Abstract
The T cell receptor complex (TCR) ζ chain is constitutively tyrosine phosphorylated specifically at two of the six ζ immunoreceptor tyrosine-based activation motif (ITAM) tyrosine residues in resting peripheral T cells. Further phosphorylation of ζ is induced by both agonist and antagonist ligands of the TCR, with agonists inducing complete phosphorylation of the ζ ITAM tyrosines. After antagonist stimulation, ζ phosphorylation is incomplete and generates discrete forms of partially phosphorylated ITAMs. Here, we mutate specific tyrosines in chimeric human CD8-ζ molecules to reflect phosphorylation in resting T cells as well as phosphorylation induced by agonist and antagonist ligands. We demonstrate that such partially phosphorylated TCR-ζ species can inhibit IL-2 production in T cell hybridomas and proliferation in T cell clones. This reveals a previously unrecognized, inhibitory function of partially phosphorylated ITAMs. These findings support the concept that TCR antagonism can arise through the generation of an inhibitory signal within the TCR complex and that constitutive ζ phosphorylation in resting T cells is an inhibitory signaling environment.
Keywords: T cell signaling, ITAM, CD3 complex phosphorylation, TCR antagonism, altered peptide ligand
The TCR of resting mature T cells is constitutively tyrosine phosphorylated within the TCR ζ chain, the function of which has not been established. Phosphorylation of ζ occurs at the tyrosines of its three immunoreceptor tyrosine-based activation motifs (ITAMs; for immunoreceptor-based activation motif with the consensus sequence D/ExxYxxL/Ix6–8YxxL/I).1 ITAMs are necessary and sufficient to mediate T cell activation, as has been unambiguously revealed by the incorporation of isolated ITAMs into chimeric proteins 1 2 3. All evidence gathered thus far indicates that both tyrosines in ITAMs are required for functional activity 4. Truncated or mutated ITAMs cannot mediate T cell activation 1 2 5 6 7; however, it has not been assessed whether ITAMs that can only be partially phosphorylated could possibly inhibit T cell activation.
The TCR complex contains a total of ten ITAMs within the CD3 ∈, γ, δ, and ζ chains. The presence of multiple ITAMs is thought to amplify signals generated by the TCR. Mice expressing ζ protein without functional ITAMs can provide the necessary signals for T cell development 8 9, because other components of the TCR complex can substitute for ζ ITAMs. The ITAMs of the ζ chain are therefore not essential for T cell maturation. However, when present, the multiple ζ ITAMs determine the selection of specific T cells during both positive and negative selection 10. Thus, the ζ chain plays an important role in shaping the TCR repertoire.
We have recently examined the actual sites of phosphorylation of the six tyrosines of ζ 11. We found that the six tyrosines were not phosphorylated to an equal extent. In resting T cells, only two of the six ζ ITAM tyrosines were phosphorylated. Stimulation of the TCR with antagonist ligands augmented the phosphorylation of these two ζ tyrosines. In addition, two other tyrosines were weakly phosphorylated. Antagonist ligands thus led to an accumulation of partially but specifically phosphorylated ζ species. In contrast, agonists induced complete phosphorylation of all six ζ tyrosines in a specific order. Thus, the quality of ζ phosphorylation was different in resting and antagonist-stimulated T cells versus fully activated T cells. This raised the question of whether the different types of ζ phosphorylation have functional consequences for T cell activation. One possibility is that ζ phosphorylation of resting or antagonist-stimulated T cells simply reflects a low level of activation below the threshold for inducing full T cell activation. Alternatively, it is possible that this type of phosphorylation actively inhibits T cell activation.
TCR antagonism occurs when T cells are simultaneously exposed to stimulatory and antagonist ligands 12 13 14 15 16. The mechanism of TCR antagonism is unclear. One hypothesis is that the two different ligands compete for binding to the TCR. Another hypothesis is that nonstimulatory ligands induce an inhibitory signal and therefore actively inhibit activation by the stimulatory TCR ligand. Antagonist ligands can induce early intracellular signaling events, with the phosphorylation of the CD3 ζ chain being the most prominent 17 18 19 20. However, signals downstream of ζ phosphorylation are greatly diminished, including the activation of Zap-70. The Zap-70 kinase is known to bind to fully phosphorylated ζ ITAMs through its tandem Src homology (SH)2 domains before its activation 21 22. ζ Phosphorylation induced by antagonist ligands with incompletely phosphorylated ITAMs hence does not support recruitment of Zap-70 and its subsequent activation.
For this report, we directly tested whether the distinct phosphorylation of ζ in resting T cells induced by antagonist ligands has functional consequences for T cell activation. To this end, we mutated tyrosines of ζ ITAMs to reflect phosphorylation of resting T cells or phosphorylation induced by antagonist or agonist ligands. Whereas partially phosphorylated ζ species cannot induce T cell activation by themselves, we found that their presence can inhibit TCR-mediated T cell activation.
Materials and Methods
Cells and Antibodies.
The 3.L2 T cell clone and hybridoma and their maintenance and specificity were previously described 23 24. The T cell hybridoma 3A9 was described in reference 25. Cells from the murine B cell line CH27, or Hi7, an I-Ek–transfected fibroblast line, were used as APCs. Anti-human (h)CD8α antibodies were RPA-T8 (PharMingen) for FACS® analysis and OKT-8 (American Type Culture Collection [ATCC]) for cross-linking. OKT-8 was purified from tissue culture supernatants using protein A. Rabbit anti–Zap-70 was the gift of A.C. Chan (Washington University, St. Louis, MO). Anti-ζ polyclonal antiserum 777 11 and biotinylated 4G10 (Upstate Biotechnology Inc.) were used for immunoblotting. Phoenix E cells were obtained from ATCC with permission from G. Nolan (Stanford University, Palo Alto, CA). T cell lines were established from splenocytes from 2.102 TCR–transgenic mice bred to recombination activating gene (RAG)-1−/− mice 26. For maintenance of 2.102 cell lines, 2 × 105 2.102 T cells were restimulated biweekly with 5 × 106 irradiated B6.AKR splenocytes, 1 μM peptide Hb(64–76), and 33 U/ml IL-2. For subcloning of 2.102 lines, 2.5 × 105 B6.AKR splenocytes, 1 μM Hb(64–76), and 33 U/ml IL-2 was incubated in round-bottomed wells.
Tissue Culture Assays.
Antagonist assays of T hybridoma lines were performed as previously described 14 with Hi7 cells as APCs. Proliferation of 2.102 lines was performed as follows: 5 × 104 CH27 cells were prepulsed with Hb(64–76), treated with mitomycin C (Sigma Chemical Co.) for 2 h at 37°C, washed three times, and then incubated with 3 × 104 2.102 T cells. Thymidine was added after 48 h.
Transfection and Retroviral Infection.
3.L2 and 3A9 cells were transfected by electroporation and selected with Zeocin (Invitrogen Corp.). Phoenix E cells were transfected using CaCl2 for production of high titer retroviral supernatants 27. 2.102 T cells were infected with retroviral supernatants 3 d after activation 27.
Constructs.
The chimera between the extracellular and transmembrane part of human CD8α and intracellular murine ζ was made similar to that described in reference 28 by PCR using the primers T7 and acim8z (5′-TCC TGC TGA ATT TTG CTC TGT TGC AGT AAA GGG TGA TA-3′) and T3 and cim8z (5′-TAT CAC CCT TTA CTG CAA CAG AGC AAA ATT CAG CAG GA-3′). pcDNAZeo (Invitrogen Corp.) or the retroviral green fluorescent protein (GFP)-RV 27 were used as plasmids. Tyrosines were replaced by phenylalanines using PCR as in references 11 and 29. A stop codon was introduced after seven amino acids of intracellular ζ with the primers 5′-TTC AGC AGG AGT TAA GAG ACT GCT GCC-3′ and 5′-AAG TCG TCC TCA ATT CTC TGA CGA CGG-3′.
Phosphotyrosine Blotting.
Transfected 3.L2 hybridoma cells (2 × 107) were incubated with 5 μg OKT-8 on ice and activated at 37°C for 5 min. Cells were lysed in 500 μl lysis buffer 17. hCD8-ζ was precipitated using OKT-8 and protein A–Sepharose and analyzed by phosphotyrosine blotting using biotin–4G10 (Upstate Biotechnology Inc.) and horseradish peroxidase–streptavidin (Southern Biotechnology Associates Inc.). The activation of 3.L2 T cell clones with peptide-pulsed Hi7 cells was previously described 17. Phosphotyrosine bands were quantitated using ImageQuant (version 1.1; Molecular Dynamics).
Results
TCR-ζ Phosphorylation in the 3.L2 T Cell Clone.
The TCR ζ chain is constitutively phosphorylated in resting peripheral T cells. To investigate constitutive as well as induced TCR-ζ phosphorylation, we first examined the 3.L2 T cell clone. 3.L2 is specific for peptide Hb(64–76)/I-Ek 23. Peptide Hb(64–76) is derived from an allelic form of murine hemoglobin protein, amino acids 64–76. In rested 3.L2 T cells, ζ was phosphorylated, as apparent in a phospho species of 21 kD termed p21 (Fig. 1 a). Stimulation of 3.L2 with fully stimulating agonist peptide Hb(64–76) presented on APCs augmented ζ phosphorylation and led to the appearance of an additional phospho species of 23 kD called p23 (Fig. 1 a). The 3.L2 TCR can also interact with a spectrum of peptide ligands related to Hb(64–76) that have a one–amino acid substitution at position 72 or 73 of the original protein sequence 23 24. The response of the 3.L2 TCR to peptide Hb(64–76) can be antagonized by such specific analogue ligands (reference 24; Fig. 1 b). We used four analogue peptide ligands of Hb(64–76), termed D73, I72, A72, and G72. These peptides had the ability to inhibit proliferation of 3.L2 in a dose-dependent manner, as previously observed (reference 24; Fig. 1 b). This was a specific effect, as the control peptide E72 did not inhibit proliferation, although it can bind to I-Ek as well as peptides D73, I72, A72, and G72. Exposure of 3.L2 to the four antagonist ligands D73, I72, A72, and G72 also induced an increase in ζ phosphorylation (Fig. 1 a). However, more phosphorylation of p21 than p23 was induced, resulting in a change in the ratio of p23/p21. This type of ζ phosphorylation has previously been termed altered ζ phosphorylation and is induced by many antagonist ligands. Lastly, the control peptide E72 induced no augmentation in ζ phosphorylation of the 3.L2 T cell (Fig. 1 a).
Figure 1.
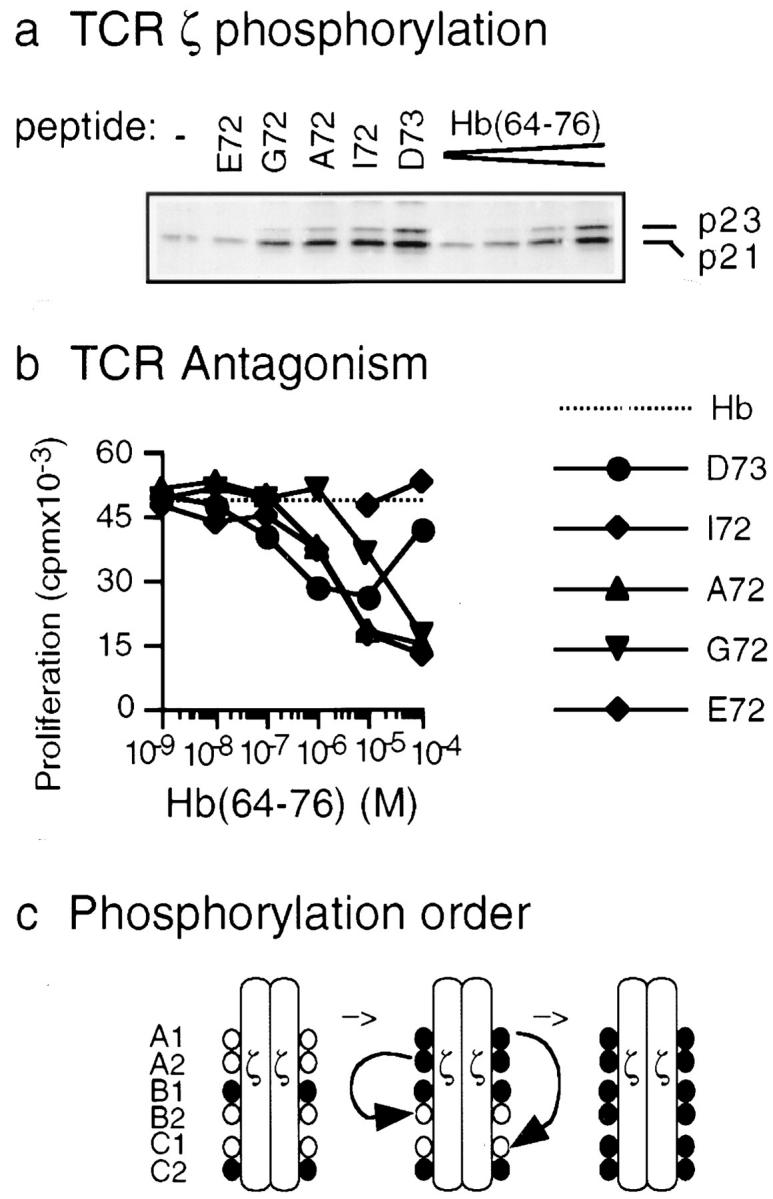
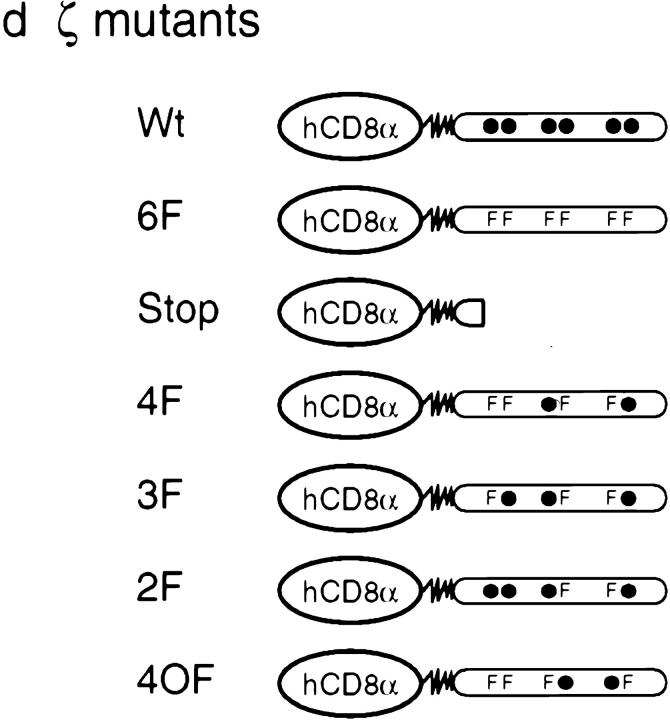
TCR-ζ phosphorylation in the 3.L2 T cell clone. (a) TCR-ζ phosphorylation in the 3.L2 T cell clone. 107 3.L2 cells were stimulated with analogue peptides E72, G72, A72, I72, and D73 or with the stimulatory peptide Hb(64–76) and 2 × 106 APCs. Peptide concentrations were 10−4 M for E72, G72, A72, I72, and D73 and 10−7, 10−6, 10−5, and 10−4 M for Hb(64–76). Cells were lysed, the TCR was precipitated using anti–Zap-70 antibodies, and tyrosine phosphorylation was examined by immunoblotting using 4G10 (PharMingen). The ratio of p23/p21 at 10−4 M was 0.75 for Hb(64–76), 0.55 for D73, 0.15 for I72, 0.11 for A72, and 0.05 for G72. (b) The 3.L2 T cell can be antagonized with peptides D73, I72, A72, and G72. B6.AKR splenocytes were prepulsed with 10−7 M Hb(64–76) peptide and washed. 3.L2 T cells were then added, as were increasing doses of the analogue peptides D73, I72, A72, G72, and E72. 3.L2 T cells proliferated in response to Hb(64–76) (dotted line). D73, I72, A72, and G72 antagonized proliferation in a dose-dependent manner, but the control peptide E72 did not (solid lines). Data represent the mean of triplicate cultures with SD < 15% of the mean. (c) Phosphorylation of ζ tyrosines A1–C2 proceeds in a specific order. In resting peripheral T cells, B1 and C2 are phosphorylated (left; • indicates phosphorylation). Upon activation with agonist ligands, phosphorylation proceeds in the indicated order through an intermediate species (center) to full phosphorylation (right). Antagonist ligands augment the phosphorylation of B1 and C2, whereas the subsequent steps are greatly diminished. (d) TCR-ζ was mutated to only allow for phosphorylation found in resting T cells or phosphorylation after activation with antagonist or agonist ligands. Chimeric proteins were prepared with the extracellular and transmembrane regions of human CD8α and the intracellular portion of murine ζ. F, tyrosine→phenylalanine substitution; •, unmodified tyrosines. The 4F mutant represents ζ found in resting T cells. After activation with antagonists, 4F is the predominant species, whereas 3F and 2F are minor, intermediate forms. For the Stop mutant, a stop codon was introduced after seven amino acids of intracellular ζ.
Antagonism of the 3.L2 T cell only occurs at certain concentrations of agonist and antagonist ligands. For the experiment shown in Fig. 1 b, the agonist Hb(64–76) was used at the low but stimulating concentration of 10−7 M. At this dose, only very little ζ phosphorylation was induced by Hb(64–76) (Fig. 1 a). Peptides G72, A72, I72, and D73 strongly induced antagonism when they were used at higher concentrations of 10−5–10−4 M. At such concentrations, the antagonist peptides induced very strong ζ phosphorylation (Fig. 1 a). Thus, at concentrations required for antagonism, antagonist peptides induced more ζ phosphorylation than the agonist peptide. This correlated with inhibition of T cell activation rather than activation. In addition, stronger antagonist peptides induced more ζ phosphorylation than weaker antagonist peptides at the same concentration (Fig. 1 a, e.g., compare D73 and G72). Therefore, the strength of the altered ζ phosphorylation signal correlated with the strength of T cell inhibition.
We recently reported that the six tyrosines of the TCR ζ chain are not phosphorylated to an equivalent degree 11. Two main tyrosines, B1 and C2, are specifically phosphorylated in resting T cells (Fig. 1 c). Upon activation with fully stimulating ligands, A1 and A2 next become phosphorylated. Tyrosines B2 and C1 are phosphorylated last, resulting in the full phosphorylation of ζ. In contrast, after stimulation with antagonist ligands, only phosphorylation of the basal sites B1 and C2 is markedly increased. A1 and A2 are also inducibly phosphorylated, albeit only slightly. The final phosphorylation of B2 and C1 is absent. Thus, A1 and A2 phosphorylation is necessary but not sufficient for the subsequent phosphorylation of C1 and B2, respectively. In conclusion, the majority of phosphorylated ζ molecules induced by antagonist ligands have only B1 and C2 phosphorylated. These findings raise the question of whether the distinct phosphorylated ζ species found in resting T cells or after stimulation with either agonist or antagonist ligands have an effect on T cell activation.
To directly test whether the partially phosphorylated species found in T cells can inhibit T cell activation, we substituted multiple tyrosines in ζ with phenylalanine to prevent phosphorylation of these sites. This resulted in proteins that could only be phosphorylated in the pattern reflecting ζ phosphorylation as identified in T cells 11. In particular, we designed mutants that allowed for the precise phosphorylation found in resting T cells or phosphorylation induced by antagonist or agonist peptides (Fig. 1 d). We designed these mutants as chimeric proteins with hCD8α to provide a specific extracellular means for antibody cross-linking and for quantifying expression 28.
The construct with four substitutions from tyrosine to phenylalanine was termed 4F, and it retained only two intact tyrosines for phosphorylation, B1 and C2 (Fig. 1 d). In resting T cells, these two main tyrosines were found to be phosphorylated with a high degree of specificity. In addition, stimulation with antagonist peptides led to a marked augmentation of phosphorylation at B1 and C2 when compared with resting T cells. Therefore, the 4F mutant not only reflected the phosphorylation pattern of resting T cells but also the main pattern induced by antagonist ligands.
The construct 3F, with three tyrosine mutations, reflected an intermediate species. It allowed for the additional phosphorylation of tyrosine A2. A2 has been found to be phosphorylated to a minor degree in resting T cells. This was most likely because phosphorylation of A2 varied depending on the resting state of T cells 11. Phospho-A2 was also induced by antagonist ligands. Thus, we could test a possible function of A2, B1, and C2 phosphorylation with the 3F mutant.
The 2F mutant allowed the additional phosphorylation of A1. One ITAM, ITAM A, could now be fully phosphorylated, but B2 and C1 were mutated. A1 and A2 phosphorylation is required for further phosphorylation of B2 and C1. However, after stimulation with antagonist peptides, A1 and A2 are found to be slightly phosphorylated without the subsequent phosphorylation of B2 and C1. Thus, the 2F mutant represents a minor species present in antagonist-activated cells but not in resting cells, and it was important to test its function. 2F also reflects an intermediate species in cells activated with agonist ligands, where A1 and A2 are phosphorylated to an extensive degree before ζ then becomes fully phosphorylated.
Several control proteins were designed: a protein without mutations in ζ, termed Wt, and two proteins with no tyrosines available for phosphorylation, termed 6F and Stop. 4OF was another control that reflected a pattern of phosphorylation not found in T cell activation.
Partially Phosphorylated TCR-ζ Can Inhibit T Cell Activation of the 3.L2 T Cell Hybridoma.
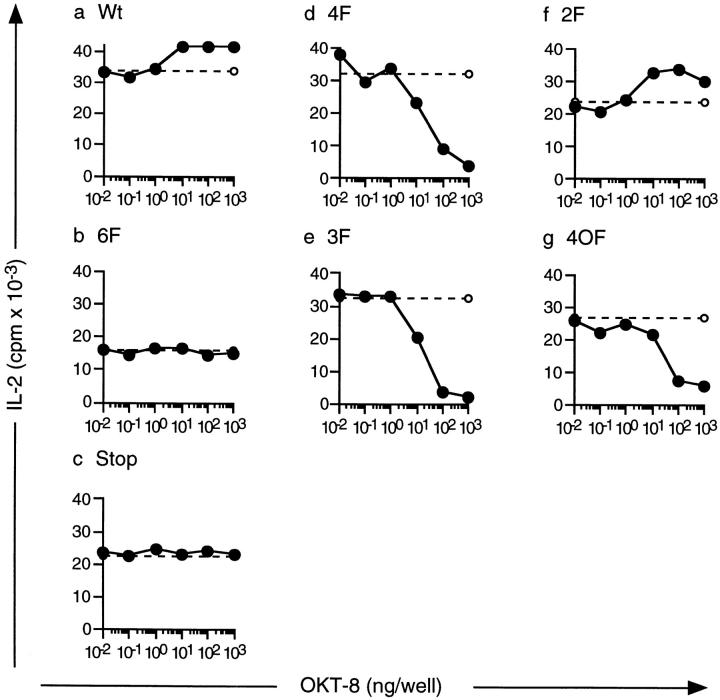
The mutants were transfected into a hybridoma derived from the 3.L2 T cell. Several clones for each construct were positive for surface expression of hCD8α. Transfected 3.L2 cells with similar hCD8-ζ expression levels were further analyzed (Fig. 2, a–g, left panels). All tested transfectants retained their ability to respond to the Hb(64–76)/I-Ek peptide in a dose-dependent manner by IL-2 production (Fig. 2, a–g, right panels), indicating that their endogenous 3.L2 receptor was intact after the transfection procedure. Transfection with the different hCD8-ζ mutants did not significantly affect the sensitivity of TCR-mediated IL-2 production for any of the transfectant clones tested. We also determined an intermediate dose of Hb(64–76) that stimulated IL-2 production within the linear part of the dose–response curve for each transfectant (Fig. 2, a–g, right panels, dashed lines).
Figure 2.
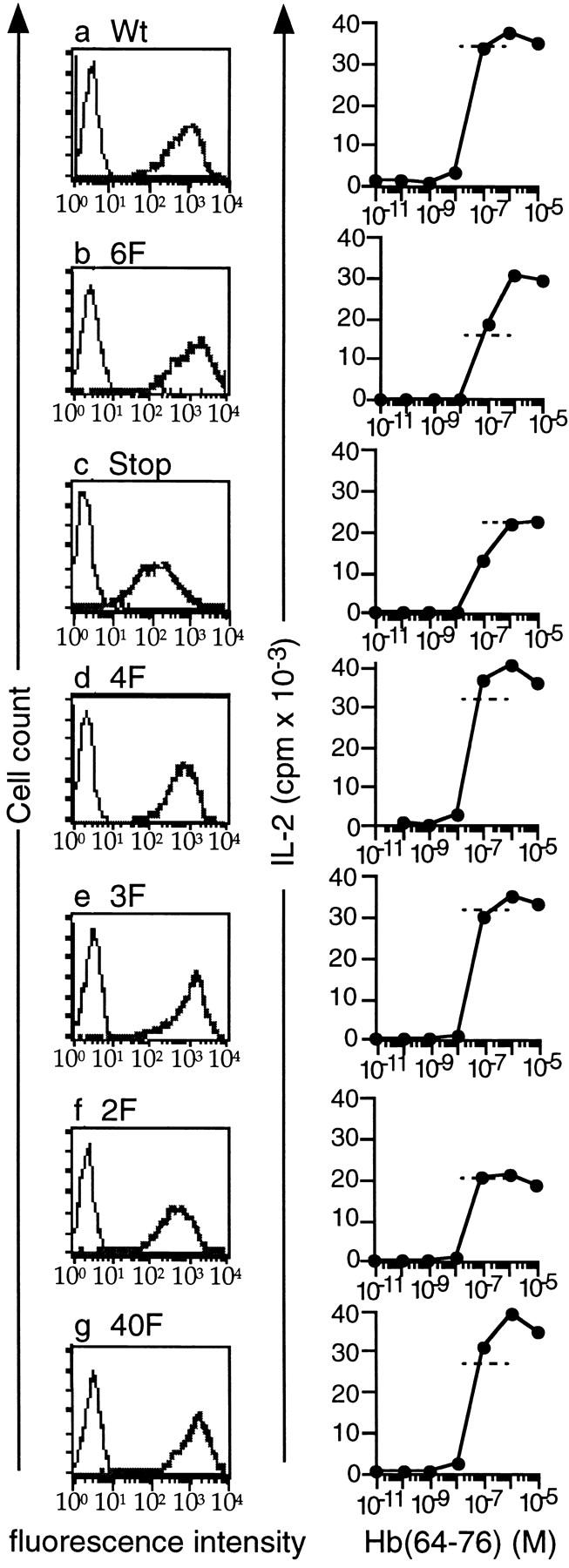
Expression of hCD8-ζ mutants in the murine 3.L2 T cell hybridoma. (a–g) Left panels: the murine 3.L2 T cell hybridoma was transfected with different hCD8-ζ mutants. Three to five independent transfectants were identified for each construct by FACS® analysis using an antibody to human CD8α, RPA-T8–PE (PharMingen). One representative subclone for each mutant is shown. Right panels: TCR-mediated IL-2 production was measured to demonstrate that the endogenous 3.L2 receptor is intact after the transfection procedure. 2 × 104 Hi7 cells were incubated with 105 3.L2 cells and increasing doses of the Hb(64–76) peptide. IL-2 was measured by growth of the IL-2–dependent cell line CTLL. All transfected clones responded to Hb(64–76)/I-Ek with similar sensitivity. The experiments were performed at the same time as the experiments shown in Fig. 3 for each transfectant. Dashed lines represent the amount of IL-2 production that resulted from exposure to peptide-pulsed APCs in Fig. 3 for each experiment shown. Two independent transfectants were analyzed with identical results for 6F, 4F, and 3F; one for Wt, Stop, and 2F; and three for 4OF. One transfectant for each construct was then subcloned, and the experiments were performed nine times. The data represent the mean of triplicate cultures with SD < 15% of the mean.
Next, we stimulated the transfected cell lines through their endogenous 3.L2 TCRs with an intermediate dose of Hb(64–76) peptide and APCs. This resulted in TCR-mediated IL-2 production within the linear portion of the dose–response curve (compare Fig. 2, a–g, right panels, dashed lines and Fig. 3, a–g, dashed lines). At the same time, we cross-linked the hCD8-ζ proteins by adding anti-hCD8α antibodies and measured IL-2 production (Fig. 3, a–g, solid lines). The experiments shown in Fig. 2, right panels, and Fig. 3 were performed on the same day for each transfectant, so the degree of peptide stimulation indicated with the dotted lines in Fig. 3 can be directly compared with the dose–response curve in Fig. 2.
Figure 3.
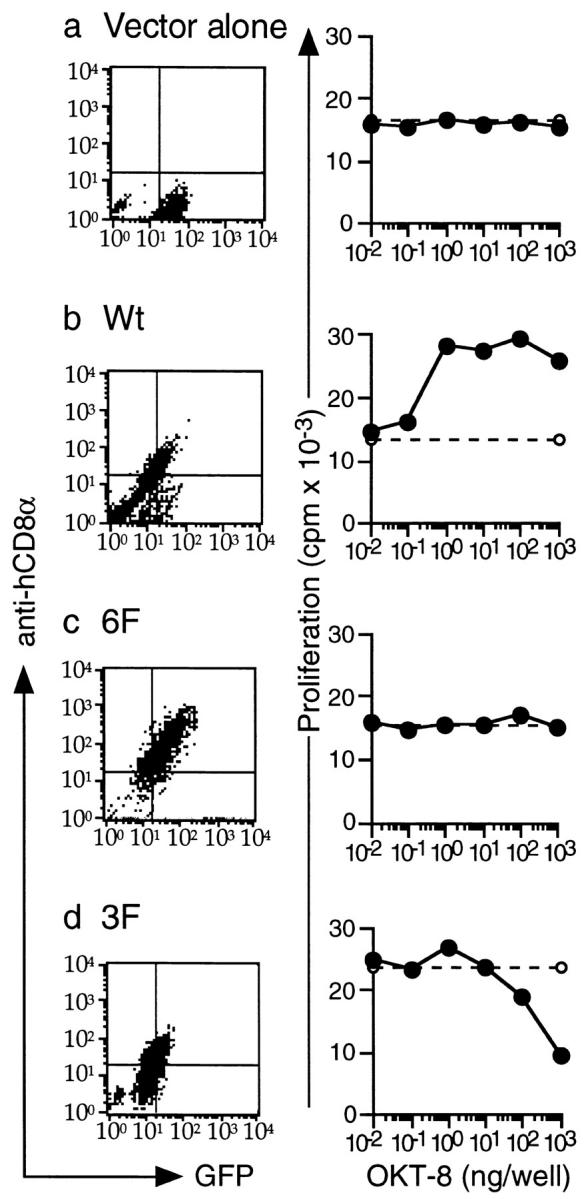
The hCD8-ζ mutants 3F, 4F, and 4OF have an inhibitory effect on TCR-mediated IL-2 production. Hi7 cells were prepulsed with a stimulating dose of Hb(64–76). 3.L2 cells were then incubated with the prepulsed APCs alone (dashed lines) or with prepulsed APCs and increasing doses of anti-hCD8α antibody OKT-8 to cross-link the mutants (solid lines). 4F, 3F, and 4OF inhibited TCR-mediated IL-2 production (d, e, and g); Wt and 2F had an augmenting effect on IL-2 production (a and f), and 6F and Stop had no effect (b and c). The peptide dose necessary for intermediate IL-2 production was determined for each transfectant in previous experiments. The peptide doses were 0.5 × 10−6 M Hb(64–76) for Wt, 6F, 3F, 2F, and 4OF; 10−6 M for Stop; and 0.5 × 10−7 M for 4F. The experiments shown were performed at the same time as the dose–response curves shown in Fig. 2 for each transfectant. Two independent transfectants were analyzed for 6F, 4F, and 3F; one for Wt, Stop, and 2F; and three for 4OF with identical results. One transfectant for each construct was then subcloned, and the experiments were performed nine times. The data represent the mean of triplicate cultures with SD < 15% of the mean.
Such treatment led to an augmentation of IL-2 production in the Wt-transfected cell lines (Fig. 3 a). IL-2 production was also induced when the cell line was stimulated through the Wt hCD8-ζ mutant alone (data not shown). This indicated that unmutated intracellular ζ induced T cell activation, consistent with previous observations 1 2 3. No effect was observed when the 6F or Stop mutants were engaged (Fig. 3b and Fig. c), as tested in two independent clones for 6F and one clone for Stop. Thus, the presence of the hCD8-ζ molecule without tyrosines had no effect on T cell activation. Interestingly, the IL-2 response to the TCR ligand was inhibited when the 4F protein was cross-linked in two independent clones (Fig. 3 d). Inhibition of the 3.L2 response was dose dependent, with 100% inhibition occurring at the highest concentration of OKT-8. The 3F mutant was also inhibitory in two independent transfectants (Fig. 3 e). Cross-linking of 2F augmented the IL-2 response induced through the endogenous TCR (Fig. 3 f). It also induced IL-2 production when cross-linked alone (data not shown). Thus, this mutant had an activating effect on T cell activation, consistent with previous studies of single intact ITAMs 1. This indicated that the presence of one intact ITAM in 2F overcame the inhibitory effect of tyrosines B1 and C2. The effect of all hCD8-ζ mutants was maximal when TCR-mediated IL-2 production was intermediate and within the linear portion of the dose–response curve. When TCR-mediated stimulation was saturated, inhibition as well as augmentation of IL-2 production was not noticeable (data not shown). Thus, the concentration requirements for stimulation were similar when inhibition was mediated by TCR antagonism and by hCD8-ζ mutants. In conclusion, we identified two inhibitory ζ mutants, 4F and 3F. These proteins contained two or three partially impaired ITAMs and lacked intact ITAMs. Thus, we demonstrated that the ζ species that represented phosphorylation in resting T cells can inhibit T cell activation. Such species are also strongly induced by antagonist ligands.
Next, we tested whether the inhibitory effect was specifically due to tyrosines B1 and C2 or whether the combination of other tyrosines could also inhibit T cell activation. B1 and C2 were mutated to phenylalanine, as was ITAM A. This left tyrosines B2 and C1 intact (Fig. 1 d, mutant 4OF). Three independent transfectants were examined. The presence of hCD8-ζ 4OF did not alter the sensitivity of the 3.L2 TCR (Fig. 2 g). Cross-linking of 4OF could also inhibit TCR-mediated T cell activation (Fig. 3 g) in all three clones. Inhibition was as efficient as with the 4F and 3F mutants. This indicated that the inhibitory effect was not dependent on tyrosines B1 and C2. Rather, other hemi-ITAMs could also inhibit T cell activation when intact ITAMs were absent.
To test whether the inhibitory effect of ζ mutants was also observed in other cell lines, we transfected 4F and 6F into the 3A9 T cell hybridoma. 3A9 is specific for peptide HEL(48–62)/I-Ak 25, a peptide derived from hen egg lysozyme, amino acids 48–62. Two positive clones for each construct were identified by expression of hCD8α, and their responsiveness to HEL/I-Ak was confirmed (not shown). Cross-linking of 4F but not 6F led to an inhibition of TCR-induced IL-2 production in a dose-dependent fashion (data not shown) in both independent clones tested for each construct. Thus, the inhibitory effect of partially phosphorylated ζ species was not limited to the 3.L2 T cell.
Inhibitory TCR-ζ Mutants Are Tyrosine Phosphorylated.
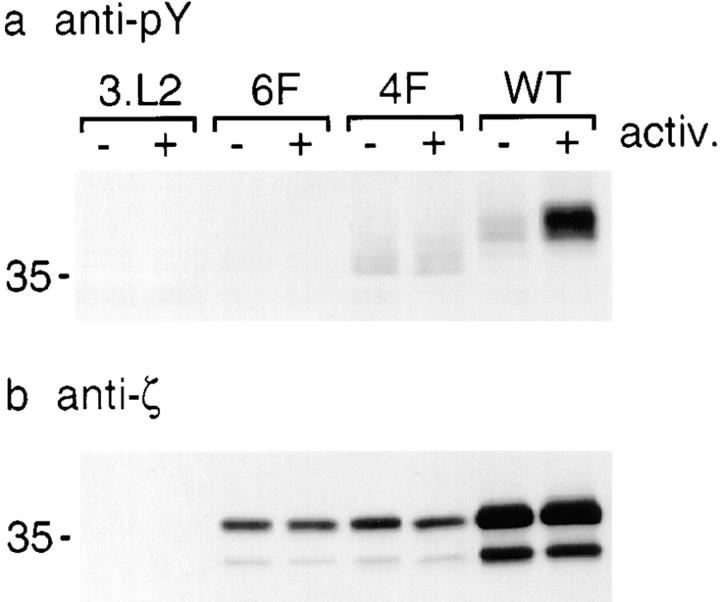
We next examined the tyrosine phosphorylation state of the hCD8-ζ 6F, 4F, and Wt proteins in the transfected 3.L2 hybridoma lines. Untransfected 3.L2 or 3.L2 transfected with 6F, 4F, or Wt was incubated with anti-hCD8α antibodies and left on ice or antibody cross-linked (Fig. 4). Cells were then lysed, the hCD8-ζ mutants were precipitated, and tyrosine phosphorylation of the chimeric hCD8-ζ protein was analyzed. In contrast to 6F, both 4F and Wt were tyrosine phosphorylated. Interestingly, 4F and Wt were both constitutively tyrosine phosphorylated to a similar extent before cross-linking of hCD8 (Fig. 4 a). This was reminiscent of constitutive phosphorylation of ζ in resting T cells. The electrophoretic mobilities of 4F and Wt differed from each other. This might be because point mutations in ζ can affect its mobility significantly 11. Alternatively, hCD8-ζ Wt could be phosphorylated to a higher degree than 4F, giving rise to a lesser mobility. In this case, the extent of phosphorylation of the hCD8-ζ Wt protein in the hybridoma cell line might be higher than that of endogenous TCR-ζ in resting nonimmortalized T cells. Phosphorylation of hCD8-ζ Wt significantly increased with activation, whereas phosphorylation of 4F was unchanged. This might be due to the binding of Zap-70 to fully phosphorylated ITAMs in hCD8-ζ Wt, which can protect tyrosines from dephosphorylation 30. Binding of Zap-70 to phospho-4F might be impaired because no fully phosphorylated ITAMs are present, resulting in the rapid dephosphorylation of phospho-4F. Alternatively, the effect of cross-linking of the constitutively phosphorylated 4F mutant might be exerted through aggregation or association with the endogenous TCR and not through an increase in phosphorylation. All transfected cell lines expressed hCD8-ζ protein (Fig. 4 b). hCD8-ζ appeared as two separate bands in immunoblotting, most likely due to glycosylation 28. In conclusion, the remaining intact tyrosines in the inhibitory mutant 4F were phosphorylated. As 6F had no effect on T cell activation and was not phosphorylated, the inhibitory effect of 4F was most likely exerted through phosphorylated tyrosines.
Figure 4.
Inhibitory TCR-ζ mutants are tyrosine phosphorylated. (a) hCD8-ζ mutants 4F and Wt are tyrosine phosphorylated before and after cross-linking of hCD8α. 2 × 107 untransfected 3.L2 hybridoma cells or 3.L2 cells transfected with 6F, 4F, or Wt were incubated with 5 μg of anti-hCD8α OKT-8 on ice. Samples were then activated and cross-linked at 37°C (+) or left on ice (−). Cells were lysed, and the mutants were immunoprecipitated using OKT-8 and analyzed by phosphotyrosine blotting using biotin–4G10 (Upstate Biotechnology Inc.) and horseradish peroxidase–streptavidin. The position of a 35-kD molecular mass marker is indicated at left. (b) 3.L2 cells transfected with 6F, 4F, and Wt express hCD8-ζ protein. An immunoblot was performed on the samples described in panel a using 777, an antibody against murine ζ that also recognizes mutant ζ 11. Two bands with different molecular sizes were apparent, most likely due to glycosylation of the extracellular portion of hCD8α. Representative of four experiments.
Partially Phosphorylated TCR-ζ Can Inhibit T Cell Proliferation.
We next examined whether ζ mutants could also inhibit proliferation of nonimmortalized T cell lines. We used T cells from 2.102 TCR–transgenic mice on a RAG-1−/− background 26. 2.102 T cells are specific for Hb(64–76)/I-Ek but express a different α/β TCR than 3.L2. We transduced such splenocytes by retroviral infection techniques using a bicistronic retrovirus coexpressing hCD8-ζ mutants and GFP 27. This enabled us to identify transfected T cells by the expression of GFP without antibody cross-linking of the hCD8α mutants. We infected activated 2.102 splenocytes with retroviral supernatants containing empty vector or hCD8-ζ Wt, 6F, and 3F on two independent occasions. GFP-positive splenocytes were FACS® sorted, expanded, and subcloned. Cells were then analyzed for the expression of hCD8α and GFP by FACS® (Fig. 5, a–d, left panels). Cells transduced with vector alone expressed GFP, indicating that the infection procedure was successful. Cells transduced with hCD8-ζ Wt, 6F, and 3F expressed both GFP and hCD8α. All cell cultures responded to Hb(64–76)/I-Ek by proliferating with similar responsiveness, indicating that the endogenous 2.102 receptor was still intact (data not shown).
Figure 5.
3F can inhibit proliferation in 2.102 TCR-transgenic splenocytes. (a–d) Left panels: expression of Wt, 6F, and 3F in 2.102 T cells. hCD8-ζ mutants Wt, 6F, and 3F were cloned into a bicistronic retrovirus coexpressing GFP. Retrovirus-containing supernatants were produced using the packaging cell line Phoenix E in two independent infections. T cells from the spleens of 2.102 TCR–transgenic, RAG-1−/− mice were harvested, activated in vitro, and infected with retroviral supernatants containing vector alone (a), Wt (b), 6F (c), or 3F (d). T cells were infected on two separate occasions. GFP-positive cells were FACS® sorted, restimulated, and subcloned. Two cell cultures for each construct were then analyzed by FACS® for expression of GFP and hCD8α using RPA-T8–PE (PharMingen). All cell cultures were positive for the expression of GFP, and the Wt-, 6F-, and 3F-transduced T cells also expressed hCD8α. Right panels: 3F can inhibit TCR-mediated proliferation. 3 × 104 2.102 T cells were stimulated with 5 × 104 CH27 cells prepulsed with Hb(64–76). This led to T cell proliferation as measured with thymidine incorporation after 48 h (dashed lines). Alternatively, increasing doses of OKT-8 were added to the experiment to cross-link the hCD8 mutants (solid lines). This led to an inhibition of T cell proliferation when 2.102 T cells were transduced with 3F (d). OKT-8 had no effect on T cells transduced with empty vector (a) or with 6F (c). The presence of Wt augmented TCR-mediated proliferation (b). The Hb(64–76) doses necessary to obtain intermediate levels of proliferation were determined in previous experiments. Doses were 3 × 10−6 M for vector alone, Wt, and 3F and 10−6 M for 6F. The data represent the mean of triplicate cultures with SD < 15% of the mean. The experiment was performed three times with similar results.
We next examined the effect of the hCD8-ζ mutants. T cells were either exposed to a stimulating dose of Hb(64–76)/I-Ek alone (Fig. 5, a–d, right panels, dashed lines) or simultaneously exposed to Hb(64–76)/I-Ek and increasing doses of anti-hCD8α (Fig. 5, a–d, right panels, solid lines). Such treatment had no effect on the vector alone or 6F-transduced cells (Fig. 5, a and c). Thus, neither the presence of anti-hCD8α antibodies nor of extracellular hCD8α had an effect on T cell proliferation. Cross-linking of hCD8-ζ Wt induced an increase in proliferation, indicating an activating effect (Fig. 5 b). When the 3F mutant was cross-linked, proliferation to peptide-pulsed APCs was inhibited in a dose-dependent manner (Fig. 5 d). This demonstrated that partially phosphorylated ζ species could also inhibit T cell activation in nonimmortalized T cells.
Discussion
Here we demonstrate that the type of TCR-ζ phosphorylation present in resting T cells can inhibit T cell activation. Thus, the constitutive phosphorylation of the TCR complex may not reflect a low level of ongoing T cell activation. Rather, it may serve to prevent any unintended T cell activation. TCR-ζ phosphorylation induced by antagonist ligands can also inhibit T cell activation. Our analysis therefore also supports the concept that TCR antagonism can occur through the generation of an inhibitory signal within the TCR complex.
Our findings raise questions about the mechanism of T cell inhibition. We think that the inhibitory effect can be exerted through the action of proteins that specifically bind partially phosphorylated ζ. Such proteins could associate with ζ through single SH2 domains or through other protein–protein interactions. During full T cell activation, the stronger, cooperative binding of the tandem SH2 domains of Zap-70 to doubly phosphorylated ITAMs might displace these proteins. One possibility is that binding proteins could be inhibitory phosphatases that are brought into the vicinity of the endogenous TCR when inhibitory hCD8-ζ mutants are antibody cross-linked. Alternatively, binding proteins could be positively acting kinases present in limiting amounts. Through their association with ζ, kinases might become sequestered and then might no longer be available to mediate T cell activation. A similar mechanism has been identified for antagonist inhibition of the high-affinity receptor for the Fc portion of IgE, Fc∈RI 31.
We overexpressed chimeric proteins and antibody cross-linked their extracellular portion to study the role of partially phosphorylated ITAMs. Similar approaches have been widely used to establish the function and importance of intact ITAMs and their signal amplification ability 1 2 3. In such studies, a multitude of ζ ITAM mutants were examined for their potential to stimulate T cells 1 2 5 6 7. Here we further extended the use of this experimental system and expressed such chimeras in cells with functional endogenous TCRs of known specificity. This enabled us to study the previously unaddressed question of whether mutant ITAMs can also inhibit T cell activation. We used hCD8-ζ chimeras because similar proteins with human intracellular ζ have been previously employed 1 28. hCD8-ζ does not associate with components of the endogenous TCR complex before antibody cross-linking 28. It therefore does not interfere with regulation of TCR signaling in the absence of cross-linking. In addition, the chimeric protein could homodimerize, much like endogenous ζ.
Ardouin et al. 9 have recently published a report that T cells from a P14 TCR–transgenic mouse lacking full ζ ITAMs functioned normally. The mutant ζ, a1-,b1-,c1-, had the amino terminal tyrosine of each ITAM converted to a phenylalanine. These findings clearly showed that intact ζ ITAMs are not needed for T cell development and function and that the four CD3 ITAMs are sufficient. In our system, we did not examine the identical mutant, because we have previously not found a TCR-ζ species phosphorylated simultaneously at tyrosines A2, B2, and C2 in T cells 11. Our 3F mutant was the most similar but with different tyrosines remaining. Based upon our findings, we would have predicted that the a1-,b1-,c1- mutant would be inhibitory; however, this was not observed. One possible explanation was that only certain tyrosine residues were inhibitory. Arguing against this is our result with the 4OF mutant. An alternative explanation is that the remaining tyrosines in the ζ molecule must be phosphorylated to be inhibitory. Consistent with this, the a1-,b1-,c1- mutant was not found to be phosphorylated, even upon pervanadate stimulation.
We studied the function of TCR-ζ, although TCR-ζ ITAMs are not essential for T cell development and function, and CD3ζ−/− mice reconstituted with ζ lacking all three ITAMs appear largely normal. Clearly, the 10 TCR/CD3 ITAMs have overlapping functions, but we contend that their number and ordered phosphorylation collectively provide the TCR with a mechanism by which it can discriminate between closely related ligands. In the ζ ITAM-less mice mentioned, the selection of T cells with specific TCRs is altered, and autoreactive T cells can be found 10. One difference between the components of the TCR complex is that CD3∈, CD3γ, or CD3δ is not constitutively phosphorylated in resting peripheral T cells, whereas CD3ζ is. T cells from mice expressing normal CD3∈, CD3γ, and CD3δ but mutated CD3ζ without ITAMs would therefore lack the inhibitory signal from partially phosphorylated ζ. Thus, the autoreactivity found in such mice might not only be due to altered thymic selection but also to increased peripheral reactivity.
To elucidate the mechanism of TCR antagonism, two different known TCRs have recently been expressed on the same T cell 32 33 34. “Cross-antagonism” of the two TCRs would indicate that an inhibitory signal leads to TCR antagonism. The outcome of the experiment varied, and cross-antagonism was observed in some but not all cases. This can be explained if TCR antagonism is induced by differing mechanisms 35. Alternatively, depending on the expression level and spatial separation of the two expressed TCRs, a local inhibitory signal through one α/β TCR may or may not affect signaling through a second TCR.
TCR antagonism can occur in the absence of functional ζ 9 36. We think that CD3∈, CD3γ, or CD3δ can substitute for ζ ITAMs in the absence of ζ, as these proteins can also substitute for ζ in T cell development. It remains to be addressed whether CD3∈, CD3γ, or CD3δ can become partially phosphorylated after stimulation with antagonist ligands. CD3∈ was not phosphorylated after stimulation with altered peptide ligands 17 18 19, but we recently found that stimulation of the 3.L2 TCR with antagonist ligands induces phosphorylation of CD3∈ (Kersh, E.N., and P.M. Allen, unpublished results). Different cell lines might therefore differ in their efficiency to induce CD3∈ phosphorylation, or certain antagonist ligands might be more effective in inducing CD3∈ phosphorylation.
Our analysis of the 2F mutant provided insight into the hierarchy of stimulatory versus inhibitory ITAMs. In the 2F mutant, functional ITAM A mediated T cell activation despite the presence of inhibitory tyrosines B1 and C2. This result was unanticipated, because in the 4F mutant, B1 and C2 alone had the ability to inhibit TCR-mediated IL-2 production. TCR-mediated IL-2 production is most likely also the result of the phosphorylation of functional ITAMs but within the TCR complex. However, the effect of such phospho-ITAMs in the TCR complex could be inhibited by the presence of B1 and C2 in the 4F mutant. We think that the decision over activation or inhibition is made based on a concentration difference of fully or partially phosphorylated ITAMs: for inhibition, partially phosphorylated ITAMs must far outnumber fully phosphorylated ITAMs. By overexpression of 3F and 4F, fully phosphorylated ITAMs in the endogenous TCR complex were outnumbered. However, in the 2F mutant, B1 and C2 could not outnumber ITAM A, and therefore activation resulted.
In conclusion, we find that basal TCR-ζ phosphorylation in resting peripheral T cells inhibits T cell activation, as do the subsequent first phosphorylation events and ζ phosphorylation induced by antagonist ligands. These findings add to our understanding of the complexity of signal initiation at the TCR and strengthen our earlier interpretation that sequential ζ phosphorylation steps increase the fidelity of T cell activation 11.
Acknowledgments
We thank Arash Grakoui for 2.102 RAG-1−/− mice; Kenneth Murphy and Bill Sha for GFP-RV; Sheila Ranganath, Wenjun Ouyang, and Dom Fenoglio for help with retroviral infections; Andrey Shaw for critically reviewing the manuscript; Andy Chan for helpful discussions; Brian Evavold for sharing unpublished results; Jerri Smith for secretarial assistance; and members of the Allen lab for stimulating discussion of this work.
This work was supported by grants from the National Institutes of Health.
G.J. Kersh's present address is Dept. of Pathology, Emory University, Atlanta, GA 30322.
Footnotes
GFP, green fluorescent proteinITAMs, immunoreceptor tyrosine-based activation motifsRAG, recombination activating gene
References
- Irving B.A., Chan A.C., Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor ζ chain. J. Exp. Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo C., Amiot M., Seed B. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor zeta chain. Cell. 1992;68:889–897. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Klausner R.D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 ∈. Science. 1992;255:79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- Weiss A. T cell antigen receptor signal transductiona tale of tails and cytoplasmic protein-tyrosine kinases. Cell. 1993;73:209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Klausner R.D. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc. Natl. Acad. Sci. USA. 1991;88:8905–8909. doi: 10.1073/pnas.88.20.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S.J., Cenciarelli C., Niklinska B.B., Letourneur F., Ashwell J.D., Weissman A.M. Mutagenesis of T cell antigen receptor ζ chain tyrosine residues. J. Biol. Chem. 1992;267:13656–13660. [PubMed] [Google Scholar]
- Koyasu S., McConkey D.J., Clayton L.K., Abraham S., Yandava B., Katagiri T., Moingeon P., Yamamoto T., Reinherz E.L. Phosphorylation of multiple CD3ζ tyrosine residues leads to formation of pp21 in vitro and in vivo. J. Biol. Chem. 1992;267:3375–3381. [PubMed] [Google Scholar]
- Shores E.W., Huang K., Tran T., Lee E., Grinberg A., Love P.E. Role of TCR ζ chain in T cell development and selection. Science. 1994;266:1047–1050. doi: 10.1126/science.7526464. [DOI] [PubMed] [Google Scholar]
- Ardouin L., Boyer C., Gillet A., Trucy J., Bernard A.-M., Nunes J., Delon J., Trautmann A., He H.-T., Malissen B. Crippling of CD3-ζ ITAMs does not impair T cell receptor signaling. Immunity. 1999;10:409–420. doi: 10.1016/s1074-7613(00)80041-2. [DOI] [PubMed] [Google Scholar]
- Shores E.W., Tran T., Grinberg A., Sommers C.L., Shen H., Love P.E. Role of the multiple T cell receptor (TCR)-ζ chain signaling motifs in selection of the T cell repertoire. J. Exp. Med. 1997;185:893–900. doi: 10.1084/jem.185.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh E.N., Shaw A.S., Allen P.M. Fidelity of T cell activation through multistep T cell receptor ζ phosphorylation. Science. 1998;281:572–575. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- De Magistris M.T., Alexander J., Coggeshall M., Altman A., Gaeta F.C.A., Grey H.M., Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Jameson S.C., Carbone F.R., Bevan M.J. Clone-specific T cell receptor antagonists of major histocompatibility complex class I–restricted cytotoxic T cells. J. Exp. Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold B.D., Sloan-Lancaster J., Allen P.M. Antagonism of superantigen-stimulated helper T-cell clones and hybridomas by altered peptide ligand. Proc. Natl. Acad. Sci. USA. 1994;91:2300–2304. doi: 10.1073/pnas.91.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Alexander J., Ruppert J., Snoke D., Franco A., Ishioka G., Grey H.M. Antigen analogs/MHC complexes as specific T cell receptor antagonists. Annu. Rev. Immunol. 1994;12:413–431. doi: 10.1146/annurev.iy.12.040194.002213. [DOI] [PubMed] [Google Scholar]
- Jameson S.C., Bevan M.J. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Shaw A.S., Rothbard J.B., Allen P.M. Partial T cell signalingaltered phospho-ζ and lack of Zap 70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Madrenas J., Wange R.L., Wang J.L., Isakov N., Samelson L.E., Germain R.N. ζ Phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C., Levine E.H., Germain R.N. Partial signaling by CD8+ T cells in response to antagonist ligands. J. Exp. Med. 1996;184:149–157. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Face D.M., Couture C., Anderson K., Shih G., Alexander J., Sette A., Mustelin T., Altman A., Grey H.M. Differential T cell signaling induced by antagonist peptide-MHC complexes and the associated phenotypic responses. J. Immunol. 1997;158:2057–2064. [PubMed] [Google Scholar]
- Wange R.L., Malek S.N., Desiderio S., Samelson L.E. Tandem SH2 domains of ZAP-70 bind to T cell antigen receptor ζ and CD3∈ from activated Jurkat T cells. J. Biol. Chem. 1993;268:19797–19801. [PubMed] [Google Scholar]
- Hatada M.H., Lu X., Laird E.R., Green J., Morgenstern J.P., Lou M., Marr C.S., Phillips T.B., Ram M.K., Theriault K. Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature. 1995;377:32–38. doi: 10.1038/377032a0. [DOI] [PubMed] [Google Scholar]
- Evavold B.D., Williams S.G., Hsu B.L., Buus S., Allen P.M. Complete dissection of the Hb(64-76) determinant using Th1, Th2 clones, and T cell hybridomas. J. Immunol. 1992;148:347–353. [PubMed] [Google Scholar]
- Kersh G.J., Allen P.M. Structural basis for T cell recognition of altered peptide ligandsa single T cell receptor can productively recognize a large continuum of related ligands. J. Exp. Med. 1996;184:1259–1268. doi: 10.1084/jem.184.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P.M., Unanue E.R. Differential requirement for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J. Immunol. 1984;132:1077–1079. [PubMed] [Google Scholar]
- Grakoui A., Donermeyer D.L., Kanagawa O., Murphy K.M., Allen P.M. TCR-independent pathways mediate the effects of antigen dose and altered peptide ligands on Th cell polarization. J. Immunol. 1999;162:1923–1930. [PubMed] [Google Scholar]
- Ouyang W., Ranganath S.H., Weindel K., Bhattacharya D., Murphy T.L., Sha W.C., Murphy K.M. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- Irving B.A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Combadière B., Freedman M., Chen L., Shores E.W., Love P., Lenardo M.J. Qualitative and quantitative contributions of the T cell receptor ζ chain to mature T cell apoptosis. J. Exp. Med. 1996;183:2109–2117. doi: 10.1084/jem.183.5.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D., Mollenauer M.N., Weiss A. Dominant-negative zeta-associated protein 70 inhibits T cell antigen receptor signaling. J. Exp. Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe C., Inman J.K., Metzger H. An unusual mechanism for ligand antagonism. Science. 1998;281:568–572. doi: 10.1126/science.281.5376.568. [DOI] [PubMed] [Google Scholar]
- Daniels M.A., Schober S.L., Hogquist K.A., Jameson S.C. Cutting edgea test of the dominant negative signal model for TCR antagonism. J. Immunol. 1999;162:3761–3764. [PubMed] [Google Scholar]
- Stotz S.H., Bolliger L., Carbone F.R., Palmer E. T cell receptor (TCR) antagonism without a negative signalevidence from T cell hybridomas expressing two independent TCRs. J. Exp. Med. 1999;189:253–264. doi: 10.1084/jem.189.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J.M., Evavold B.D. Cutting edgedueling TCRs: peptide antagonism of CD4+ T cells with dual antigen specificities. J. Immunol. 1999;163:1750–1754. [PubMed] [Google Scholar]
- Sloan-Lancaster J., Allen P.M. Altered peptide ligand-induced partial T cell activationmolecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- Liu H., Vignali D.A.A. Differential CD3ζ phosphorylation is not required for the induction of T cell antagonism by altered peptide ligands. J. Immunol. 1999;163:599–602. [PubMed] [Google Scholar]