Abstract
Group B Streptococcus (GBS) is a major cause of newborn sepsis and meningitis and induces systemic release of tumor necrosis factor alpha (TNF-α), believed to play a role in morbidity and mortality. While previous studies have shown that GBS can induce TNF-α release from monocytes and macrophages, little is known about the potential modulating effect of plasma or serum on GBS-induced TNF-α release, and there are conflicting reports as to the host receptors involved. In a human whole-blood assay system, GBS type III COH-1 potently induced substantial monocyte TNF-α release in adult peripheral blood and, due to a higher concentration of monocytes, 10-fold-greater TNF-α release in newborn cord blood. Remarkably, GBS-induced TNF-α release from human monocytes was enhanced ∼1,000-fold by heat-labile serum components. Experiments employing C2-, C3-, or C7-depleted serum demonstrated that C3 activation via the alternative pathway is crucial for potent GBS-induced TNF-α release. Accordingly, whole blood from C3-deficient mice demonstrated significantly reduced GBS-induced TNF-α release. Preincubation with human serum enhanced the TNF-α-inducing activity of GBS in a C3- and factor B-dependent manner, implying deposition of complement components via the alternative pathway. GBS-induced TNF-α release was inhibited by monoclonal antibodies directed against each of the components of CR3 and CR4: the common integrin β subunit CD18 and the α subunits CD11b (of CR3) and CD11c (of CR4). Blood derived from CR3 (CD11b/CD18)-deficient mice demonstrated a markedly diminished TNF-α response to GBS. We conclude that the ability of plasma and serum to greatly amplify GBS-induced TNF-α release reflects the activity of the alternative complement pathway that deposits fragments on GBS and thereby enhances CR3- and CR4-mediated monocyte activation.
Group B Streptococcus (Streptococcus agalactiae, or GBS) is a major cause of neonatal sepsis and meningitis leading to significant morbidity and mortality (39). GBS also infects pregnant and parturient women and is a growing cause of infections in nonpregnant adults with underlying chronic illness (18). Both acquired and innate immunity are important to host defense against GBS. The role of antibody in facilitating opsonophagocytosis is well described (28). In addition, the complement system contributes to opsonophagocytosis of GBS in both the presence and the absence of specific antibodies (5, 50). However, the role of complement activation with respect to GBS-induced cytokine release has not been defined. Innate immune responses of newborns to GBS infection are also believed to include receptor-mediated recognition of this pathogen by host leukocytes, resulting in the release of inflammatory mediators, including tumor necrosis factor alpha (TNF-α), into the systemic circulation (30).
Several lines of evidence suggest that GBS-induced TNF-α release is relevant to the pathophysiology of neonatal sepsis. Isolates of GBS that cause sepsis in human newborns have a relatively high inflammatory potential as reflected by enhanced induction of cytokines, including TNF-α, in vitro (6). Moreover, human newborns infected with GBS have been shown to release TNF-α into the systemic circulation (51). In a rat model of GBS infection, TNF-α release was greater in newborn than in adult rats, the extent of TNF-α release correlated with mortality, and an antibody to TNF-α was protective against early death (43). Despite the growing appreciation of the importance of GBS-induced TNF-α release to the pathophysiology of infection, the mechanisms by which this agent of neonatal bacteremia induces TNF-α release in whole blood have not yet been defined.
Human plasma and serum greatly potentiate the inflammatory activity of gram-negative bacteria via lipopolysaccharide (LPS)-binding protein-mediated delivery of LPS to the monocyte LPS receptor complex (CD14/MD2/TLR4) (45). Less is known of the mechanisms by which soluble host components might amplify responses to gram-positive bacteria. Studies of monocytes in culture have demonstrated that heat-labile factors in serum potentiate the ability of gram-positive bacteria to induce cytokine release (15, 24). Addition of fibronectin (34) or LPS-binding protein (32) to cell culture media can modestly enhance GBS-induced TNF-α release from monocytes. However, the extent to which either of these factors may account for the enhancing effect of human serum and whether these mechanisms are operative in whole blood are unknown.
GBS is thought to induce TNF-α synthesis via engagement of leukocyte innate immune receptors and subsequent intracellular signaling, resulting in activation of the transcription factors NF-κB and activator protein 1 (48). Several innate immune receptors are candidates for host cell recognition of GBS, but the literature in support of a role for one or another innate immune receptor has been conflicting. In addition, to our knowledge, none of these studies have addressed the potential role of plasma or serum in modulating GBS-induced inflammatory responses.
CD14 is a glycosylphosphatidylinositol-linked surface receptor expressed abundantly on monocytes that has affinity for certain microbial surface components, including LPS, peptidoglycan, and lipoteichoic acids (37, 45). CD14 functions as a coreceptor with other pattern recognition receptors such as Toll-like receptors (TLRs) for a number of microbial products. Some studies of GBS activation of monocytes in vitro suggested a role for CD14 in triggering the release of TNF-α in response to whole GBS particles (32) or GBS-derived cell wall components (16), whereas others have suggested that CD14 is not required for cell activation by gram-positive bacteria, including GBS (7, 14).
The TLRs are another family of receptors that may be involved in responses to GBS (1, 25, 33). Although TLR2 is involved in the detection of gram-positive bacterial cell wall components (e.g., lipoteichoic acid and peptidoglycan) (40) as well as whole gram-positive bacteria such as Listeria monocytogenes (20) and Staphylococcus aureus (46), it does not appear to be involved in activation of monocytes by whole heat-killed GBS (20). However, GBS apparently elaborates a heat-labile soluble factor capable of activating monocytes in a TLR2- and CD14-dependent fashion (23). Thus, TLR2 may participate in host recognition of GBS in vivo.
Among the receptors proposed to contribute to inflammatory signaling by GBS are the leukocyte β-integrins. β-Integrins are heterodimeric surface receptors that bind microbe- and host-derived ligands and contribute to both phagocytosis of microbial particles and microbe-induced leukocyte signaling (17). The C-terminal lectin domain of CR3 can bind directly to microbial surface constituents including LPS, mycobacterial polysaccharides, and fungal β-glucan (52). Another mechanism by which integrin receptors can bind microbial particles occurs when microbes are opsonized by complement, in particular the opsonic fragment C3bi, and subsequently bind to the A (or I) domain of CD11b/CD18 (CR3). In addition to CR3, monocytes also express CR4 (CD11c/CD18), an integrin receptor that binds many of the same ligands as CR3 does. Overall, little is known of the potential role of CR4 in bacterial activation of host cells.
Several studies have suggested a role for CD11b/CD18 in mediating cytokine induction in cultured cells in response to heat-killed GBS in vitro (15, 16, 32). However, more recent studies have suggested that TLRs (and CD14) may be central to GBS-induced activation and that CR3, although important for phagocytosis of GBS, does not contribute to GBS-induced cytokine release (22, 23). Among studies that support a role for CR3 in GBS-induced cytokine release, some employing serum-free conditions demonstrate lectin-like interactions between GBS and CR3 (2) that can trigger cytokine release (3). Such studies raise the possibility that host-derived soluble extracellular components do not play a role in the activation of monocytes by GBS. To our knowledge, however, none of these studies have directly addressed the potential role of plasma or serum in modulating GBS-induced TNF-α release.
The immature immune system of newborns manifests impaired production and priming of neutrophils, relatively lower complement activity, decreased production of antibodies, and a high percentage of naïve T lymphocytes (38). Studies comparing the relative capacities of adult and newborn peripheral blood mononuclear cells (PBMCs) or adherent monocytes to mount cytokine responses to microbes or their surface components have yielded conflicting results (7, 51). As GBS is an important cause of neonatal bacteremia (39) and because TNF-α released upon bloodstream infection with GBS appears to play a significant role in clinical outcomes of GBS infection (6, 43, 51), we sought to define host determinants of TNF-α induction in newborn and adult whole blood and from blood-derived monocytes. We have found that both newborn and adult plasma or serum dramatically enhance GBS-induced TNF-α release from human monocytes. Studies employing human serum depleted of specific complement components and mice deficient in complement component C3 demonstrated the critical role of the alternative complement pathway in such potentiation. Experiments employing neutralizing monoclonal antibodies (MAbs) and mice deficient in complement receptor CR3 demonstrated the importance of CR3 and CR4. These studies provide evidence that the alternative complement pathway accounts for the ability of plasma and serum to markedly enhance the inflammatory activity of GBS via deposition of complement fragments and subsequent activation of monocytes via CR3 and CR4.
MATERIALS AND METHODS
Bacterial strains.
The clinical isolate GBS type III COH-1 was stored as frozen stocks (−80°C). Prior to each experiment, GBS was cultured overnight on 5% sheep's blood agar (tryptic soy agar II; BBL Becton Dickinson). Three colonies were picked, subcultured in 10 ml of Todd-Hewitt broth (Difco), and grown to mid-exponential phase (A650 of ∼0.25). Bacteria were collected by centrifugation and resuspended to the desired concentration in minimal essential medium (MEM; Gibco BRL) immediately prior to assay. To prepare heat-killed (HK) bacteria, subcultures were washed twice in sterile, pyrogen-free saline, resuspended in saline, and incubated at 80°C for 60 min. Lack of viability of HK bacteria was verified by subculture. The HK bacteria were washed twice with sterile saline before storage at −80°C.
Blood.
Peripheral blood was collected from adult volunteers (mean age, 27 years), and newborn cord blood (mean gestational age, 39 weeks) was collected from placentas immediately after cesarean section delivery. Those births at which antibiotics were administered during labor and/or delivery were excluded. Blood was anticoagulated with 129 mM sodium citrate (Becton Dickinson, Franklin Lakes, N.J.) or with a recombinant form of the thrombin-inactivating peptide hirudin (HV1 peptide; Calbiochem) used at a final concentration of 50 U/ml. For some experiments, 2 ml of blood was also collected into sterile tubes containing K2-EDTA (Vacutainer; Becton Dickinson) in order to obtain an automated total white blood cell count and differential (Technion H3 RTX automated cell counter; Miles, Tarrytown, N.Y.). All blood collections were in accordance with protocols approved by the Brigham and Women's Hospital Institutional Review Board.
Murine peripheral blood was derived from tail bleeds of age (∼7 weeks)- and sex (male)-matched control C57BL/6 mice or from C3-deficient (C3−/−) mice, whose derivation has been previously described (50). For analysis of CR3-deficient mice, whose derivation was previously described (9), mice were sedated by intraperitoneal injection of ketamine and xylazine and subsequently bled via intracardiac puncture. Mouse blood was collected into sterile tubes containing 129 mM citrate buffer and kept on ice before use in the GBS-induced TNF-α release assay. Absence of C3 in the blood of C3-deficient mice was verified by serum enzyme-linked immunosorbent assay (ELISA). Handling and bleeding of mice were in accordance with the guidelines of the Harvard Medical Area Standing Committee on Animals.
Bacterial growth assay.
Survival and growth of GBS strains in whole blood were measured in a 100-μl microassay format: 60 μl of blood, 5 to 25 μl of antibody (for experiments in which blocking MAbs were added), MEM (as needed to maintain constant volumes), and 10 μl of bacteria, added last. Bacteria were added to a final concentration of 102 to 105 bacteria per ml. Tubes were incubated at 37°C with end-over-end mixing for 5 h. At the indicated times (15, 60, 180, or 300 min), 10 μl of a sample inoculated with 104 bacteria/ml (or 10 μl of the sample diluted 100-fold in MEM) was plated onto blood agar plates and grown overnight before quantitation of CFU.
Monocyte isolation.
Heparinized blood was layered onto Ficoll-Hypaque gradients, the PBMC layer was collected, and the PBMC fraction was subjected to hypotonic lysis to remove red blood cells. For studies of monocytes in suspension, monocytes were isolated from PBMCs by using magnetic microbeads coupled to an anti-CD14 MAb according to the instructions of the manufacturer (Miltenyi Biotec). Monocyte suspensions were prepared in 10% autologous serum-MEM prior to addition of HK-GBS. For experiments employing adherent monocytes, PBMCs were incubated in 24-well plates, and monocytes were allowed to adhere during a 1-h incubation at 37°C. Nonadherent cells were removed by two washes with MEM. To measure the number of monocytes per well, monocytes were incubated with trypsin-EDTA for 5 min and then scraped off with the plunger of a tuberculin syringe. Monocyte viability was monitored by trypan blue exclusion.
Measurement of TNF-α.
After incubation of GBS in blood or with isolated monocytes for 5 h (as described above), samples were diluted with 4 volumes of ice-cold RPMI medium (Gibco BRL) and centrifuged at 1,000 × g at 4°C for 5 min. The extracellular medium was recovered and stored at −20°C. Human and murine TNF-α was assayed by ELISA according to the instructions of the manufacturer (R & D Systems, Minneapolis, Minn.). For analysis of TNF-α release in experiments employing sera depleted of specific complement components or in the presence of blocking antibodies (see below), TNF-α release was expressed as a percentage, relative to the bacterial dose-response curve, of that found under normal conditions (i.e., repleted sera or in the absence of added antibody, respectively).
Flow cytometry of intracellular TNF-α.
HK-GBS was added to citrated blood at a final concentration of 2.5 × 106 to 5.0 × 106 bacteria/ml. In some experiments, 10 μg of brefeldin A (Sigma)/ml was added to the blood before the bacteria in order to inhibit TNF-α secretion. Blood samples were incubated at 37°C with end-over-end rotation for 4 h. For samples in which surface expression of CD14 was to be measured, phycoerythrin- or fluorescein isothiocyanate-conjugated CD14 MAb (eBiosciences, San Diego, Calif.) was added, and samples were incubated at room temperature for 30 min. After red blood cell lysis with 1× FACSLyse solution (BD Biosciences) and permeabilization with 1× FACSPerm Solution (BD Biosciences), samples were washed with 1× phosphate-buffered saline (PBS)-0.5% human serum albumin. To determine which blood leukocytes synthesize TNF-α in response to GBS, cells were stained for intracellular TNF-α according to the protocol of the manufacturer (Becton Dickinson Immunocytometry Systems). TNF-α was stained with a phycoerythrin-conjugated TNF-α MAb with murine immunoglobulin G1 (IgG1) as control. Flow cytometry was performed using a MoFlo cytometer with a 488-nm laser. Data were analyzed with Summit version 7.19 software (Cytomation, Inc.).
Of note, the relative concentrations of leukocyte populations in blood vary between donors, necessitating a correction for their relative abundance. In addition, flow cytometry provides both the percentage of a leukocyte population that is positive (e.g., the percentage of neutrophils that are TNF-α positive) and the mean fluorescence intensity (MFI) as a measure of the relative signal intensity of the positive cells (e.g., the quantity of TNF-α per positive neutrophil). Thus, the relative contribution of neutrophils, for example, to GBS-induced TNF-α release should be proportional to the percentage of total leukocytes that are neutrophils, the percentage of neutrophils that are positive for TNF-α, and the MFI per neutrophil. A relative contribution product (RCP) of these three numbers was calculated: for example, RCPneutrophils = (% of total leukocytes that are neutrophils) × (% of neutrophils that are positive) × (MFI of positive neutrophils/MFI of negative neutrophils). These data were normalized by dividing the RCP for each of the populations (i.e., neutrophils, monocytes, or lymphocytes) by RCPtotal (i.e., the RCP for the total leukocyte population was defined as 100%).
Complement analysis.
We evaluated the role of the complement system in GBS-induced TNF-α release by comparing the activity of autologous serum to that of heat-treated (56°C for 30 min) autologous serum. C2-, C3-, and C7-depleted human sera and purified human C2, C3, C7, factor B, and factor D (for repletion experiments) were obtained from Advanced Research Technologies (San Diego, Calif.). All complement-depleted sera were tested at a 10% (vol/vol) final concentration diluted in MEM. For repletion experiments, purified complement components were added back to a final concentration equivalent to that present in 10% normal serum.
Antibody blocking experiments.
To evaluate the contributions of innate immune receptors to GBS-induced TNF-α release, whole-blood assays were performed after preincubation for 1 h with specific neutralizing MAbs. For analysis of the contribution of CD18, a murine anti-human CD18 IgG1 MAb (7E4; Beckman Coulter) or, as a control, a murine IgG1 MAb to the unrelated antigen trinitrophenol (antitrinitrophenol clone 107.3; BD PharMingen) was employed. The CD11a MAb clone 38 and CD11c MAb BU15 were from Serotec. CD11b MAbs OKM-1, M1/70 [both Fc and F(ab′)2 fragment], and m60.1, an Fab fragment of a monoclonal murine IgG directed against a nonlinear epitope of the A domain of CD11b (49), were generously provided by Michele Mariscalco (Baylor College of Medicine, Houston, Tex.). CD11b MAb ICRF44 was from PharMingen. For experiments evaluating the role of TLR2, we employed a known TLR2 agonist, synthetic bacterial lipopeptide (sBLP) consisting of palmitylated N-acyl-S-diacylglyceryl cysteine (Pam3CysSerLys4; Bachem) and the neutralizing murine IgG1 MAb 2392 to human TLR2 (4) or a murine IgG1 control. To determine the possible role of CD14 in bacterium-induced TNF-α release, we employed a neutralizing mouse anti-human CD14 MAb (clone 61D3; eBiosciences) and a murine IgG1 isotypic control (clone P3; eBiosciences).
RESULTS
GBS induces TNF-α release from monocytes in whole newborn or adult blood.
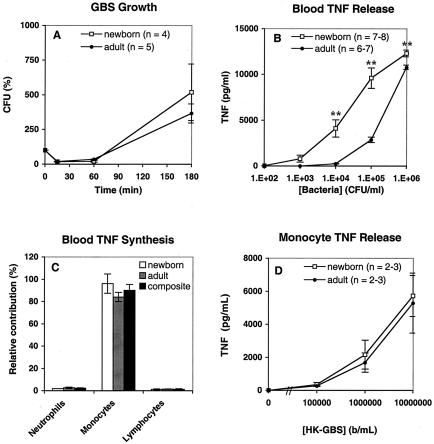
To determine the relative abilities of newborns and adults to mount a rapid inflammatory response to GBS, we compared GBS-induced TNF-α release in newborn cord and adult whole blood. The clinical isolate GBS type III COH-1 (102 to 105 bacteria/ml) was added to citrated newborn cord blood or adult blood and incubated in vitro for 5 h. Bacterial viability, measured as CFU, initially declined and then increased by 3 h in both newborn and adult blood (Fig. 1A). Analysis of the extracellular medium by ELISA at 5 h demonstrated significant TNF-α release with inocula as low as 104 bacteria/ml (Fig. 1B). Of note, despite similar growth curves (Fig. 1A), GBS was at least 10-fold more potent in inducing TNF-α release (per bacterial inoculum) in newborn than in adult blood (Fig. 1B), which suggests an inherently greater responsiveness of newborn blood to GBS. The relatively high GBS-induced TNF-α release in neonatal blood was also noted with blood samples that were anticoagulated with the thrombin-inhibitory peptide hirudin, indicating that the marked TNF-α release in neonates was independent of the means of anticoagulation (data not shown).
FIG. 1.
GBS induces TNF-α release from monocytes in newborn and adult blood. (A) Growth of GBS type III COH-1 added at 104 bacteria/ml to citrated adult blood (closed symbols) or newborn cord blood (open symbols) and incubated at 37°C. Bacterial growth was assessed by quantitative culture at the time indicated. (B) Extracellular medium was collected at 5 h, and TNF-α was measured by ELISA. TNF-α release is plotted relative to input inoculum (102 to 106 bacteria/ml). The total number of subjects studied (n) is indicated in each graph. Data points represent means ± standard errors of the means. P values were calculated for the comparison of TNF-α release in newborn and adult blood (**, P < 0.01). (C) Analysis of TNF-α-positive leukocytes derived from newborn blood (n = 2) and adult blood (n = 2) revealed that monocytes accounted for the greatest proportion of TNF-α synthesis. Differences between the relative contributions of composite monocytes and composite neutrophils or lymphocytes were significant (P < 0.001). (D) HK-GBS was added to neonatal or adult monocytes suspended to 105 bacteria/ml in 10% autologous serum. After a 5-h incubation at 37°C, the supernatants were collected for TNF-α measurement.
Relatively high GBS-induced TNF-α release corresponded to the newborn cellular (i.e., hemocyte) blood fraction rather than the humoral (plasma) fraction (unpublished data), suggesting that the robust TNF-α release of newborns is due to differences between the cellular fractions of newborn and adult blood. To define which leukocytes are responsible for GBS-induced TNF-α release in whole blood, intracellular TNF-α was assayed by flow cytometry as described in Materials and Methods. This analysis revealed that GBS-induced TNF-α synthesis in whole blood occurred predominantly in monocytes (Fig. 1C).
Enhanced TNF-α release in newborn blood compared to that in adult blood could be due to greater cellular reactivity of neonatal monocytes or to the relatively higher monocyte concentration in newborn cord blood. To distinguish these possibilities, we directly compared the abilities of newborn and adult monocytes, tested at 105 monocytes/ml in 10% autologous serum, to release TNF-α in response to HK-GBS. HK-GBS-induced TNF-α releases from neonatal and adult monocytes were indistinguishable (Fig. 1D). In addition, for a subset of the blood samples analyzed, we measured both automated differential white blood cell counts and GBS-induced TNF-α release, allowing for correction of TNF-α release per blood monocyte. This analysis indicated that, in whole blood, adult monocytes release at least as much TNF-α per cell as do newborn blood monocytes (data not shown). Overall, these results suggest that the greater GBS-induced TNF-α release in newborn cord blood (Fig. 1B) is due to the higher concentration of monocytes in newborn blood.
Human serum markedly enhances the TNF-α-inducing activity of GBS.
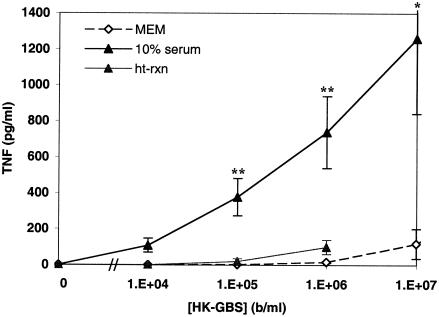
GBS-induced TNF-α release from adult peripheral blood- or newborn cord blood-derived hemocytes that were extensively washed with Hanks balanced salt solution was dependent on the presence of serum (unpublished data). In order to determine whether the crucial role of extracellular components noted with washed hemocytes was also manifest with isolated monocytes, HK-GBS was added to human monocytes in the presence of MEM, autologous serum, or heat-treated autologous serum. Remarkably, addition of autologous serum resulted in an ∼1,000-fold enhancement (relative to the bacterial dose curve) of HK-GBS-induced TNF-α release from both newborn and adult monocytes (Fig. 2). The enhancing effect of serum was greatly diminished by heat treatment, raising the possibility that the complement system participates in this enhancement.
FIG. 2.
Heat-labile serum components potentiate GBS-induced TNF-α release from human monocytes in culture. PBMC-derived monocytes were cultured in MEM, 10% fresh autologous serum (in MEM), or 10% heat-treated autologous serum, after which HK-GBS was added at the indicated concentrations. TNF-α release was measured by ELISA. Similar results were obtained with newborn cord blood- and adult peripheral blood-derived monocytes, and data were pooled. Data represent the means ± standard errors of the means of 2 to 22 independent determinations. Differences between serum and MEM (*, P < 0.05; **, P < 0.01) as well as differences between serum and heat-treated serum (P < 0.01) were significant.
Serum enhancement of GBS-induced TNF-α release from monocytes depends on the presence of C3, but not C2 or C7.
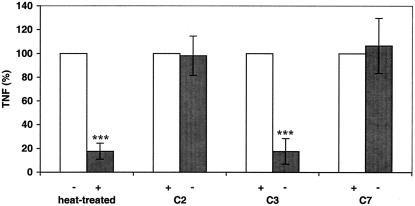
To evaluate the potential role of complement in serum enhancement of GBS-induced TNF-α release, HK-GBS was added to human monocytes cultured in autologous serum, heat-treated autologous serum, or serum depleted of (or repleted with) C2, C3, or C7. Similar results were obtained with newborn and adult monocytes, and the results were pooled (Fig. 3). Heat treatment and depletion of C3, but not of C2 or C7, significantly reduced HK-GBS-induced TNF-α release. Similar results were obtained with live GBS (L-GBS) (data not shown). These results implicate C3 as essential to GBS-induced TNF-α release and suggest that activation can proceed via the alternative complement pathway since C2 is not required.
FIG. 3.
Heat treatment or depletion of C3, but not of C2 or C7, reduces the ability of human serum to potentiate GBS-induced TNF-α release from human monocytes. HK-GBS (106 bacteria/ml) was added to cultured monocytes in the presence of 10% heat-treated autologous serum or 10% C2-, C3-, or C7-depleted heterologous pooled serum. After a 5-h incubation, the extracellular medium was harvested for TNF-α measurement. The data are presented as pairwise comparisons to the “normal” or repleted condition that is normalized to 100% TNF-α release. Similar results were obtained with newborn and adult monocytes, and the data were pooled (n = 3 to 9). Differences between normal serum and heat-treated serum and between C3-depleted and C3-repleted serum were significant (***, P < 0.001).
Blood from C3-deficient mice demonstrates reduced TNF-α release in response to GBS.
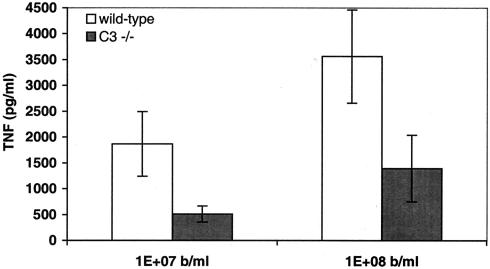
To verify the role of complement component C3 in GBS-induced TNF-α release in whole blood, we compared GBS-induced TNF-α release in whole blood derived from C3-deficient mice and sex- and age-matched C57BL/6 controls. In agreement with the important role of C3 in GBS-induced TNF-α release from human monocytes in culture (Fig. 3), blood derived from C3-deficient mice demonstrated a marked impairment in GBS-induced TNF-α release (Fig. 4).
FIG. 4.
Whole blood from mice deficient in complement component C3 exhibits reduced GBS-induced TNF-α release. Peripheral blood of healthy sex- and age-matched control C57BL/6 mice or C3-deficient mice was incubated with the indicated concentrations of HK-GBS for 5 h before collection of the extracellular fluid for measurement of murine TNF-α by ELISA. Background TNF-α release in control (i.e., no GBS) samples was subtracted to obtain GBS-induced TNF-α release. Data represent the means ± standard errors of the means (n = 6). Differences between wild-type and C3−/− mice were significant (P < 0.05).
Preincubation of GBS with serum enhances TNF-α induction in a C3- and factor B-dependent fashion.
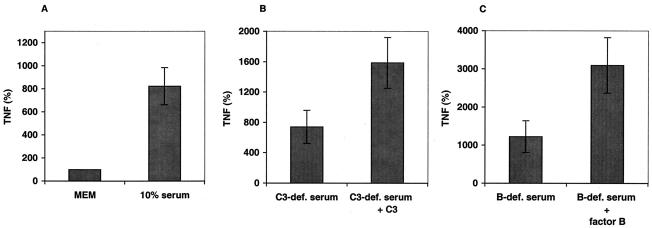
Complement activation results in both deposition of opsonic factors onto bacterial surfaces and generation of soluble inflammatory mediators known to induce and/or prime for TNF-α release (i.e., the anaphylotoxins C3a and C5a). To distinguish between these mechanisms, we preincubated HK-GBS for 1 h in medium alone (MEM), MEM supplemented with 10% autologous serum, 10% C3-deficient serum with or without C3, or 10% factor B-deficient serum with or without factor B (a key component of the alternative complement pathway). After this preopsonization, bacteria were washed three times with PBS to remove unbound components before addition to cultured monocytes to measure TNF-α release. Data are expressed in relation to a dose curve of control bacteria that were preincubated with medium (MEM) alone, defined as 100% TNF-α release (Fig. 5). Preincubation of GBS with 10% serum greatly enhanced its TNF-α-inducing activity, suggesting that serum components deposited on GBS enhance its inflammatory potential. The potentiating activity of serum was C3 and factor B dependent, implicating deposition of complement components through the alternative pathway as a mechanism by which complement enhances GBS-induced TNF-α release from human monocytes.
FIG. 5.
TNF-α-inducing activity of GBS is markedly enhanced by preexposure to serum in a C3- and factor B-dependent manner. HK-GBS was preincubated for 1 h at 37°C in MEM alone (MEM) or MEM supplemented with 10% serum (A), 10% C3-depleted serum with or without repletion of C3 (B), or 10% factor B-depleted serum with or without repletion of factor B (C). Purified complement components were added to final concentrations found in 10% serum. After preincubation, bacteria were washed three times with PBS and added to cultured monocytes at a concentration of 106 or 107 bacteria per ml. After a 5-h incubation, the extracellular medium was harvested for TNF-α measurement. Data are normalized as percentages of the TNF-α-inducing activity of HK-GBS incubated in MEM alone (defined as 100%) and represent the means ± standard errors of the means. Similar results were obtained with newborn and adult monocytes, and the data were pooled (n = 6 to 15). Differences between MEM and 10% serum (P < 0.01), C3-depleted serum and C3-repleted serum (P < 0.05), and factor B-depleted and factor B-repleted serum (P < 0.05) were significant.
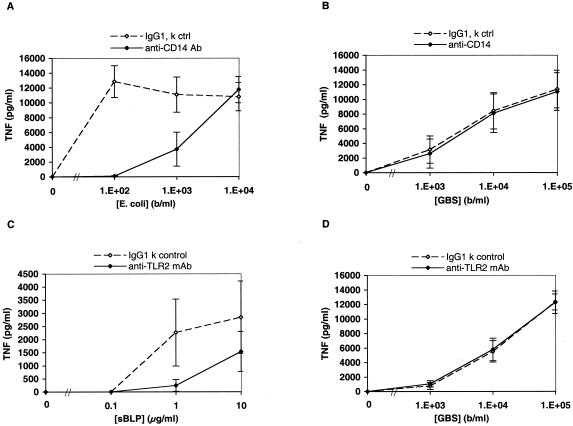
GBS-induced TNF-α release in whole blood is apparently CD14 and TLR2 independent.
In further experiments, we attempted to define the roles of monocyte innate immune receptors that may be activated upon addition of GBS to blood. As some studies have suggested a role for CD14 and TLR2 in host responses to GBS (23), we evaluated the effect of neutralizing antibodies to these innate immune receptors in the whole-blood assay system. To assess the possible role of CD14 in GBS-induced TNF-α release, whole blood was preincubated with a neutralizing MAb to human CD14. Although the antibody substantially inhibited TNF-α induction by live Escherichia coli K1/r (Fig. 6A), it had no effect on TNF-α induction by L-GBS type III COH-1 (Fig. 6B). To assess the potential contribution of TLR2 to GBS-induced TNF-α release, we employed MAb 2392, a neutralizing murine IgG1 against human TLR2. As a positive control, we tested the ability of this antibody to inhibit TNF-α induction by an sBLP known to signal via TLR2 (4). The TLR2 MAb markedly inhibited sBLP-induced TNF-α release in whole blood (Fig. 6C). However, this antibody had no effect on L-GBS-induced TNF-α release (Fig. 6D).
FIG. 6.
Neutralizing MAbs to CD14 and TLR2 inhibit ligand-induced TNF-α release in whole blood but do not inhibit GBS-induced TNF-α release. The figure shows the effect of preincubation of newborn or adult whole blood (pooled data) with an isotype control antibody or a neutralizing MAb to CD14 on TNF-α release induced by live E. coli K1/r (A) or GBS type III COH-1 (B). Differences in the effects of the CD14 MAb between E. coli and GBS were significant (n = 4 to 8; P < 0.01). To evaluate TLR2, newborn or adult blood was preincubated with 150 μg of an isotype control MAb or the neutralizing TLR2 MAb 2392 per ml, after which the known TLR2 agonist sBLP (C) or L-GBS (D) was added. Differences in the effects of the TLR2 MAb between sBLP and GBS were significant (n = 4, P < 0.001).
GBS-induced TNF-α release is mediated via CR3 (CD11b/CD18) and CR4 (CD11c/CD18).
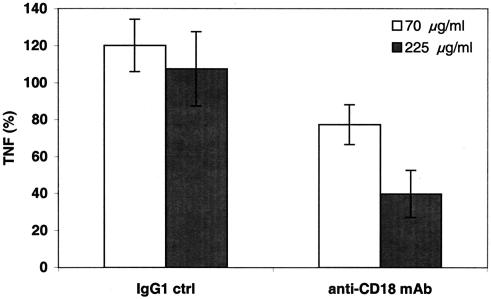
CD18-containing β-integrins are known to bind particles that have been opsonized by complement component C3bi (17). To investigate the potential participation of the leukocyte β2-integrin CD18 in induction of TNF-α release by GBS, we assessed the effect of a blocking anti-human CD18 murine IgG1 MAb (clone 7E4; Beckman Coulter) and isotype control on GBS-induced TNF-α release. The CD18 MAb exerted a marked dose-dependent inhibitory effect on GBS-induced TNF-α release, reducing it by as much as 60% (Fig. 7). Control experiments confirmed that this CD18 MAb did not inhibit bacterial viability and that its inhibitory activity was also manifest towards HK-GBS-induced TNF-α release (data not shown), indicating that the inhibitory effect on GBS-induced TNF-α release is likely to be mediated via blocking CD18 on host leukocytes.
FIG. 7.
A neutralizing CD18 MAb inhibits GBS-induced TNF-α release. Newborn or adult blood was preincubated for 1 h with either a control murine IgG1 MAb to an unrelated antigen (antitrinitrophenol) or a neutralizing murine IgG1 MAb to CD18 before addition of L-GBS type III COH-1 (104 bacteria/ml). TNF-α release was measured at 5 h by ELISA and is expressed as a percentage of control. Data represent the means ± standard errors of the means (n = 2 to 4). Differences between control samples and those containing 225 μg of CD18 MAb/ml were significant (P < 0.05).
The β-integrin CD18 is known to heterodimerize with one of several α chains to form distinct integrin receptors: CD11a/CD18 (LFA-1), CD11b/CD18 (CR3), or CD11c/CD18 (CR4). To determine which of these CD18-containing heterodimeric receptors may participate in GBS-induced TNF-α release, we tested an array of MAbs directed against CD11a, CD11b, or CD11c for inhibitory activity in the whole-blood assay (Table 1). Of the antibodies tested, two mediated significant inhibition. An F(ab′)2 fragment (m60.1) directed against a nonlinear epitope of the A domain of the α-integrin CD11b (31) resulted in 22% ± 6% inhibition (P < 0.01) of L-GBS-induced TNF-α release and a 20% ± 5% inhibition (P < 0.001) of HK-GBS-induced TNF-α induction (Table 1). A neutralizing MAb to human CD11c (clone BU15; Serotec) markedly inhibited both L-GBS- and HK-GBS-induced TNF-α release (94% ± 4% [P < 0.001] and 71% ± 11% [P < 0.01], respectively; Table 1). The ability of the CD11c antibody to markedly inhibit GBS-induced TNF-α release was also confirmed with isolated human monocytes in culture (unpublished data).
TABLE 1.
Effects of MAbs to CD11 on GBS-induced TNF-α releasea
| CD11 molecule | MAb clone | % Inhibition (P)
|
|
|---|---|---|---|
| L-GBS | HK-GBS | ||
| CD11c | BU15 | 94 ± 4 (0.00007) | 71 ± 11 (0.004) |
| CD11b | m60.1 | 22 ± 6 (0.01) | 20 ± 5 (0.001) |
| ICRF44 | None | None | |
| OKM-1 | None | None | |
| M1/70 (Fc) | None | None | |
| M1/70 [F(ab′)2] | None | None | |
| CD11a | Clone 38 | None | None |
MAbs were tested in whole human blood at final concentrations ranging from ∼100 to 500 μg/ml. Data represent maximal inhibition of L-GBS- # or HK-GBS-induced TNF-α release obtained with anti-CD11c clone BU15 and anti-CD11b m60.1 at 250 μg/ml. For each anti-CD11 MAb evaluated, an isotype control antibody was tested at an identical concentration and had no inhibitory effect. Experiments with newborn and adult blood yielded similar results, and the data were pooled.
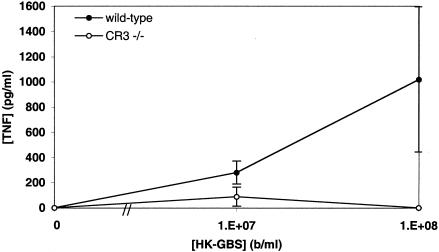
To further investigate the importance of CD18-based integrins to GBS-induced TNF-α release in whole blood, we measured GBS-induced TNF-α release in blood from mice deficient in the complement receptor CR3. CR3-deficient mice released significantly less TNF-α in response to HK-GBS than did age- and sex-matched control mice (Fig. 8). Taken together with the CD18, CD11b, and CD11c antibody blocking results described above, these data indicate an important role for CD11b/CD18 (CR3) and CD11c/CD18 (CR4) in GBS-induced TNF-α release in whole human and murine blood.
FIG. 8.
Blood derived from mice deficient in the complement receptor CR3 demonstrates markedly reduced GBS-induced TNF-α release. Blood from CR3-deficient or age- and sex-matched control C57/129 mice was incubated with HK-GBS at 107 or 108 bacteria/ml before measurement of TNF-α by ELISA. Data represent the means ± standard errors of the means (n = 4 to 6). Differences between CR3-deficient and control mice were significant (P < 0.05, Fisher's exact test).
DISCUSSION
Several studies have demonstrated the ability of GBS to induce cytokine release from monocytes or macrophages cultured in vitro (6, 23, 27, 47). Such studies, often employing phagocytes cultured in serum-free medium, heat-treated serum, or heterologous serum (e.g., fetal bovine serum with murine macrophages), have identified candidate host receptors that may be involved in GBS recognition (2, 23, 32) as well as intracellular signaling pathways that are subsequently activated (3, 48). However, to our knowledge, the potential modulatory effect of plasma or serum on GBS-induced cytokine release has not been previously addressed. In addition, controversy exists as to the relevant host receptors involved in GBS-induced cytokine release. Some studies suggest that lectin-like interactions between GBS and CR3, which occur in the absence of serum opsonins, are sufficient to mediate cytokine release (3). Other studies have suggested that TLR- (and CD14-) mediated activation is crucial for GBS-induced cytokine release (23) and that CR3, although important for opsonophagocytosis, does not contribute to GBS-induced cytokine release (22).
In our study, GBS-induced TNF-α release was measured in response to both live (Fig. 1B) and HK bacteria added to whole human blood. In the presence of plasma (i.e., whole blood) a GBS inoculum of as little as 103 live bacteria per ml (Fig. 1B) was sufficient to induce detectable TNF-α release. The potency of GBS-induced TNF-α release was markedly diminished when blood hemocytes were cultured in the absence of plasma or serum (i.e., artificial medium [data not shown]). Accordingly, the ability of GBS to induce TNF-α release from human monocytes was dramatically amplified by the presence of autologous serum such that, in the presence of serum, bacteria were able to induce equivalent amounts of TNF-α release at ∼1,000-fold-lower bacterial concentrations (Fig. 2). Under such conditions (i.e., in the presence of fresh autologous serum) as few as 104 HK-GBS particles per ml were capable of inducing detectable TNF-α release (Fig. 2).
The pathway by which human plasma or serum potentiates the inflammatory activity of whole gram-negative bacteria by LPS-binding protein-facilitated delivery of LPS to a monocyte receptor complex containing CD14, TLR4, and MD2 is increasingly well defined (8, 26, 45). However, the mechanism(s) by which plasma or serum enhances the inflammatory activity of gram-positive bacteria and the relevant host receptors are less well established. With respect to GBS, the role of the complement system in enhancing antibody-mediated opsonophagocytosis is well described (5, 50), but the potential role of complement in GBS-induced cytokine release has not been addressed. Our study provides strong evidence that complement activation via the alternative pathway largely accounts for the marked enhancement by human serum of GBS-induced TNF-α release from human monocytes. Multiple observations support the central role of the alternative complement pathway as a central mechanism by which plasma or serum greatly amplifies GBS-induced TNF-α release: (i) heat treatment (56°C) eliminated the ability of autologous serum to enhance GBS-induced TNF-α release from hemocytes (data not shown) or isolated monocytes (Fig. 2), (ii) depletion of C3 (but not of C2 or C7) substantially reduced the potentiating effect of serum (Fig. 3), (iii) blood from mice deficient in the soluble complement component C3 released much less TNF-α in response to GBS than did blood from control mice (Fig. 4), (iv) preincubation of GBS with serum enhanced its ability to induce TNF-α in a C3- and factor B-dependent manner (Fig. 5), and (v) MAb m60.1, known to bind the CR3 A domain that mediates interaction with C3bi-coated particles (53), inhibited GBS-induced TNF-α release in whole blood (Table 1).
Our study implicates CD18, a β2-integrin, as critical for GBS-induced TNF-α release in human whole blood (Fig. 7). Using blocking MAbs (Table 1), we identified two integrin α chains that apparently contribute to such GBS-induced cytokine release: (i) MAb m60.1 (which binds a nonlinear epitope in the A domain of CD11b) (49) mediated a modest but significant inhibition of GBS-induced TNF-α release in whole blood and (ii) a MAb to CD11c mediated a marked inhibition. The marked inhibition mediated by the anti-CD11c MAb (Table 1) demonstrates the importance of CR4 to the recognition of GBS and is of particular interest as the roles of CR4 in innate immunity are not as well defined as those of CR3. Mice deficient in the receptor CR3, and which express little CR4 as well (9), demonstrated markedly diminished GBS-induced TNF-α release (Fig. 8). Taken together, these data support important roles for both CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in mediating GBS-induced TNF-α release in whole human blood.
Overall, our data implicate the deposition, via the alternative pathway, of complement components onto the GBS surface and consequent CR3- and CR4-mediated activation as a central mechanism by which plasma or serum potentiates GBS-induced TNF-α release from human monocytes in culture and in whole blood. Of note, the ability of GBS to activate the human complement system has been demonstrated in vitro (11, 12) and measured in vivo in infected human newborns (19). It has been shown that, upon exposure to human serum, the C3-derived fragments C3b and C3bi are deposited on the surface of GBS type III COH-1 (11). However, these prior studies of GBS-induced complement activation have not addressed its impact on GBS-induced cytokine release. As both CR3 and CR4 are known to bind C3bi (17), such deposition provides a mechanism by which complement can enhance CR3- and CR4-mediated cell activation. Importantly, engagement of integrins can trigger signaling cascades culminating in activation of activator protein 1 (21), a transcription factor known to participate in GBS-induced TNF-α release (48).
The present study did not find evidence for a contribution by CD14 to GBS-induced TNF-α release in whole blood (Fig. 6). Although CD14 can contribute to monocyte detection of a number of gram-positive bacterial cell wall products in isolated form (e.g., peptidoglycan and lipoteichoic acid) (37), signaling by both L-GBS (Fig. 6) and HK-GBS (unpublished data) was apparently CD14 independent. Some other studies have also found that CD14 is not necessary for signaling by whole gram-positive bacteria (7, 14).
Henneke and coworkers recently reported that whole GBS cells activate murine macrophages in a MyD88-dependent fashion (23), suggesting the participation of an as-yet-unidentified TLR and/or activation of pathways that induce secretion of autocrine factors (e.g., interleukin-1 or interleukin-18) that could signal through MyD88. A heat-labile soluble factor released from GBS has been shown to signal via TLR2-TLR6 along with the CD14 coreceptor (23). In our study, preincubation of whole blood with neutralizing CD14 or TLR2 MAbs blocked TNF-α induction by the known corresponding agonists (E. coli and sBLP, respectively) but not by L-GBS (Fig. 6). These results suggest that any soluble GBS-derived TLR2 agonists bind epitopes on CD14 and/or TLR2 distinct from those that recognize LPS or sBLP, respectively, or that the relative contribution of the GBS soluble factor is minor under our assay conditions (i.e., live, proliferating bacteria in whole human blood). Similarly, our results are apparently contradictory to an in vitro study of thioglycolate-induced peritoneal macrophages from CD11b-deficient mice employing fetal bovine serum that demonstrated nearly normal TNF-α responses to HK-GBS, leading the authors to conclude that CD11b (and therefore CR3) does not contribute to GBS-induced TNF-α release (22). However, these results are not directly comparable to ours in that (i) our murine studies focused on GBS-induced TNF-α release in fresh, whole peripheral blood and (ii) our studies of GBS-induced TNF-α release both in whole human blood and with isolated human monocytes were performed in the presence of fresh autologous plasma or serum.
It is important to note that the participation of CR3 and CR4 in GBS-induced TNF-α release does not exclude a contribution by TLRs to this process. Although our data demonstrate that CD18-based β-integrins, specifically CR3 and CR4, are necessary for GBS-induced TNF-α release, they may not be sufficient. Additional signals may be required for TNF-α induction, such as integrin receptor clustering, endogenous or exogenous (i.e., microbial) coactivating signals, or cooperation with another receptor type (17). Of note, integrins can form signaling complexes that incorporate diverse receptors (36, 44), including TLRs and their signaling intermediates (42). For example, CR3 acts in concert with TLR4 to elicit full LPS-inducible gene expression from murine macrophages (35). As some TLRs can function intracellularly (46), it is also possible that blocking of opsonophagocytosis by neutralizing CR3-CR4 prevents whole GBS particles from engaging their putative TLR (23) within the cell.
Although human newborns are known to have relatively lower levels of complement components (38), neonatal levels of alternative pathway components are apparently sufficient for effective amplification of GBS-induced TNF-α release as evidenced by the marked TNF-α release from neonatal monocytes in both autologous plasma (i.e., whole blood; Fig. 1B) and serum (Fig. 1D). A relatively high concentration of CD18-bearing monocytes in newborn blood positions the newborn to mount a vigorous inflammatory response to GBS (Fig. 1B). This marked TNF-α response may allow the human newborn to optimize innate immune defense against GBS in the absence of specific antibody. Consistent with this hypothesis, TNF-α has been shown to enhance opsonophagocytosis of GBS by human neutrophils (13), which can occur in the absence of specific antibody (10). While TNF-α may augment bacterial clearance by its action on neutrophils, it may also trigger the deleterious changes in host physiology associated with sepsis syndrome. As excessive TNF-α release in response to high-grade bacteremia with GBS appears to contribute to the pathophysiology of this infection (43), our results suggest that adjunctive immunomodulatory therapy aimed at reducing the inflammatory activity of GBS may prove to be particularly beneficial in human newborns with invasive GBS infections. Based on our study and other published work, such strategies might include blocking inflammatory microbial surface components (41), the alternative pathway of complement activation (29), CR3- and CR4-activated intracellular signaling pathways (48), or the bioactivity of released TNF-α (43).
Acknowledgments
We thank Michele Mariscalco and Celetta Callaway for helpful advice and for sharing MAbs to CD11; Tanya Mayadas for developing and sharing CR3-deficient mice; Holden Maker (BD Biosciences) for advice regarding flow cytometry analysis; William Kolb (Advanced Research Technologies) for advice regarding complement-depleted sera; Dennis Kasper for helpful discussions; the labor and delivery staff of the Brigham and Women's Hospital for their assistance with cord blood collections; and Kathleen McGovern, Dimitri Sigounas, Rene Roy, Michelle Ocana, and April Burton for expert technical assistance.
This work was supported by the Aventis Pasteur Fellowship grant from the Infectious Disease Society of America, NIH KO8 AI50583, and an unrestricted research grant from Baxter Corp (to O.L.) and by NIH R01 A142940 and contract NIH-NIAID-DMID-02-13 (to M.R.W.).
Editor: V. J. DiRita
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Albanyan, E. A., and M. S. Edwards. 2000. Lectin site interaction with capsular polysaccharide mediates nonimmune phagocytosis of type III group B streptococci. Infect. Immun. 68:5794-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albanyan, E. A., J. G. Vallejo, C. W. Smith, and M. S. Edwards. 2000. Nonopsonic binding of type III group B streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen-activated protein kinase pathway. Infect. Immun. 68:2053-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 5.Baker, C. J., M. S. Edwards, B. J. Webb, and D. L. Kasper. 1982. Antibody-independent classical pathway-mediated opsonophagocytosis of type Ia, group B streptococcus. J. Clin. Investig. 69:394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berner, R., J. Csorba, and M. Brandis. 2001. Different cytokine expression in cord blood mononuclear cells after stimulation with neonatal sepsis or colonizing strains of Streptococcus agalactiae. Pediatr. Res. 49:691-697. [DOI] [PubMed] [Google Scholar]
- 7.Berner, R., P. Welter, and M. Brandis. 2002. Cytokine expression of cord and adult blood mononuclear cells in response to Streptococcus agalactiae. Pediatr. Res. 51:304-309. [DOI] [PubMed] [Google Scholar]
- 8.Beutler, B., X. Du, and A. Poltorak. 2001. Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. J. Endotoxin Res. 7:277-280. [PubMed] [Google Scholar]
- 9.Bhat, N., P. Y. Perera, J. M. Carboni, J. Blanco, D. T. Golenbock, T. N. Mayadas, and S. N. Vogel. 1999. Use of a photoactivatable taxol analogue to identify unique cellular targets in murine macrophages: identification of murine CD18 as a major taxol-binding protein and a role for Mac-1 in taxol-induced gene expression. J. Immunol. 162:7335-7342. [PubMed] [Google Scholar]
- 10.Butko, P., A. Nicholson-Weller, and M. R. Wessels. 1999. Role of complement component C1q in the IgG-independent opsonophagocytosis of group B streptococcus. J. Immunol. 163:2761-2768. [PubMed] [Google Scholar]
- 11.Campbell, J. R., C. J. Baker, and M. S. Edwards. 1991. Deposition and degradation of C3 on type III group B streptococci. Infect. Immun. 59:1978-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell, J. R., C. J. Baker, and M. S. Edwards. 1992. Influence of serotype of group B streptococci on C3 degradation. Infect. Immun. 60:4558-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell, J. R., and M. S. Edwards. 2000. Cytokines enhance opsonophagocytosis of type III group B streptococcus. J. Perinatol. 20:225-230. [DOI] [PubMed] [Google Scholar]
- 14.Cauwels, A., E. Wan, M. Leismann, and E. Tuomanen. 1997. Coexistence of CD14-dependent and independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect. Immun. 65:3255-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuzzola, M., G. Mancuso, C. Beninati, C. Biondo, F. Genovese, F. Tomasello, T. H. Flo, T. Espevik, and G. Teti. 2000. Beta 2 integrins are involved in cytokine responses to whole Gram-positive bacteria. J. Immunol. 164:5871-5876. [DOI] [PubMed] [Google Scholar]
- 16.Cuzzola, M., G. Mancuso, C. Beninati, C. Biondo, C. von Hunolstein, G. Orefici, T. Espevik, T. H. Flo, and G. Teti. 2000. Human monocyte receptors involved in tumor necrosis factor responses to group B streptococcal products. Infect. Immun. 68:994-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlers, M. R. 2000. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2:289-294. [DOI] [PubMed] [Google Scholar]
- 18.Farley, M. M. 2001. Group B streptococcal disease in nonpregnant adults. Clin. Infect. Dis. 33:556-561. [DOI] [PubMed] [Google Scholar]
- 19.Fenton, L. J., and R. C. Strunk. 1977. Complement activation and group B streptococcal infection in the newborn: similarities to endotoxin shock. Pediatrics 60:901-907. [PubMed] [Google Scholar]
- 20.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 21.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 22.Henneke, P., O. Takeuchi, R. Malley, E. Lien, R. R. Ingalls, M. W. Freeman, T. Mayadas, V. Nizet, S. Akira, D. L. Kasper, and D. T. Golenbock. 2002. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J. Immunol. 169:3970-3977. [DOI] [PubMed] [Google Scholar]
- 23.Henneke, P., O. Takeuchi, J. A. van Strijp, H. K. Guttormsen, J. A. Smith, A. B. Schromm, T. A. Espevik, S. Akira, V. Nizet, D. L. Kasper, and D. T. Golenbock. 2001. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J. Immunol. 167:7069-7076. [DOI] [PubMed] [Google Scholar]
- 24.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imler, J. L., and J. A. Hoffmann. 2001. Toll receptors in innate immunity. Trends Cell Biol. 11:304-311. [DOI] [PubMed] [Google Scholar]
- 26.Ingalls, R. R., H. Heine, E. Lien, A. Yoshimura, and D. Golenbock. 1999. Lipopolysaccharide recognition, CD14, and lipopolysaccharide receptors. Infect. Dis. Clin. N. Am. 13:341-353, vii. [DOI] [PubMed] [Google Scholar]
- 27.Joyner, J. L., N. H. Augustine, K. A. Taylor, T. R. La Pine, and H. R. Hill. 2000. Effects of group B streptococci on cord and adult mononuclear cell interleukin-12 and interferon-gamma mRNA accumulation and protein secretion. J. Infect. Dis. 182:974-977. [DOI] [PubMed] [Google Scholar]
- 28.Kasper, D. L., L. C. Paoletti, M. R. Wessels, H. K. Guttormsen, V. J. Carey, H. J. Jennings, and C. J. Baker. 1996. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J. Clin. Investig. 98:2308-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirschfink, M. 2001. Targeting complement in therapy. Immunol. Rev. 180:177-189. [DOI] [PubMed] [Google Scholar]
- 30.Kwak, D. J., N. H. Augustine, W. G. Borges, J. L. Joyner, W. F. Green, and H. R. Hill. 2000. Intracellular and extracellular cytokine production by human mixed mononuclear cells in response to group B streptococci. Infect. Immun. 68:320-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, J. O., P. Rieu, M. A. Arnaout, and R. Liddington. 1995. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18). Cell 80:631-638. [DOI] [PubMed] [Google Scholar]
- 32.Medvedev, A. E., T. Flo, R. R. Ingalls, D. T. Golenbock, G. Teti, S. N. Vogel, and T. Espevik. 1998. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-κB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J. Immunol. 160:4535-4542. [PubMed] [Google Scholar]
- 33.Modlin, R., H. Brightbill, and P. Godowski. 1999. The toll of innate immunity on microbial pathogens. N. Engl. J. Med. 340:1834-1835. [DOI] [PubMed] [Google Scholar]
- 34.Peat, E. B., N. H. Augustine, W. K. Drummond, J. F. Bohnsack, and H. R. Hill. 1995. Effects of fibronectin and group B streptococci on tumour necrosis factor-alpha production by human culture-derived macrophages. Immunology 84:440-445. [PMC free article] [PubMed] [Google Scholar]
- 35.Perera, P. Y., T. N. Mayadas, O. Takeuchi, S. Akira, M. Zaks-Zilberman, S. M. Goyert, and S. N. Vogel. 2001. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 166:574-581. [DOI] [PubMed] [Google Scholar]
- 36.Porter, J. C., and N. Hogg. 1998. Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 8:390-396. [DOI] [PubMed] [Google Scholar]
- 37.Pugin, J., I. D. Heumann, A. Tomasz, V. V. Kravchenko, Y. Akamatsu, M. Nishijima, M. P. Glauser, P. S. Tobias, and R. J. Ulevitch. 1994. CD14 is a pattern recognition receptor. Immunity 1:509-516. [DOI] [PubMed] [Google Scholar]
- 38.Schelonka, R., and A. Infante. 1998. Neonatal immunology. Semin. Perinatol. 22:2-14. [DOI] [PubMed] [Google Scholar]
- 39.Schuchat, A. 1999. Group B streptococcus. Lancet 353:51-56. [DOI] [PubMed] [Google Scholar]
- 40.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 41.Scott, M., M. Gold, and R. Hancock. 1999. Interaction of cationic peptides with lipoteichoic acid and gram-positive bacteria. Infect. Immun. 67:6445-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi, C., X. Zhang, Z. Chen, M. K. Robinson, and D. I. Simon. 2001. Leukocyte integrin Mac-1 recruits toll/interleukin-1 receptor superfamily signaling intermediates to modulate NF-κB activity. Circ. Res. 89:859-865. [DOI] [PubMed] [Google Scholar]
- 43.Teti, G., G. Mancuso, and F. Tomasello. 1993. Cytokine appearance and effects of anti-tumor necrosis factor alpha antibodies in a neonatal rat model of group B streptococcal infection. Infect. Immun. 61:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Triantafilou, M., and K. Triantafilou. 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 23:301-304. [DOI] [PubMed] [Google Scholar]
- 45.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 46.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 47.Vallejo, J. G., C. J. Baker, and M. S. Edwards. 1996. Roles of the bacterial cell wall and capsule in induction of tumor necrosis factor alpha by type III group B streptococci. Infect. Immun. 64:5042-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallejo, J. G., P. Knuefermann, D. L. Mann, and N. Sivasubramanian. 2000. Group B streptococcus induces TNF-alpha gene expression and activation of the transcription factors NF-kappa B and activator protein-1 in human cord blood monocytes. J. Immunol. 165:419-425. [DOI] [PubMed] [Google Scholar]
- 49.Violette, S. M., J. R. Rusche, S. R. Purdy, J. G. Boyd, J. Cos, and S. Silver. 1995. Differences in the binding of blocking anti-CD11b monoclonal antibodies to the A-domain of CD11b. J. Immunol. 155:3092-3101. [PubMed] [Google Scholar]
- 50.Wessels, M. R., P. Butko, M. Ma, H. B. Warren, A. L. Lage, and M. C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams, P. A., J. F. Bohnsack, N. H. Augustine, W. K. Drummond, C. E. Rubens, and H. R. Hill. 1993. Production of tumor necrosis factor by human cells in vitro and in vivo, induced by group B streptococci. J. Pediatr. 123:292-300. [DOI] [PubMed] [Google Scholar]
- 52.Xia, Y., V. Vetvicka, J. Yan, M. Hanikyrova, T. Mayadas, and G. D. Ross. 1999. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 162:2281-2290. [PubMed] [Google Scholar]
- 53.Yalamanchili, P., C. Lu, C. Oxvig, and T. A. Springer. 2000. Folding and function of I domain-deleted Mac-1 and lymphocyte function-associated antigen-1. J. Biol. Chem. 275:21877-21882. [DOI] [PubMed] [Google Scholar]