Abstract
1α,25-dihydroxyvitamin D3 (D3) promotes the maturation of myeloid cells and surface expressions of CD14 and CD11b, markers of cell differentiation in response to D3. To examine how these responses are regulated, THP-1 cells were grown in serum-free medium and incubated with D3. This was associated with rapid and transient increases in phosphatidylinositol 3-kinase (PI 3-kinase) activity. Furthermore, induction of CD14 expression in response to D3 was abrogated by (a) the PI 3-kinase inhibitors LY294002 and wortmannin; (b) antisense oligonucleotides to mRNA for the p110 catalytic subunit of PI 3-kinase; and (c) a dominant negative mutant of PI 3-kinase. In THP-1 cells, induction of CD11b expression by D3 was also abrogated by LY294002 and wortmannin. Similarly, LY294002 and wortmannin inhibited D3-induced expression of both CD14 and CD11b in peripheral blood monocytes. In contrast to CD14 and CD11b, hormone-induced expression of the Cdk inhibitor p21 in THP-1 cells was unaffected by either wortmannin or LY294002. These findings suggest that PI 3-kinase selectively regulates D3-induced monocyte differentiation, independent of any effects on p21.
Keywords: myeloid cell, differentiation, vitamin D3, phosphatidylinositol 3-kinase
Pretreatment of THP-1 cells with antisense oligonucleotides to the vitamin D receptor (VDR) mRNA abrogated both activation of PI 3-kinase in response to D3 and hormone-induced CD14 expression. Moreover, both Western blots and in vitro kinase assays carried out on immunoprecipitates of the VDR showed that D3 treatment brought about formation of a complex containing both PI 3-kinase and the VDR. These findings reveal a novel, nongenomic mechanism of hormone action regulating monocyte differentiation, in which vitamin D3 activates a VDR- and PI 3-kinase–dependent signaling pathway.
The steroid hormone 1α,25-dihydroxyvitamin D3 (D3) plays critical roles in regulating numerous cellular and physiological responses. In addition to its well-established importance in plasma calcium homeostasis 1 2 and bone resorption 3 4, D3 also plays a functional role in the hemopoietic system. D3 modulates the expression of several genes in promonocytic cell lines, thereby regulating their differentiation 5. Indeed, when myeloid leukemia cells such as HL60, U937, THP-1, and M1 are incubated with D3, they differentiate into cells expressing functional properties and differentiation markers of monocytes and/or macrophages, including CD14 and CR3 6 7 8 9.
The pleiotropic effects of D3 are principally mediated through the intracellular vitamin D receptor (VDR). The VDR is a member of the superfamily of nuclear steroid, thyroid, and retinoic acid receptors. As such, it functions as a ligand-dependent transcription factor, regulating the activation of vitamin D–responsive target genes 10. At the molecular level, the VDR activates its target genes via interactions with specific DNA sequences designated as vitamin D–responsive elements (VDRE) 11 12 13, a mode of signaling referred to as “genomic action.”
CD14 is a 55-kD glycoprotein originally described as a differentiation marker of monocytes 14. Extensive research has demonstrated that CD14 is involved in mediating responses to several bacterial cell wall products, most notably bacterial LPS 15 16 17 18. CD14 has other pleiotropic effects including, but not limited to, regulating IL-2–induced monocyte tumoricidal activity 19, monocyte resistance to HIV 20, and the adhesive capacity of the molecule LFA-1 21 22. As CD14 is inserted into the cell membrane through a glycosylphosphatidylinositol anchor 23, the mode of signal transduction through this molecule has been intensively investigated. Recent reports indicate a Drosophila Toll–like membrane protein acts as a cell surface coreceptor to mediate signaling after ligand binding to CD14 24 25.
CD14 is undetectable on the surface of monocytic precursors, and increases dramatically during their differentiation into monocytes 26 27 28. Therefore, surface expression of CD14 is an excellent model in which to study the mechanisms of myeloid cell maturation regulated by D3. Induction of CD14 expression in response to D3 occurs at the level of gene transcription and requires new protein synthesis 28 29. However, a curious paradox of this and other systems is that VDRE sequences have not been identified in the promoter regions of many D3-inducible genes, including CD14 10 28. Hence, the regulatory events leading to CD14 expression in response to D3 remain largely unknown.
The β2 integrin CR3 is another classical marker of monocyte differentiation that is involved in cell adhesion and also functions as a complement receptor 6 7 8 9. CD11b, the α subunit of CR3, is a 160-kD glycoprotein that associates noncovalently with a β2 subunit partner, CD18. CD11b–CD18 is expressed by mature myeloid cells, and is widely used as an early monocyte differentiation marker 30. Vitamin D3 has been shown to increase cell surface expression of CD11b by HL-60 30 31, U937 31, and THP-1 cells 8.
In various attempts to define the molecular mechanisms regulating cellular responses to D3, efforts have been made to identify alternative signaling pathways to the classical mode of genomic action. Indeed, during the past decade, experimental evidence for “nongenomic” signaling has challenged the concept of exclusive VDR-mediated genomic action. For example, D3 has been shown to stimulate the rapid formation of second messengers including ceramides, cAMP, inositols, and calcium, and to activate a variety of protein kinases including protein kinase C, Raf, mitogen-activated protein (MAP) kinase, and Src family kinases 10 32 33 34. However, the physiological importance of nongenomic signaling by itself or relative to VDR-mediated genomic action is still unclear. Moreover, it is not known whether D3 uses the classical VDR for nongenomic signaling, or whether an alternative receptor system is involved.
Phosphatidylinositol 3-kinase (PI 3-kinase), a lipid kinase composed of a Src homology 2 domain–containing regulatory subunit (p85) and a 110-kD catalytic subunit (p110), catalyzes the formation of inositol phospholipids phosphorylated at the D3 position of PI. Although PI 3-kinase is known to be important in a wide variety of cellular processes, including intracellular trafficking, organization of the cytoskeleton, cell growth and transformation, and prevention of apoptosis 35 36, its potential role in D3-induced monocyte differentiation remains largely unknown. In this regard, in a recent series of experiments from this laboratory that examined monocyte differentiation, it was observed that expression of a dominant negative mutant of PI 3-kinase in U937 cells abrogated D3-induced CD14 expression (Herrera-Velit, P., Z. Hmama, and N. Reiner, unpublished data). This finding provided direct evidence to suggest that monocyte differentiation in response to D3 may be PI 3-kinase dependent. This study investigated the role of PI 3-kinase and the VDR in regulating monocyte differentiation in response to D3. The results obtained show that D3-induced expression of the monocyte differentiation markers CD14 and CD11b requires PI 3-kinase, and that hormone treatment induces formation of a signaling complex in which the VDR associates with PI 3-kinase. Activation of PI 3-kinase represents a novel pathway for D3 signaling regulating monocyte differentiation, and suggests a mechanism of action for the VDR distinct from classical genomic signaling.
Materials and Methods
Reagents and Chemicals.
RPMI 1640, HBSS, and penicillin/streptomycin were from StemCell Technologies, Inc. Wortmannin, l-α-phosphatidylinositol, PMSF, leupeptin, pepstatin A, and aprotinin were purchased from Sigma Chemical Co. LY294002 and microcystin were from Calbiochem Corp. Protein A–agarose and electrophoresis reagents were purchased from Bio-Rad Laboratories. [γ-32P]ATP was from Nycomed Amersham Plc.
mAbs.
The following mAbs were used: 3C10 (mouse IgG2b, anti-CD14 mAb; a gift from Dr. W.C. Van Voorhis, University of Washington, Seattle, WA); W6/32 (mouse IgG2a, anti–HLA class I mAb; American Type Culture Collection); UB93-3 (mouse mAb to PI 3-kinase; Upstate Biotechnology Inc.) and 9A7 (rat IgG2b, anti-VDR; Chemicon International). Other Abs used included anti-CD14 and anti-CD11b, both murine IgG1 (SC-7328 and SC-1186, respectively; Santa Cruz Biotechnology, Inc.). Murine IgG1 isotype control MG100 was from Caltag Laboratories.
Cell Lines.
The promonocytic cell line THP-1 was from the American Type Culture Collection. The promonocytic cell line U937 transfected with cDNA, encoding the entire coding region of either wild-type bovine PI 3-kinase subunit p85α (Wp85α) or mutant bovine p85α, (Δp85α) has been described 37. The mutant has a deletion of 35 amino acids from residues 479–513 of bovine p85α and the insertion of two other amino acids (Ser-Arg) in the deleted position. Mutant p85α competes with native p85 for binding to essential signaling proteins, thereby acting as a dominant negative mutant 38. THP-1 and U937 cell lines were cultured in RPMI 1640 supplemented with 10% FCS (HyClone), 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cell density was maintained at a concentration of <5 × 105/ml.
Isolation and Culture of Human Monocytes.
Peripheral blood mononuclear cells were isolated as described previously 39. Monocytes were allowed to adhere for 1 h at 37°C in a humidified atmosphere with 5% CO2. Nonadherent cells were removed by three washes with HBSS. Adherent cells were immediately treated and used for cell surface phenotype analysis.
Cell Surface Phenotype Analysis.
To measure the expression of surface molecules, cells were incubated with specific mouse mAb or irrelevant isotype-matched IgG (10 μg/ml) for 30 min, then washed twice and labeled with FITC-conjugated F(ab′)2 sheep anti–mouse IgG (Sigma Chemical Co.) for 30 min. Cells were then washed twice and fixed in 2% paraformaldehyde in staining buffer. All staining and washing procedures were performed at 4°C in HBSS containing 0.1% NaN3 and 1% FCS. Cell fluorescence was analyzed using a Coulter Elite flow cytometer. Relative fluorescence intensities of 5,000–10,000 cells were recorded as single-parameter histograms (log scale, 1,024 channels, 4 log decades), and the mean fluorescence intensity (MFI) was calculated for each histogram. Results are expressed as MFI indices which correspond to MFI of cells + specific Ab/MFI of cells + irrelevant isotype-matched IgG.
In Vitro PI 3-Kinase Assay.
Cell lysates for analysis of PI 3-kinase were prepared in 20 mM Tris, pH 8.0, 1% Triton X-100, 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM Na3VO4, 5 mM NaF, 100 nM microcystin, 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 10 μg/ml aprotinin. Aliquots of lysates adjusted for protein concentration (300–500 μg protein) were incubated for 2–4 h at 4°C with UB93-3 mAb (anti–PI 3-kinase), and immune complexes were adsorbed onto protein A–agarose for 30–60 min. The complexes were washed twice with lysis buffer and three times with 10 mM Tris-HCl, pH 7.4. PI 3-kinase activity was measured as described 22 40. In brief, immunoprecipitates were incubated for 10 min at 4°C with 10 μg of sonicated (3 times for 20 s in an ultrasonic cell disrupter; Branson Sonic Power Co.) l-α-phosphatidylinositol in 10 μl of 30 mM Hepes, to which was added 40 μl of kinase assay buffer (30 mM Hepes, 30 mM MgCl2, 200 μM adenosine, 50 μM ATP, and 10 μCi of [γ-32P]ATP). Reactions were carried out for 15 min at room temperature, and stopped by the addition of 100 μl of 1 N HCl and 200 μl of chloroform/methanol (1:1, vol/vol). Lipids were separated on oxalate-treated silica TLC plates using a solvent system of chloroform/methanol/water/28% ammonia (45:35:7.5:2.5, vol/vol/vol/vol). Plates were exposed to X-ray film at −70°C. Incorporation of radioactivity into lipids was measured by excising the corresponding portions of the TLC plate, followed by liquid scintillation counting. Alternatively, cell lysates were incubated overnight at 4°C with 9A7 mAb (anti-VDR), and immune complexes were adsorbed onto protein A–agarose, washed in lysis buffer containing 200 mM NaCl, and assayed for PI 3-kinase activity as described above.
RNA Isolation and Reverse Transcription PCR.
RNA isolation, cDNA synthesis, and PCR conditions were as described previously 41. Sequences (5′ to 3′) of oligonucleotide primers used in PCR amplifications were as follows: CD14 sense, CCC AAG CTT GGG CAG AGG TTC GGA AGA CTT ATC G; CD14 antisense, GGG GTA CCC CTT GAC CGT GTC AGC ATA CTG CC 29; Cdk inhibitor p21 sense, TTC TCC TTT TCC TCT CTC C; p21 antisense, TCT ACT CCC CCA TCA TAT ACC; β-actin sense, CAC CCC GTG CTG CTG ACC GAG GCC; β-actin antisense, CCA CAC GGA GTA CTT GCG CTC AGG 41. Controls included in the reverse transcription (RT)-PCR reactions were no RNA and RNA without RT, and different cycle numbers of PCR reactions were performed to ensure linear cDNA amplification.
Sense and Antisense Oligonucleotides.
Phosphorothioate-modified oligonucleotides (S-oligos) to VDR and p110 subunit of PI 3-kinase were synthesized by Life Technologies, Inc. The oligonucleotides were phosphorothioate-modified to limit intracellular degradation, and purified by high-performance liquid chromatography to remove incomplete synthesis products. 21-mer sequences, including the presumed translation initiation site of human cDNA sequences corresponding to the VDR 42 and the α isoform of the p110 subunit of PI 3-kinase 43, were made in both sense and antisense orientations with the following sequences: VDR sense, 5′-ATG GAG GCA ATG GCG GCC AGC-3′; VDR antisense, 5′-GCT GGC CGC CAT TGC CTC CAT-3′; p110 sense, 5′-ATG CCT CCA AGA CCA TCA TCA-3′; and p110 antisense, 5′-TGA TGA TGG TCT TGG AGG CAT-3′. The sequences were selected by screening for uniqueness using Blast 227, and were also tested for lack of secondary structure and pairing by using Primer Software (v. 2.0). 3–7 × 106 THP-1 cells were suspended in 500 μl RPMI containing 2.5% lipofectAMINE (Life Technologies, Inc.) and 5 μM S-oligo, and incubated on a rotary shaker for 4 h at 37°C. In addition, fluorescent-modified antisense and sense and flow cytometry were used to monitor S-oligo incorporation into cells. After this incubation, the medium was brought up to 5–10 ml, and cells were cultured for an additional 18 h at 37°C and 5% CO2.
Immunoprecipitation and Western Blotting.
To examine a potential association between the VDR and PI 3-kinase, D3-treated cells were lysed in the buffer used to prepare cell lysates for PI 3-kinase assay, then immunoprecipitated with specific mAb to VDR. Immunoprecipitates were washed with lysis buffer containing 200 mM NaCl to minimize nonspecific protein–protein interactions. Samples were then analyzed by SDS-PAGE and immunoblotting with Abs to the p85 PI 3-kinase subunit as described previously 40. To measure the effects of cell treatments with antisense S-oligos on protein levels of VDR, whole-cell lysates prepared by boiling in Laemmli buffer were subjected to SDS-PAGE and immunoblotting with the mAb 9A7. Membranes were developed by enhanced chemiluminescence (ECL) as described 44.
Results
D3-induced CD14 Expression in THP-1 Cells.
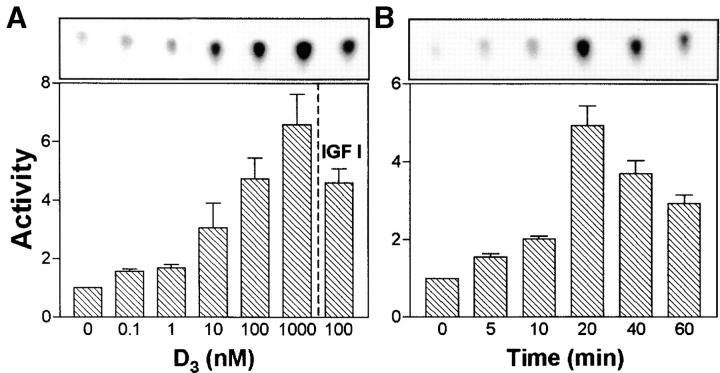
Initial experiments involving immunofluorescence and flow cytometric analysis defined the experimental conditions for D3-induced CD14 expression by THP-1 cells. THP-1 cells maintained in complete medium expressed nearly undetectable levels of CD14 on the surface, and treatment with hormone caused a dose- and time-dependent increase in CD14 expression (Fig. 1). A response was obtained in the presence of as little as 0.1 nM D3 (Fig. 1 A), and at 100 nM, virtually all cells expressed CD14 at an ∼50-fold increase in MFI. The kinetics of induction of CD14 in response to 100 nM D3 is shown in Fig. 1 B. CD14 expression increased progressively with time and was maximal (55-fold increase in MFI index, average of 2 separate experiments) after 48 h. Since a 24-h exposure to 100 nM D3 was sufficient to induce a high level of surface CD14 (53-fold increase in MFI index, average of 2 separate experiments) in nearly 100% of cells, subsequent experiments were carried out under these conditions.
Figure 1.
Induction of CD14 expression in response to D3. (A) THP-1 cells were incubated in RPMI 1640 containing 10% FCS for 24 h with the indicated concentrations of D3. (B) Cells were incubated in the same medium with 100 nM D3 for the indicated times. (C) Serum-starved cells were incubated for 24 h either in serum-free medium, or in medium supplemented with either 10% FCS, IGF-I (100 ng/ml), D3 (100 nM), 10% FCS + D3, or IGF-I + D3. At the end of incubation, cells were washed with staining buffer and labeled for 30 min at 4°C with either 3C10 (anti-CD14) mAb or irrelevant IgG2b. Cells were washed and stained with FITC-conjugated F(ab)′2 sheep anti–mouse IgG. Samples were washed and fixed in 2% paraformaldehyde before flow cytometric analysis. Results in (A) and (B) are expressed as histograms of fluorescence intensity. Histograms displaced to the right represent cells stained with 3C10, and histograms on the left represent cells stained with irrelevant IgG2b. Data presented correspond to one of two independent experiments yielding similar results. In C, results are means ± SEM (n = 3 independent experiments) of MFI indices which correspond to the following ratio: MFI of cells incubated with specific Ab/MFI of cells stained with irrelevant Ab.
Other experiments examined whether the CD14 response of cells to D3 (100 nM, 24 h) was serum dependent. Results obtained in three independent experiments (Fig. 1 C) using RPMI 1640 alone showed that THP-1 cells were fully responsive for CD14 induction (MFI index = 104.4 ± 5.1, mean ± SEM). Addition of FCS had only a limited additive effect (MFI index = 125.0 ± 14.1). Conversely, addition of insulin growth factor (IGF)-I a serum component known to influence cell differentiation 30, to D3 minimally inhibited CD14 expression. These observations suggest that in THP-1 cells, D3 is capable of inducing CD14 in the absence of any serum factors, and that D3 delivery to cell surface is independent of serum vitamin D binding protein 45 46.
Treatment of THP-1 Cells with D3 Activates PI 3-Kinase.
In light of the important roles played by PI 3-kinase in regulating the differentiation of a variety of cell types 47 48 49, the possibility that D3-induced monocytic differentiation involves PI 3-kinase was investigated. As shown in Fig. 2 A, incubation of serum-starved THP-1 cells with 10 nM D3 brought about a significant increase in PI 3-kinase activity (3.07 ± 1.17 fold increase, mean ± SEM, n = 3), with a maximum response observed at 1 μM (6.59 ± 1.47 fold increase). Although PI 3-kinase activity was also significantly increased in IGF-I (100 ng/ml)–treated cells (Fig. 2 A), this was not associated with increased CD14 expression (Fig. 1 C). Time course experiments using 100 nM D3 (Fig. 2 B) showed that PI 3-kinase activation was detectable by as early as 5 min and reached a maximum level (4.94 ± 0.72 fold increase) by 20 min.
Figure 2.
D3 induces PI 3-kinase activation in THP-1 cells. Serum-starved (6 h) cells were stimulated with D3, followed by detergent lysis and immunoprecipitation with anti–PI 3-kinase Ab. PI 3-kinase activity was assayed as described in Materials and Methods. Spots corresponding to phosphatidylinositol 3-phosphate (PIP) were cut and subjected to scintillation counting. In A, cells were stimulated with a range of concentrations of D3 (0–1,000 nM) or with IGF-I (100 ng/ml) for 20 min. In B, cells were stimulated with 100 nM D3 for 0–60 min. The upper rectangles in both panels show PIP spots obtained in one representative experiment. The graphical data shown are the means ± SEM of values obtained in three separate experiments. Activities are expressed as fold increase relative to control (untreated) cells.
D3-induced Expression of CD14 and CD11b are PI 3-Kinase Dependent.
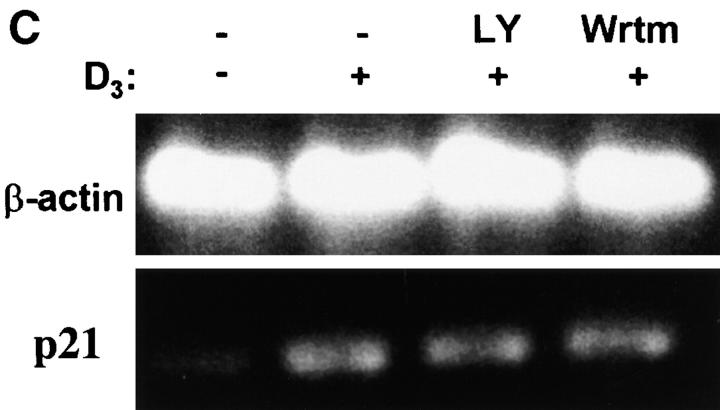
To address whether PI 3-kinase activation is required for D3-induced monocyte differentiation, serum-starved THP-1 cells were incubated with either wortmannin or LY294002 for 20 min before addition of hormone. Inhibitors were used at concentrations known to be relatively selective for inhibition of PI 3-kinase 48 50. Incubation with inhibitors alone had no effect on basal expression of CD14 (data not shown). Preincubation with 5 nM wortmannin led to 65.7 ± 5.6% inhibition of D3-induced CD14 expression (mean ± SEM, n = 3; Fig. 3 A). Increasing the concentration of wortmannin to 50 nM inhibited CD14 expression by 86.0 ± 8.5%. LY294002, an inhibitor of PI 3-kinase that acts via a distinct mechanism from that of wortmannin, when used at 1.5 μM reduced D3-induced CD14 expression by 66.8 ± 11.9%. CD14 expression decreased further in the presence of 15 μM LY294002 (88.1 ± 5.1%). In contrast to abrogation of D3-induced CD14, neither wortmannin nor LY294002 had significant effects on the expression of HLA class I molecules (data not shown), indicating that the effects of neither wortmannin nor LY294002 were due to nonspecific toxicity. The PI 3-kinase inhibitors LY294002 and wortmannin also attenuated D3-induced cell surface expression of CD11b by THP-1 cells. While incubation with inhibitors alone had no effect on basal levels of CD11b expression, preincubation with 5 nM wortmannin inhibited the response to D3 by 41 ± 7% (Table ). A higher concentration of 50 nM increased inhibition to 100 ± 12%. LY294002 inhibited D3-induced CD11b surface expression by 48 ± 10% at a concentration of 1.5 μM, and by 88 ± 14% at a concentration of 15 μM (Table ). Surface expression of CD14 and CD11b was also examined in human peripheral blood monocytes. As shown in Table , LY294002 and wortmannin markedly inhibited D3-induced expression of both CD14 and CD11b in these cells.
Figure 3.
Wortmannin and LY294002 attenuate D3-induced CD14 expression, but not expression of Cdk inhibitor p21. Serum-starved THP-1 (4 × 106) cells were incubated for 20 min at 37°C and 5% CO2 in medium alone, with wortmannin (Wrtm), or with LY294002 (LY). D3 (100 nM) was then added for 24 h at 37°C. (A) Aliquots of cells (∼0.5 × 106) from each treatment were washed with staining buffer and labeled with anti-CD14 mAb or irrelevant mAb, followed by FITC-conjugated secondary Abs. Flow cytometric analyses were performed, and MFI indices were determined as described in the legend to Fig. 1. The results shown are the means ± SEM of values obtained in three separate experiments. (B and C) Total RNA was extracted from the cells remaining, and RT-PCR was carried out for CD14 (B) and p21 (C) as described (reference 41). β-actin was analyzed to control for loading. Negative controls consisting of no RNA and RNA without RT were included, and these produced no signals (data not shown). The data shown are from one of two independent experiments that yielded similar results.
Table 1.
Effects of PI 3-Kinase Inhibitors on Vitamin D3–induced Surface Expression of CD11b by THP-1 Cells
| Treatment | CD11b (MFI index) |
|---|---|
| Control | 1.19 ± 0.17 |
| Vit D3 | 2.77 ± 0.78 |
| 15 μM LY alone | 1.17 ± 0.17 |
| 50 nM wrtm alone | 1.13 ± 0.15 |
| 1.5 μM LY + vit D3 | 2.01 ± 0.41 |
| 5.0 nM wrtm + vit D3 | 2.13 ± 0.37 |
| 15 μM LY + vit D3 | 1.38 ± 0.22 |
| 50 nM wrtm + vit D3 | 1.19 ± 0.14 |
Serum-starved THP-1 cells were incubated for 20 min at 37°C and 5% CO2 in either medium alone, wortmannin (wrtm), or LY294002 (LY). D3 (vit D3 [100 nM]) was then added for 24 h at 37°C. Cells from each treatment were washed with staining buffer, labeled with anti-CD11b mAb or irrelevant mAb followed by FITC-conjugated secondary Abs, and flow cytometric analyses were performed. The results shown are the means ± SEM of values obtained in three separate experiments. Results are expressed as MFI indices which correspond to MFI of cells + specific Ab/MFI of cells + irrelevant isotype-matched IgG.
Table 2.
Effects of PI 3-kinase Inhibitors on Vitamin D3–induced Surface Expression of CD14 and CD11b by Peripheral Blood Monocytes
| Treatment | CD14 (MFI index) | CD11b (MFI index) |
|---|---|---|
| Control | 1.09 | 1.27 |
| Vit D3 | 1.98 | 1.80 |
| 15 μM LY alone | 1.03 | 1.08 |
| 50 nM wrtm alone | 1.21 | 1.25 |
| 1.5 μM LY + vit D3 | 1.37 | 1.63 |
| 5.0 nM wrtm + vit D3 | 1.54 | 1.54 |
| 15 μM LY + vit D3 | 1.07 | 1.24 |
| 50 nM wrtm + vit D3 | 1.20 | 1.20 |
Peripheral blood monocytes were allowed to adhere to plastic dishes for 1 h at 37°C and 5% CO2 in serum-free medium. Cells were then washed with warm HBSS, and the remaining adherent monocytes were incubated for 20 min at 37°C and 5% CO2 in either medium alone, wortmannin (wrtm), or LY294002 (LY). D3 (vit D3 [100 nM]) was then added for 24 h at 37°C. Cells from each treatment were washed with staining buffer and labeled with either anti-CD14 mAb, anti-CD11b mAb, or irrelevant mAb, followed by FITC-conjugated secondary Abs. Flow cytometric analyses were performed, and MFI indices were determined as described in Materials and Methods. Results are expressed as MFI indices which correspond to MFI of cells + specific Ab/MFI of cells + irrelevant isotype-matched IgG. The results shown are based on 10,000 recorded events for each treatment condition performed on cells from 1 individual donor.
Inhibition of PI 3-kinase has been shown to affect the transport of some cell surface molecules 51, and thus was a possible mechanism to explain the effects of PI 3-kinase inhibitors on D3-induced CD14 expression. However, when total RNA was isolated and RT-PCR was performed using primers for CD14 and β-actin, the results showed that mRNA levels for CD14 were markedly reduced in cells incubated with either wortmannin or LY294002 (Fig. 3 B). These findings indicate that inhibition of PI 3-kinase results in attenuation of D3-induced CD14 gene expression at a pretranslational level.
Treatment of immature myeloid cells with D3 induces the expression of the Cdk inhibitor p21, and the latter has been shown to regulate gene expression and to promote myleoid cell differentiation 52. Experiments were carried out to examine whether the induction of p21 in response to hormone also involved PI 3-kinase. As shown in Fig. 3 C, THP-1 cells expressed low levels of p21 mRNA in the basal state. As expected, p21 expression was significantly induced in response to incubation of cells with D3. However, in contrast to the findings with CD14 and CD11b, preincubation of cells with either wortmannin or LY294002 had no effect on hormone-induced expression of p21. These findings suggest that PI 3-kinase selectively regulates monocyte differentiation, independent of any effects on p21.
The role of PI 3-kinase in regulating monocyte differentiation was investigated further by inhibiting the synthesis of the p110 catalytic subunit of PI 3-kinase. THP-1 cells were incubated in the presence of antisense S-oligo complementary to the p110 translation initiation region (including the ATG initiation codon), and then assayed for both PI 3-kinase activation and CD14 expression in response to D3. Flow cytometric analysis of cells exposed to fluorescein-modified antisense S-oligo, under the same conditions used for unmodified S-oligos, revealed that THP-1 cells readily incorporated foreign DNA (data not shown). As shown in Fig. 4 A, antisense S-oligo to p110 mRNA significantly attenuated D3-induced PI 3-kinase activity (% inhibition = 87.1 ± 5.6, mean ± SEM, n = 3), whereas at the same concentration, the control sense S-oligo had no apparent effect on the PI 3-kinase response. In parallel with this effect on PI 3-kinase activation, pretreatment with antisense S-oligo (and not with the control, sense oligo) almost completely inhibited hormone-induced surface expression of CD14 (% inhibition = 91.7 ± 3.9, mean ± SEM of three experiments; Fig. 4 B). In addition, RT-PCR results shown in Fig. 4 C demonstrated that D3-induced mRNA levels for CD14 were markedly diminished in cells treated with antisense S-oligo to p110 mRNA.
Figure 4.
Antisense S-oligo to the p110 subunit of PI 3-kinase inhibits D3-induced CD14 expression. 7 × 106 THP-1 cells were suspended in 500 μl of 2.5% lipofectAMINE/RPMI 1640 alone (Control), or containing 5 μM of either sense (S) or antisense (AS) S-oligo to p110. Cells were then incubated on a rotary shaker for 4 h at 37°C. The incubation was brought up to 10 ml of medium, and cells were cultured for an additional 18 h at 37°C and 5% CO2. (A) 3 × 106 cells were stimulated with 100 nM D3 for 20 min and assayed for PI 3-kinase assay as described in Materials and Methods. The upper rectangle shows PIP spots obtained in one representative experiment. The graphical data shown below represent PI 3-kinase activities (means ± SEM of values obtained in three separate experiments), calculated as described in the legend to Fig. 2. (B) Fractions of control or S-oligo–treated cells (∼1 × 106) were incubated in medium alone or with D3 (100 nM, 24 h), washed with staining buffer, and then labeled with anti-CD14 mAb or irrelevant mAb, followed by FITC-conjugated secondary Abs. Flow cytometric analyses were performed, and MFI indices were determined as described in the legend to Fig. 1. Results are expressed as histograms of fluorescence intensity. Histograms displaced to the right represent cells stained with anti-CD14, and histograms on the left represent cells stained with irrelevant IgG2b. Bold, italicized values are means ± SEM (n = 3) of MFI indices, calculated as described in the legend to Fig. 1. (C) Control or S-oligo–treated cells (∼3 × 106) were incubated with D3 (100 nM, 24 h). Total RNA was extracted and RT-PCR was carried out for CD14 and β-actin as described (reference 41). RT-PCR controls, as described in the legend to Fig. 3, were included. The data shown are from one of two independent experiments that yielded similar results.
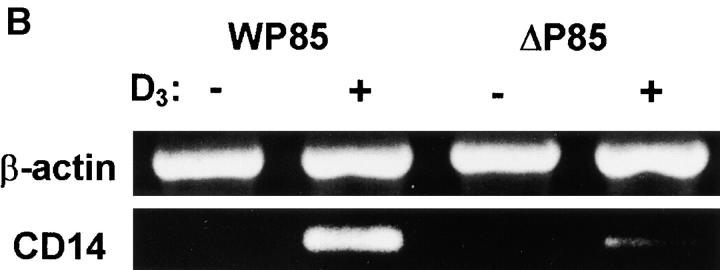
The PI 3-kinase requirement for D3-induced CD14 expression was also examined in U937 cells transfected with a dominant negative mutant of p85 (Δp85). Stable transfection of these cells with Δp85 resulted in a significant reduction of stimulated PI 3-kinase activity 22 37. Exposure of Δp85 U937 to D3 (100 nM, 48 h) led to only a marginal increase in surface expression of CD14 above baseline. In contrast, cells transfected with wild-type p85 showed significant induction of CD14 expression (Fig. 5 A). In addition, as observed with THP-1 cells, RT-PCR experiments showed that attenuation of PI 3-kinase in U937 cells resulted in markedly reduced response to D3 for induction of CD14 mRNA (Fig. 5 B). Taken together, these findings strongly suggest that PI 3-kinase plays a central role in D3-induced monocyte differentiation, and indicate that PI 3-kinase activation is required to induce CD14 expression.
Figure 5.
CD14 response to D3 in U937 cells transfected with wild-type bovine p85α (WP85) or dominant negative mutant Δp85α (Δp85). (A) Cells were stimulated with either 100 nM D3 or medium alone for 48 h. Cells were washed with staining buffer, and then labeled with anti-CD14 mAb or irrelevant mAb, followed by secondary FITC-conjugated Abs. Flow cytometric analyses were performed, and MFI indices were determined as described in the legend to Fig. 1. The data shown are the means ± SEM of values obtained in three separate experiments. (B) Cells were stimulated with D3 or medium alone for 24 h, then total RNA was extracted and RT-PCR was carried out for CD14 and β-actin as described (reference 41). No signals were obtained with controls consisting of no RNA and RNA without RT (data not shown). The data shown are from one of two independent experiments that yielded similar results.
Induction of CD14 in Response to D3 Requires the VDR.
Many responses to D3, such as induction of expression of osteopontin, osteocalcin, calbindin, and 24-hydroxylase, are brought about by a mechanism involving binding of the VDR to a specific VDRE in the corresponding promoters 53. Since no VDRE has been identified within the CD14 gene promoter 28, this raised the question as to whether the VDR plays any role in regulating D3-induced CD14 expression. To address this question, THP-1 cells were incubated overnight with antisense S-oligo specific to VDR mRNA. This resulted in significant attenuation of the level of VDR protein in THP-1 cells as detected by Western blotting (Fig. 6 A). In contrast, pretreatment of cells with the control, sense S-oligo had no apparent effect on the level of VDR protein. In parallel with the reduction of VDR protein, D3-induced surface expression of CD14 was markedly attenuated (Fig. 6 B; in two separate experiments an average decrease of 86.8% was observed). In addition, cells were treated with S-oligo antisense specific for VDR mRNA before D3, followed by RNA extraction and RT-PCR. The results showed that the induction of CD14 mRNA was markedly attenuated in antisense-treated cells (Fig. 6 C). Taken together, these findings strongly suggest that the VDR is essential for D3-induced CD14 gene expression.
Figure 6.
VDR antisense S-oligo inhibits D3-induced CD14 expression. 5 × 106 THP-1 cells were suspended in 500 μl of 2.5% lipofectAMINE/RPMI 1640 alone (control), or containing 5 μM of either sense (S) or antisense (AS) S-oligo to the VDR. Cells were then incubated on a rotary shaker for 4 h at 37°C. The volume was then brought up to 7 ml, and cells were cultured for an additional 18 h at 37°C and 5% CO2. (A) 0.5 × 106 cells were boiled in Laemmli buffer and subjected to SDS-PAGE and immunoblotting with mAb to VDR. Membranes were developed by ECL as described (reference 44). The data shown are from one of two independent experiments that yielded similar results. (B) Aliquots of control or S-oligo–treated cells (∼1 × 106) were incubated with D3 (100 nM, 24 h), washed with staining buffer, and then labeled with anti-CD14 mAb or irrelevant mAb followed by FITC-conjugated, secondary Abs. Flow cytometric analyses were performed, and MFI indices were determined as described in the legend to Fig. 1. Results are expressed as histograms of fluorescence intensity. Histograms displaced to the right represent cells stained with anti-CD14, and histograms on the left represent cells stained with irrelevant IgG2b. Bold, italicized values in each frame are averages (n = 2) of MFI indices, determined as in the legend to Fig. 1. (C) Control or S-oligo–treated cells (∼3 × 106) were incubated with D3 (100 nM, 24 h). Total RNA was extracted and RT-PCR was carried out for CD14 and β-actin as described (reference 41). Controls consisting of no RNA and RNA without RT were included, and no signals were obtained (data not shown). The data shown are from one of two independent experiments that yielded similar results.
Association of PI 3-Kinase with the VDR.
Given the evidence that both PI 3-kinase and the VDR were essential for induction of CD14 in response to D3, the question examined next was whether the VDR is required for activation of PI 3-kinase. THP-1 cells were treated with antisense S-oligos as described above to reduce VDR protein expression before incubation with D3 (100 nM, 20 min). Cell lysates were then prepared and assayed for PI 3-kinase activity. The results in Fig. 7 A show that pretreatment with antisense to VDR almost completely abrogated (92.9% inhibition, average of two separate experiments) PI 3-kinase activation in response to D3. In contrast, treatment with control sense S-oligos to VDR had no inhibitory effect.
Figure 7.
The VDR is required for D3-induced PI 3-kinase activation. (A) 3 × 106 THP-1 cells were treated overnight with 5 μM of either sense (S) or antisense (AS) S-oligo to VDR as described in the legend to Fig. 6. Cells were then stimulated for 20 min with 100 nM D3, and lysates were prepared and assayed for PI 3-kinase activities as described in Materials and Methods. The upper rectangle shows PIP spots obtained in one representative experiment. The data shown below are PI 3-kinase activities (averages of values obtained in two separate experiments), calculated as described in the legend to Fig. 2. (B) Cells were stimulated with D3 as in A and cell lysates were prepared and incubated overnight at 4°C either with 9A7 mAb (anti-VDR) or with mAb specific for the p85 subunit of PI 3-kinase. Immune complexes were adsorbed onto protein A and assayed for PI 3-kinase activities as described in Materials and Methods. The upper rectangle shows PIP spots obtained in one representative experiment. The data shown below are PI 3-kinase activities (average of two separate experiments), calculated as described in the legend to Fig. 2. (C) Cell lysates prepared from either control cells or D3-treated cells were immunoprecipitated with specific mAbs to either the VDR or the p85 subunit of PI 3-kinase. Immunoprecipitates were then analyzed by SDS-PAGE and immunoblotting with Abs to p85 (reference 40). Immunoprecipitates with normal mouse IgG (MIgG) and normal rat IgG (RIgG) were used to control for the specificity of anti-p85 and anti-VDR Abs, respectively.
The evidence that the D3 receptor was required for both PI 3-kinase activation and CD14 expression in response to D3 suggested the possibility that this might involve a signaling complex containing both the VDR and PI 3-kinase. To test this hypothesis, THP-1 cells were incubated with D3, and immunoprecipitates prepared with mAb to the VDR were assayed for PI 3-kinase activity. The results shown in Fig. 7 B indicate that immunoprecipitates of the VDR prepared from cells activated with D3 contained PI 3-kinase activity. To examine further whether the VDR associates with PI 3-kinase upon D3 stimulation, aliquots from anti-VDR and anti-p85 (PI 3-kinase) immunoprecipitates of D3-treated cells were subjected to SDS-PAGE and Western blotting. Blots were probed with mAb to p85 and developed by ECL. The results shown in Fig. 7 C indicate that Abs to p85 reacted in anti-VDR immunoprecipitates with a band that presumably corresponds to the p85 subunit of PI 3-kinase. This association was only observed in cells treated with hormone, and not in untreated cells. Taken together, the findings suggest that D3 treatment induces the formation of a signaling complex containing the VDR and PI 3-kinase. This results in activation of the lipid kinase, and is required for monocyte differentiation and induction of CD14 expression.
Discussion
The steroid hormone vitamin D3 is known to induce immature, myeloid precursor cells to differentiate into mature monocytic cells. This process is accompanied by high-level expression of mRNA and protein for CD14, and other markers such as CD11b 27 28 29. How these D3-initiated events are regulated is not completely understood. Cellular responses to D3 have primarily been attributed to activation of the VDR, which acts as a transcription factor modulating the expression of a variety of genes 53. In contrast to genomic signaling, several reports have implicated alternative, nongenomic mechanisms of action for D3 involving initiation of signaling at the cell membrane 54 55 56. The objective of this study was to examine signaling events in response to D3 in order to define further how this hormone regulates myeloid cell differentiation.
The principal conclusions drawn from the experiments reported are that D3-induced expression of CD14 and CD11b requires both PI 3-kinase and the VDR. Moreover, this process appears to involve the formation of a PI 3-kinase–VDR signaling complex. The conclusion that D3-induced CD14 expression is PI 3-kinase dependent is based on several lines of evidence, including: (a) in vitro kinase assays with immunoprecipitated PI 3-kinase, showing that incubation of cells with D3 leads to activation of the enzyme (Fig. 2); (b) D3-induced CD14 expression is abrogated in THP-1 cells incubated with the PI 3-kinase inhibitors, wortmannin and LY294002 (Fig. 3); (c) an antisense strategy to downregulate PI 3-kinase also attenuated D3-induced CD14 expression (Fig. 4); and (d) expression of a dominant negative mutant of PI 3-kinase (Δp85) in U937 cells also completely abrogated D3-induced expression of both CD14 mRNA and protein (Fig. 5). Similarly, CD11b induction in response to D3 was also attenuated by wortmannin and LY294002 in both THP-1 cells and in human peripheral blood monocytes. Taken together, these findings establish that D3-induced CD14 and CD11b expression are regulated by PI 3-kinase, and that they are consistent with a nongenomic mechanism of hormone action.
Expression of the Cdk inhibitor p21 is induced by D3, and this is associated with the differentiation of immature myeloid cells, including the expression of CD14 52. Therefore, it was possible that inhibition of PI 3-kinase may have abrogated hormone-induced expression of p21 proximally, leading to secondary, indirect effects on the expression of CD14 and CD11b. However, the findings that neither wortmannin nor LY294002 inhibited the induction of p21 while at the same time they inhibited the expression of CD14 and CD11b (Fig. 3, Table and Table ) indicated that this was not the case. These findings suggest that PI 3-kinase selectively regulates these monocyte differentiation markers independent of any effects on p21. They also suggest that D3-induced signaling initiated through the VDR appears to involve at least two separate pathways, one leading to p21 induction that is independent of PI 3-kinase, and a second pathway that is PI 3-kinase dependent and which regulates at least the induction of CD14 and CD11b. Moreover, although p21 has been shown to be involved in regulating monocyte differentiation 52, results from the present study suggest that it is not sufficient by itself for induction of monocyte differentiation in response to D3, and that other, PI 3-kinase– and VDR-regulated elements are required.
Induction of CD14 expression in response to D3 is regulated at the level of gene transcription 28 29 and the CD14 promoter, particularly from bp −128 to −70, has been shown to be critical for CD14 expression in response to D3 28. However, this region does not contain a canonical VDRE 28. Rather, the CD14 promoter contains two GC boxes that bind the nonreceptor, transcription factor Sp1, and this interaction is believed to be essential for CD14 expression 28 57. In light of this molecular data, activation of CD14 transcription would not be expected to be a direct response to D3 signaling through the VDR. Nevertheless, experiments that used VDR antisense S-oligo to downregulate expression of the endogenous VDR before D3 stimulation showed that the VDR is required for D3-induced expression of CD14 mRNA (Fig. 6). The obligate requirement for the VDR in this response was also supported by the finding that activation of PI 3-kinase in response to D3 was markedly attenuated in cells treated with VDR antisense (Fig. 7 A). Moreover, a signaling complex containing both the VDR and PI 3-kinase was identified in D3-treated cells (Fig. 7b and Fig. c), suggesting that a functional cytosolic VDR is a prerequisite for PI 3-kinase activation in response to D3. These findings appear to identify a novel, nongenomic mechanism of action for the VDR. It is of interest to note that the hormone-induced formation of a VDR–PI 3-kinase signaling complex is reminiscent of the finding that activation of the Raf-MAP kinase pathway by D3 in keratinocytes involves an association of the VDR with the adaptor protein p66shc 58. Together, these findings suggest that other models of nongenomic signaling involving the VDR may yet be identified.
The observations that PI 3-kinase regulates CD14 expression in response to D3 and that the VDR is directly involved in this process do not exclude the possibility of a component of genomic action by the VDR, despite the absence of a canonical VDRE within the CD14 promoter. One possibility to consider is that upon incubation of cells with D3, the VDR may translocate to the nucleus towards D3-responsive elements, and enhance the activity of the known CD14 transcription factor Sp1. Two observations lend support to this hypothesis: (a) the finding of transcriptional synergism between the VDR and Sp1 using a construct of the VDRE, the binding sites for Sp1, and a luciferase reporter gene 59; and (b) the presence of a VDRE-like sequence at the distal Sp1 site within the CD14 gene 60.
The findings that D3-induced monocyte differentiation for CD14 and CD11b expression requires PI 3-kinase are consistent with previous reports in which this lipid kinase has been implicated in regulating the differentiation of various cell types, including immature myeloid cells 49 61 62 63. For example, indirect data, based solely on the use of inhibitors, suggested a potential role for PI 3-kinase in regulating the differentiation of the myeloid leukemia cells HL-60 63. In addition, when FDC-P1 myeloid cells that express the M-CSF receptor c-fms were treated with M-CSF, there occurred the rapid formation of a complex between c-fms and several signal transduction proteins, including PI 3-kinase 49. Other studies have suggested that the differentiation of FDC-P1 cells is regulated by the activities of both phospholipase C-γ and PI 3-kinase in response to M-CSF stimulation 61. In addition, stimulation of type III receptor tyrosine kinases in the same cell line was observed to lead to PI 3-kinase–dependent monocytic differentiation 62. Thus, the steroid hormone D3 appears to share with M-CSF the property of PI 3-kinase activation during the induction of monocyte differentiation.
It is of interest to compare D3 with phorbol esters that also act to promote monocyte differentiation. For example, PMA induces the expression of the monocyte differentiation markers CR3 (CD11b/CD18) and p150,95 64, and modulates several functional properties of myeloid precursor cells, such as intracellular adhesion molecule 1–dependent adhesion 22, phagocytosis 44 65, and bactericidal activity 66. However, PMA does not activate PI 3-kinase (Hmama, Z., and N. Reiner, unpublished data), and PMA-induced cell differentiation is resistant to PI 3-kinase inhibition 22. Consistent with the findings presented above, although low concentrations of D3 induce an ∼50-fold increase in CD14 surface expression, optimal doses of PMA resulted in only marginal increases (Hmama, Z., and N. Reiner, unpublished data). Regarding signaling mechanisms regulating PMA-induced cell differentiation, several reports have implicated the protein kinase C–Raf-MAP kinase pathway 33 67 68. MAP kinase activity has been also shown to be activated by D3 in keratinocytes, enterocytes, and in the promyelocytic cell lines HL60 and NB4 34 58 69. To examine whether the MAP kinase pathway may be involved in THP-1 cell differentiation, D3-treated cells were examined for tyrosine phosphorylation of p42 and p44 MAP kinase isoforms, and assayed for MAP kinase activity using myelin basic protein as substrate. The results obtained (data not shown) indicated that MAP kinase is not activated in response to D3 in THP-1 cells.
The mechanism by which PI 3-kinase becomes activated in a complex with the VDR is not presently known. At least one well-established mechanism for activation of PI 3-kinase is known to involve interactions of the Src homology 2 domain of the p85 regulatory subunit with tyrosine phosphorylated proteins, including both receptor- and nonreceptor-protein tyrosine kinases 35. In the VDR, the only potential sites of phosphorylation correspond to Ser51 70 and Ser208 71, and the relevance of these residues in VDR-mediated D3 signal transduction is unknown. Thus, it is not presently clear how a direct interaction of the VDR with the p85 subunit may lead to activation of PI 3-kinase. On the other hand, a multimolecular complex involving the VDR and a tyrosine-phosphorylated adaptor protein could potentially be involved in D3-induced activation of PI 3-kinase.
At present, it is not clear how VDR-activated PI 3-kinase would be targeted to the membrane compartment to mediate cellular responses, given the cytosolic and nuclear localization of the VDR. One possibility to consider is that there is a small, membrane-associated population of VDR molecules that induces translocation of PI 3-kinase to the membrane compartment. A second possibility is that once activated, the kinase may simply diffuse to the vicinity of the inner leaflet of the plasma membrane. Third, it is also possible that VDR-activated PI 3-kinase may act on nonmembrane-associated substrates to mediate its effects.
During the course of these studies, it was observed that IGF-I failed to induce CD14 surface expression (Fig. 1 C), despite it ability to activate PI 3-kinase (Fig. 2). Therefore, PI 3-kinase activation in response to D3 appears to be necessary, but not sufficient to bring about CD14 expression. These findings suggest the possibility that induction of CD14 in response to D3 may involve VDR-mediated signals that bifurcate to involve nongenomic effects of PI 3-kinase, perhaps acting in concert with a genomic mechanism of action. In this respect, cross-talk between nuclear receptor–mediated signaling and nongenomic actions have been described in D3-treated osteoblasts 72.
In summary, the findings presented demonstrate a novel pathway in which D3 signaling for myeloid cell differentiation involves PI 3-kinase activation and signal complex formation with the VDR, which is itself shown to be required for cell differentiation. This pathway is essential for D3-induced expression of CD14 and CD11b, and attributes important functional roles to PI 3-kinase and the VDR in monocyte differentiation.
Acknowledgments
We thank Dr. W.C. Van Voorhis for antibody to CD14, and Mr. Raymond Lo for technical assistance.
This work was supported by grant MT-8633 from the Medical Research Council of Canada.
Footnotes
Abbreviations used in this paper: D3, 1α,25-dihydroxyvitamin D3; ECL, enhanced chemiluminescence; IGF, insulin-like growth factor; MAP, mitogen-activated protein; MFI, mean fluorescence intensity; PI 3-kinase, phosphatidylinositol 3-kinase; PIP, phosphatidylinositol 3-phosphate; RT, reverse transcription; S-oligo, phosphorothioate-modified oligonucleotide; VDR, vitamin D receptor; VDRE, vitamin D–responsive element.
References
- Boyle I.T., Miravet L., Gray R.W., Holick M.F., DeLuca H.F. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxyvitamin D in nefrectomized rats. Endocrinology. 1972;90:605–608. doi: 10.1210/endo-90-3-605. [DOI] [PubMed] [Google Scholar]
- Nemere I. Nongenomic effects of 1,25-dihydroxyvitamin D3potential relation of a plasmalemmal receptor to the acute enhancement of intestinal calcium transport in chick. J. Nutr. 1995;125:1695S–1698S. doi: 10.1093/jn/125.suppl_6.1695S. [DOI] [PubMed] [Google Scholar]
- Holick M.F., Garabedian M., DeLuca H.F. 1,25-dihydroxycholecalciferolmetabolite of vitamin D3 active on bone in anepheric rats. Science. 1972;176:1146–1147. doi: 10.1126/science.176.4039.1146. [DOI] [PubMed] [Google Scholar]
- Raisz L.G., Trummel C.L., Holick M.F., DeLuca H.F. 1,25-dihydroxyvitamina potent stimulator of bone resorption in tissue culture. Science. 1972;175:768–769. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nakamura K., Iho S. Differentiation of myeloid cells and 1,25-dihydroxyvitamin D3 . Leuk. Lymphoma. 1997;27:25–33. doi: 10.3109/10428199709068268. [DOI] [PubMed] [Google Scholar]
- Brackman D., Lund-Johansen F., Aarskog D. Expression of leukocyte differentiation antigens during the differentiation of HL-60 cells induced by 1,25-dihydroxyvitamin D3comparison with the maturation of normal monocytic and granulocytic bone marrow cells. J. Leukoc. Biol. 1995;58:547–555. doi: 10.1002/jlb.58.5.547. [DOI] [PubMed] [Google Scholar]
- Oberg F., Botling J., Nilsson K. Functional antagonism between vitamin D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U-937 cells. J. Immunol. 1993;150:3487–3495. [PubMed] [Google Scholar]
- Schwende H., Fitzke E., Ambs P., Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3 . J. Leukoc. Biol. 1996;59:555–561. [PubMed] [Google Scholar]
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1α,25-dihydroxyvitamin D3 . Proc. Natl. Acad. Sci. USA. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R., Okamura W.H., Norman A.W. Structure-function relationships in the vitamin D endocrine system. Endocr. Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- Ozono K., Liao J., Kerner S.A., Scott R.A., Pike J.W. The vitamin D-responsive element in the human osteocalcin gene. Association with a nuclear proto-oncogene enhancer. J. Biol. Chem. 1990;265:21881–21888. [PubMed] [Google Scholar]
- Darwish H.M., DeLuca H.F. Identification of a 1,25-dihydroxyvitamin D3-response element in the 5′-flanking region of the rat calbindin D-9k gene. Proc. Natl. Acad. Sci. USA. 1992;89:603–607. doi: 10.1073/pnas.89.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierold C., Darwish H.M., DeLuca H.F. Identification of a vitamin D-response element in the rat calcidiol (25-hydroxyvitamin D3) 24-hydroxylase gene. Proc. Natl. Acad. Sci. USA. 1994;91:900–902. doi: 10.1073/pnas.91.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R.F., Nadler L.M., Schlossman S.F. Antigens on human monocytes identified by monoclonal antibodies. J. Immunol. 1981;126:1435–1439. [PubMed] [Google Scholar]
- Wright S.D., Ramos R.A., Tobias P.S., Ulevitch R.J., Mathison J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Medvedev A.E., Flo T., Ingalls R.R., Golenbock D.T., Teti G., Vogel S.N., Espevik T. Involvement of CD14 and complement receptor CR3 and CR4 in nuclear factor-kappa B activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J. Immunol. 1998;160:4535–4542. [PubMed] [Google Scholar]
- Wooten R.M., Morrison T.B., Weis J.H., Wright S.D., Thieringer R., Weis J.J. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi . J. Immunol. 1998;160:5485–5492. [PubMed] [Google Scholar]
- Sellati T.J., Bouis D.A., Kitchens R.L., Darveau R.P., Pugin J., Ulevitch R.J., Gangloff S.C., Goyert S.M., Norgard M.W., Radolf J.D. Treponem treponema and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 1998;160:5455–5464. [PubMed] [Google Scholar]
- Bosco M.C., Espinosa-Delgado I., Rowe T.K., Malabarba M.G., Longo D.L., Varesio L. Functional role for the myeloid differentiation antigen CD14 in the activation of human monocytes by Il-2. J. Immunol. 1997;159:2922–2931. [PubMed] [Google Scholar]
- Zybarth G., Reiling N., Schmidtmayerova H., Sherry B., Bukrinsky M. Activation-induced resistance of human macrophages to HIV-1 infection in vitro. J. Immunol. 1999;162:400–406. [PubMed] [Google Scholar]
- Beekhuizen H., Blokland I., Corsel-van Tilburg A.J., Koning F., van Furth R. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J. Immunol. 1991;147:3761–3767. [PubMed] [Google Scholar]
- Hmama Z., Knutson K.L., Herrera-Velit P., Nandan D., Reiner N.E. Monocyte adherence induced by lipopolysaccharide involves CD14, LFA-1, and cytohesin-1. Regulation by Rho and phosphatidylinositol 3-kinase. J. Biol. Chem. 1999;274:1050–1057. doi: 10.1074/jbc.274.2.1050. [DOI] [PubMed] [Google Scholar]
- Haziot A.S., Chen S., Ferrero E., Low M., Silber R., Goyert S.M. The monocyte differentiation antigen CD14 is anchored to the cell surface by a phosphatidylinositol linkage. J. Immunol. 1986;141:547–552. [PubMed] [Google Scholar]
- Kirschning C.J., Wesche H., Merrill A.T., Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J.C., Young D.W., Golenbock D.T., Christ W.J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H.W.L., Ulevitch R.J. CD14cell surface receptor and differentiation marker. Immunol. Today. 1994;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- Antal-Szalmas P., Strip J.A., Weersink A.J., Verhoef J., Van Kessel K.P. Quantitation of surface CD14 on human monocytes and neutrophils. J. Leukoc. Biol. 1997;61:721–728. doi: 10.1002/jlb.61.6.721. [DOI] [PubMed] [Google Scholar]
- Zhang D.E., Hetherington C.J., Gonzalez D.A., Chen H.M., Tenen D.G. Regulation of CD14 expression during monocytic differentiation induced with 1α,25-dihydroxyvitamin D3 . J. Immunol. 1994;153:3276–3284. [PubMed] [Google Scholar]
- Martin T.R., Mongovin S.M., Tobias P.S., Mathison J.C., Moriarty A.M., Letrucq D.J., Ulevitch R.J. The CD14 differentiation antigen mediates the development of endotoxin responsiveness during differentiation of mononuclear phagocytes. J. Leukoc. Biol. 1994;56:1–9. doi: 10.1002/jlb.56.1.1. [DOI] [PubMed] [Google Scholar]
- Liu Q., Ning W., Dantzer R., Freund G.G., Kelly K.W. Activation of protein kinase C-ζ and phosphatidylinositol 3′-kinase and promotion of macrophage differentiation by insulin-like growth factor-I. J. Immunol. 1998;160:1393–1401. [PubMed] [Google Scholar]
- James S.Y., Williams M.A., Kelsey S.M., Newland A.C., Colston K.W. The role of vitamin D derivatives and retinoids in the differentiation of human leukaemia cells. Biochem. Pharmacol. 1997;54:625–634. doi: 10.1016/s0006-2952(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Gniadecki R. Nongenomic signaling by vitamin D.A new face of Src. Biochem. Pharmacol. 1998;56:1273–1277. doi: 10.1016/s0006-2952(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Kharbanda S., Saleem A., Emoto Y., Stone R.U., Kufe D. Activation of Raf-1 and mitogen-activated protein kinases during monocytic differentiation of human myeloid leukemia cells. J. Biol. Chem. 1994;269:872–878. [PubMed] [Google Scholar]
- Marcinkowska E., Wiedlocha A., Radzikowski C. 1,25-Dihydroxyvitamin D3 induced activation and subsequent nuclear translocation of MAPK is upstream regulated by PKC in HL-60 cells. Biochem. Biophys. Res. Commun. 1997;241:419–426. doi: 10.1006/bbrc.1997.7832. [DOI] [PubMed] [Google Scholar]
- Fry M.J. Structure, regulation and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta. 1994;1226:237–268. doi: 10.1016/0925-4439(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Toker A., Cantley L.C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Herrera-Velit P., Knutson K.L., Reiner N.E. Phosphatidylinositol 3-kinase-dependent activation of protein kinase C-ζ in bacterial lipopolysaccharide-treated human monocyte. J. Biol. Chem. 1997;272:16445–16452. doi: 10.1074/jbc.272.26.16445. [DOI] [PubMed] [Google Scholar]
- Hara K., Yonezawa K., Sakaue H., Ando A., Kotani K., Kitamura T., Kitamura Y., Ueda H., Stephens L., Jackson T.R. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl. Acad. Sci. USA. 1994;91:7415–7419. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.K., Herrera-Velit P., Brownsey R.W., Reiner N.E. CD14-dependent activation of protein kinase C and mitogen-activated protein kinases (p42 and p44) in human monocytes treated with bacterial lipopolysaccharide. J. Immunol. 1994;153:2642–2652. [PubMed] [Google Scholar]
- Herrera-Velit P., Reiner N.E. Bacterial lipopolysaccharide induces the association and coordinate activation of p53/56lyn and phosphatidylinositol 3-kinase in human monocytes. J. Immunol. 1996;156:1157–1165. [PubMed] [Google Scholar]
- Nandan D., Reiner N.E. TGF-β attenuates the class II transactivator and reveals an accessory pathway of IFN-γ action. J. Immunol. 1997;158:1095–1101. [PubMed] [Google Scholar]
- Baker A.R., McDonnell D.P., Hughes M., Crisp T.M., Mangelsdorf D.J., Haussler M.R., Pike J.W., Shine J., O'Malley B.W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc. Natl. Acad. Sci. USA. 1988;85:3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S., Hiles I., Ormondroyd E., Nizetic D., Antonacci R., Rocchi M., Waterfield M.D. Molecular cloning, cDNA sequence, and chromosomal localization of the human phosphatidylinositol 3-kinase p110α (PIK3CA) gene. Genomics. 1994;24:472–477. doi: 10.1006/geno.1994.1655. [DOI] [PubMed] [Google Scholar]
- Nandan D., Reiner N.E. Attenuation of gamma interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovaniselective inhibition of signaling through Janus kinases and Stat1. Infect. Immun. 1995;63:4495–4500. doi: 10.1128/iai.63.11.4495-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baelen H., Allewaert K., Bouillon R. New aspects of the plasma carrier protein for 25-dihydroxycholecalciferol in vertebrates. Ann. NY Acad. Sci. 1988;538:60–68. doi: 10.1111/j.1749-6632.1988.tb48850.x. [DOI] [PubMed] [Google Scholar]
- Cooke N.E., Haddad J.G. Vitamin D binding protein (Gc-globulin) Endocr. Rev. 1989;10:294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- Kimura K., Hattori S., Kabuyama Y., Shizawa Y., Takayanagi J., Nakamura S., Toki S., Matsuda Y., Onodera K., Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J. Biol. Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- Kaliman P., Vinals F., Testar X., Palacin M., Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J. Biol. Chem. 1996;271:19146–19151. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L.R., Bourette R.P., Lioubin M.N., Algate P.A., Myles G.M., Carlberg K. Growth and differentiation signals regulated by the M-CSF receptor. Mol. Reprod. Dev. 1997;46:96–103. doi: 10.1002/(SICI)1098-2795(199701)46:1<96::AID-MRD15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wymann M.P., Bulgarelli-Leva G., Zvelebil M.J., Pirola L., Vanhaesebroeck B., Waterfield M.D., Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundra R., Kornfeld S. Wortmannin retards the movement of the mannose 6-phosphate/insulin-like growth factor II receptor and its ligand out of endosomes. J. Biol. Chem. 1998;273:3848–3853. doi: 10.1074/jbc.273.7.3848. [DOI] [PubMed] [Google Scholar]
- Liu M., Lee M.H., Cohen M., Bommakanti M., Freedman L.P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- Christakos S., Raval-Pandya M., Wernyj P.P., Yang W. Genomic mechanisms involved in the pleiotropic actions of 1,25-dihydroxyvitamin D3 . Biochem. J. 1996;316:361–371. doi: 10.1042/bj3160361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D.M., Anyochi R., Bhatia M., Meckling-Gill K.A. 1,25-dihydroxyvitamin D3 stimulates expression and translocation of protein kinase Cα and Cδ via a nongenomic mechanism and rapidly induces phosphorylation of a 33-kDa protein in acute promyelocytic NB4 cells. J. Biol. Chem. 1996;271:16090–16096. doi: 10.1074/jbc.271.27.16090. [DOI] [PubMed] [Google Scholar]
- Bhatia M., Kirkland J.B., Meckling-Gill K.A. Monocytic differentiation of acute promyelocytic leukemia cells in response to 1,25-dihydroxyvitamin D3 is independent of nuclear receptor binding. J. Biol. Chem. 1995;270:15962–15965. doi: 10.1074/jbc.270.27.15962. [DOI] [PubMed] [Google Scholar]
- Darwish H.M., DeLuca H.F. Recent advances in the molecular biology of vitamin D action. Prog. Nucleic Acid Res. Mol. Biol. 1996;53:321–344. doi: 10.1016/s0079-6603(08)60149-x. [DOI] [PubMed] [Google Scholar]
- Zhang D.E., Hetherington C.J., Tan S., Dziennis S.E., Gonzalez D.A., Chen H.M., Tenen D.G. Sp1 is a critical factor for the monocytic specific expression of human CD14. J. Biol. Chem. 1994;269:11425–11434. [PubMed] [Google Scholar]
- Gniadecki R. Activation of Raf-mitogen-activated protein kinase signaling pathway by 1,25-dihydroxyvitamin D3 in normal human keratinocytes. J. Invest. Dermatol. 1996;106:1212–1217. doi: 10.1111/1523-1747.ep12348498. [DOI] [PubMed] [Google Scholar]
- Liu M., Freedman L.P. Transcriptional synergism between the vitamin D3 receptor and other nonreceptor transcription factors. Mol. Endocrinol. 1994;8:1593–1604. doi: 10.1210/mend.8.12.7708050. [DOI] [PubMed] [Google Scholar]
- Perez-Fernandez R., Arce V., Freedman L.P. Delineation of a DNA recognition element for the vitamin D3 receptor by binding site selection. Biochem. Biophys. Res. Commun. 1993;192:728–737. doi: 10.1006/bbrc.1993.1475. [DOI] [PubMed] [Google Scholar]
- Bourette R.P., Myles G.M., Choi J.L., Rohrschneider L.R. Sequential activation of phosphatidylinositol 3-kinase and phospholipase C-γ2 by the M-CSF receptor is necessary for differentiation signaling. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5880–5893. doi: 10.1093/emboj/16.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Angelotti T., Niederfellner G., Herbst R., Ullrich A. Activation of phosphatidylinositol 3-kinase is necessary for differentiation of FDC-P1 cells following stimulation of type III receptor tyrosine kinases. Cell Growth Differ. 1998;9:247–256. [PubMed] [Google Scholar]
- Marcinkowska E., Wiedlocha A., Radzikowski C. Evidence that phosphatidylinositol 3-kinase and p70S6K protein are involved in differentiation of HL-60 cells induced by calcitriol. Anticancer Res. 1998;18:3507–3514. [PubMed] [Google Scholar]
- Noti J.D., Reinemann B.C. The leukocyte integrin gene CD11c is transcriptionally regulated during monocyte differentiation. Mol. Immunol. 1995;32:361–369. doi: 10.1016/0161-5890(94)00164-v. [DOI] [PubMed] [Google Scholar]
- Hmama Z., Gabathuler R., Jefferies W.A., de Jon G., Reiner N.E. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. J. Immunol. 1998;161:4882–4893. [PubMed] [Google Scholar]
- Caron E., Liautard J.P., Kohler S. Differentiated U937 cells exhibit increased bactericidal activity upon LPS activation and discriminate between virulent and avirulent Listeria and Brucella species. J. Leukoc. Biol. 1994;56:174–181. doi: 10.1002/jlb.56.2.174. [DOI] [PubMed] [Google Scholar]
- Kang C.D., Lee B.K., Kim K.W., Kim C.M., Kim S.H., Chung B.S. Signaling mechanism of PMA-induced differentiation of K562 cells. Biochem. Biophys. Res. Commun. 1996;221:95–100. doi: 10.1006/bbrc.1996.0551. [DOI] [PubMed] [Google Scholar]
- Herrera R., Hubbell S., Decker S., Petruzzelli L. A role for the MEK/MAPK pathway in PMA-induced cell cycle arrestmodulation of megakaryocytic differentiation of K562 cells. Exp. Cell Res. 1998;238:407–414. doi: 10.1006/excr.1997.3847. [DOI] [PubMed] [Google Scholar]
- de Boland A.R., Norman A.W. 1α,25(OH)2-vitamin D3 signaling in chick enterocytesenhancement of tyrosine phosphorylation and rapid stimulation of mitogen-activated protein (MAP) kinase. J. Cell. Biochem. 1998;69:470–482. doi: 10.1002/(sici)1097-4644(19980615)69:4<470::aid-jcb8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Hsieh J.C., Jurutka P.W., Galligan M.A., Terpening C.M., Haussler C.A., Samuels D.S., Shimizu Y., Shimizu N., Haussler M.R. Human vitamin D receptor is selectively phosphorylated by protein kinase C on serine 51, a residue crucial to its trans-activation function. Proc. Natl. Acad. Sci. USA. 1991;88:9315–9319. doi: 10.1073/pnas.88.20.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurutka P.W., Hsieh J.C., Nakajima S., Haussler C.A., Whitfield G.K., Haussler M.R. Human vitamin D receptor phosphorylation by casein kinase II at Ser-208 potentiates transcriptional activation. Proc. Natl. Acad. Sci. USA. 1996;93:3519–3524. doi: 10.1073/pnas.93.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farach-Carson M.C., Ridal A.L. Dual 1,25-dihydroxyvitamin D3 signal response pathways in osteoblastscross-talk between genomic and membrane-initiated pathways. Am. J. Kidney Dis. 1998;31:729–742. doi: 10.1053/ajkd.1998.v31.pm9531195. [DOI] [PubMed] [Google Scholar]