. Taken together, these findings suggest that an exogenous form of S. mansoni paramyosin inhibits activation of the terminal pathway of complement and thus has an important immunomodulatory role in schistosomiasis.
Schistosomiasis is one of the most prevalent parasitic diseases, affecting over 200 million people who live in areas of endemicity in Africa, South America, and Asia (12). Schistosoma mansoni, one of the causative agents of this disease, resides within patients' mesenteric and portal blood systems and successfully evades host immune responses (43). During skin penetration into the mammalian host and shortly afterwards, the larvae convert from exhibiting sensitivity to complement-mediated killing to bearing resistance to complement-mediated killing (32, 49). The first step in that conversion is the removal of the glycocalyx coat that contains strong complement activators. Subsequently, the transformed schistosomula and the adult worms employ several strategies to evade the host's complement system (14), thus enabling the parasite to reside for years within the host's circulatory system in direct contact with complement proteins. This parasite contains proteins that bind in vitro to the complement proteins C1 (25) and C2 (22) and C8 and C9 (40) and may thus block the complement cascade at multiple steps. Paramyosin, one of the inhibitory proteins, was shown to bind in vitro to C1q and inhibit C1 and the classical pathway of complement activation (25). Paramyosin is in essence an invertebrate muscle protein (11) that serves as a major component of the thick filament and is thought to play an important role in certain specialized contractile states (7, 23). It has also been found to be an immunogen during infection of mice and humans with helminth parasites (27, 37, 47). A nonfilamentous membrane-bound form of paramyosin was also found in the tegumental outer layer (16, 33) and on the surface (18, 31) of S. mansoni schistosomes. Analyzed on the basis of its capacity to bind to Fc (31), it has been suggested to have immunomodulatory activities. Paramyosin served in experimental systems as a vaccine molecule potent against infection with S. mansoni and Schistosoma japonicum (27, 46) and is currently under investigation as a candidate vaccine against schistosomiasis in humans (2).
Parizade et al. (40) reported that schistosomula, lung stage worms, and adult worms of S. mansoni express on their surface a 94-kDa complement inhibitor (SCIP-1) that has characteristics similar to those of human CD59. CD59 is an 18-kDa membrane glycoprotein expressed in most vertebrate tissues (9, 10) that protects homologous cells from damage by complement. It binds to C8 and C9 (30, 39) and inhibits assembly of the C5b-9n membrane attack complex (MAC) (36, 48). As described here, sequencing and specific antibody binding data indicate that SCIP-1 is a tegumental form of paramyosin. Our data further prove that paramyosin binds in vitro to C8 and C9 and inhibits MAC formation. This finding extends the range of potential immunomodulatory activities of paramyosin.
MATERIALS AND METHODS
Parasites and parasite extracts.
An Egyptian strain of S. mansoni was used in this work. The life cycle was maintained in Puerto Rican Biomphalaria glabrata snails and outbred ICR mice (Charles River Laboratories, Wilmington, Mass.). Cercariae were collected from infected snails, mechanically transformed into schistosomula by repeated transfers through a syringe needle (5, 32), and fractionated over a Percoll gradient to separate the bodies from the tails (28). The schistosomula were then incubated in defined synthetic medium (DSM) composed of RPMI 1640 and nutrient F12 (GIBCO, Paisley, United Kingdom) (1:1) for 3 h or overnight at 37°C in a 6% CO2 incubator. Outbred ICR mice were infected by subcutaneous infection with 400 to 450 cercariae. Adult worms were collected by perfusion of the hepatic portal system of infected mice at 6 to 8 weeks postinfection (52). Nonidet P-40 (NP-40)-released material (NPRM) was extracted from 24-h-old schistosomula by treatment with 1% NP-40 in phosphate-buffered saline (PBS) for 2 h on ice, and particulate debris was removed by centrifugation for 1 min at 20,000 × g.
SCIP-1 purification and sequencing.
NPRM from schistosomula was fractionated over a DEAE-cellulose (DE-52; Whatman, Maidstone, Kent, United Kingdom) column. Bound proteins were eluted with a salt concentration gradient (0 to 0.6 M NaCl in 0.01 M sodium acetate buffer, pH 6.0) in the presence of protease inhibitors (Inhibitor Cocktail [catalog no. P8340]; Sigma, St. Louis, Mo.). Protein concentrations were determined using a protein assay kit (Bio-Rad, Richmond, Va.). Fractions containing SCIP-1 were identified in Western blot assays with rabbit anti-human CD59 antibodies (received from Peter Lachmann, Cambridge, United Kingdom). Partially purified SCIP-1 was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 8% acrylamide gels under nonreducing conditions. The SCIP-1 band was excised from the gel and submitted to the Protein Center of Technion (Haifa, Israel) for mass spectrometry analysis.
Purification of native and recombinant paramyosin.
Mouse monoclonal anti-paramyosin antibodies (4B1 and immunoglobulin G2a [IgG2a] [obtained from Edward Pearce]) were coupled to cyanogen bromide-activated Sepharose (Sigma) according to Sigma's recommended protocol. NPRM from schistosomula (50,000 total) was passed through a column of packed anti-paramyosin-Sepharose (0.5 ml). The bound protein was eluted with glycine-HCl (pH 2.3), immediately titrated to pH 7.0 with carbonate buffer (pH 8.6), and dialyzed with PBS. Recombinant paramyosin was expressed as inclusion bodies in Escherichia coli transformed with an expression vector, BL-21-DE3 pLys (obtained from Alan Sher and John Anderson). The recombinant paramyosin was purified in inclusion bodies, renatured by dialysis with a high salt buffer, and purified over a W-pore C4 high-pressure liquid chromatography column (Phenomenex, Torrance, Calif.).
Western blot analysis.
NPRM or purified protein was subjected to SDS-PAGE on an 8% acrylamide gel and transferred onto a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). The membrane was blocked with a 5% skim milk solution (Tnuva, Rehovot, Israel) in Tris-buffered saline containing 0.05% Tween 20 (Sigma) (TBST) (pH 8.0) for 1 h at room temperature. Next, the membrane was treated with rabbit anti-CD59 or anti-paramyosin antibody (1:300), washed, and treated with peroxidase-conjugated goat anti-rabbit IgG (Sigma) (1:5,000) as a second antibody. Bands were developed with an enhanced chemiluminescence reagent (Pierce, Rockford, Ill.) and exposed to a BioMax film (Kodak, Rochester, N.Y.).
Immunofluorescence of schistosomula and adult worms.
Schistosomula (100 total, 24 h old) or fresh adult worms (20 mixed male and female worms) were treated with a blocking solution containing 0.5% bovine serum albumin (BSA), 0.1% γ-globulin, and 10% heat-inactivated goat serum (Sigma) in DSM (50 μl) for 30 min at room temperature. They were then incubated with polyclonal rabbit anti-paramyosin antibodies or normal rabbit serum (diluted 1:50 in 50 μl of DSM) for 30 min at 37°C. After three washes with DSM on ice, they were treated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Sigma) (1:100 in DSM) for 30 min on ice. The schistosomes were washed with DSM and examined under a fluorescence microscope (Olympus, Hamburg, Germany).
Paramyosin binding to C8 and C9.
Purified human C8 and C9 (Advanced Research Technologies, San Diego, Calif.) and BSA (Sigma) (1 μg each) were subjected to SDS-PAGE (8% acrylamide gel) and transferred onto a nitrocellulose membrane. After blocking with 5% milk in TBST for 1 h at room temperature, the membrane was incubated with recombinant paramyosin (4 μg/ml) or with paramyosin purified from NPRM of schistosomula over a DEAE-cellulose column in 5% milk in TBST for 2 h at 37°C. After two washes with TBST, the membrane was reacted with monoclonal anti-paramyosin antibody (1:4 in TBST) for 1 h at room temperature, washed with TBST, and incubated with peroxidase-conjugated goat anti-mouse IgG (Sigma) (1:5,000) for 1 h at room temperature. Bands were visualized with an enhanced chemiluminescence reagent.
To show paramyosin binding competition between native C9 and blotted C9, recombinant paramyosin (1 μg) was premixed with native C9 (1:5 [wt/wt]) for 1 h at 37°C before it was added to the membrane. For determination of the binding site of paramyosin within C9, purified human C9 was cleaved into C9a and C9b by treatment with thrombin (Sigma) for 4 h at 37°C or into C9a′ and C9b′ by treatment with trypsin (Sigma) for 35 min at 37°C (3). The resultant fragments were separated by SDS-12% PAGE, blotted onto a nitrocellulose membrane, and reacted with recombinant paramyosin (data shown) or purified paramyosin from NPRM of schistosomula (data not shown) for 2 h at 37°C and then reacted with monoclonal anti-paramyosin antibodies and goat anti-mouse IgG (1:5,000) as described above.
In competition assays between paramyosin and human CD59 for binding to human C9, C9 (1 μg) was blotted onto a nitrocellulose membrane that was then blocked with 5% milk-TBST. The membrane was pretreated with purified human CD59 (kindly provided by Paul Morgan, Cardiff, United Kingdom) (0, 0.33, or 1 μg) for 1.5 h and then with recombinant paramyosin (2 μg) for 1.5 h at 37°C. Next, the membrane was incubated with monoclonal anti-paramyosin antibodies overnight at 4°C and then reacted with goat anti-mouse IgG as described above.
C9 polymerization assays.
Purified human C9 (2 μg) was incubated with 42 μM ZnCl2 in 20 mM Tris (pH 7.2) for 2 h at 37°C (54). To test the effect of paramyosin on C9 polymerization, C9 was pretreated with various amounts of recombinant paramyosin at 37°C for 30 min. The samples were subjected to SDS-PAGE on a 3 to 10% acrylamide gradient gel under reducing conditions and stained with Coomassie blue.
C9 polymerization on rabbit erythrocytes (ER) was performed as previously described (55). Briefly, erythrocytes (2 × 108/ml) were mixed with 100 μl of normal human serum (NHS) supplemented with 6 μg of C9 and were incubated for 1 h at 37°C. To examine the effect of paramyosin on C9 polymerization, the NHS supplemented with 6 μg of C9 was premixed for 30 min at 37°C with 40 μg of recombinant paramyosin. The lysed cells were washed twice with 3 ml of TBS and sedimented for 20 min at 4,800 × g at 4°C, once with 3 ml of 5 mM EDTA (pH 8.0), and once with 3 ml of 0.5 mM phosphate buffer (pH 8.0). The lysed cell pellet was dissolved in 30 μl of reducing sample buffer and heated for 5 min at 95°C. The samples were analyzed by SDS-PAGE on a 3 to 10% gradient acrylamide gel under reducing conditions and stained with Coomassie blue.
Hemolytic assays. Lysis of ER by the alternative complement pathway (15).
Fresh, washed erythrocytes (2 × 107) were incubated with NHS (6%) as a source of complement in the presence of Mg-EGTA (2.5 mM MgCl2, 10 mM EGTA) and various amounts of recombinant paramyosin (0, 2, 10, or 20 μg [premixed with NHS for 15 min at 37°C]) in 0.1 ml of GVB (Veronal-buffered saline [pH 7.4] containing 0.1% gelatin and 0.02% NaN3) for 30 min at 37°C. A total of 1 ml of cold GVB containing 10 mM EDTA was added to stop lysis. Following centrifugation at 4,400 × g for 10 min at 4°C, light absorption of the supernatant at 412 nm was measured and percent lysis (relative to cells completely lysed by water) was calculated. Percent inhibition of lysis by paramyosin was calculated relative to samples containing no paramyosin.
Lysis of antibody-sensitized sheep erythrocytes by the classical complement pathway.
To produce antibody-sensitized erythrocytes (EAs), fresh washed sheep erythrocytes (109 cells/ml) were incubated with rabbit hemolysin (DIFCO) (1:2,000 in GVB) for 30 min at 37°C and washed with GVB containing 0.5 mM MgCl2 and 0.15 mM CaCl2 (34). A total of 15 μl of EAs (1.5 × 107) was incubated with 15 μl of C8-deficient human serum (1:10 in GVB) (41) for 15 min at 37°C, and the resultant EAC5b-7 cells were washed with GVB. Concomitantly, various amounts of recombinant paramyosin (0, 1, 2, and 4 μg) were added to 30 μl of C7-deficient human serum (diluted 1:4,000 in GVB containing 10 mM EDTA) for 20 min at 37°C. The treated C7-deficient serum was then added to EAC5b-7 cells for 30 min at 37°C. A total of 1 ml of cold GVB containing 10 mM EDTA was added to stop lysis, the cells were subjected to centrifugation at 4,400 × g for 10 min at 4°C, and light absorption of the supernatants at 412 nm was measured. Percent lysis (relative to cells completely lysed by water) was calculated. Percent inhibition of lysis by paramyosin was calculated relative to samples containing no paramyosin.
Complement-mediated killing of schistosomula in vitro.
Schistosomula (200 total [3 h old] in 15 μl of DSM) were pretreated with heat-inactivated polyclonal rabbit anti-CD59 or anti-paramyosin antiserum or normal rabbit serum (2, 10, or 40 μl) in wells of a 96-well microtiter plate (Corning Incorporated, New York, N.Y.) for 30 min at room temperature. Then, 100 μl of normal or C4-depleted human serum (32) was added into each well and the plates were incubated overnight in a 6% CO2 incubator at 37°C. Heat-inactivated serum served as a control. The mortality of the schistosomula was assessed under an inverted microscope on the basis of motility and granularity data (19). Experiments were run in triplicate. Percent mortality values for control cultures were subtracted from those for experimental cultures, and percent net mortality was calculated according to the following formula: percent mortality = [(E − C) × 100]/(100 − C), where C represents the percentage of dead schistosomula in control cultures and E represents the percentage of dead schistosomula in experimental cultures.
Statistical analysis.
Student's unpaired and paired t tests were used to determine the statistical significance of differences between various data sets. Results are expressed as arithmetic means ± standard deviations (SDs). Statistical significance was assumed when P < 0.05.
RESULTS
Identification of SCIP-1 as paramyosin.
As explained in Materials and Methods, a DEAE-cellulose column was used to partially purify SCIP-1 from NPRM of S. mansoni schistosomula. Bound proteins were eluted from the DEAE-cellulose by a salt concentration gradient (0 to 0.6 M NaCl) in a 0.01 M NaAc buffer (pH 6.0). A large protein peak eluted between 0.1 and 0.2 M NaCl. Fractions containing SCIP-1 were identified by Western blot analysis with polyclonal rabbit anti-CD59 antibodies, pooled, and concentrated. The SCIP-1 pool was subjected to SDS-PAGE under nonreducing conditions on two lanes of an 8% gel (0.1 mg per lane). The 94- to 97-kDa SCIP-1 band was identified by Western blotting with polyclonal rabbit anti-CD59 antibodies in one lane, while the second lane was stained with Coomassie blue. The SCIP-1 band was cut from the stained gel and subjected to trypsin digestion and mass spectrometry analysis of the peptides. A total of 15 peptides were identified; 10 of them were identical to sequences from S. mansoni paramyosin (SwissProt accession no. P06198). A similar analysis performed with adult worm NPRM also identified paramyosin in the SCIP-1 band. Frequently, SCIP-1 ran on SDS-PAGE gels as a doublet. Analysis of the upper and lower bands demonstrated that both contained paramyosin sequences.
To further demonstrate that paramyosin, like SCIP-1, can bind to anti-CD59 antibodies, paramyosin purified from NPRM over an affinity column of monoclonal anti-paramyosin antibody was analyzed by SDS-PAGE and Western blotting with polyclonal rabbit anti-CD59 antibodies. As shown in Fig. 1, affinity-purified native paramyosin (running here as a doublet) bound the polyclonal anti-human CD59 antibodies. The two bands perhaps represented isoforms generated by phosphorylation of paramyosin (50) or products of an alternative splicing or of proteolysis.
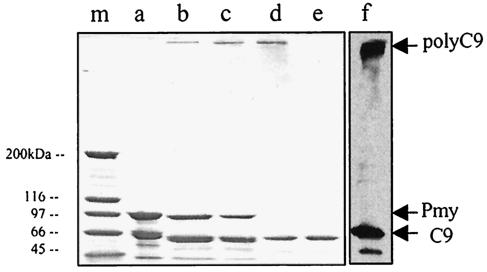
FIG. 1.
Binding of anti-CD59 antibody (1:300) to paramyosin (Pmy). (Lanes a to c) NPRM from schistosomula or affinity purified protein were subjected to SDS-8% PAGE and transferred onto a nitrocellulose membrane. After blocking with a 5% milk solution in TBST for 1 h at room temperature, the membrane was treated with rabbit anti-CD59 antibody or normal rabbit serum (NRS) (1:300) and then washed and treated with peroxidase-conjugated goat anti-rabbit IgG as a second antibody (1:5,000). Bands were developed by enhanced chemiluminescence. Lane a, NPRM of schistosomula; lane b, effluent of affinity column; lane c, eluate of affinity column; lane d, SCIP-1 (arrowhead) eluted from DEAE-cellulose (peak tube) was subjected to SDS-PAGE and the gel was stained with Coomassie blue; lane m, stained molecular mass markers (200, 120, 97, and 67 kDa).
Immunofluorescence of schistosomula and adult worms with anti-paramyosin antibody.
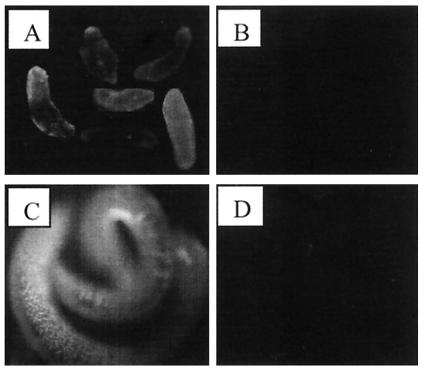
To examine whether paramyosin is located on the surface of the parasite, live, intact schistosomula and adult worms were analyzed by immunofluorescence with anti-paramyosin antibodies. Labeling was performed with rabbit anti-paramyosin antibodies as explained in Materials and Methods. Examination under a fluorescence microscope showed that paramyosin is present on the surface of schistosomula and adult worms (males and females) (Fig. 2). Paramyosin was distributed over the tegument with patches of darker and brighter regions.
FIG. 2.
Immunofluorescence analysis showing the presence of paramyosin on the surface of schistosomula and adult worms. After treatment with blocking solution, 100 24-h-old schistosomula (A and B) or 20 fresh adult worms (C and D) were incubated with polyclonal rabbit anti-paramyosin antibodies (A and C) or normal rabbit serum (B and D) for 30 min at 37°C. After three washes with DSM on ice, they were treated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (1:100 in DSM) for 30 min on ice. The schistosomes were washed with DSM and examined under a fluorescence microscope.
Paramyosin binds to C8 and C9.
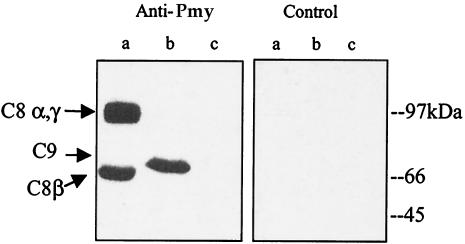
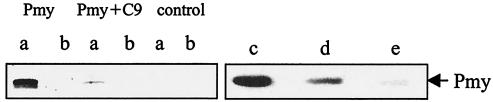
Purified human C8 and C9 and BSA were subjected to SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking, the membrane was reacted with recombinant paramyosin (4 μg/ml) or paramyosin purified from NPRM of schistosomula over a DEAE-cellulose column for 2 h at 37°C. Western blot analysis with monoclonal anti-paramyosin antibody showed that both recombinant paramyosin (Fig. 3) and purified paramyosin (data not shown) bound to C8 and C9 on the membrane but not to BSA. When identical amounts of native and recombinant paramyosin (as determined by immunoblotting with anti-paramyosin antibodies) were reacted with C9, recombinant paramyosin produced a stronger band than native paramyosin (data not shown). This suggested that recombinant paramyosin binds to C9 with a higher affinity or that the native paramyosin preparation contains an inhibitor of C9 binding. Paramyosin bound to both α-γ and β chains of C8. To test whether native C9 can compete with blotted C9 in binding to paramyosin, recombinant paramyosin was premixed with native C9 (1:5) for 1 h at 37°C before it was added to the membrane containing C9 and BSA. As shown in Fig. 4 lanes a, soluble C9 almost completely blocked paramyosin binding to blotted C9, suggesting the involvement of native epitopes on C9 in the binding. Furthermore, soluble CD59 blocked paramyosin binding to blotted C9 (Fig. 4, lanes c to e), implying that paramyosin binds to C9 roughly to the same site where CD59 binds to it (21), i.e., between Cys359 and Cys384.
FIG. 3.
Binding of paramyosin to C8 and C9. Purified human C8 and C9 and BSA (1 μg each) were subjected to SDS-8% PAGE and transferred onto a nitrocellulose membrane. After blocking with 5% milk in TBST, the membrane was incubated with recombinant paramyosin (4 μg/ml) in blocking solution for 2 h at 37°C. After two washes with TBST, the membrane was reacted with monoclonal anti-paramyosin antibody (1:4) (Anti-Pmy) or an isotype-matched, unrelated monoclonal antibody (Control) and then incubated with peroxidase-conjugated goat anti-mouse IgG (1:5,000) for 1 h at room temperature. Bands were visualized by enhanced chemiluminescence. Lane a, C8α and C8γ (upper band) and C8β (lower band); lane b, C9; lane c, BSA.
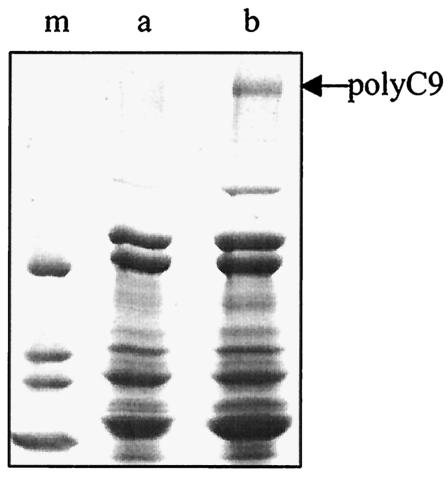
FIG. 4.
Binding of paramyosin to blotted C9: competition with soluble C9 or CD59. (Lanes a and b) Purified human C9 (lanes a) or BSA (lanes b) (1 μg each) was blotted onto nitrocellulose membrane. The membrane was reacted with paramyosin (Pmy) or paramyosin preincubated with C9 (1:5 [wt/wt]) (Pmy+C9) or buffer (control) and then with monoclonal anti-paramyosin antibody, peroxidase-conjugated goat anti-mouse IgG, and enhanced chemiluminescence reagent. (Lanes c to e) Purified human C9 was blotted onto nitrocellulose membrane and reacted with recombinant paramyosin preincubated with or without purified human CD59. Further details are provided in Materials and Methods. Bound paramyosin was detected with monoclonal anti-paramyosin antibodies, goat anti-mouse IgG, and enhanced chemiluminescence reagent. Lane c, 2 μg of paramyosin without CD59; lane d, 2 μg of paramyosin plus 0.33 μg of CD59; lane e, 2 μg paramyosin plus 1 μg of CD59.
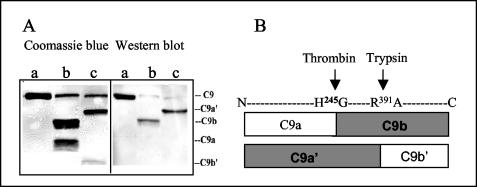
To further characterize the region in C9 involved in binding to paramyosin, purified human C9 was cleaved into C9a and C9b with human thrombin or into C9a′ and C9b′ with trypsin (3). The resultant fragments were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. After blocking with milk, the membrane was incubated with recombinant paramyosin and then with monoclonal anti-paramyosin antibody as described above. Paramyosin bound to the C9b fragment of thrombin cleavage and to the C9a′ fragment of trypsin cleavage (Fig. 5A). The C9 cleavage sites are shown schematically in Fig. 5B. The data indicate that the paramyosin binding site in C9 is located between Gly245 and Arg391, the sequence overlapping C9b and C9a′.
FIG. 5.
Location of the paramyosin binding site in C9. C9 was cleaved into C9a (molecular weight, 34,000) and C9b (37,000) by incubation with α-thrombin for 4 h at 37°C or into C9a′ (47,000) and C9b′ (24,000) by incubation with trypsin for 35 min at 37°C. The fragments were separated by SDS-12% PAGE and stained with Coomassie blue or blotted onto nitrocellulose membrane. The membrane was incubated with recombinant paramyosin for 2 h at 37°C and then reacted with monoclonal anti-paramyosin and goat anti-mouse IgG (1:5,000) as described above. (A) Profile of the C9 and C9 fragments bands after staining (left panel) or Western blotting (right panel) with paramyosin and anti-paramyosin. Lanes a, C9 plus PBS; lanes b, C9 plus thrombin; lanes c, C9 plus trypsin. (B) Schematic presentation of cleavage sites in C9. The C9 fragments that bind paramyosin are shown in boxes shaded in gray.
Inhibition of Zn2+-induced C9 polymerization by paramyosin.
Human C9 has a tendency to polymerize on prolonged incubation (for 3 days) at 37°C to a circular polymer of C9 (polyC9), a process that is accelerated by the presence of Zn2+ ions and that occurs in their presence within 2 h (54). PolyC9 has a tubular structure resistant to dissociation by boiling in SDS (44). To examine whether paramyosin can inhibit Zn2+-induced C9 polymerization, C9 was mixed with various amounts of paramyosin for 30 min at 37°C and then incubated with ZnCl2 (at a final concentration of 42 μM) for 2 h at 37°C. The samples were analyzed by SDS-PAGE on a 3 to 10% acrylamide gradient gel under reducing conditions, and the gel was stained with Coomassie blue. As shown in Fig. 6, paramyosin inhibited the Zn2+-induced C9 polymerization in a dose-dependent manner. At about threefold molar excess (8 μg), paramyosin completely inhibited polymerization of C9.
FIG. 6.
Inhibition of Zn2+-induced C9 polymerization by recombinant paramyosin (Pmy). C9 (2 μg) was mixed with various amounts of paramyosin (0, 2, 4, or 8 μg) for 30 min at 37°C and then incubated with 42 μM ZnCl2 for 2 h at 37°C. The samples were subjected to a SDS-PAGE gradient gel (3 to 10% acrylamide). Lane a, C9 plus 8 μg of paramyosin; lane b, C9 plus 4 μg of paramyosin; lane c, C9 plus 2 μg of paramyosin; lane d, C9 without paramyosin; lane e, C9 without ZnCl2; lane f, Western blot of C9 without paramyosin (from lane d) with anti-C9 antibody (1:300). The 97-kDa bands seen in lanes a to c represent paramyosin. The 67-kDa bands in lanes d and e represent monomeric C9 and in lanes a to c represent a mixture of C9 with a 67-kDa fragment of recombinant paramyosin. Lower weak bands probably represent fragments of paramyosin (lanes a to c) or C9 (lanes d and e).
Inhibition of polyC9 formation on ER by paramyosin.
Complement activation by cells like ER culminates in formation of the complement MAC composed of C5b, C6, C7, C8, and C9 and cell lysis (4). The C9 within the MAC expresses a hydrophobic domain and polymerizes and inserts into the plasma membrane of the target cell (45). To further test whether paramyosin can inhibit polyC9 formation on ER, recombinant paramyosin was added to 100 μl of NHS supplemented with 6 μg of C9, incubated for 30 min at 37°C, and then incubated with ER for 1 h at 37°C. The ER were washed, dissolved in reducing sample buffer, and analyzed by SDS-PAGE on a 3 to 10% gradient gel. As shown in Fig. 7, lane a, paramyosin inhibited polyC9 deposition on ER.
FIG. 7.
Inhibition of polyC9 deposition on ER by paramyosin. Fresh ER were treated with 100 μl of NHS supplemented with 6 μg of C9 preincubated for 30 min at 37°C with (lane a) or without (lane b) 40 μg of recombinant paramyosin. The lysed ER were sedimented, and the pellet was dissolved in reducing sample buffer and analyzed on a SDS-PAGE gradient gel (3 to 10% acrylamide) stained with Coomassie blue. Lane m, molecular mass markers (200, 120, 97, and 67 kDa). The arrow indicates a band of polyC9. Other bands represent proteins originated from the red blood cells and serum proteins adhering to blood cells.
Inhibition of complement-mediated hemolysis by paramyosin.
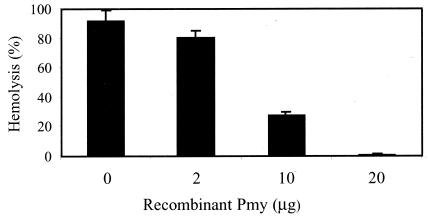
That paramyosin inhibits C9 polymerization suggested that paramyosin might block complement-mediated lysis of target cells. The alternative and classical pathways of human complement are activated by ER and EAs (antibody-coated sheep erythrocytes), respectively. This leads to lysis of the red blood cells by the complement MAC. To test whether paramyosin can inhibit the lysis of ER by the alternative pathway, NHS was incubated with recombinant paramyosin (0, 2, 10, or 20 μg) for 15 min at 37°C and then with ER in GVB containing 2.5 mM MgCl2 and 10 mM EGTA for 30 min at 37°C. As shown in Fig. 8, paramyosin significantly (P < 0.05) inhibited lysis of ER by the complement in a dose-dependent manner. Lysis of ER was reduced to 30% of that of the control by 10 μg of paramyosin and completely blocked by 20 μg of paramyosin.
FIG. 8.
Inhibition of complement-mediated lysis of ER by recombinant paramyosin (Pmy). Fresh ER (2 × 107 cells) were incubated with NHS (6%) in the presence of Mg-EGTA and various amounts (0, 2, 10, or 20 μg) of recombinant paramyosin for 30 min at 37°C. Cold GVB containing 10 mM EDTA was added to stop lysis. Following centrifugation, light absorption of the supernatant was measured at 412 nm and percent lysis was calculated. Results shown are means ± SD representative of three independent experiments.
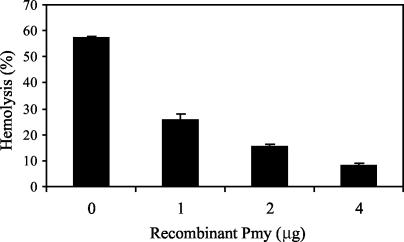
Next, we tested whether paramyosin can inhibit lysis of EAs by binding to C8 and C9. EAs were treated with C8-deficient human serum for 15 min at 37°C to generate EAs with bound C5b-7 complexes (EAC5b-7 cells) and washed. Next, C7-deficient human serum (as a source for C8 and C9) preincubated with or without recombinant paramyosin for 20 min at 37°C was added to EAC5b-7 cells in GVB containing 10 mM EDTA and incubated for 30 min at 37°C. EDTA was added to block further classical pathway activation. As shown in Fig. 9, paramyosin significantly (P < 0.005) inhibited lysis of EAC5b-7 by C8 and C9 in a dose-dependent manner. This clearly demonstrates that paramyosin can block cell lysis at the stage of MAC formation.
FIG. 9.
Inhibition of complement-mediated lysis of EAs by paramyosin (Pmy). EAs (1.5 × 107 cells) were incubated with C8-deficient human serum (1:10) for 15 min at 37°C. After washes with GVB containing EDTA, C7-deficient human serum (1:4,000) premixed with recombinant paramyosin (0, 1, 2, or 4 μg) was added to EAC5b-7 in the presence of 10 mM EDTA. After 30 min of incubation at 37°C, the cells were sedimented and the light absorption of the supernatant was measured at 412 nm. Percent hemolysis was calculated. Results shown are means ± SD representative of three independent experiments.
Paramyosin on schistosomula is protective from complement-mediated killing.
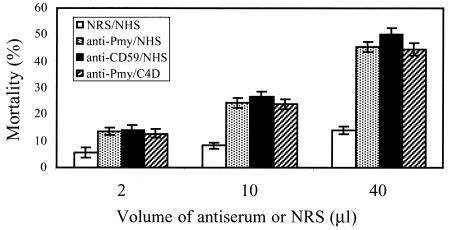
To block the postulated complement inhibitory activity of paramyosin, schistosomula were treated with heat-inactivated rabbit anti-paramyosin or anti-CD59 antibodies for 30 min at room temperature. Normal rabbit serum served as a control. Next, 100 μl of normal or C4-depleted human serum was added as a source of complement for an overnight incubation at 37°C. As shown in Fig. 10, preincubation with anti-paramyosin or with anti-CD59 antibodies significantly (P < 0.05) enhanced the in vitro killing of schistosomula by human complement in a dose-dependent manner even in the absence of an active classical complement pathway. In contrast, normal rabbit serum produced only a small enhancement of schistosomula killing. No significant difference was observed between the results obtained with anti-paramyosin and anti-CD59 antibodies.
FIG. 10.
Enhanced complement-mediated killing of schistosomula by anti-CD59 and anti-paramyosin (anti-Pmy) antibodies. Schistosomula (3 h old) were treated with rabbit anti-CD59 or anti-paramyosin antisera or normal rabbit serum (NRS) for 30 min at room temperature and then with NHS or C4-depleted human serum (C4D) overnight at 37°C. Percent mortality of schistosomula was determined under the microscope on the basis of motility and granularity data. Results shown are means ± SD representative of three independent experiments.
DISCUSSION
Results shown here demonstrate that paramyosin of the blood fluke S. mansoni is the inhibitor of the MAC C5b-9 of human complement, previously named SCIP-1 (40). SCIP-1 was originally referred to as a CD59-like protein on the basis of two criteria: (i) binding to anti-CD59 antibodies and (ii) binding to and inhibition of complement C9. By using column chromatography and acrylamide gel fractionation, SCIP-1 has been partially isolated. This permitted its identification as paramyosin by mass spectrometry. Purified, native paramyosin fulfills the two above-mentioned criteria. In addition, recombinant paramyosin is shown to bind C8 and C9, inhibit formation of the MAC, and block complement-mediated cell lysis. That anti-paramyosin antibodies sensitize schistosomula to killing by human complement strongly supports the claim that paramyosin acts as a complement inhibitor in its native form on the surface of schistosomes.
Paramyosin, a muscle protein of invertebrates, assembles with myosin to form the thick muscle filaments (7, 11). Our data confirm previous reports (18, 31, 33) that paramyosin can be found in schistosomes of S. japonicum and S. mansoni not only within muscles but also within the tegument and on the surface. Antibodies directed to paramyosin label schistosomula, as well as adult worms, by immunofluorescence (31) (Fig. 2). External paramyosin can be removed from schistosomula by detergent solubilization of the tegument (Fig. 1) and, at least partly, by treatment with a phosphatidylinositol-specific phospholipase C (unpublished data). In addition, paramyosin can be exogenously biotinylated on the surface of live S. mansoni adult worms (31). Adults of the Asian schistosome S. japonicum also contain a surface form of paramyosin (31). Paramyosin was also detected in the tegument of other parasitic worms such as Echinococcus granulosus (37) and Taenia solium (26). It is likely that the external and intrategumental paramyosin is produced by the subtegumental cells within cell bodies situated beneath the outer muscle fibers (20). Alternatively, the paramyosin may be produced within the muscle fibers and secreted into the tegument coating it and may reach the external surface through it. Another source for surface paramyosin, at least in larvae, could be secretions of the postacetabular glands, as shown for S. japonicum cercariae (18).
Human CD59 is an 18-kDa glycosylphosphatidylinositol-linked membrane glycoprotein, widely expressed in all circulating cells and most tissues, that serves as an inhibitor of the C5b-9 MAC of human complement (9, 17, 39, 48). The complement inhibitory activity of CD59 depends on its ability to bind to C8 in the C5b-8 complex (30) and to C9 between Cys359 and Cys384 (21), thereby inhibiting ion channel formation by C5b-8 (13) and generation of the polymerized C9 responsible for MAC cytolytic activity (36, 39, 48). In contrast to CD59 that binds to C8α (30), paramyosin appears to bind both C8α and C8β chains (Fig. 3). As shown here, paramyosin binds to C9 at a site located between Gly245 and Arg391 that overlaps with the postulated CD59 binding site. It is suggested that CD59 and paramyosin bind to the same location in C9 or in close proximity. This is supported by the competition between purified human CD59 and paramyosin for binding to C9 blotted onto nitrocellulose membrane (Fig. 4, right panel). The fact that paramyosin shares no continuous sequence homology with CD59, as concluded from alignment trials, is perhaps surprising in light of the fact that rabbit anti-CD59 antibodies bind specifically to paramyosin. However, monoclonal antibodies directed to CD59 do not bind to paramyosin and rabbit anti-paramyosin polyclonal antibodies do not bind to human CD59 (unpublished data). Therefore, it is suggested that the rabbit antiserum raised against human CD59 contains antibodies directed to a specific conformational peptide epitope or to a modifying nonpeptide residue of CD59 (e.g., carbohydrate or lipid) present also on paramyosin.
Paramyosin is considered a vaccine candidate against schistosomiasis mansoni (42), schistosomiasis japonicum (24, 46), filariasis (29, 38), and cysticercosis (57). It acts as one of the major schistosome immunogens during infection with S. mansoni in mice (27) and humans (47). Vaccination trials performed with S. mansoni (16, 42) or S. japonicum (6, 35, 53) infections in mice, pigs, or water buffaloes demonstrated that immunization with native or recombinant paramyosin can reduce the worm burden and liver or fecal egg counts in infected animals. It will soon become clear whether or not paramyosin exerts a similar vaccination activity in humans (2).
The relationship between the paramyosin B cell epitopes contributing to protection from infection and the complement modulatory sites in paramyosin is unclear. It is conceivable that protective antibodies targeted at the paramyosin active sites would be more effective. Such antibodies would mediate activation of humoral and cellular immune responses, and at the same time inhibit the capacity of paramyosin to serve as an immune evasion molecule. Remarkably, paramyosin is capable of inhibiting complement activation by binding to at least three complement components: C1q (25), C8, and C9 (Fig. 3 to 5). By binding to complement C1 it may inhibit initial activation of the classical pathway, and by binding to C8 and C9 it may inhibit generation of the membranolytic terminal complement complexes. In addition, by binding to Fc of IgG (31) it may mask the surface and block binding of immune antibodies. Paramyosin is a multifunctional alpha-helical coiled-coil muscle protein (8, 11) and has various zones of positive and negative charges on the outer surface of the alpha-helices (23). These highly charged zones have been suggested to account for the ability of paramyosin to bind to ligands (31) such as Fc, collagen, and C1. Possibly, they are also involved in the binding of paramyosin to C8 and C9.
In summary, these results suggest a novel immunomodulatory role for paramyosin in inhibition of the terminal pathway of complement. It is anticipated that paramyosin of other parasitic worms, e.g., S. japonicum (1), T. solium (56) and Opisthorchis felineus (51), which share a high degree of homology with S. mansoni paramyosin, also have a C9 inhibitory activity. To date, the complement system has not been counted among the major immune effector mechanisms involved in limiting infection by parasitic worms. This is largely because of the most efficient control of complement activation by these parasites. Yet, as can be shown in vitro, the complement system has all of the arsenal required to execute killing of schistosomes. Future attempts to establish passive or active parasite humoral immunotherapy will have to consider and cope with the multitude of complement evasion molecules, such as the surface paramyosin.
Acknowledgments
This work was supported in part by the U.S.-Israel Binational Science Foundation, the Cooperation Program of the Deutsches Krebsforschungszentrum (DKFZ) and the Israeli Ministry of Science (MOS), and the National Institutes of Health (AI 18867 and TW006280).
We are grateful to Sir Peter Lachmann for the anti-CD59 antibodies and for his continuous interest and support, to Edward Pearce for the anti-paramyosin monoclonal antibodies, and to Alan Sher and John Anderson for the plasmid expressing paramyosin.
Editor: J. M. Mansfield
REFERENCES
- 1.Becker, M. M., B. H. Kalinna, W. Yang, S. A. Harrop, J. C. Scott, G. J. Waine, J. D. Kurtis, and D. P. McManus. 1995. Gene cloning and complete nucleotide sequence of Philippine Schistosoma japonicum paramyosin. Acta Trop. 59:143-147. [DOI] [PubMed] [Google Scholar]
- 2.Bergquist, R., M. Al-Sherbiny, R. Barakat, and R. Olds. 2002. Blueprint for schistosomiasis vaccine development. Acta Trop. 82:183-192. [DOI] [PubMed] [Google Scholar]
- 3.Biesecker, G., C. Gerard, and T. E. Hugli. 1982. An amphiphilic structure of the ninth component of human complement. Evidence from analysis of fragments produced by alpha-thrombin. J. Biol. Chem. 257:2584-2590. [PubMed] [Google Scholar]
- 4.Biesecker, G., E. R. Podack, C. A. Halverson, and H. J. Muller-Eberhard. 1979. C5b-9 dimer: isolation from complement lysed cells and ultrastructural identification with complement-dependent membrane lesions. J. Exp. Med. 149:448-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brink, L. H., D. J. McLaren, and S. R. Smithers. 1977. Schistosoma mansoni: a comparative study of artificially transformed schistosomula and schistosomula recovered after cercarial penetration of isolated skin. Parasitology 74:73-86. [DOI] [PubMed] [Google Scholar]
- 6.Chen, H., T. Nara, X. Zeng, M. Satoh, G. Wu, W. Jiang, F. Yi, S. Kojima, S. Zhang, and K. Hirayama. 2000. Vaccination of domestic pig with recombinant paramyosin against Schistosoma japonicum in China. Vaccine 18:2142-2146. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, C., A. G. Szent-Gyorgyi, and J. Kendrick-Jones. 1971. Paramyosin and the filaments of molluscan “catch” muscles. I. Paramyosin: structure and assembly. J. Mol. Biol. 56:223-227. [DOI] [PubMed] [Google Scholar]
- 8.Cowgill, R. W. 1974. Location and properties of sulfhydryl groups on the muscle protein paramyosin from Mercenaria mercenaria. Biochemistry 13:2467-2474. [DOI] [PubMed] [Google Scholar]
- 9.Davies, A., and P. J. Lachmann. 1993. Membrane defence against complement lysis: the structure and biological properties of CD59. Immunol. Res. 12:258-275. [DOI] [PubMed] [Google Scholar]
- 10.Davies, A., D. L. Simmons, G. Hale, R. A. Harrison, H. Tighe, P. J. Lachmann, and H. Waldmann. 1989. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J. Exp. Med. 170:637-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elfvin, M., R. J. Levine, and M. M. Dewey. 1976. Paramyosin in invertebrate muscles. I. Identification and localization. J. Cell Biol. 71:261-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels, D., L. Chitsulo, A. Montresor, and L. Savioli. 2002. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 82:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farkas, I., L. Baranyi, Y. Ishikawa, N. Okada, C. Bohata, D. Budai, A. Fukuda, M. Imai, and H. Okada. 2002. CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9. J. Physiol. 539:537-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishelson, Z. 1991. Complement evasion by parasites: search for “Achilles' heel.” Clin. Exp. Immunol. 86(Suppl. 1):47-52. [PMC free article] [PubMed] [Google Scholar]
- 15.Fishelson, Z., R. D. Horstmann, and H. J. Muller-Eberhard. 1987. Regulation of the alternative pathway of complement by pH. J. Immunol. 138:3392-3395. [PubMed] [Google Scholar]
- 16.Flanigan, T. P., C. H. King, R. R. Lett, J. Nanduri, and A. A. Mahmoud. 1989. Induction of resistance to Schistosoma mansoni infection in mice by purified parasite paramyosin. J. Clin. Investig. 83:1010-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher, C. M., R. A. Harrison, P. J. Lachmann, and D. Neuhaus. 1994. Structure of a soluble, glycosylated form of the human complement regulatory protein CD59. Structure 2:185-199. [DOI] [PubMed] [Google Scholar]
- 18.Gobert, G. N. 1998. The role of microscopy in the investigation of Pmy as a vaccine candidate against Schistosoma japonicum. Parasitol. Today 14:115-118. [DOI] [PubMed] [Google Scholar]
- 19.Gold, D., and E. Flescher. 2000. Influence of mechanical tail-detachment techniques of schistosome cercariae on the production, viability, and infectivity of resultant schistosomula: a comparative study. Parasitol Res. 86:570-572. [DOI] [PubMed] [Google Scholar]
- 20.Hockley, D. J. 1973. Ultrastructure of the tegument of Schistosoma. Adv. Parasitol. 11:233-305. [DOI] [PubMed] [Google Scholar]
- 21.Husler, T., D. H. Lockert, and P. J. Sims. 1996. Role of a disulfide-bonded peptide loop within human complement C9 in the species-selectivity of complement inhibitor CD59. Biochemistry 35:3263-3269. [DOI] [PubMed] [Google Scholar]
- 22.Inal, J. M., and R. B. Sim. 2000. A Schistosoma protein, Sh-TOR, is a novel inhibitor of complement which binds human C2. FEBS Lett. 470:131-134. [DOI] [PubMed] [Google Scholar]
- 23.Kagawa, H., K. Gengyo, A. D. McLachlan, S. Brenner, and J. Karn. 1989. Paramyosin gene (unc-15) of Caenorhabditis elegans. Molecular cloning, nucleotide sequence and models for thick filament structure. J. Mol. Biol. 207:311-333. [DOI] [PubMed] [Google Scholar]
- 24.Kalinna, B. H., and D. P. McManus. 1997. A vaccine against the Asian schistosome, Schistosoma japonicum: an update on paramyosin as a target of protective immunity. Int. J. Parasitol. 27:1213-1219. [DOI] [PubMed] [Google Scholar]
- 25.Laclette, J. P., C. B. Shoemaker, D. Richter, L. Arcos, N. Pante, C. Cohen, D. Bing, and A. Nicholson-Weller. 1992. Paramyosin inhibits complement C1. J. Immunol. 148:124-128. [PubMed] [Google Scholar]
- 26.Laclette, J. P., P. J. Skelly, M. T. Merchant, and C. B. Shoemaker. 1995. Aldehyde fixation dramatically alters the immunolocalization pattern of paramyosin in platyhelminth parasites. Exp. Parasitol. 81:140-143. [DOI] [PubMed] [Google Scholar]
- 27.Lanar, D. E., E. J. Pearce, S. L. James, and A. Sher. 1986. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science 234:593-596. [DOI] [PubMed] [Google Scholar]
- 28.Lazdins, J. K., M. J. Stein, J. R. David, and A. Sher. 1982. Schistosoma mansoni: rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp. Parasitol. 53:39-44. [DOI] [PubMed] [Google Scholar]
- 29.Li, B. W., R. Chandrashekar, and G. J. Weil. 1993. Vaccination with recombinant filarial paramyosin induces partial immunity to Brugia malayi infection in jirds. J. Immunol. 150:1881-1885. [PubMed] [Google Scholar]
- 30.Lockert, D. H., K. M. Kaufman, C. P. Chang, T. Husler, J. M. Sodetz, and P. J. Sims. 1995. Identity of the segment of human complement C8 recognized by complement regulatory protein CD59. J. Biol. Chem. 270:19723-19728. [DOI] [PubMed] [Google Scholar]
- 31.Loukas, A., M. K. Jones, L. T. King, P. J. Brindley, and D. P. McManus. 2001. Receptor for Fc on the surfaces of schistosomes. Infect. Immun. 69:3646-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marikovsky, M., F. Levi-Schaffer, R. Arnon, and Z. Fishelson. 1986. Schistosoma mansoni: killing of transformed schistosomula by the alternative pathway of human complement. Exp. Parasitol. 61:86-94. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto, Y., G. Perry, R. J. Levine, R. Blanton, A. A. Mahmoud, and M. Aikawa. 1988. Paramyosin and actin in schistosomal teguments. Nature 333:76-78. [DOI] [PubMed] [Google Scholar]
- 34.Mayer, M. M. 1961. Complement and complement fixation, p. 133-240. In E. A. Kabat and M. M. Mayer (ed.), Experimental immunochemistry, 2nd ed. Charles C. Thomas, Springfield, Ill.
- 35.McManus, D. P., J. Y. Wong, J. Zhou, C. Cai, Q. Zeng, D. Smyth, Y. Li, B. H. Kalinna, M. J. Duke, and X. Yi. 2001. Recombinant paramyosin (rec-Sj-97) tested for immunogenicity and vaccine efficacy against Schistosoma japonicum in mice and water buffaloes. Vaccine 20:870-878. [DOI] [PubMed] [Google Scholar]
- 36.Meri, S., B. P. Morgan, A. Davies, R. H. Daniels, M. G. Olavesen, H. Waldmann, and P. J. Lachmann. 1990. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology 71:1-9. [PMC free article] [PubMed] [Google Scholar]
- 37.Muhlschlegel, F., L. Sygulla, P. Frosch, P. Massetti, and M. Frosch. 1993. Paramyosin of Echinococcus granulosus: cDNA sequence and characterization of a tegumental antigen. Parasitol. Res. 79:660-666. [DOI] [PubMed] [Google Scholar]
- 38.Nanduri, J., and J. W. Kazura. 1989. Paramyosin-enhanced clearance of Brugia malayi microfilaremia in mice. J. Immunol. 143:3359-3363. [PubMed] [Google Scholar]
- 39.Ninomiya, H., and P. J. Sims. 1992. The human complement regulatory protein CD59 binds to the alpha-chain of C8 and to the “b” domain of C9. J. Biol. Chem. 267:13675-13680. [PubMed] [Google Scholar]
- 40.Parizade, M., R. Arnon, P. J. Lachmann, and Z. Fishelson. 1994. Functional and antigenic similarities between a 94-kD protein of Schistosoma mansoni (SCIP-1) and human CD59. J. Exp. Med. 179:1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parizade, M., Y. Ghendler, R. Arnon, and Z. Fishelson. 1990. Resistance of the parasite Schistosoma mansoni to immune attack. FASEB J. 4:2228. [Google Scholar]
- 42.Pearce, E. J., S. L. James, S. Hieny, D. E. Lanar, and A. Sher. 1988. Induction of protective immunity against Schistosoma mansoni by vaccination with schistosome paramyosin (Sm97), a nonsurface parasite antigen. Proc. Natl. Acad. Sci. USA 85:5678-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce, E. J., and A. Sher. 1987. Mechanisms of immune evasion in schistosomiasis. Contrib. Microbiol. Immunol. 8:219-232. [PubMed] [Google Scholar]
- 44.Podack, E. R., and J. Tschopp. 1982. Circular polymerization of the ninth component of complement. Ring closure of the tubular complex confers resistance to detergent dissociation and to proteolytic degradation. J. Biol. Chem. 257:15204-15212. [PubMed] [Google Scholar]
- 45.Podack, E. R., and J. Tschopp. 1982. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc. Natl. Acad. Sci. USA 79:574-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez, B. L., J. D. Kurtis, P. M. Wiest, P. Arias, F. Aligui, L. Acosta, P. Peters, and G. R. Olds. 1996. Paramyosin: a candidate vaccine antigen against Schistosoma japonicum. Parasite Immunol. 18:49-52. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro de Jesus, A., I. Araujo, O. Bacellar, A. Magalhaes, E. Pearce, D. Harn, M. Strand, and E. M. Carvalho. 2000. Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect. Immun. 68:2797-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rollins, S. A., J. Zhao, H. Ninomiya, and P. J. Sims. 1991. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J. Immunol. 146:2345-2351. [PubMed] [Google Scholar]
- 49.Samuelson, J. C., and J. P. Caulfield. 1986. Cercarial glycocalyx of Schistosoma mansoni activates human complement. Infect. Immun. 51:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt, J., O. Bodor, L. Gohr, and W. Kunz. 1996. Paramyosin isoforms of Schistosoma mansoni are phosphorylated and localized in a large variety of muscle types. Parasitology 112:459-467. [DOI] [PubMed] [Google Scholar]
- 51.Shustov, A. V., A. T. Kotelkin, A. V. Sorokin, V. A. Ternovoi, and V. B. Loktev. 2002. The Opisthorchis felineus paramyosin: cDNA sequence and characterization of its recombinant fragment. Parasitol. Res. 88:724-730. [DOI] [PubMed] [Google Scholar]
- 52.Smithers, S. R., and R. J. Terry. 1965. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 55:695-700. [DOI] [PubMed] [Google Scholar]
- 53.Taylor, M. G., M. C. Huggins, F. Shi, J. Lin, E. Tian, P. Ye, W. Shen, C. G. Qian, B. F. Lin, and Q. D. Bickle. 1998. Production and testing of Schistosoma japonicum candidate vaccine antigens in the natural ovine host. Vaccine 16:1290-1298. [DOI] [PubMed] [Google Scholar]
- 54.Tschopp, J. 1984. Circular polymerization of the membranolytic ninth component of complement. Dependence on metal ions. J. Biol. Chem. 259:10569-10573. [PubMed] [Google Scholar]
- 55.Tschopp, J., E. R. Podack, and H. J. Muller-Eberhard. 1985. The membrane attack complex of complement: C5b-8 complex as accelerator of C9 polymerization. J. Immunol. 134:495-499. [PubMed] [Google Scholar]
- 56.Vargas-Parada, L., and J. P. Laclette. 2003. Gene structure of Taenia solium paramyosin. Parasitol. Res. 89:375-378. [DOI] [PubMed] [Google Scholar]
- 57.Vázquez-Talavera, J., C. F. Solis, L. I. Terrazas, and J. P. Laclette. 2001. Characterization and protective potential of the immune response to Taenia solium paramyosin in a murine model of cysticercosis. Infect. Immun. 69:5412-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]