Abstract
The cause of many autoimmune and inflammatory diseases is unresolved, although dysregulated production of tumor necrosis factor (TNF) family members appears to be important in many cases. BAFF, a new member of the TNF family, binds to B cells and costimulates their growth in vitro. Mice transgenic for BAFF have vastly increased numbers of mature B and effector T cells, and develop autoimmune-like manifestations such as the presence of high levels of rheumatoid factors, circulating immune complexes, anti–DNA autoantibodies, and immunoglobulin deposition in the kidneys. This phenotype is reminiscent of certain human autoimmune disorders and suggests that dysregulation of BAFF expression may be a critical element in the chain of events leading to autoimmunity.
Keywords: autoantibodies, tumor necrosis factor–related, B cell, rheumatoid factors, transgenic
Self-reactive B cells constantly emerge during lymphopoiesis. Fortunately, the immune system has several powerful means of eliminating or neutralizing autoreactive B cells, both centrally as they emerge from the stem cell pool and peripherally as mature B cells undergo somatic mutation 1 2. Despite the diversity of systems that allow discrimination between self and nonself, failure to eliminate autoreactive lymphocytes occurs, for instance, in genetically predisposed individuals, or as a consequence of infection or cytokine imbalance, leading to autoimmunity 3 4. Autoimmune diseases can be divided into three categories, T cell–dominant, B cell–dominant, or combinational types. Direct roles for B cells and immunoglobulin production have also been proposed in autoimmune disorders more traditionally thought to be primarily T cell–mediated, such as rheumatoid arthritis 5 6 and inflammatory bowel disease 7. In animal models, such involvement can be readily demonstrated 6 8 9.
Most members of the tumor necrosis factor family control aspects of immune function such as organogenesis of secondary lymphoid organs 10, lymphocyte activation, and effective inflammatory/immune responses 11. Interestingly, imbalance in the production of some of these ligands has been shown to contribute to inflammatory and autoimmune disorders 12. The Fas/FasL system is probably the most striking example of a pathway directly involved in the elimination of some subsets of autoreactive B and T lymphocytes by apoptosis. Mice lacking functional expression of Fas (lpr) or FasL (gld) develop severe autoimmune lymphoproliferative disorders, leading to tissue destruction 13. Another member of the TNF superfamily, CD30L, seems to have a role of protection against autoimmunity, as signaling through its receptor was shown to protect against autoimmune diabetes mediated by CD8 T cells 14. More often, a reverse situation is seen by which abnormal levels rather than absence of expression directly contributes to the pathophysiology of autoimmune diseases 12. TNF is the most extensively studied member of this family, and its participation in several autoimmune disorders such as rheumatoid arthritis, inflammatory bowel disease, and autoimmune encephalomyelitis is now well established 15. Participation or abnormal expression in autoimmune disease has been described for other ligands of this family such as OX40 16, CD27 17, and lymphotoxin 18.
Early studies on the CD40/CD40L pathway revealed specific roles in B cell biology 19 promoting B cell activation, proliferation, survival, isotype switching, germinal center formation, and generation of memory B cells 20. It was, therefore, not surprising that interrupting this pathway inhibited disease in rodent models of rheumatoid arthritis and systemic lupus nephritis, in which B cell tolerance is severely altered 21 22. Recent studies have shown that the role of the CD40/CD40L pathway in disease is not solely directed towards B cell regulation, but extends to many other cell types 20.
We have recently identified a new TNF-like ligand, called BAFF (for B cell activating factor belonging to the TNF family), which is expressed by dendritic cells and possibly T cells and binds primarily to B cells 23. BAFF is secreted from transfected cells and has the potential to act as a soluble mediator. BAFF was also independently identified as TALL-1 24, as THANK, which regulated apoptosis, NF-κB and c-Jun NH2-terminal kinase in a human myelocytic cell line 25, and, more recently, as BlyS, a factor that, when administrated to normal mice, disrupted splenic B and T cell zones and resulted in elevated serum IgM concentrations 26. Similar to CD40L, BAFF promotes B cell proliferation in the presence of an anti–IgM antibody in vitro 23 26. We have generated transgenic mice overexpressing BAFF under the control of a liver-specific promoter. These mice have excessive numbers of mature B cells, spontaneous germinal center reactions, secrete autoantibodies, high plasma cell numbers in secondary lymphoid organs, elevated numbers of effector T cells, and Ig deposition in the kidney.
Materials and Methods
Generation of BAFF Transgenic Mice.
A PCR fragment encoding full-length murine BAFF was generated by reverse transcription–PCR using previously described sequence information 23. First strand cDNA was synthesized from mouse lung polyA+ (Clontech) using oligo dT according to the manufacturer's protocol (GIBCO BRL). The PCR reaction contained 1× pfu buffer (Stratagene Inc.), 0.2 mM dNTPs, 10% DMSO, 12.5 pM primers, 5 units pfu enzyme (Stratagene Inc.), and the following primers with Not1 restriction sites 5′-TAAGAATGCGGCCGCGGAATGGATGAGTCTGCAAA-3′ and 5′-TAAGAATGCGGCCGCGGGATCACGCACTCCAGCAA-3′. The template was amplified for 30 cycles at 94°C for 1 min, 54°C for 2 min, and 72°C for 3 min, followed by a 10-min extension at 72°C. This sequence corresponds to nucleotides 214–1171 of the GenBank file AF119383. The PCR fragment was digested with Not1 and cloned into a modified pCEP4 vector (Invitrogen Corp.). The resulting vector was then digested with Xba1 to remove BAFF plus the SV40 polyA addition site sequence. This fragment was cloned into a pUC-based vector in which the promoter, a 1-kb blunt Bgl2–Not1 fragment containing the human ApoE enhancer and AAT (alpha antitrypsin) previously purified from the plasmid clone 540B (a gift from Dr. Katherine Parker Ponder, Washington University, St. Louis, MO) was further inserted at the EcoRV site. An EcoRV/Bgl2 fragment was purified from the final vector and used for the generation of transgenic mice. The injected offspring of C57BL/6J female × DBA/2J male F1 (BDF1) mice were backcrossed onto C57BL/6 mice. Techniques of microinjection and generation of transgenic mice have been previously described 27. Animal experiments were approved by the Institutional Animal Care and Use Committee.
Analytical Methods.
Serum samples were subjected to reducing SDS-PAGE analysis using a linear 12.5% gel. MOPC-21 mouse IgG1 standard antibody was obtained from PharMingen. Total RNA from mouse liver was prepared and processed for Northern blot analysis using an isolation kit from Promega Corp. according to the manufacturer's guidelines. BAFF transgene-specific mRNA was detected using a probe spanning the SV40 polyA tail of the transgene construct and obtained by digestion of the modified pCEP4 vector with Xba1 and BamH1. The probe recognizes a 1.8–2-kd band corresponding to mRNA from the BAFF transgene. PCR analysis of tail DNA from BAFF transgenic (Tg) mice used 12.5 pM of the primers 5′-GCAGTTTCACAGCGATGTCCT-3′ and 5′-GTCTCCGTTGCGTGAAATCTG-3′ in a reaction containing 1× Taq polymerase buffer (Stratagene Inc.), 0.2 nM dNTPs, 10% DMSO, and 5 U Taq polymerase (Stratagene Inc.). 719 bp of the transgene was amplified for 35 cycles at 94°C for 30 s, 54°C for 1 min, and 72°C for 1.5 min, followed by a 10-min extension at 72°C.
The presence of proteins in mouse urine was measured using Multistix 10 SG reagent strips for urinalysis (Bayer Corp., Diagnostics Division).
Cellular Analysis.
Differential white blood cell counts of fresh EDTA-anticoagulated whole blood were performed with an Abbott Cell Dyne 3500 apparatus. For FACS® analysis (Becton Dickinson & Co.), fluorescein–(FITC), CyChrome™–, and phycoerythrin (PE)-labeled rat anti–mouse antibodies: anti–B220, anti–CD4, anti–CD8, anti–CD43, anti–IgM, anti–CD5, anti–CD25, anti–CD24, anti–CD38, anti–CD21, anti–CD44, anti–MHC class II, anti–l-selectin, and hamster anti–Bcl-2/control hamster Ig kit were purchased from PharMingen. Production of recombinant Escherichia coli, as well as mammalian cell-derived mouse Flag-tagged BAFF, was performed as previously described for human BAFF 23. All antibodies were used according to the manufacturer's specifications. PBL were isolated by density gradient centrifugation of EDTA-treated mouse blood over lymphocyte M (Cedarlane). FACS® analysis of spleen, bone marrow, and mesenteric lymph nodes was performed effectively as described previously 28.
Detection of Total Mouse Ig and Rheumatoid Factors in Mouse Sera by ELISA Assays.
ELISA plates (Corning Glass Works) were coated overnight at 4°C with a solution of 10 μg/ml goat anti–total mouse Ig (Southern Biotechnology Associates, Inc.) in 50 mM sodium bicarbonate buffer, pH 9.6. Plates were washed three times with PBS/0.1% Tween and blocked overnight with 1% gelatin in PBS. 100 μl/well of serum serial dilutions or standard dilutions was added to the plates for 30 min at 37°C. Mouse Ig were detected using 100 μl/well of a 1-μg/ml solution of an alkaline phosphatase (AP)–labeled goat anti–total mouse Ig (Southern Biotechnology Associates, Inc.) for 30 min at 37°C. After a last wash with PBS/0.1% Tween, the enzymatic reaction was developed using a solution of 10 μg/ml of p-nitrophenyl phosphate (Boehringer Mannheim Biochemicals) in 10% diethanolamine. The reaction was stopped by adding 100 μl of 3 N NaOH/well. The optical density was measured at 405 nm using a spectrophotometer from Molecular Devices. Standard curves were obtained using purified mouse Ig purchased from Southern Biotechnology Associates, Inc. In the case of detection of rheumatoid factors, the plates were coated with normal goat Ig (Jackson ImmunoResearch Laboratories, Inc.) instead of goat anti–mouse Ig, and detection of mouse Ig was performed as described above. Detection of mouse isotypes in the RF assay was done using AP-labeled goat anti–mouse IgA, IgM, IgG2a, IgG2b, IgG1, and IgG3, as well as purified mouse IgA, IgM, IgG2a, IgG2b, IgG1, and IgG3 for standard curves (Southern Biotechnology Associates, Inc.). All statistical comparisons were performed by analysis of variance.
Detection of Circulating Immune Complexes and Precipitation of Cryoglobulins in Mouse Sera.
The assay was performed as previously described 29 30 with the following modifications: ELISA plates (Corning Glass Works) were coated overnight at 4°C with 5 μg/ml of human C1q (Quidel) in 50 mM sodium bicarbonate buffer, pH 9.6. The plates were washed three times with PBS/0.1% Tween. 50 μl/well of 0.3 M EDTA was added to the plates plus 50 μl/well of serum serial dilutions or solutions of known concentrations of a standard immune complex (peroxidase-mouse antiperoxidase) from DAKO Corp. The plates were incubated 30 min at 37°C. The plates were washed three times with PBS/0.1% Tween. Mouse Ig in the immune complexes were detected using 100 μl/well of a 1 μg/ml solution of an AP-labeled goat anti–mouse Ig (Southern Biotechnology Associates, Inc.) as described above for the ELISA assays. Cryoglobulins were detected by incubating overnight at 4°C mouse serum diluted 1/15 in water and precipitates were scored visually.
Anti–double- and –single-stranded DNA Assays.
Detection of anti–single-stranded (ss) DNA antibodies was performed using NUNC-immuno Plate MaxiSorp plates (NUNC A/S). Plates were coated overnight at 4°C first with 100 μg/ml methylated BSA (Calbiochem Corp.), then with 50 μg/ml grade I calf thymus DNA (Sigma Chemical Co.) that was previously sheared by sonication, and finally digested with S1 nuclease. This DNA was used to coat plates for the anti–double-stranded (ds) DNA assays, but was additionally boiled 10 min and chilled on ice before coating plates for the anti–ssDNA assays. After blocking, serial dilutions of the serum samples were added and incubated at room temperature for 2 h. Autoantibodies were detected with goat anti–mouse IgG-AP (Sigma Chemical Co.) and developed as described above for the ELISA assays. Standard curves were obtained using known quantities of anti–DNA mAb 205, which is specific for both ss- and dsDNA 31.
Immunohistochemistry.
Frozen sections of spleen and lymph nodes were subjected to immunohistochemical analysis as previously described 32. Biotin-labeled antibodies rat anti–B220, anti–CD11c, and anti–syndecan-1, as well as unlabeled rat anti–CD4, anti–CD8α, and anti–CD8β were purchased from PharMingen. Biotin-labeled peanut agglutinin was obtained from Vector Laboratories, Inc. Horseradish peroxidase (HRP)–labeled mouse anti–rat Ig and HRP-streptavidin were purchased from Jackson ImmunoResearch Laboratories, Inc., and AP-labeled streptavidin from Southern Biotechnology Associates, Inc. In the case of immunohistochemistry on kidney tissue to detect Ig deposition, paraffin sections were used, dewaxed, and blocked using diluted horse serum from Vector Laboratories, Inc., followed by staining with HRP goat anti–mouse Ig from Jackson ImmunoResearch Laboratories, Inc. Detection was performed as previously described for frozen sections 32.
Results
BAFF-Tg Mice.
Full-length murine BAFF was expressed in transgenic mice using the liver-specific alpha-1 antitrypsin promoter with the APO E enhancer. The full-length version was chosen with the expectation that BAFF would be cleaved and act systemically or, if retained in a membrane-bound form, that local liver-specific abnormalities would be observed, possibly providing functional clues. We obtained 13 founder mice positive for the BAFF transgene. BAFF overexpression in the liver of transgenic mice was confirmed by Northern blot analysis (data not shown). An ELISA assay for murine BAFF is not available; however, we found that 2% serum from BAFF-Tg mice, but not from control mice, blocked the binding of mammalian cell-derived mouse soluble Flag-tagged BAFF to BJAB cells. Moreover, 5% serum from BAFF-Tg mice but not from control mice increased the proliferation of human B cells from PBL in the presence of anti–μ (data not shown). These data suggest that soluble BAFF is present in the blood of BAFF-Tg mice. In all BAFF-Tg mice examined histologically, the livers showed no abnormalities, indicating that local overexpression of BAFF did not induce any immunological or pathological events (data not shown).
Expansion of the Peripheral Blood B Cell Compartment in BAFF-Tg Mice.
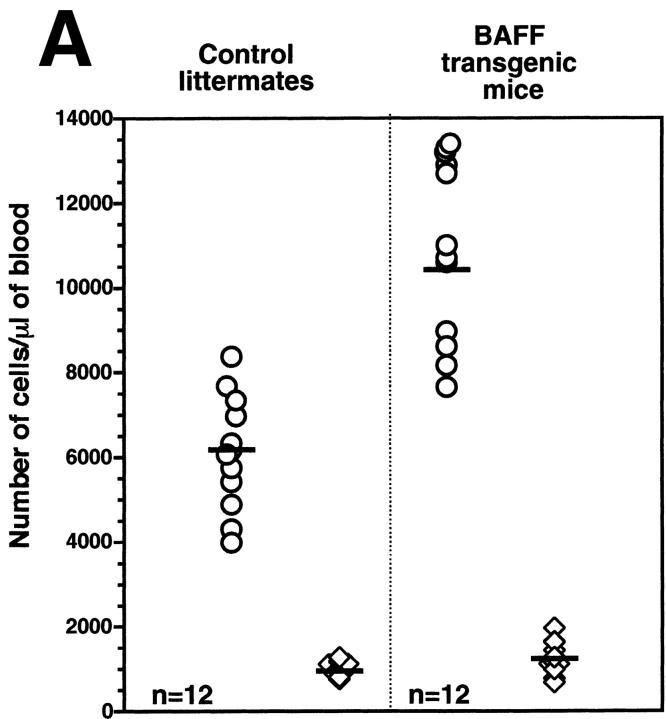
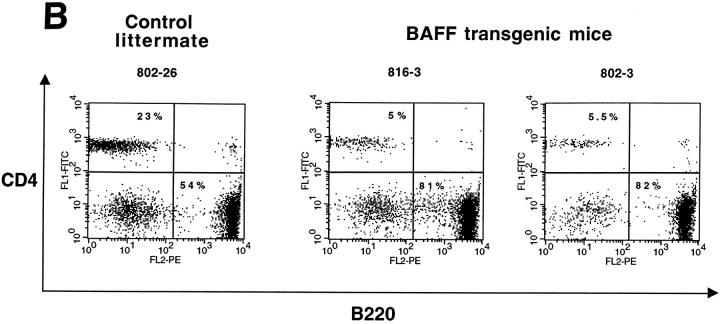
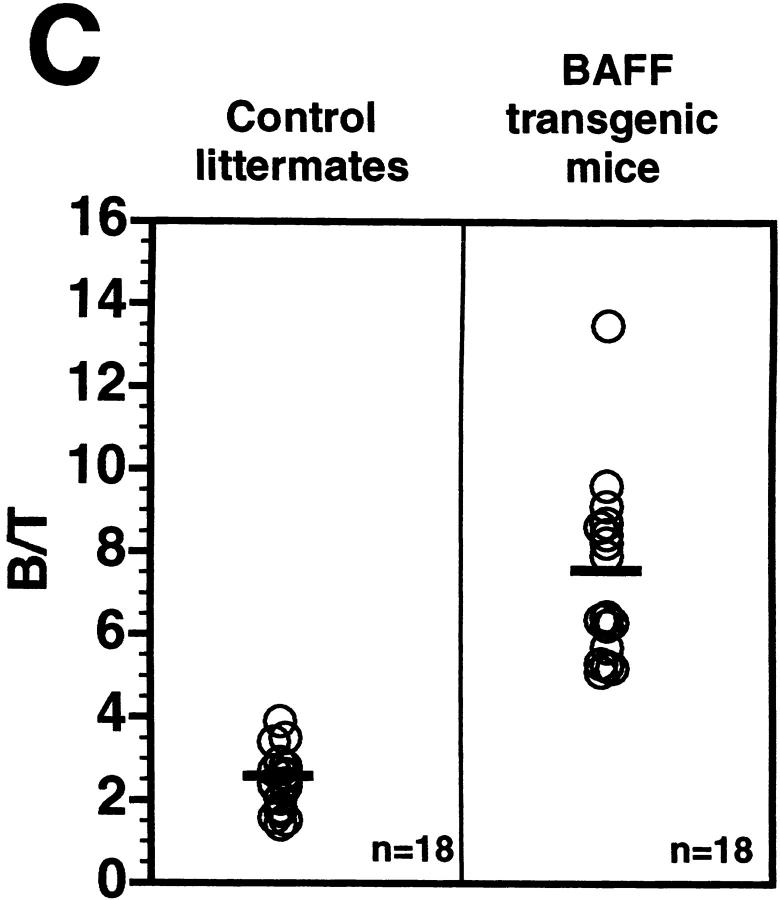
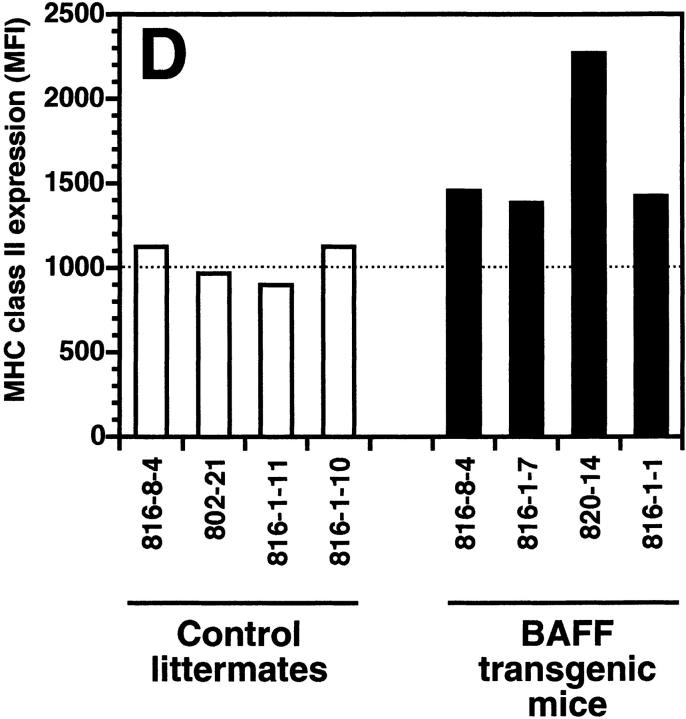
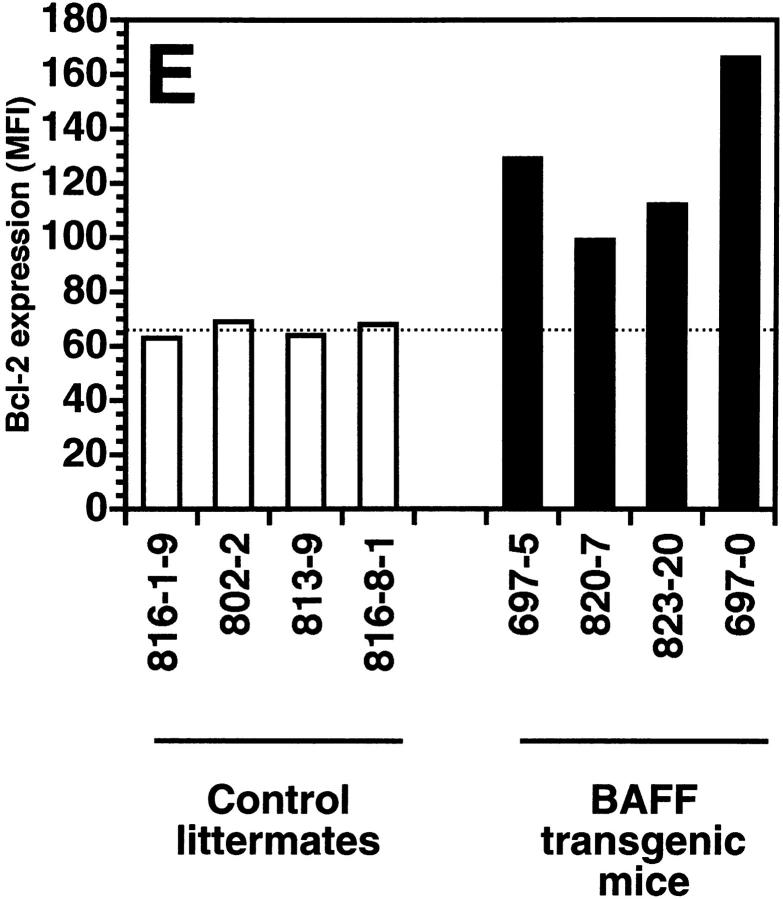
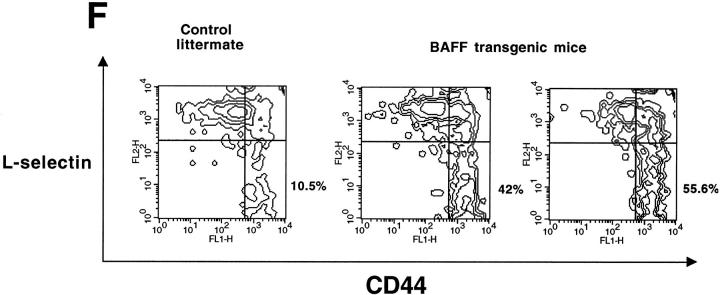
The transgenic mouse population was found to have more lymphocytes in the blood when compared with control negative littermates, reaching values as high as 13,000 lymphocytes/μl of blood (Fig. 1 A). In contrast, the number of granulocytes per microliter of blood in both BAFF-Tg and control mice remained within normal limits (Fig. 1 A). The elevated lymphocyte levels resulted from an expanded B cell subset, since FACS® analysis, using anti–CD4 and –B220 antibodies, of peripheral blood cells (PBL) from 18 BAFF-Tg mice issued from six different founders showed increased B/T ratios (Fig. 1b and Fig. c). Likewise, combining the number of lymphocytes per microliter of blood with the percentage of circulating T cells, calculation of absolute numbers of CD4 circulating T cells revealed a 50% reduction of this T cell subset in BAFF-Tg mice when compared with control mice, and the same observation was made for the CD8 T cell subset (data not shown). All peripheral blood B cells from BAFF-Tg mice had increased MHC class II and Bcl-2 expression when compared with B cells from control mice (Fig. 1d and Fig. e, respectively), indicating some level of B cell activation in PBL of BAFF-Tg mice. T cells in the blood of BAFF-Tg mice did not express the early activation markers CD69 or CD25; however, 40–56% of CD4 or CD8 T cells were activated effector T cells with a CD44hi, l-selectinlo phenotype versus only 8–12% in control littermates (Fig. 1 F). Thus, BAFF-Tg mice clearly show signs of an expanded peripheral blood B cell compartment and global B cell activation along with T cell alterations.
Figure 1.
Increased B cell numbers in BAFF-Tg mice. (A) Increased lymphocyte counts in BAFF-Tg mice. The graph compares 12 control littermates (left) with 12 BAFF-Tg mice (right). Lymphocyte counts (○) and granulocytes (including neutrophils, eosinophils, basophils; ⋄) are shown. (B) Increased proportion of B cells in PBL from BAFF-Tg mice. PBL were stained with both anti–B220-FITC and anti–CD4-PE for FACS® analysis and gated on live cells using the forward and side scatter profile. Percentages of CD4- and B220-positive cells are indicated. One control mouse (left) and two BAFF-Tg mice (right) are shown and the results were representative of seven animals analyzed in each group. (C) FACS® analysis of the ratio of B to T cells in PBL. The difference between control animals and BAFF-Tg mice in A and C was statistically significant (P < 0.001). (D) Increased MHC class II expression on B cells from BAFF-Tg mice PBL. MHC class II expression was analyzed by FACS®. (E) Increased Bcl-2 expression in B cells from BAFF-Tg mice PBL. Bcl-2 expression was measured by intracytoplasmic staining and cells were analyzed by FACS®. In both D and E, B220-positive cells were gated. Four control littermates (white bars) and four BAFF-Tg mice are shown and are representative of at least 12 animals analyzed for each group. MFI, mean of fluorescence intensity. The dotted line represents the average MFI for the control animals. The difference between control animals and BAFF-Tg mice was statistically significant (P < 0.005). (F) Increased expression of effector T cells in BAFF-Tg mice. PBL were stained with anti–CD4-CyChrome™, anti–CD44-FITC and anti–l-selectin-PE. Shown are CD4+-gated cells. Percentages of CD44hi/l-selectinlo cells are indicated. One control mouse (left) and two BAFF-Tg mice (right) are shown and the results were representative of eight animals analyzed in each group.
Expanded B Cell Compartments Are Composed of Mature Cells.
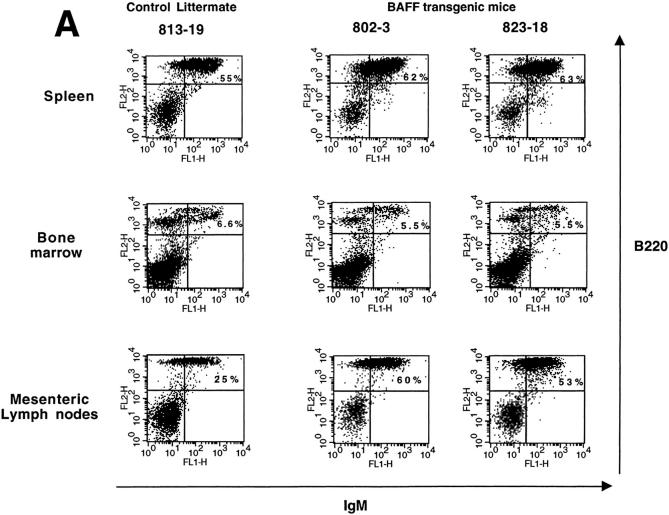
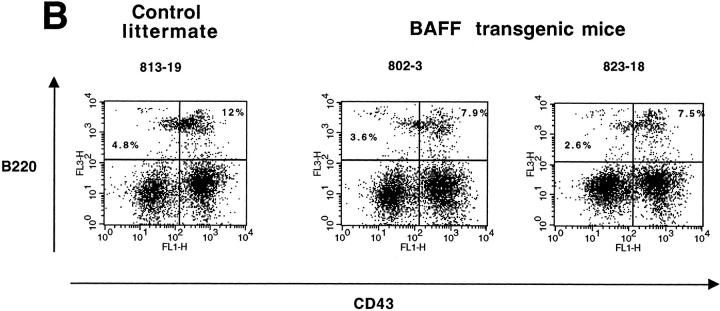
To see whether overexpression of BAFF in the transgenic mice was affecting the B cell compartment centrally in the bone marrow and peripherally in secondary lymphoid organs, we examined the spleen, bone marrow (BM), and mesenteric lymph nodes (MLN) from a total of seven BAFF-Tg mice and seven control littermates derived from four different founder mice by FACS® analysis. The mature B cell compartment was analyzed by dual staining with anti–B220 and –IgM antibodies. Two representative BAFF-Tg mice and one control littermate are shown in Fig. 2. The mature B cell compartment (IgM+/B220+) was increased in both the spleen and the MLN (Fig. 2 A, top and bottom panels, respectively). Analysis of B220+/IgM+ B cells (Fig. 2 A, middle) or the proB cell (CD43+/B220+) and the preB cell (CD43−/B220+) compartments in the BM (Fig. 2 B) showed that BAFF-Tg mice and control littermates were similar. The slight difference in the mature B cell percentage seen in Fig. 2 A (middle) was not consistent in all seven mice analyzed. These data indicate that overexpression of BAFF is affecting the mature B cell compartment in the periphery, but not progenitor B cells in the BM.
Figure 2.
Increased proportion of B cells in the spleen, MLN, but not in the bone marrow of BAFF-Tg mice. (A) FACS® staining for mature B cells using both anti–IgM-FITC and anti–B220-PE, in spleen (top), bone marrow (middle), and MLN (bottom). Percentages of B220+/IgM+ mature B cells are indicated. (B) FACS® staining for pre–B cells (B220+/CD43−) and pro–B cells (B220+/CD43+) in the bone marrow using anti–CD43-FITC, anti–B220-CyChrome™, and anti–IgM-PE simultaneously. Shown are cells gated on the IgM negative population. Percentages of pre–B cells (B220+/CD43−) and pro–B cells (B220+/CD43+) are indicated. For all panels (A and B), one control mouse (left) and two BAFF-Tg mice (right) are shown and results are representative of seven animals analyzed for each group.
We calculated the total number of B and T cells in the spleen, BM, and MLN (Table ). The total number of B cells was at least sevenfold higher in the spleen and MLN of BAFF-Tg mice. The total number of T cells was also increased twofold in the spleen and MLN of these mice (Table ). Total numbers of B and T cells in the BM in BAFF-Tg mice were similar to that of control mice (Table ). The population of CD5+ B1 cells in the spleen, BM, and peritoneal lavages of BAFF-Tg mice was similar to that of control mice and only marginally increased in MLN (data not shown).
Table 1.
Total Number of T and B Cells in the Spleen, Mesenteric Lymph Nodes, and Bone Marrow
| Mouse number | Number of B cells (×107) | Number of T cells (×107) | ||||
|---|---|---|---|---|---|---|
| Spleen | BM | MLN | Spleen | BM | MLN | |
| BAFF-Tg mice | ||||||
| 816-8-34 | 18 | 4 | 1.4 | 3.5 | 0.4 | 1.1 |
| 802-43 | 34 | 1.3 | 5.6 | 3.5 | 0.15 | 1.7 |
| 823-3-23 | 16 | 3.1 | 3 | 3.8 | 0.46 | 3.6 |
| 802-41 | 46 | 3 | 5 | 5.3 | 0.3 | 0.9 |
| 816-1-29 | 23 | 1.4 | 1.2 | 4 | 0.07 | 0.3 |
| Mean ± SD | 27.4 ± 11 | 2.6 ± 1.04 | 3.2 ± 1.8 | 4 ± 0.6 | 0.28 ± 0.14 | 1.5 ± 1.2 |
| Control littermates | ||||||
| 816-1-20 | 4.9 | 3.5 | 0.12 | 1.8 | 0.15 | 0.27 |
| 802-51 | 7 | 2.6 | 0.26 | 3.1 | 0.08 | 0.61 |
| 823-2-3 | 5.5 | 1.3 | 0.33 | 3 | 0.14 | 0.05 |
| 823-2-6 | 2.3 | 1.6 | 0.1 | 1.5 | 0.23 | 0.3 |
| 816-1-24 | 3.9 | 1.9 | 0.17 | 1.9 | 0.21 | 0.32 |
| Mean ± SD | 4.7 ± 1.5 | 2.2 ± 0.8 | 0.19 ± 0.08 | 2.2 ± 0.6 | 0.16 ± 0.05 | 0.31 ± 0.2 |
| Controls/BAFF-TgP values | P < 0.01 | P > 0.1 | P < 0.01 | P < 0.01 | P > 0.1 | P < 0.05 |
Analysis by FACS® of B cell subpopulations in the spleen revealed an increased proportion of marginal zone (MZ) B cells in BAFF-Tg mice when compared with control mice (Table ). The population of follicular B cells remained equivalent in both BAFF Tg and control mice, whereas the fraction of newly formed B cells was slightly decreased in BAFF-Tg mice (Table ). This result was also confirmed on B220+ splenic B cells using anti–CD38 vs. anti–CD24 antibodies or anti–IgM vs. anti–IgD antibodies and analyzing the CD38hi/CD24+ and IgMhi/IgDlo MZ B cell population, respectively, as previously described 33 (data not shown). Immunohistochemical analysis using an anti–mouse IgM antibody revealed the expansion of the IgM-bright MZ B cell area in the spleen of BAFF-Tg mice when compared with control mice (data not shown). All BAFF-Tg B220+ splenic B cells also expressed higher levels of Bcl-2 (data not shown) and also MHC class II (Table ) compared with splenic B cells from control mice, indicating that splenic B cells as well as peripheral blood B cells are in an activated state. At equal cell concentration, splenocytes isolated from BAFF-Tg mice survived longer in culture medium when compared with control splenocytes and the thymidine incorporation after 6 d of culture relative to the incorporation on day 0 was only decreased 20% in cultures with BAFF-Tg–derived splenocytes vs. 60% reduction with control splenocytes (data not shown). In vivo 5-bromo-2′-deoxyuridine (BrdU) incorporation for 4 d did not reveal any higher BrdU intake in BAFF Tg–splenic B cells when compared with control mice (data not shown).
Table 2.
Increased MHC Class II Expression on B Cells and Enlarged Proportion of MZ B Cells in the Spleen of BAFF-Tg Mice
| Levels of MHC class II expression on B220+ B cells (MFI) | Percentage of follicular B cells* | Percentage of MZ B cells‡ | Percentage of newly formed B cells§ | ||
|---|---|---|---|---|---|
| Control mice | |||||
| 816-1-10 | 1170 | 45 | 6 | 12 | |
| 802-21 | 1029 | 48 | 10.5 | 9 | |
| 823-1 | 1240 | 39 | 9 | 6.5 | |
| BAFF-Tg mice | |||||
| 802-6 | 1707 | 49 | 18 | 5.9 | |
| 820-7 | 1900 | 39 | 23 | 6.3 | |
| 816-1-1 | 2088 | 40 | 23 | 5.8 | |
Splenocytes were analyzed by FACS® and gated on the B220+ population. A representative experiment is shown. MFI, mean of fluorescence intensity.
*B220+/ΙγΜlo/CD21int.
‡B220+/IgMhi/CD21hi.
§B220+/IgMhi/CD21lo.
BAFF-Tg Mice Have Enlarged B Cell Follicles, Numerous Germinal Centers, and Reduced Dendritic Cell Numbers and Increased Plasma Cell Numbers in Both the Spleen and MLN.
BAFF-Tg mice had large spleens (Fig. 3 A), Peyer's patches (B), and lymph nodes (C). Immunohistochemistry showed the presence of enlarged B cell follicles and reduced periarteriolar lymphocyte sheath (or T cell area) in BAFF-Tg mice (Fig. 4 B). Interestingly, few germinal centers were observed in nonimmunized control littermates (and is typical of this colony in general) and those present were small (Fig. 4 C), whereas BAFF-Tg mice possessed numerous and large germinal centers in the absence of immunization (Fig. 4 D). Staining with anti–CD11c for dendritic cells in the T cell zone and the marginal zone of control mice (Fig. 4 E) was considerably reduced in BAFF-Tg mice (Fig. 4 F). Syndecan-1–positive plasma cells were almost undetectable in the spleen from control littermates (Fig. 4 G), yet the red pulp of BAFF-Tg mice was strongly positive for syndecan-1 (Fig. 4 H). Very similar observations were made with MLN (Fig. 5). In the MLN of BAFF-Tg mice, the B cell areas were dramatically expanded (Fig. 5 B), in contrast to the normal appearance, where B cell follicles were easily recognizable at the periphery of the node under the capsule with a typical paracortical T cell zone (Fig. 5 A). The medulla of MLN from BAFF-Tg mice were filled with syndecan-1–positive cells that presumably are plasma cells (Fig. 5 H). Therefore, analysis of secondary lymphoid organs in BAFF-Tg mice was consistent with the expanded B cell compartment seen by FACS® analysis and indicates multiple cellular abnormalities and intense immune activity.
Figure 3.
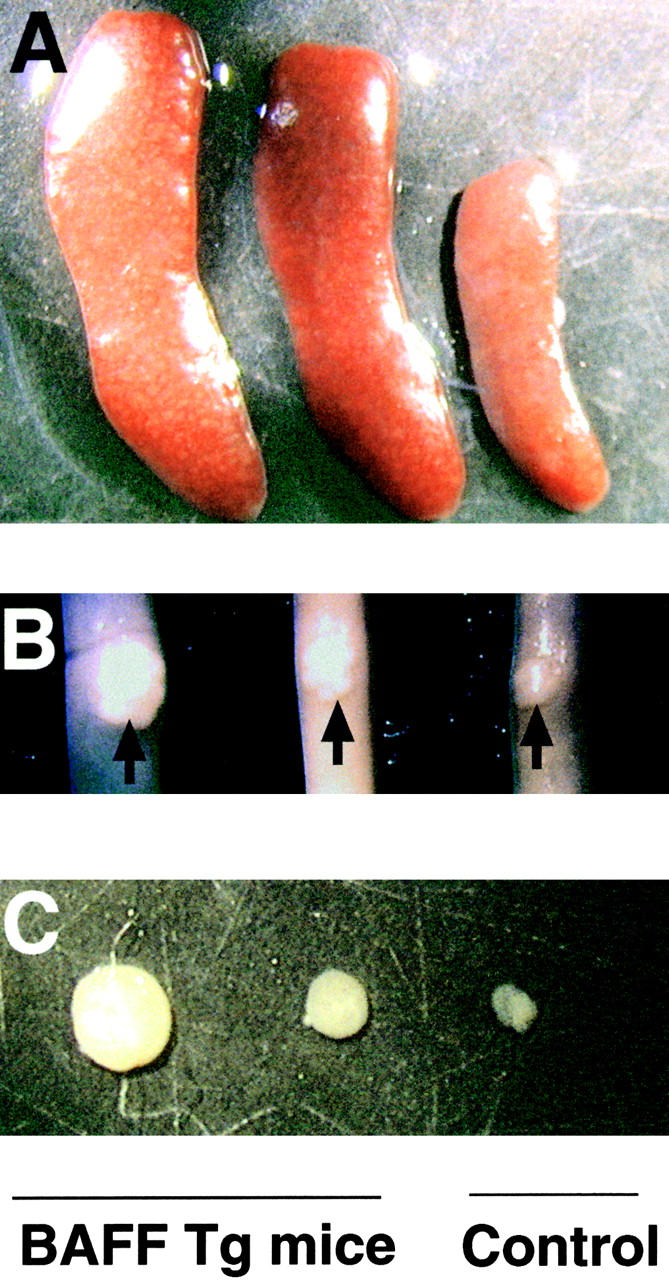
Enlarged spleen, Peyer's patches, and lymph nodes in BAFF-Tg mice. Photograph of (A) spleen, (B) Peyer's patches (indicated with an arrow) on the small intestine, and (C) inguinal lymph nodes of a control mouse (right) and two BAFF-Tg mice (left). Pictures (5×) are representative of at least 12 mice killed for each group.
Figure 4.
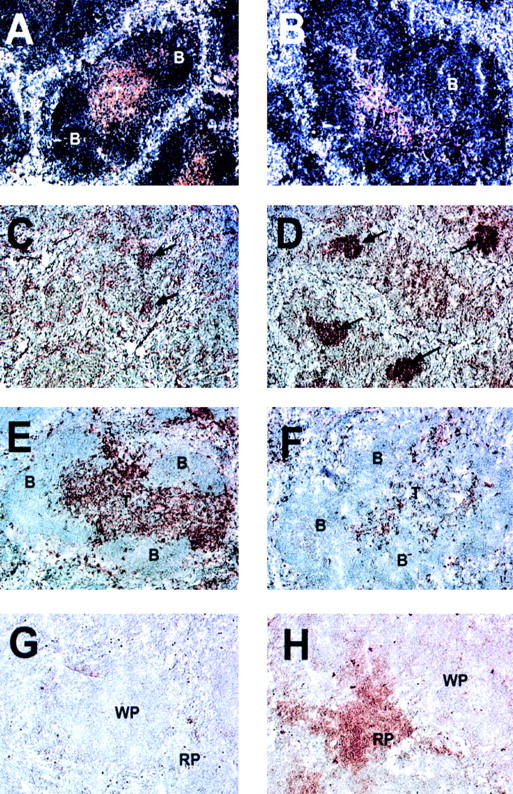
Altered T and B cell organization, intense germinal center reactions, decreased number of dendritic cells, and increased number of plasma cells in the spleen of BAFF-Tg mice. A control mouse is shown in A, C, E, and G and a BAFF-Tg mouse in B, D, F, and H. B cells are blue and T cells brown (A and B). Germinal centers are marked with an arrow (C and D). Only a few residual germinal centers are seen in control mice (C). CD11c-positive dendritic cells are brown and appear in the T cell zone, bridging channels and the marginal zone (E). Very few are present in BAFF-Tg mice (F). Syndecan-1–positive plasma cells were only detectable in the red pulp of BAFF-Tg mice (H) but not control mice (G). These pictures are representative of at least 12 BAFF-Tg mice analyzed and 12 control mice. 100× except C and D (50×). B, B cell follicle; T, periarteriolar lymphocyte sheath; WP, white pulp; RP, red pulp.
Figure 5.
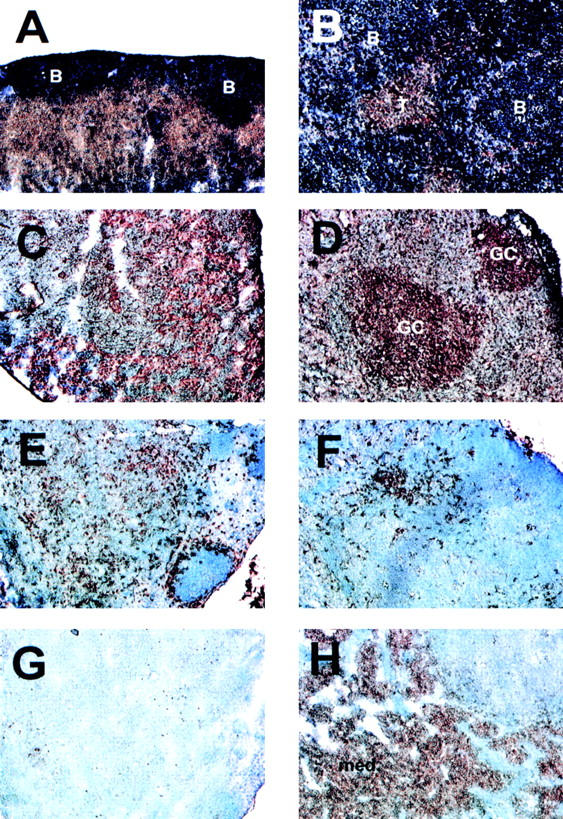
Disrupted T and B cell organization, intense germinal center reactions, and large numbers of plasma cells in the MLN of BAFF-Tg mice. The control mouse is shown in A, C, E, and G and the BAFF-Tg mouse is shown in B, D, F, and H. The immunohistochemistry was performed as described in Fig. 6. T and B cell staining is shown in A and B, germinal centers in C and D, dendritic cells in E and F, and plasma cells in G and H. The background is slightly more intense in C, but no germinal centers were detectable. GC, germinal center. Original magnifications: ×100.
BAFF-Tg Mice Have High Levels of Total Immunoglobulins, Rheumatoid Factors, and Circulating Immune Complexes in Their Serum.
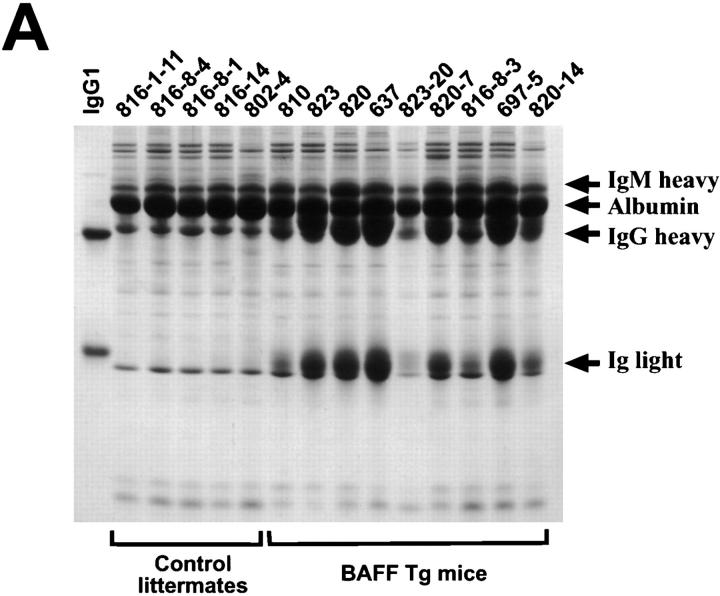
The increased B cell compartment in BAFF-Tg mice suggested that the level of total Ig in the blood of these animals might also be increased. SDS-PAGE analysis of the serum showed that IgG levels were elevated in all BAFF-Tg mice, while the nontransgenic littermates displayed a normal pattern of serum proteins (Fig. 6 A). By comparison with an IgG1 standard antibody, the levels of IgG in a nontransgenic mouse were ∼5–8 mg/ml, and these levels increased to at least 50 mg/ml in some BAFF-Tg mice (quantification was done with underloaded gels). In normal mice, the light chain band is smeared due to the polyclonal nature of the Ig and on this basis the elevated Ig levels in BAFF-Tg mice were also polyclonal in nature. IgM levels were visibly increased in these mice, albeit not as much as IgG, and this band is seen as a smear on top of a transferrin band in this gel.
Figure 6.
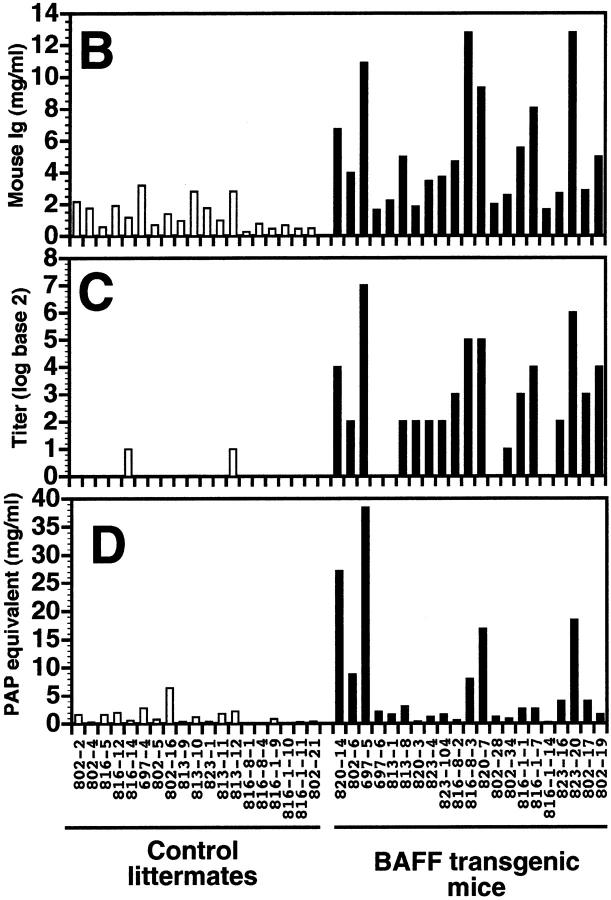
Increased Ig, RF, and CIC levels in BAFF-Tg mice. (A) Reduced SDS-PAGE of sera from five control littermates and nine BAFF-Tg mice showing that BAFF increases IgG levels. For comparison, mouse IgG1 (MOPC-21) was included as a standard: loading per lane was 5 μg of MOPC-21 and 0.5 μl of the serum. The sharp band slightly below the Ig light chain is not an immunoglobulin and the IgM heavy chain comigrates with transferrin. ELISA-based analysis of total mouse Ig (B), RF (C), and CIC (D) in the sera of 19 control littermates (white bars) and 21 BAFF-Tg mice (black bars). The titer (log base 2) for RF is defined as the dilution of the sera giving an OD three times higher than that of background. The quantity of CIC is defined as the quantity of peroxidase-mouse antiperoxidase required to generate an OD equivalent to that obtained with the tested serum. The difference between control animals and BAFF-Tg mice was statistically significant (P < 0.001 in B and C, P < 0.003 in D).
High serum Ig levels in BAFF-Tg mice were confirmed by ELISA (Fig. 6 B), and the high levels of Ig seen in these mice led us to suspect the presence of rheumatoid factors, or autoantibodies directed against antigenic determinants on the Fc domain of IgG 34. These antibodies could bind to the goat anti–mouse Ig used to coat the ELISA plates and give erroneously high values. ELISA plates were, therefore, coated with normal goat Ig and the binding of BAFF Tg Ig to normal goat Ig was measured. Fig. 6 C shows that sera from most BAFF-Tg mice contained Ig reacting with normal goat Ig, whereas only 2 of 19 control mice exhibited reactivity in the same assay. These RF were mainly of the IgM, IgA, and IgG2a isotypes (data not shown).
Presence of RF can be associated with the presence of high levels of circulating immune complexes (CIC) and cryoglobulin in the blood 34. To verify whether or not BAFF-Tg mice have abnormal serum levels of CIC, a C1q-based binding assay was used to detect CIC in the 21 BAFF-Tg mice analyzed above. Only five BAFF-Tg mice showed significantly high levels of CIC when compared with control mice; nonetheless, these mice corresponded to the animals having the highest total serum Ig and rheumatoid factor levels (Fig. 6 D). We also observed precipitate formation when sera from BAFF-Tg mice, but not control sera, were diluted 1/15 in water, indicating the presence of cryoglobulin in these mice (data not shown). Thus, in addition to B cell hyperplasia, BAFF-Tg mice display severe hyperglobulinemia associated with the presence of RF and CIC.
Some BAFF-Tg Mice Have High Levels of Anti–ss and –dsDNA Autoantibodies, Ig Deposition in the Kidneys, and Proteinuria.
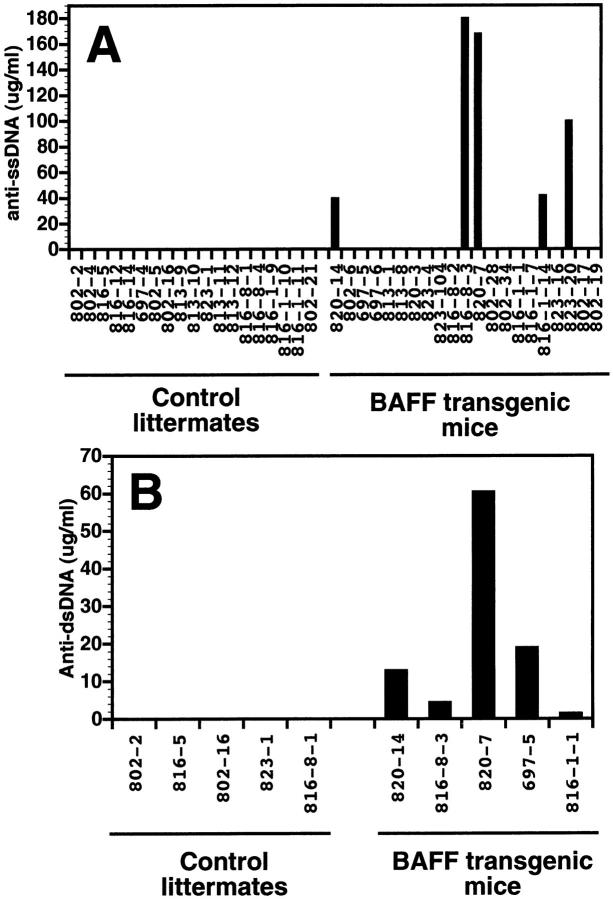
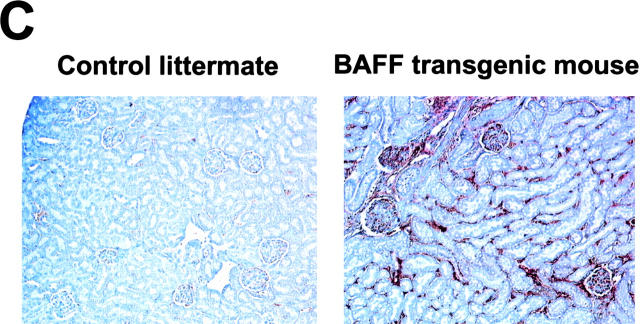
Initially, we observed kidney abnormalities reminiscent of a lupus-like disease in two of our founder mice. The presence of anti–DNA autoantibodies has also been described in SLE patients or the SLE-like (SWR × NZB)F1 (SNF1) mouse 31. Anti–ssDNA autoantibody levels were detected in BAFF-Tg mice previously shown to have the highest level of total serum Ig (Fig. 7 A). We analyzed the serum of two BAFF-Tg mice negative for antibodies against ssDNA (697-5 and 816-1-1) and three transgenic mice secreting anti–ssDNA antibodies (820-14, 816-8-3, and 820-7) for the presence of anti–dsDNA antibodies in parallel with five control littermates. BAFF-Tg mice also secreted anti–dsDNA; however, the levels of secretion did not always correlate with that of anti–ssDNA antibodies, as serum from BAFF-Tg mouse 697-5, which did not contain detectable levels of anti–ssDNA antibodies, was clearly positive for the presence of anti–dsDNA (Fig. 7 B). Therefore, BAFF-Tg mice showing the most severe hyperglobulinemia secrete high levels of anti–DNA autoantibodies. Additionally, and also reminiscent of lupus-like nephritis, we detected immunoglobulin deposition in the kidney of six BAFF-Tg mice analyzed (Fig. 7 C), three of these mice did not secrete detectable levels of anti–DNA antibodies (data not shown). All BAFF-Tg mice have proteinuria (Table ). Sera from all BAFF-Tg mice, but not control mice, diluted 1/100, stained the nuclei of lymph node cells on tissue sections, indicating the presence of antinuclear antibodies in the serum of BAFF-Tg mice (data not shown).
Figure 7.
Presence of anti–ssDNA and –dsDNA autoantibodies in some BAFF-Tg mice, and Ig deposition in the kidneys. (A) Analysis by ELISA of anti–ssDNA autoantibodies in 19 control littermates (left) and 21 BAFF-Tg mice (black bars). (B) Analysis by ELISA of anti–ssDNA autoantibodies in five control littermates and the five animals showing levels of anti–ssDNA autoantibodies from A (black bars). (C) Paraffin sections of kidneys from a control mouse (left) and a BAFF-Tg mouse (right), stained with goat anti–mouse Ig HRP. Ig deposition is shown by a brown staining. These pictures are representative of six BAFF-Tg mice analyzed. Original magnifications.
Table 3.
Levels of Proteinuria* in BAFF-Tg Mice
| Control littermates | BAFF-Tg mice | ||
|---|---|---|---|
| 816-1-20 | − | 802-43 | +++ |
| 802-51 | + | 823-3-23 | +++ |
| 823-2-3 | +/− | 816-1-33 | +++ |
| 823-2-6 | − | 816-1-29 | ++++ |
| 816-1-24 | − | 802-11 | +++ |
| 802-64 | − | 802-13 | ++++ |
| 802-68 | − | 802-27 | +++ |
| 823-14-13 | − | 816-6-2 | +++ |
| 823-14-19 | +/− | 816-8-15 | +++ |
| 816-6-4 | + | 816-8-20 | ++ |
*Proteinuria was measured using medical color strips dipped in mouse urine and is defined as follows: −, no proteinuria; +/−, trace; +, 30 mg/dl; ++, 100 mg/dl; +++, 300 mg/dl; ++++, >2,000 mg/dl.
Discussion
BAFF is a powerful cytokine affecting B cells, and has consequences for T cell and dendritic cell status. The nature of the expanded B cell subset in BAFF-Tg mice is still unclear, but seems to be restricted to mature B cells that have been activated. Overexpression of BAFF led to the emergence of autoimmune manifestations such as production of autoantibodies, proteinuria, Ig deposition in kidneys, and intense germinal center formation. Thus, BAFF ligand and its receptor on B cells form a novel immunoregulatory system.
Whether a ligand is secreted or membrane-bound has profound biological ramifications. These mice were designed to express high levels of BAFF in the liver and, while it cannot be excluded that low level of expression somewhere in the immune system accounts for this unusual biology, we view it more likely that BAFF is secreted from the liver and acts at a distant site. We have indirect evidence for the presence of BAFF in the serum of transgenic mice and, moreover, injection of recombinant BAFF in normal mice led to some of the effects described here 26. Well-defined secretion of a TNF family ligand with functional consequences in vivo has been observed only infrequently; e.g., TNF and lymphotoxin-α. BAFF, TWEAK, and APRIL are three relatively new ligands that possess canonical furin cleavage motifs in the stalk region and are readily secreted from transfected cell systems 23 35 36. Whether such secretion in vitro actually predicts for a soluble ligand system is not clear, yet this BAFF-Tg mouse would indicate that secretion can occur at least from the liver and, thus, soluble BAFF ligand is capable of mediating biological events in vivo. Alternatively, facile cleavage may represent a mechanism that ensures a very transient localized signaling event.
The in vitro analysis using recombinant soluble BAFF protein showed that BAFF costimulated B cell growth in conjunction with B cell receptor activation, yet by itself it did not stimulate proliferation of resting B cells 23. If BAFF is truly a soluble mediator, then this observation is similar to that made originally for IL-2 and T cell growth, and prompts the question of whether BAFF is a B cell growth factor. The present data do not allow one to distinguish between a costimulatory action (e.g., analogous to the activity of CD28) and a true B cell growth factor-like activity. Regardless of the mechanism, these data suggest that expansion of the B cell compartment in these mice is the result of BAFF-induced proliferative stimuli, yet negative results with 4 d in vivo BrdU incorporation and increased ex vivo survival of splenocytes raised the possibility that this observation stems from an increased output from the bone marrow and/or a decreased death rate.
The CD40 pathway clearly plays a major role in B cell regulation, inviting a comparison with the BAFF system. An increase in the size of the B cell population, enlarged spleens, lymph nodes, and Ig deposition in the kidney were also observed in CD40L-Tg mice 37. Several aspects clearly distinguish these two mice; for example, CD40L-Tg mice develop inflammatory bowel disease (IBD), which was not observed in BAFF-Tg mice, and the alterations in the organization of the secondary lymphoid organs are very different 37. In CD40L-Tg mice, but not BAFF-Tg mice, the organization of the thymus is altered, which presumably impairs proper T cell selection leading to IBD, as seen in other mouse models with thymic disfunction 38. The difference between these two transgenic mice may be due in large part to the distinct distribution of these two ligands and their corresponding receptors. Transgene-expressed CD40L is a membrane-bound ligand primarily expressed on thymocytes and activated T cells, whereas transgenic liver-expressed BAFF has the characteristic of a soluble ligand and, therefore, can diffuse into multiple compartments. CD40 is expressed on a wide variety of cell types 20, whereas expression of BAFF receptor is restricted to B cells and possibly monocytic cells 23 24 25 26. Given the disparate phenotypes of the BAFF- and CD40L-Tg mice, it is fair to predict that BAFF and CD40L probably play distinct roles in normal animals as well. Our results on splenic architecture and elevated Ig levels in the serum of BAFF-Tg mice are consistent with those described in a recent study using short-term injection of soluble BAFF in normal mice 26 and tend to minimize possible developmental disturbances related to the expression of the BAFF transgene in our mice. However, chronic exposure of the transgenic mice to BAFF led to changes not paralleled in mice injected for 4 d with recombinant BAFF 26. In both cases, serum IgM levels were elevated, yet the BAFF-Tg mice exhibited vastly increased IgG and IgA levels. The effects of short-term injections were interpreted as possibly stemming from activation of T-independent B cell events, whereas here the elevated IgG and IgA levels, the presence of non-IgM RF isotypes, and the extensive germinal center formation clearly indicate ongoing T cell–dependent B cell events.
It is unclear at this point whether BAFF induces the expansion of both naive and activated B cells, as all B cells in these mice exhibit elevated expression of both MHC class II and Bcl-2 and hence show signs of activation. In preliminary experiments, anti–SRBC antibody titers after primary immunization were similar in BAFF-Tg mice and control littermates, suggesting that the original pool of naive SRBC-specific B cells was not expanded compared with that of control mice. In contrast, SRBC-specific IgG levels after a secondary response to SRBC were significantly higher in BAFF-Tg mice when compared with control mice (data not shown). This result supports a model where BAFF induces the proliferation and/or survival of B cells that had received an activating B cell receptor signal and this effect might only be detectable after secondary immunization or long-term monitoring of the immune response. The enlarged proportion of MZ B cells in the spleen is interesting as these cells are described as B cells in an activated state 39 and as such may be preferential targets for BAFF-induced proliferative/survival signals. Since the MZ contains memory B cells 40, it is conceivable that memory B cells may be specific responders to BAFF-induced signals; this interpretation would be consistent with the stronger secondary response seen with SRBC in BAFF-Tg mice. Additional experiments will be required to define this aspect accurately. These results also raise the fundamental question of the physiological role of BAFF in normal individuals, and whether examining its function may answer remaining questions such as the nature of the mechanisms governing differentiation of B cells into plasma cells vs. germinal center B cells or plasma cells vs. memory B cells.
Among the increased B cell populations in BAFF-Tg mice are emerging autoreactive B cells, secreting RF and anti–DNA autoantibodies. It is well known that tolerance to self antigens is never complete and autoreactive B cells, as well as low levels of rheumatoid factors, can be detected in normal individuals 3. These autoreactive B cells are referred to as natural autoreactive B cells. Therefore, one possibility in BAFF-Tg mice is that the emergence of a large number of autoreactive B cells may reflect the expansion of occasional natural autoreactive B cells in response to BAFF-proliferative stimuli. If this was the case, one would predict that only higher levels of IgM RF would be detected 34, yet high levels of IgA and IgG2a RF were observed indicating isotype switching in the RF-specific B cells. RF other than IgM are found in patients with autoimmune diseases such as rheumatoid arthritis 34. Therefore, the population of RF-specific autoreactive B cells in BAFF-Tg mice is not only expanded but also activated, indicating a dynamic antigen-specific process leading to autoimmune manifestations rather than passive expansion. It cannot be excluded that BAFF-Tg mice have larger numbers of nonnatural self-reactive B cells that have not been identified or possibly larger numbers of foreign antigen-specific activated B cells.
A number of studies have shown that the escape of autoreactive B cells from clonal deletion or functional inactivation (clonal anergy) alone is not enough to develop autoimmune disease 1 41. Additional factors such as infection, cytokines, and costimulatory help from T cells are required. The presence of large germinal centers in secondary lymphoid organs of BAFF-Tg mice, higher total T cell numbers in the spleen and MLN, as well as the increased proportion of both CD4 and CD8 effector T cells in the periphery, and the quality of the RF isotypes strongly suggest the active participation of T cells in the immune reactions triggered in BAFF-Tg mice. Whether the enlarged population of activated effector T cells contains autoreactive T cells remains to be determined. One may question why BAFF-Tg mice do not show more severe pathological manifestations. Potential explanations include the expression of nonlethal BAFF levels in surviving BAFF-Tg founders, the absence of either pathogenic B cells or tissue-destructing antibodies as seen in some murine models of lupus and rheumatoid arthritis, respectively 6 42 and, finally, the H-2b background of the BAFF-Tg mice, which may not favor the emergence of severe autoimmune manifestations.
If overexpression of BAFF indeed initiates an active autoimmune reaction in our transgenic mice, we need to question why tolerance to self has failed. Downregulation of Bcl-2 expression in autoreactive B cells has been shown to be one way to sensitize these cell to cell death signals 43. B cells in BAFF-Tg mice express higher levels of Bcl-2, indicating a possible protection against apoptotic signals, and also suggesting that BAFF, like CD40L, provides survival signals to B cells. One can speculate that this event coupled with a BAFF proliferative signal may explain the accumulation of autoreactive B cells in these mice. However, these changes alone are probably not sufficient to generate an autoreactive response and one potential answer may reside in the role of T cells. Increased numbers of effector T cells were directly observed in the periphery and there was an apparent reduction in the numbers of dendritic cells in the spleen of BAFF-Tg mice. Dendritic cells are believed to be essential for T cell tolerance to self 44, and their deficit in BAFF-Tg mice may promote the emergence of autoreactive T cells. Thus, we hypothesize that the role of BAFF overexpression in impairing self-tolerance may rely on two mechanisms: promoting enhanced survival and proliferation of activated autoreactive B cells and suppression of the protective effects of dendritic cells against the emergence of autoreactive T cells.
These experiments demonstrate that ectopic overexpression of BAFF was sufficient to initiate the expansion of the mature B cell compartment, resulting in lupus-like autoimmune manifestations. This transgenic mouse model potentially brings new insight into the etiology of autoimmune disorders, provides a novel framework for the investigation of autoreactivity, and potentially opens the door to new therapeutic strategies both for the treatment of some autoimmune disorders and the stimulation of humoral responses.
Acknowledgments
We thank Beth Atanian for maintaining the colony of BAFF-Tg mice, Jennifer Beane and Sukumari Mohan for technical assistance, and Susan Kalled, Paula Hochman, Linda Burkly, Paul Rennert, and Chris Benjamin for critical reading of the manuscript. We also thank Ann Marshak-Rothstein and Ron Corley for useful advice.
Footnotes
Abbreviations used in this paper: AP, alkaline phosphatase; BAFF, B cell activating factor belonging to the TNF family; BM, bone marrow; BrdU, 5-bromo-2′-deoxyuridine; CIC, circulating immune complexes; ds, double stranded; HRP, horseradish peroxidase; MLN, mesenteric lymph node; MZ, marginal zone; ss, single stranded; Tg, transgenic.
References
- Goodnow C.C., Cyster J.G., Hartley S.B., Bell S.E., Cooke M.P., Healy J.I., Akkaraju S., Rathmell J.C., Pogue S.L., Shokat K.P. Self-tolerance check points in B cell development. Adv. Immunol. 1995;59:279–369. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- Miller J.F., Basten A. Mechanisms of tolerance to self. Curr. Opin. Immunol. 1996;8:815–821. doi: 10.1016/s0952-7915(96)80010-0. [DOI] [PubMed] [Google Scholar]
- McDevitt H.O., Wakeland E.K. Autoimmunity. Curr. Opin. Immunol. 1998;10:647–648. doi: 10.1016/s0952-7915(98)80083-6. [DOI] [PubMed] [Google Scholar]
- Rose N.R. The role of infection in the pathogenesis of autoimmune disease. Semin. Immunol. 1998;10:5–13. doi: 10.1006/smim.1997.0100. [DOI] [PubMed] [Google Scholar]
- Fox D. The role of T cells in the immunopathogenesis of rheumatoid arthritis. Arthritis Rheum. 1997;40:598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- Korganow A.S., Ji H., Mangialiao S., Duchatelle V., Pelanda R., Martin T., Degott G., Kikutani H., Rajewski K., Pasquali J.L. From systemic T cell reactivity to organ-specific autoimmune disease via immunoglobulin. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- Elson C.E., Sartor R.B., Tennyson G.S., Riddel R.H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Dohi T., Fujihashi K., Rennert P., Iwatani K., Kiyono H., McGhee J.R. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J. Exp. Med. 1999;189:1169–1179. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant M., Fuss I.J., Chu A., Strober W. Oxazolone colitisa murine model of T helper cell type 2 colitis treatable with antibodies to interleukin-4. J. Exp. Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert P.D., Browning J.L., Mebius R., Mackay F., Hochman P.S. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravestein L.A., Borst J. Tumor necrosis factor receptor family members in the immune system. Semin. Immunol. 1998;10:423–434. doi: 10.1006/smim.1998.0144. [DOI] [PubMed] [Google Scholar]
- Wang J., Lenardo M.J. Molecules involved in cell death and peripheral tolerance. Curr. Opin. Immunol. 1997;9:818–825. doi: 10.1016/s0952-7915(97)80184-7. [DOI] [PubMed] [Google Scholar]
- Moulian N., Berrih-Aknin S. Fas/APO-1/CD95 in health and autoimmune diseasethymic and peripheral aspects. Semin. Immunol. 1998;10:449–456. doi: 10.1006/smim.1998.0155. [DOI] [PubMed] [Google Scholar]
- Kurts C., Carbone F.R., Krummel M.F., Koch K.M., Miller J.F.A.P., Heath W.R. Signaling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature. 1999;398:341–344. doi: 10.1038/18692. [DOI] [PubMed] [Google Scholar]
- Cope A.P. Regulation of autoimmunity by proinflammatory cytokines. Curr. Opin. Immunol. 1998;10:669–676. doi: 10.1016/s0952-7915(98)80087-3. [DOI] [PubMed] [Google Scholar]
- Weinberg A.D., Vella A.T., Croft M. OX-40life beyond the effector T cell stage. Semin. Immunol. 1998;10:471–480. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- Brugnoni D., Airo P., Marino R., Notarangelo L.D., van Lier R.A., Attaneo R. CD70 expression on T-cell subpopulationsstudy of normal individuals and patients with chronic immune activation. Immunol. Lett. 1997;55:99–104. doi: 10.1016/s0165-2478(96)02693-4. [DOI] [PubMed] [Google Scholar]
- Mackay F., Browning J.L., Lawton P., Shah S.A., Comiskey M., Bhan A.K., Mizoguchi E., Terhorst C., Simpson S.J. Both lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology. 1998;115:1464–1475. doi: 10.1016/s0016-5085(98)70025-3. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Bazan F., Blanchard D., Briere F., Galizzi J.P., van Kooten C., Liu Y.J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu. Rev. Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- van Kooten C., Banchereau J. Function of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- Kalled S.L., Cutler A.H., Datta S.K., Thomas D.W. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritispreservation of kidney function. J. Immunol. 1998;160:2158–2165. [PubMed] [Google Scholar]
- Vogel L.A., Noelle R.J. CD40 and its crucial role as a member of the TNFR family. Semin. Immunol. 1998;10:435–442. doi: 10.1006/smim.1998.0145. [DOI] [PubMed] [Google Scholar]
- Schneider P., Mackay F., Steiner V., Hofmann K., Bodmer J.L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H. BAFF, a novel ligand of the tumor necrosis factor (TNF) family, stimulates B-cell growth. J. Exp. Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H.-B., Hu W.-H., Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J. Leukoc. Biol. 1999;65:680–683. [PubMed] [Google Scholar]
- Mukhopadhyay A., Ni J., Zhai Y., Yu G.-L., Aggarwal B.B. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-κB, and c-jun NH2-terminal kinase. J. Biol. Chem. 1999;274:15978–15981. doi: 10.1074/jbc.274.23.15978. [DOI] [PubMed] [Google Scholar]
- Moore P.A., Belvedere O., Orr A., Pieri K., LaFleur D.W., Feng P., Soppet D., Charters M., Gentz R., Parmelee D. BlySmember of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- Mcknights G.S., Hammer R.E., Kuenzel E.A., Brinster R.L. Expression of chicken transferrin gene in transgenic mice. Cell. 1983;34:335–341. doi: 10.1016/0092-8674(83)90368-9. [DOI] [PubMed] [Google Scholar]
- Browning J.L., Dougas Sizing I., Lawton P., Bourdon P.R., Rennert P.D., Majeau J.R., Ambrose C.M., Hession C., Miatkowski K., Griffith D.A. Characterization of lymphotoxin-αβ complexes on the surface of mouse lymphocytes. J. Immunol. 1997;159:3288–3298. [PubMed] [Google Scholar]
- Singh V.K., Tingle A.J. Detection of circulating immune complexes by a C1q-microplate ELISA system. J. Immunol. Methods. 1982;50:109–114. doi: 10.1016/0022-1759(82)90308-8. [DOI] [PubMed] [Google Scholar]
- June C.H., Contreras C.E., Perrin L.H., Lambert P.H. Improved detection of immune complexes in human and mouse serum using a microassay adaptation of the C1q binding test. J. Immunol. Methods. 1979;31:23–29. doi: 10.1016/0022-1759(79)90282-5. [DOI] [PubMed] [Google Scholar]
- Datta S.K., Patel H., Berry D. Induction of a cationic shift in IgG anti–DNA autoantibodiesrole of helper T cells with classical and novel phenotypes in three murine models of lupus nephritis. J. Exp. Med. 1987;165:1252–1261. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Majeau G.R., Lawton P., Hochman P.S., Browning J.L. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur. J. Immunol. 1997;27:2033–2042. doi: 10.1002/eji.1830270830. [DOI] [PubMed] [Google Scholar]
- Oliver A.M., Martin F., Gartland G.L., Carter R.H., Kearney J.F. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory molecules. Eur. J. Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- Jefferis R. Rheumatoid factors, B cells and immunoglobulin genes. Br. Med. Bull. 1995;51:312–331. doi: 10.1093/oxfordjournals.bmb.a072963. [DOI] [PubMed] [Google Scholar]
- Chicheportiche Y., Bourdon P.R., Xu H., Hsu Y.M., Scott H., Hession C., Garcia I., Browning J.L. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- Hahne M., Kataoka T., Schroter M., Hofmann K., Irmler M., Bodmer J.L., Schneider P., Bornand T., Holler N., French L. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor growth. J. Exp. Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C.H., Rulffes J.T., Haugen H.S., Hoggatt I.H., Aruffo A., Durham S.K., Farr A.G., Hollenbaugh D. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int. Immunol. 1996;9:1111–1122. doi: 10.1093/intimm/9.8.1111. [DOI] [PubMed] [Google Scholar]
- Hollander G.A., Simpson S.J., Mizoguchi E., Nichogiannopoulou A., She S., Gutierrez-Ramos J.-C., Bhan A., Burakoff S.J., Wang B., Terhorst C. Severe colitis resulting from aberrant thymic selection. Immunity. 1995;3:27–38. doi: 10.1016/1074-7613(95)90156-6. [DOI] [PubMed] [Google Scholar]
- Kraal G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Oldfield S., MacLennan I.C. Memory B cells in T cell-dependent antibody responses colonize the splenic marginal zones. Eur. J. Immunol. 1988;18:355–362. doi: 10.1002/eji.1830180306. [DOI] [PubMed] [Google Scholar]
- Fang W., Weintraub B.C., Dunlap B., Garside P., Pape K.A., Jenkins M.K., Goodnow C.C., Mueller D.L., Behrens T.W. Self-reactive B lymphocytes overexpressing Bcl-XL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity. 1998;9:35–45. doi: 10.1016/s1074-7613(00)80586-5. [DOI] [PubMed] [Google Scholar]
- Chan O.T.M., Hannum L.G., Haberman A.M., Madaio M.P., Schlomchik M.J. A novel mouse with B cells lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 1999;189:1639–1647. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D.T., Korsmeyer S.J. BCL-2 familyregulators of cell death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]