Abstract
Interleukin (IL)-1β is a pleiotropic cytokine implicated in a variety of activities, including damage of insulin-producing cells, brain injury, or neuromodulatory responses. Many of these effects are mediated by nitric oxide (NO) produced by the induction of NO synthase (iNOS) expression. We report here that IL-1β provokes a marked repression of genes, such as fragile X mental retardation 1 (FMR1) and hypoxanthine phosphoribosyltransferase (HPRT), having a CpG island in their promoter region. This effect can be fully prevented by iNOS inhibitors and is dependent on DNA methylation. NO donors also cause FMR1 and HPRT gene silencing. NO-induced methylation of FMR1 CpG island can be reverted by demethylating agents which, in turn, produce the recovery of gene expression. The effects of IL-1β and NO appear to be exerted through activation of DNA methyltransferase (DNA MeTase). Although exposure of the cells to NO does not increase DNA MeTase gene expression, the activity of the enzyme selectively increases when NO is applied directly on a nuclear protein extract. These findings reveal a previously unknown effect of IL-1β and NO on gene expression, and demonstrate a novel pathway for gene silencing based on activation of DNA MeTase by NO and acute modification of CpG island methylation.
Keywords: interleukin 1β, nitric oxide, FMR1, CpG island methylation, gene repression
Methylation status of control regions in the genome plays a critical role in the regulation of gene expression 1 2 3. In susceptible genes containing 5′ CpG islands, cytosine methylation favors a repressive chromatin structure that prevents the binding of transcriptional activators to the promoter 4 5. The molecular link between methyl groups on the DNA and the positioning of nucleosomes to form an inactive chromatin configuration has been recently elucidated 6. Well-known examples of methylation-dependent gene silencing are X-linked gene inactivation 7 and genomic imprinting 8, and changes in the pattern of DNA methylation occur during cell differentiation 9 10 and tumorigenesis 11 12 13 14. Hypermethylation of CpG islands can be associated with the silencing of some genes, such as fragile X mental retardation 1 (FMR1), causing inherited mental diseases 15 16. Differential genomic DNA methylation also has the potential to influence the development of T cell cytokine production profiles 17. Here, we report that exposure to IL-1β (acting through inducible nitric oxide synthase [iNOS] induction) or direct application of NO donors induces in several cell types the suppression of the expression of FMR1 and other housekeeping genes containing a CpG island in their promoter. This effect is shown to be produced by DNA methylation resulting from activation of DNA methyltransferase (DNA MeTase). These observations demonstrate that IL-1β and NO, which are messenger molecules involved in a wide variety of pathophysiological processes, can have a direct effect on gene expression.
Materials and Methods
Materials.
IL-1β and IFN-γ were purchased from Genzyme. S-nitroso-N-acetylpenicillamine (SNAP), N-methyl arginine (L-NMA and D-NMA), actinomycin D (ActD), trichostatin A (TSA), 5-aza-2′-deoxycytidine (AzadC), dithiothreitol (DTT), β-mercaptoethanol (β-ME), and protease inhibitors were obtained from Sigma Chemical Co. Sodium nitroprusside (SNP) was from Fluka. 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine hydrochloride (AMT), S-ethylisothiourea hydrobromide (EIT), and l-N 6-(1-iminoethyl)-lysine hydrochloride (L-NIL) were from Tocris Cookson, Ltd. 3-morpholinosydnonimine hydrochloride (SIN) was from ICN Iberica. Rediprime® DNA labeling system, [32P]dCTP, and cold and 3H-labeled S-adenosylmethionine were from Nycomed Amersham plc. Glutathione (GSH) and poly deoxyinosine-deoxycytosine (poly dI-dC) were from Boehringer Mannheim. Restriction enzymes were from Promega or New England Biolabs. All other reagents were of the best quality commercially available.
Cell Culture.
Insulin-producing rat RINm5F (RIN) cell, Jurkat T cell, and mouse leukemic monocyte-macrophage cell (RAW 264 cell) lines were grown in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B under 5% CO2 at 37°C. Human lymphocytes were obtained from peripheral blood of healthy donors as reported previously 18.
Reverse Transcription PCR.
Total RNA was extracted from cell lines or fresh peripheral lymphocytes by the guanidine phenol method. RNA was reverse transcribed using random hexamers, and the cDNA was amplified using specific primers. PCR amplification of the CGG repeats at the FRAXA site and KH domains was carried as reported previously 18 19. Amplification of hypoxanthine phosphoribosyltransferase gene (HPRT) in RIN cells was assessed using murine primers. Human specific primers were used for HPRT mRNA analysis in Jurkat T cells and fresh peripheral lymphocytes 7. Reverse transcription (RT)-PCR of ATP-ase or glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) was used as control. PCR products were visualized on agarose gel stained with ethidium bromide.
Northern and Western Blots.
Northern blot of FMR1 gene was performed using 10 μg total RNA and 10 ng/ml of human FMR1 cDNA probe labeled with [α-32P]dCTP. Hybridization conditions were: 16 h at 42°C in 50% formamide, 6× saline-sodium phosphate-EDTA (SSPE), 5× Denhardt's solution, 0.5% SDS, 100 μg/ml herring sperm DNA. Wash conditions were: 2× SSPE, 0.1% SDS at room temperature and 0.1× SSPE, 0.1% SDS at 55°C. DNA MeTase expression was assayed with the same protocol using a specific 5-kb cDNA probe. Northern blot of iNOS and GAPDH was assayed by standard procedures. Western blot analysis of DNA MeTase was performed using 20–40 μg of nuclear protein extract resolved on 5% SDS-PAGE, transferred onto polyvinylidene difluoride membrane, and subjected to immunodetection using a 1:2,000 dilution of primary antibody and an enhanced chemiluminescence detection 13.
Southern Blot.
DNA samples were prepared from cultured cell lines by standard procedures. 10 μg of genomic DNA was digested overnight with the restriction enzymes EcoRI-EagI or HindIII-SacII, EagI and SacII being sensible to methylation. Restriction fragments were separated by electrophoresis on 0.8% agarose gel, Southern blotted, and hybridized with radiolabeled StB12.3 probe as described previously 20.
DNA MeTase Assay.
DNA MeTase activity was determined in nuclear protein extracts by the assay developed by Adams et al. 21 with minor modifications. Cells were lysed in buffer containing 20 mM Tris-HCl, pH 8, 137 mM NaCl, 5 mM MgCl2, 5 mM EDTA, 10% glycerol, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 100 μg/ml RNase. After centrifugation, nuclear extracts were prepared by resuspension of the crude nuclei in high salt buffer. 15–25 μg of proteins was incubated for 2 h at 37°C with 4 μg of poly (dI-dC) as template and 5.25 μM 3H-labeled S-adenosylmethionine (1 μCi; Amersham Pharmacia Biotech) as methyl donor. Reactions were stopped, proteins extracted, and DNA template was recovered by ethanol precipitation. RNA was removed by resuspension of the precipitates in NaOH; DNA was spotted on Whatman filters, dried, and then washed with trichloroacetic acid (5%) followed by ethanol, then ether. Filters were placed in the scintillation mixture, and DNA MeTase activity was determined by scintillation counting. Results were expressed as cpm per microgram of protein; all experiments were performed in duplicate. Background levels were determined in assays where poly (dI-dC) was omitted. Statistical analyses were performed using Student's t test.
Other Enzymatic Assays.
Lactate dehydrogenase (LDH) and pyruvate kinase (PK) were measured by standard procedures in the 12,000 g supernatant of Jurkat T cell homogenate as described previously 22. Hexokinase (HK) was measured in the homogenate of Jurkat T cells as reported elsewhere 23. Statistical analyses were performed using Student's t test.
Cell Proliferation Assay.
Cellular proliferation was determined by a colorimetric assay system using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) following the manufacturer's instructions (Cell Proliferation Kit I; Boehringer Mannheim).
Results and Discussion
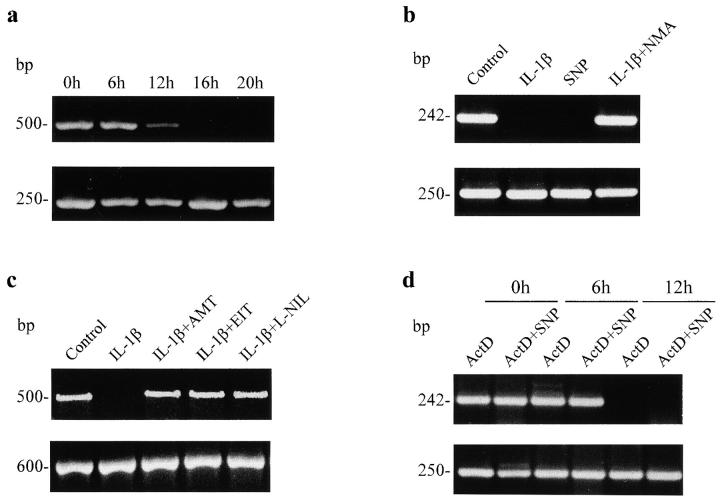
Fragile X syndrome, the most common form of hereditary mental retardation 24, results from repression of the FMR1 gene due to the expansion of the CGG repeats in its first exon and methylation of the 5′ CpG island. The latter alteration appears to be the primary cause of the disease, since hypermethylation of the CpG island in the active X chromosome is only observed in affected individuals, whereas there are cases with full expansion of the CGG repeats but with an unmethylated island that do not manifest the syndrome 25 26. Furthermore, in vitro reactivation of the FMR1 gene by demethylating agents has been reported recently 27. We have observed a marked inhibitory effect of IL-1β on FMR1 gene expression in RIN cells assessed by RT-PCR of both KH domains and CGG repeats (Fig. 1, a–c). Inhibition of FMR1 expression was appreciable after 12 h of incubation with IL-1β, and complete suppression of the gene resulted with longer exposures (Fig. 1 a). Since IL-1β is known to be a powerful stimulus for induction of NOS in RIN and other cell types 28 29, we investigated whether NO acted as a mediator of FMR1 repression. Fig. 1 b shows that SNP, an NO donor, mimics the action of IL-1β, and that the IL effect is fully prevented by the simultaneous addition of L-NMA, an inhibitor of NOS activity. This preventive effect was not observed when we used D-NMA (not shown). To further demonstrate that IL-1β exerts gene silencing via NO production, we used specific iNOS inhibitors such as AMT, EIT, and L-NIL and found that all of them also prevented the action of IL-1β (Fig. 1 c). To determine if FMR1 mRNA stability was altered by NO, ActD was used to inhibit RNA synthesis. As shown in Fig. 1 d, the time course of FMR1 mRNA degradation was not modified by the presence of SNP. Thus, production of NO by IL-1β or addition of NO precursors can produce FMR1 gene silencing. In preliminary experiments, we have observed that IFN-γ, which induces NO synthesis, as well as NO donors can also inhibit FMR1 expression in a monocyte-macrophage cell line (RAW 264 cells).
Figure 1.
FMR1 expression in RIN cells treated with IL-1β and NO donors. In this and other figures, RT-PCR of KH domains and CGG repeats of FMR1 give bands of 500 and 242 bp, respectively; Na/K ATP-ase (250 bp) and GAPDH (600 bp) were used as control. (a) Time course of IL-1β (25 U/ml) induced FMR1 repression in RIN cells. (b) 16 h treatment with IL-1β (25 U/ml), SNP (100 μM), or IL-1β (25 U/ml) plus NMA (100 μM). (c) 16 h treatment with IL-1β (25 U/ml) or IL-1β plus a specific iNOS inhibitor (20 nM AMT, 100 nM EIT, or 10 μM L-NIL). (d) Different exposure times of RIN cells with ActD alone (5 μg/ml) or added simultaneously with SNP (100 μM). Data shown in this and the other figures are representative of two to six experiments.
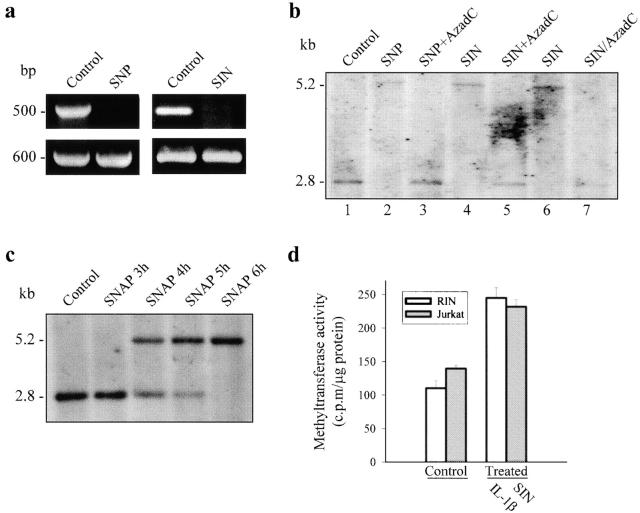
To elucidate the mechanisms underlying the effect of NO on gene regulation, we studied the expression of FMR1 in human cells (Jurkat T cells and fresh lymphocytes) where the complete map of the FMR1 promoter is known 30. Although these cells are not stimulated by IL-1β, the application of NO precursors (500 μM SNP and 100 μM SIN) resulted in complete suppression of FMR1 expression (Fig. 2 a; see also Fig. 3 b), thus allowing a more detailed analysis of the repression of FMR1 gene by NO. Since it has been reported that NO can produce DNA damage by disruption of nucleotide bonds 31, we tested if the effect of NO could be explained by direct interaction with CG sites, particularly abundant in (CGG)n repeats and CpGs of FMR1. The fact that we were unable to induce CG cleavage in the FMR1 gene by NO donors in cellular and cell-disrupted preparations (not shown) led us to hypothesize that NO could be part of a signaling pathway regulating methylation of the CpG island in the FMR1 promoter region. This was tested by Southern blot using methyl-sensitive restriction enzymes and the StB12.3 probe currently applied to study the length and the methylation status of FMR1 in fragile X patients 20 24. Fig. 2 b shows that treatment of Jurkat T cells with SNP or SIN produced full CpG island methylation (lanes 2, 4, and 6), which was totally prevented when the cells were treated with the demethylating agent AzadC in the presence of any of the NO donors (lanes 3 and 5). In the experiments where we applied an NO donor (or IL-1β) plus AzadC, we incubated the cells for 24 h as has been recommended when using the demethylating agent 7. After incubation with and washout of SIN, addition of AzadC also reverted the methylating action of NO (lane 7), indicating that the effect of AzadC was not due to direct chemical interaction with SIN. CpG methylation induced by NO donors (SIN or SNAP) progressed with time after the initial 3 h of treatment, and full methylation was observed at 6 h (Fig. 2 c).
Figure 2.
NO-induced FMR1 silencing and methylation of FMR1 CpG island in Jurkat T cells. (a) RT-PCR of FMR1 KH domains after 16 h incubation with SNP (500 μM) or SIN (100 μM). GAPDH was used as control. (b) Southern blot of genomic DNA digested to completion with EcoRI-EagI. The 2.8-kb fragment results from cleavage by both enzymes, whereas the 5.2-kb fragment corresponds to digestion with EcoRI, indicating protection from restriction by methylation. Untreated cells (lane 1); cells incubated for 24 h with SNP (500 μM) or SIN (100 μM) (lanes 2 and 4); cells treated for 24 h simultaneously with SNP (500 μM) plus AzadC (2 μg/ml) or SIN (100 μM) plus AzadC (2 μg/ml) (lanes 3 and 5); cells incubated for 16 h with SIN (100 μM) (lane 6); and cells exposed for 16 h to SIN (100 μM), washed, and incubated for 24 h with AzadC (2 μg/ml) (lane 7). (c) Methylation pattern induced by 3, 4, 5, or 6 h incubation with SNAP (100 μM). (d) DNA MeTase activity in RIN and Jurkat T cells treated for 16 h with IL-1β (25 U/ml) and for 6 h with SIN (100 μM), respectively, expressed as cpm/μg protein (100 cpm/μg protein corresponds to a specific activity of 0.015 pmol/h/μg protein).
Figure 3.
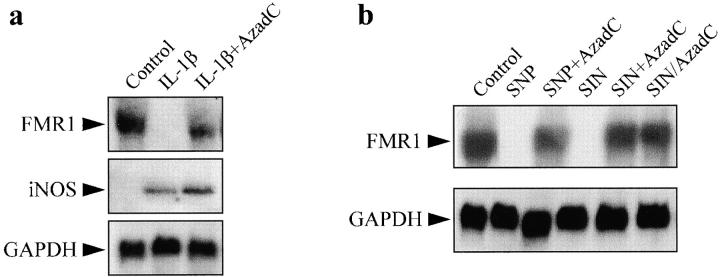
FMR1 expression in RIN and Jurkat T cells incubated with IL-1β or NO donors and the demethylating agent AzadC. (a) Northern blot of FMR1 and iNOS in RIN cells incubated for 24 h with IL-1β (25 U/ml) in the absence or presence of AzadC (2 μg/ml). (b) Northern blot of FMR1 in Jurkat T cells following the same protocol as in the legend to Fig. 2 b, except for the condition shown in lane 6. GAPDH expression was used as control.
To further investigate the NO-dependent methylation process, we measured the activity of DNA MeTase, the major DNA methylating enzyme that produces 5′ methylcytosine 32. Incubation of RIN cells with IL-1β increased DNA MeTase activity to about twice the level found in control cells (P < 0.001, n = 3). A qualitatively similar effect was observed in Jurkat T cells exposed to an NO donor (P < 0.001, n = 3; Fig. 2 d). These increases in activity are within the range of those recently published in transformed rodent cells overexpressing c-fos 33. Since NO-induced methylation of the CpG island is abolished by the presence of the demethylating agent AzadC (see Fig. 2 b), it is expected that addition of this product would prevent FMR1 silencing induced by either IL-1β or NO. Fig. 3 a illustrates that FMR1 suppression produced by IL-1β was almost completely reverted by incubation with the demethylating agent. It is also shown that AzadC did not diminish the level of expression of iNOS induced by IL-1β. Similarly, demethylation also prevented FMR1 suppression resulting from exposure of the cells to NO donors (Fig. 3 b). These data indicate that NO-dependent FMR1 gene silencing results from methylation of the CpG island, an effect mediated by activation of DNA MeTase.
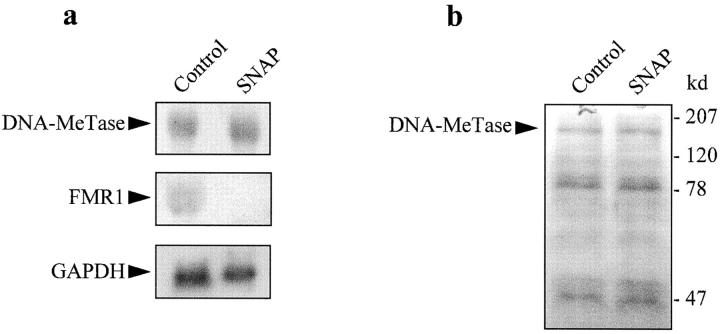
The major mammalian DNA MeTase is a large protein with an NH2-terminal putative regulatory domain comprising two thirds of the protein with eight cysteine residues, and a COOH-terminal catalytic domain with the adenosylmethionine binding region and a proline-cysteine catalytic center 34. The signals and mechanisms involved in regulation of DNA MeTase activity are poorly understood. The NH2 terminus is unnecessary for catalysis, but its cleavage from the COOH terminus causes a large stimulation of the initial velocity of methylation of unmethylated DNA 34. The NH2 terminus contains a major phosphorylation site (serine 514) although its relevance in catalysis is uncertain, since treatment of the enzyme with phosphatases and kinases results in no significant effect on the catalytic rate 35. We assayed whether the effect of NO on DNA MeTase activity was due to activation of guanyl cyclase, by incubation of Jurkat T cells with 2 mM dibutyryl cGMP for 24 h. cGMP had no effect on either DNA MeTase activity or the expression of FMR1 gene (not shown). The Ras signaling pathway has been shown to increase DNA MeTase transcription 36, and recently it has been suggested that fos may transform cells through alterations in DNA methylation 33. Therefore, we tested if the expression of DNA MeTase could be altered by exposure of Jurkat T cells to an NO donor. Northern and Western blot analyses, shown in Fig. 4, a and b, respectively, indicate that NO does not affect the expression of the major human DNA MeTase. We have not studied the expression of the recently described DNA MeTase3A and DNA MeTase3B 37; however, it is very unlikely that they mediate the effects of NO described here, since these two enzymes are present mainly in embryonic tissues 37. Moreover, NO was able to activate DNA MeTase in a nuclear protein extract (see below), thus strongly suggesting that the regulation of the enzyme by NO is not dependent on transcription.
Figure 4.
Effect of NO on the expression of DNA MeTase in Jurkat T cells. (a) Northern blot of DNA MeTase in cells incubated for 16 h with 100 μM SNAP. FMR1 and GAPDH were used as control. (b) Western blot of DNA MeTase using a polyclonal antibody in cells incubated for 16 h with 100 μM SNAP.
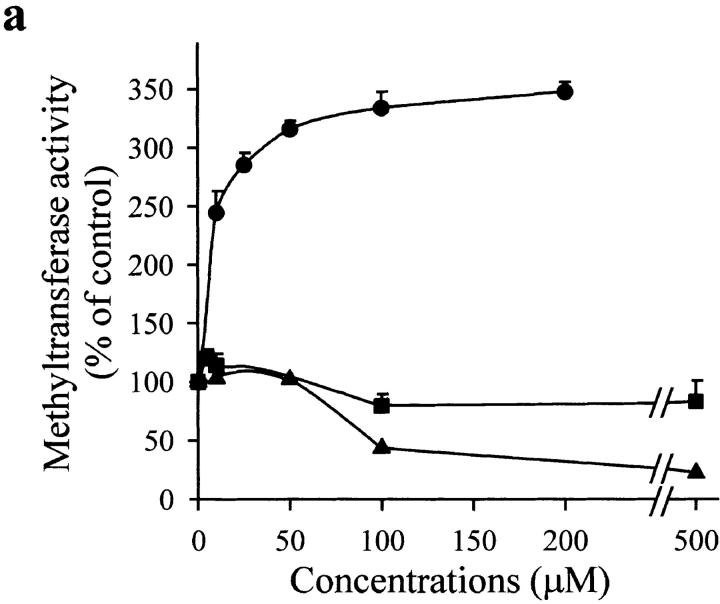
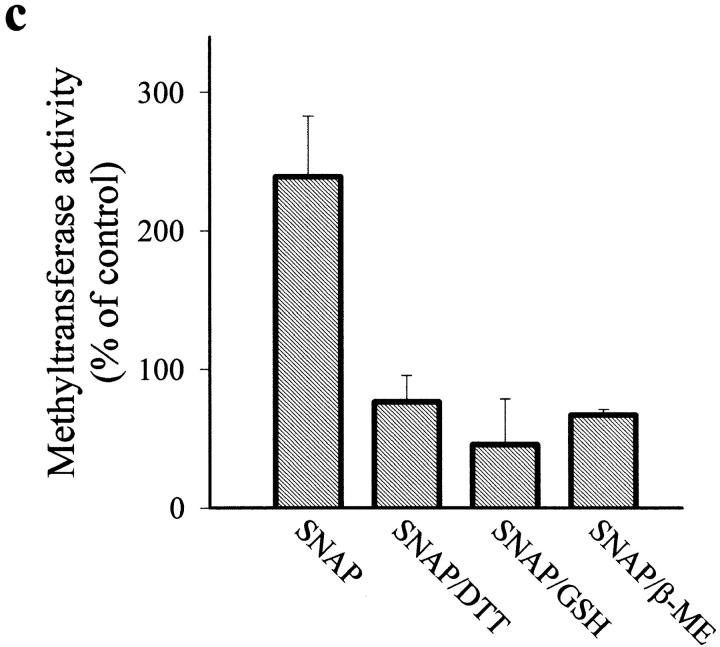
The Western blot in Fig. 4 b also shows that NO did not modify the magnitude or size of any of the bands obtained with the polyclonal antibody against the DNA MeTase, thus indicating that cleavage of the NH2-terminal regulatory domain is probably not involved in the mechanism of action of NO. However, the presence of several cysteine residues in the protein suggested the possibility of a direct reaction of NO with thiols. Fig. 5 a shows that application of an NO donor (SNAP) to a nuclear protein extract induced a dose-dependent increase of DNA MeTase activity. In addition, sodium nitrite and peroxynitrite at different concentrations did not increase the enzymatic activity. On the contrary, high concentration of peroxynitrite drastically inhibited the reaction. Fig. 5 b illustrates the time course of DNA MeTase activation induced by 50 μM of different NO donors (SNAP, SIN, and SNP) in the nuclear protein extract. In all cases, after 3 h incubation with the NO donor a statistically significative increase in the DNA MeTase activity was observed (P < 0.001, n = 3 for SNAP; P < 0.001, n = 2 for SIN and SNP); however, no change was obtained when expired SNAP or SIN was used (more than eight half-lives). Thiol oxidation independent of NO did not seem to play a role in the action of NO donors on DNA MeTase activity, since similar effects were seen when superoxide dismutase and catalase were added to the reaction mixture (not shown). Enhancement of DNA MeTase activity induced by SNAP was completely reversed by further incubation, after washout of the NO donor, with reducing agents such as DTT, GSH, or β-ME (Fig. 5 c). These results strongly suggest that NO, either directly or through mediators present in the nuclear extract, regulates DNA MeTase activity possibly by nitrosation of some cysteines present in the protein.
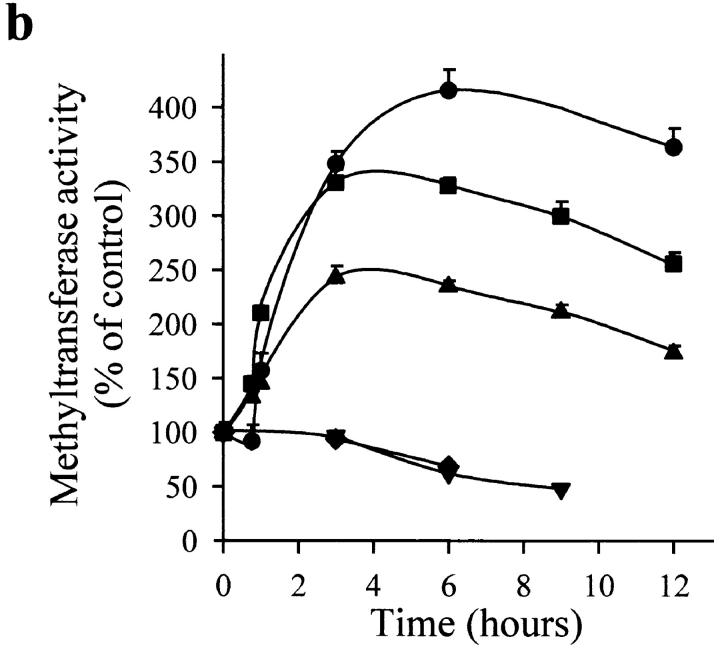
Figure 5.
Effect of NO on DNA MeTase activity in a nuclear protein extract of Jurkat T cells. (a) Dose-dependent effect of 3 h incubation with SNAP (•), sodium nitrite (▪), and peroxynitrite (▴) on the activity of DNA MeTase. (b) Time course of DNA MeTase activity in the presence of 50 μM SNAP (•), SNP (▪), SIN (▴), expired SIN (▾), or expired SNAP (♦). Values in plots a and b are expressed in percentage of control values by mean ± SEM of three or two independent experiments in duplicate. (c) Effect of reducing agents on DNA MeTase activity stimulated by 50 μM SNAP. The nuclear protein extract was incubated for 3 h without (control) or with 50 μM SNAP. Nuclear protein extracts were recovered, after washing, by centrifugation or by filtration in a Sephadex G-25 spin column. Samples were further incubated for 2 h with no addition (control and SNAP) or in the presence of 5 mM DTT (SNAP/DTT), 100 μM GSH (SNAP/GSH), or 100 μM β-ME (SNAP/β-ME). Values are expressed in percentage of control values by mean ± SEM of at least three independent experiments in duplicate. All the values of DNA MeTase activity in the presence of the reducing agents were significantly different (P < 0.001) from the value of enzymatic activity in the absence of the agents. The average DNA MeTase activity in basal conditions was 0.017 ± 0.005 (n = 15) pmol/h/μg protein. In each experiment, the basal DNA MeTase activity was set to 100%.
To evaluate the selectivity of the stimulatory effect of NO on DNA MeTase, we measured in cell extracts the activity of other enzymes, such as lactate dehydrogenase (LDH), hexokinase (HK), and pyruvate kinase (PK), encoded by genes that are constitutively expressed in all cells and are involved in housekeeping functions. Cell extracts were incubated for 3 h in the absence or presence of the stimulus. Basal activities (given by mean ± SD) were 0.02 ± 0.006 (n = 6) pmol/h/μg protein for DNA MeTase, 110 ± 22 (n = 5) pmol/min/μg protein for LDH, 12 ± 5 (n = 3) pmol/min/μg protein for HK, and 27 ± 11 (n = 3) pmol/min/μg protein for PK. Table shows that whereas 50 μM SNAP induced a marked increase in DNA MeTase activity, it had no effect on the activities of any of the other enzymes studied. Thus, in cell extracts, NO exerts a selective effect on DNA MeTase.
Table 1.
Effect of NO on the Activity of Several Cellular Enzymes
| DNA MeTase | LDH | HK | PK | |
|---|---|---|---|---|
| Activity induced by SNAP (% of control) | 366 ± 90 (n = 6) | 105 ± 17 (n = 5) | 100 ± 1 (n = 3) | 107 ± 12 (n = 3) |
Cell extracts were incubated with 50 μM SNAP for 3 h. Results are expressed by mean ± SEM of independent experiments in duplicate (number of experiments in parenthesis.
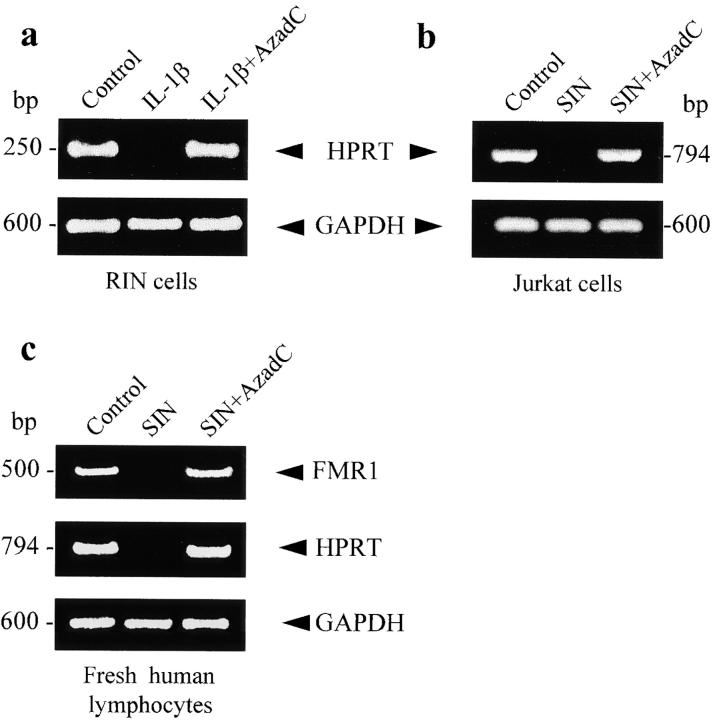
Given that the inhibitory effect of NO on FMR1 expression can be explained by activation of DNA MeTase and methylation of the CpG island, we explored if a similar action is exerted on other genes, such as HPRT, known to contain a CpG island in the promoter region. Fig. 6 shows that exposure of RIN and Jurkat T cells to IL-1β (a) and NO donors (b), respectively, resulted in abolishment of HPRT expression. In both cases, demethylation with AzadC produced recovery of gene expression. NO-dependent methylation of CpG islands observed in transformed cells (such as RIN and Jurkat T cells) was also clearly apparent in fresh human lymphocytes. Fig. 6 c illustrates the silencing of FMR1 and HPRT genes by NO donors and the reversion of this effect by AzadC. The expression of genes such as GAPDH or Na/K ATP-ase, which do not contain CpG-rich promoters, is unaffected by NO (Fig. 6, a–c; see also Fig. 1 Fig. 2 Fig. 3).
Figure 6.
HPRT expression in RIN and Jurkat T cells assessed by RT-PCR. (a) RIN cells unstimulated and treated for 24 h with IL-1β (25 U/ml) in the absence or presence of AzadC (2 μg/ml). (b) Similar protocol used with Jurkat T cells stimulated with 100 μM SIN. (c) RT-PCR of FMR1 and HPRT from fresh peripheral lymphocytes incubated for 24 h with SIN (100 μM) in the absence or presence of AzadC (2 μg/ml). RT-PCR of GAPDH was used as control.
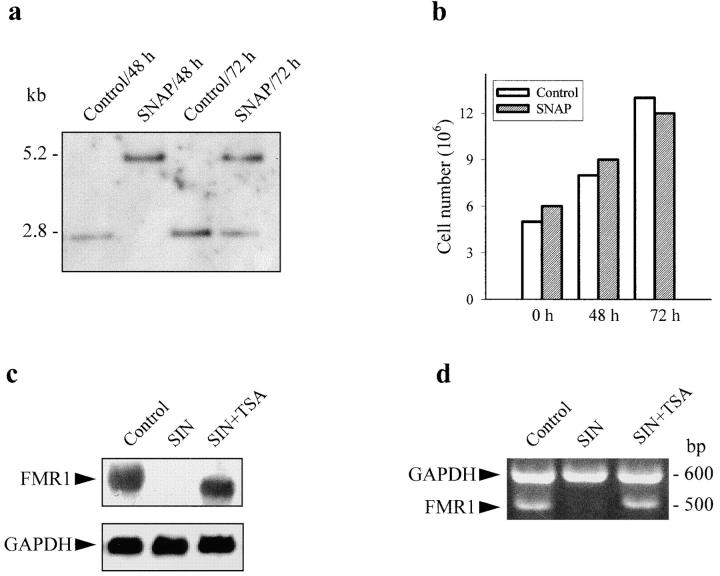
NO-induced DNA methylation was maintained for several hours after washout of the signal; however, it was a reversible phenomenon. In mitotically active cells (such as Jurkat T cells) previously exposed to NO donors, a unique and methylated band was observed by Southern blot 48 h after removal of the stimulus, whereas after 72 h two bands, methylated and unmethylated, were present (Fig. 7 a). After 48 or 72 h incubation, we observed a clear increase in the number of cells (Fig. 7 b). These results indicate that DNA methylation appears to be transient, due to either loss of methylation in the new generation of dividing cells or the existence of active DNA demethylation. This latter mechanism is suggested by the fact that reversibility of NO-induced methylation was also observed in nondividing cells such as quiescent fresh lymphocytes (not shown), and is in accordance with a recent report describing the existence of DNA demethylases in human cells 38.
Figure 7.
Reversibility of CpG island methylation in dividing cells and reversion of FMR1 expression by a deacetylase inhibitor. (a) Jurkat T cells were incubated for 16 h with 100 μM SNAP. Methylation of the CpG island of FMR1 was measured by Southern blot at 48 and 72 h after washout of the stimulus. Control cells were treated in the same way but without exposure to SNAP. (b) Cell number was measured in the same experimental conditions as in a, with the MTT cell proliferation kit. (c) Northern blot of FMR1 in Jurkat T cells incubated for 24 h in the absence or presence of 2 μM TSA, an inhibitor of deacetylases. (d) RT-PCR of KH domain of FMR1 in the same experimental conditions as in c. In both cases, GAPDH was used as control.
DNA methylation causes repression of gene expression by promoting the condensation of chromatin. Methylated sites on DNA bind the 5-methylcytosine binding protein (MeCP2) that exists in a complex with Sin3A and histone deacetylase, resulting in a compact chromatin structure 6. To investigate whether NO-mediated gene silencing requires histone deacetylation, we tested the effect of TSA, an inhibitor of deacetylases 33. Fig. 7c and Fig. d, show that suppression of FMR1 expression (determined by both Northern blot and RT-PCR, respectively) was prevented by incubation of the cells with 2 μM TSA, hence supporting the view that NO-induced gene repression is due to increased recruitment of histone deacetylases by methylated DNA.
NO is a broadly distributed signaling molecule involved in numerous physiological and pathophysiological processes 39 40 41, but its action on the genome is poorly understood. Moreover, many actions of IL-1β are mediated by the induction of iNOS and the resulting NO production 42 43. We show that by activating DNA MeTase, NO can induce methylation of 5′ CpG islands and, hence, repress gene expression. Methylation/demethylation of DNA is known to be associated with X-linked gene inactivation, imprinting, and fragile X syndrome 7 8 15. Changes in DNA methylation are also observed during development, the acquisition of T cell cytokine production profiles, and tumorigenesis 9 10 11 12 13 14 17, and several transcription factors actively promoting DNA demethylation have been reported 9 44. Our findings provide the first case of FMR1 gene silencing in situations other than fragile X syndrome, although the methylation status in the two conditions shows some differences. Methylation induced by NO was lost with time, and TSA reversed the inhibitory effect on gene expression induced by NO (see above). In contrast, it has been recently reported that TSA has no effect on transcription in cells from fragile X patients due to additional modifications in histone–DNA association 45. The marked repressive effect of IL-1β and NO on the expression of housekeeping genes, such as FMR1 and HPRT, with a CpG island in the promoter might be part of a general adaptive mechanism triggered in cells challenged by stressing situations.
It has been reported that nuclear factor κB (NF-κB) in B cells can induce specific demethylation of the Igκ locus 46, and in some cells, including RIN cells, IL-1β receptor stimulation induces a cascade that activates NF-κB 47 48. In this work, we have shown that IL-1β clearly represses gene expression by a mechanism involving methylation. Our data indicate that the possible demethylating activity of NF-κB in RIN cells does not counterbalance the increase in activity of the DNA MeTase. Similarly, in Jurkat T cells the incubation with PMA plus a calcium ionophore (A23187), which induce NF-κB 49, does not prevent the inhibitory action of NO on gene expression (results not shown). However, it could be speculated that IL-1β transitorily represses housekeeping genes having CpG islands via NO production and methylation while inducing tissue/stage-specific gene expression by activating demethylation via NF-κB. For instance, it has been reported that Ras induces an increase in demethylase activity in parallel to its induction of transcription of DNA MeTase 36.
Methylation of cytosine also gives an explanation for the high occurrence of genomic C–T transitions observed under exposure to NO 50. The abundance of 5-methylcytosine in methylated DNA favors the transition to thymine by simple deamination, which can occur spontaneously and is potentiated by NO 51. Finally, given the resemblance between certain viruses and housekeeping promoters 1, the reported antiviral action of NO 52 could be explained by methylation-dependent silencing of the viral genome. Interestingly, DNA MeTase is a housekeeping gene without 5′ CpG island and, thus, its expression is insensitive to NO (see above). In contrast, it has a specific promoter containing activating protein (AP)-1, AP-2, and glucocorticoid response elements 53, suggesting a potentially high level of regulation by cellular signal transduction pathways.
In conclusion, we report here a novel action of IL-1β mediated by NO production. NO induces a posttranscriptional increase in the activity of DNA MeTase, resulting in CpG island methylation and suppression of gene expression. These results give new insights into the pathophysiological regulation of genes with CpG-rich promoters.
Acknowledgments
We would like to express our gratitude to Dr. J. López-Barneo for encouragement and suggestions on this work, and for help in writing the manuscript. We thank Dr. M. Szyf for the generous gift of the cDNA probe and polyclonal antibodies to study DNA MeTase gene expression. We also thank Dr. J.L. Mandel for the StB12.3 probe used in the Southern blot tests, and Dr. A.J. Verkerk for the cDNA probe used to study FMR1 gene expression.
This work is supported by grants from the Comisión Interministerial de Ciencia y Tecnología (SAF96-0205) and Servicio Andaluz de Salud.
Footnotes
Abbreviations used in this paper: ActD, actinomycin D; AMT, 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine; AzadC, 5-aza-2′-deoxycytidine; β-ME, β-mercaptoethanol; DNA MeTase, DNA methyltransferase; DTT, dithiothreitol; EIT, S-ethylisothiourea; FMR1, fragile X mental retardation 1 gene; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GSH, glutathione; HK, hexokinase; HPRT, hypoxanthine phosphoribosyltransferase; iNOS, inducible NOS; LDH, lactate dehydrogenase; L-NIL, l-N 6-(1-iminoethyl)-lysine; NF-κB, nuclear factor κB; NMA, N-methyl arginine; NO, nitric oxide; NOS, NO synthase; PK, pyruvate kinase; RIN, RINm5F; RT, reverse transcription; SIN, 3-morpholinosydnonimine hydrochloride; SNAP, S-nitroso-N-acetylpenicillamine; SNP, sodium nitroprusside; SSPE, saline-sodium phosphate-EDTA; TSA, trichostatin A.
References
- Bird A.P. CpG islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Razin A., Cedar H. DNA methylation and gene expression. Microbiol. Rev. 1991;55:451–458. doi: 10.1128/mr.55.3.451-458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. The essentials of DNA methylation. Cell. 1992;70:5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- Tate P.H., Bird A.P. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr. Opin. Genet. Dev. 1993;3:226–231. doi: 10.1016/0959-437x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- Eden S., Cedar H. Role of DNA methylation in the regulation of transcription. Curr. Opin. Genet. Dev. 1994;4:255–259. doi: 10.1016/s0959-437x(05)80052-8. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing–a three-way connection. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M.D., Hansen R.S., Hornstra I.K., Gartler S.M., Yang T.P. 5-Azadeoxycytidine-induced chromatin remodeling of the inactive X-linked HPRT gene promoter occurs prior to transcription factor binding and gene reactivation. J. Biol. Chem. 1997;272:14921–14926. doi: 10.1074/jbc.272.23.14921. [DOI] [PubMed] [Google Scholar]
- Li E., Beard C., Jaenish R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Marin M., Karis A., Visser P., Grosveld F., Philipsen S. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell. 1997;89:619–628. doi: 10.1016/s0092-8674(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Silke J., Georgiev O., Marti P., Giovannini N., Rungger D. An embryonic demethylation mechanism involving binding of transcription factors to replicating DNA. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1446–1453. doi: 10.1093/emboj/17.5.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo A., Herman J.G., Mao L., Lee D.J., Gabrielson E., Burger P.C., Baylin S.B., Sidransky D. CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat. Med. 1995;1:686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Laird P.W., Jackson-Grusby L., Fazeli A., Dickinson S.L., Jung W.E., Li E., Weinberg R.A., Jaenisch R. Suppression of intestinal neoplasia by DNA hypomethylation. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Ramchandani S., MacLeod A.R., Pinard M., von Hofe E., Szyf M. Inhibition of tumorigenesis by a cytosine-DNA, methyltransferase, antisense oligodeoxynucleotide. Proc. Natl. Acad. Sci. USA. 1997;94:684–689. doi: 10.1073/pnas.94.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.Z., Pettersson U., Beard C., Jackson-Grusby L., Jaenish R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–92. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- Oberle I., Rousseau F., Heitz D., Kretz C., Devys D., Hanauer A., Boue J., Bertheas M.F., Mandel J.L. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Knight S.J., Flannery A.V., Hirst M.C., Campbell L., Christodoulou Z., Phelps S.R., Pointon J., Middleton-Price H.R., Barnicoat A., Pembrey M.E. Trinucleotide repeat amplification and hypermethylation of a CpG island in FRAXE mental retardation. Cell. 1993;74:127–134. doi: 10.1016/0092-8674(93)90300-f. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D.R., Shirley K.M., McDonald L.E., Bielefeldt-Ohmann H., Kay G.F., Kelso A. Distinct methylation of the interferon gamma (IFN-γ) and interleukin 3 (IL-3) genes in newly activated primary CD8+ T lymphocytesregional IFN-γ promoter demethylation and mRNA expression are heritable in CD44highCD8+ T cells. J. Exp. Med. 1998;188:103–117. doi: 10.1084/jem.188.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmadcha A., De Diego Y., Pintado E. Assessment of FMR1 expression by reverse transcriptase-polymerase chain reaction of KH domains. J. Lab. Clin. Med. 1998;131:170–173. doi: 10.1016/s0022-2143(98)90160-3. [DOI] [PubMed] [Google Scholar]
- Pintado E., De Diego Y., Hmadcha A., Carrasco M., Sierra J., Lucas M. Instability of the CGG repeat at the FRAXA locus and variable phenotypic expression in a large fragile pedigree. J. Med. Genet. 1995;32:907–908. doi: 10.1136/jmg.32.11.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J., Pintado E., Lotspeich L., Spiker D., McMahon W., Petersen P.B., Nicholas P., Pingree C., Kraemer H.C., Wong D.L. Molecular analysis and test of linkage between the FMR-1 gene and infantile autism in multiplex families. Am. J. Hum. Genet. 1994;55:951–959. [PMC free article] [PubMed] [Google Scholar]
- Adams R.L., Rinaldi A., Seivwright C. Microassay for DNA methyltransferase. J. Biochem. Biophys. Methods. 1991;22:19–22. doi: 10.1016/0165-022x(91)90077-a. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Gualberto A., Pintado E. Regulation of fructose 2,6-bisphosphate levels in cold-acclimated brown adipose tissue of rat. FEBS Lett. 1988;229:91–94. doi: 10.1016/0014-5793(88)80804-4. [DOI] [PubMed] [Google Scholar]
- Krebs A., Wiggings D., Stubbs M., Sols A., Bedoya F. Studies on the mechanism of the antifungal action of benzoate. Biochem. J. 1983;214:657–663. doi: 10.1042/bj2140657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boue J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M.F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N. Engl. J. Med. 1991;325:1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Hagerman R.J., Hull C.E., Safanda J.F., Carpenter I., Staley L.W., O'Connor R.A., Seydel C., Mazzocco M.M., Snow K., Thibodeau S.N. High functioning fragile X malesdemonstration of an unmethylated fully expanded FMR-1 mutation associated with protein expression. Am. J. Med. Genet. 1994;51:298–308. doi: 10.1002/ajmg.1320510404. [DOI] [PubMed] [Google Scholar]
- Stöger R., Kajimura T.M., Brown W.T., Laird C.D. Epigenetic variation illustrated by DNA methylation patterns of the fragile-X gene FMR1. Hum. Mol. Genet. 1997;6:1791–1801. doi: 10.1093/hmg/6.11.1791. [DOI] [PubMed] [Google Scholar]
- Chiurazzi P., Pomponi M.G., Willemsen R., Oostra B.A., Neri G. In vitro reactivation of the FMR1 gene involved in fragile X syndrome. Hum. Mol. Genet. 1998;7:109–113. doi: 10.1093/hmg/7.1.109. [DOI] [PubMed] [Google Scholar]
- Cetkovic-Cvrlje M., Sandler S., Eizirik D.L. Nicotinamide and dexamethasone inhibit interleukin 1-induced nitric oxide production by RINm5F cells without decreasing messenger ribonucleic acid expression for nitric oxide synthase. Endocrinology. 1993;133:1739–1743. doi: 10.1210/endo.133.4.7691579. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- Schwemmle S., de Graaff E., Deissler H., Glaser D., Wohrle D., Kennerknecht I., Just W., Oostra B.A., Dorfler W., Vogel W., Steinbach P. Characterization of FMR1 promoter elements by in vivo-footprinting analysis. Am. J. Hum. Genet. 1997;60:1354–1362. doi: 10.1086/515456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y., Matsumoto T. Nucleotide-selective cleavage of duplex DNA by nitric oxide. Biochem. Biophys. Res. Commun. 1995;211:748–753. doi: 10.1006/bbrc.1995.1876. [DOI] [PubMed] [Google Scholar]
- Li E., Bestor T.H., Jaenisch R. Targeted mutation of DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Bakin A.V., Curran T. Role of DNA 5-methylcytosine transferase in cell transformation by fos. Science. 1999;283:387–390. doi: 10.1126/science.283.5400.387. [DOI] [PubMed] [Google Scholar]
- Bestor T.H. Activation of mammalian DNA methyltransferase by cleavage of a Zn binding regulatory domain. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:2611–2617. doi: 10.1002/j.1460-2075.1992.tb05326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J.F., Pavlovich J.G., Reich N.O. Peptide mapping of the murine DNA methyltransferase reveals a major phosphorylation site and the start of translation. J. Biol. Chem. 1997;272:17851–17857. doi: 10.1074/jbc.272.28.17851. [DOI] [PubMed] [Google Scholar]
- Rouleau J., MacLeod A.R., Szyf M. Regulation of the DNA-methyltransferase by the ras-Ap-1 signaling pathway. J. Biol. Chem. 1995;270:1595–1601. doi: 10.1074/jbc.270.4.1595. [DOI] [PubMed] [Google Scholar]
- Okano M., Shaoping X., Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- Ramchandani S., Bhattacharya S.K., Cervoni N., Szyf M. DNA methylation is a reversible biological signal. Proc. Natl. Acad. Sci. USA. 1999;96:6107–6112. doi: 10.1073/pnas.96.11.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Bredt D.S., Snyder S.H. Nitric oxidea physiologic messenger molecule. Annu. Rev. Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Avila M.A., Carretero M.V., Rodriguez E.N., Mato J.M. Regulation by hypoxia of methionine adenosyltransferase activity and gene expression in rat hepatocytes. Gastroenterology. 1998;114:364–371. doi: 10.1016/s0016-5085(98)70489-5. [DOI] [PubMed] [Google Scholar]
- Flodstrom M., Chen M.C., Smismans A., Schuit F., Pipeleers D.G., Eizirik D.L. Interleukin-1β increases arginine accumulation and activates the citrulline-NO cycle in rat pancreatic beta cells. Cytokine. 1999;11:400–407. doi: 10.1006/cyto.1998.0446. [DOI] [PubMed] [Google Scholar]
- Luk W.P., Zhang Y., White T.D., Lue F.A., Wu C., Jiang C.G., Zhang L., Moldofsky H. Adenosinea mediator of interleukin-1β-induced hippocampal synaptic inhibition. J. Neurosci. 1999;19:4238–4244. doi: 10.1523/JNEUROSCI.19-11-04238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis M., Frank D., Keshet I., Siegfried Z., Mendelsohn M., Nemes A., Temper V., Razin A., Cedar H. Sp1 elements protect a CpG island from de novo methylation. Nature. 1994;371:435–438. doi: 10.1038/371435a0. [DOI] [PubMed] [Google Scholar]
- Coffee B., Zhang F., Warren S.T., Reines D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat. Genet. 1999;22:98–101. doi: 10.1038/8807. [DOI] [PubMed] [Google Scholar]
- Kirillov A., Kistler B., Mostoslavsky R., Cedar H., Wirth T., Bergman Y. A role for nuclear NF-κB in B-cell-specific demethylation of the Igκ locus. Nat. Genet. 1996;13:436–449. doi: 10.1038/ng0895-435. [DOI] [PubMed] [Google Scholar]
- Cao Z., Henzel W.J., Gao S. IRAKa kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- Cao Z., Xiong J., Takeuchi M., Kurama T., Goedel D. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- Imbert V., Rupec R.A., Livoisi A., Pahi N.L., Traenckner E.B., Mueller-Dieckmann C., Farahifar D., Rossi B., Auberger P., Baeuerle P., Peyron J. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Murata J., Tada M., Iggo R.D., Sawamura Y., Shinohe Y., Abe H. Nitric oxide as a carcinogenanalysis by yeast functional assay of inactivating p53 mutations induced by nitric oxide. Mutat. Res. 1997;379:211–218. doi: 10.1016/s0027-5107(97)00149-8. [DOI] [PubMed] [Google Scholar]
- Wink D.A., Kasprzak K.S., Maragos C.M., Elespuru R.K., Misra M., Dunams T.M., Cebula T.A., Koch W.H., Andrews A.W., Allen J.S. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- Karupiah G., Xie Q.W., Buller R.M., Nathan C., Duarte C., MacMicking J.D. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Rouleau J., Tanigawa G., Szyf M. The mouse DNA methyltransferase 5′-region. A unique housekeeping gene promoter. J. Biol. Chem. 1992;267:7368–7377. [PubMed] [Google Scholar]