Abstract
The E2A proteins, E12 and E47, are required for progression through multiple developmental pathways, including early B and T lymphopoiesis. Here, we provide in vitro and in vivo evidence demonstrating that E47 activity regulates double-positive thymocyte maturation. In the absence of E47 activity, positive selection of both major histocompatibility complex (MHC) class I– and class II–restricted T cell receptors (TCRs) is perturbed. Additionally, development of CD8 lineage T cells in an MHC class I–restricted TCR transgenic background is sensitive to the dosage of E47. Mice deficient for E47 display an increase in production of mature CD4 and CD8 lineage T cells. Furthermore, ectopic expression of an E2A inhibitor helix-loop-helix protein, Id3, promotes the in vitro differentiation of an immature T cell line. These results demonstrate that E2A functions as a regulator of thymocyte positive selection.
Keywords: E2A, positive selection, thymocyte development, helix-loop-helix
During maturation in the thymus, T lineage cells interact with peptide containing MHC proteins either to differentiate or, alternatively, to undergo programmed cell death. Immature double-positive (DP) thymocytes that express TCRs possessing high affinity for self-peptide–MHC complexes undergo apoptosis, a process referred to as negative selection. Those cells that express TCRs with sufficient affinity for self-peptide–MHC complexes are induced to differentiate into mature single-positive (SP) T cells through a process termed positive selection. Any DP thymocyte expressing a TCR that is incapable of binding self-peptide–MHC complexes or binding with too low an affinity dies “by neglect” 1 2 3.
A large number of receptor-mediated signaling pathways and downstream effector molecules that promote positive versus negative selection have been described. However, the nuclear targets of those signaling events remain largely unknown. Previous studies have shown that the helix-loop-helix (HLH) proteins, E12 and E47, are required during the early stages of T lineage development 4. E12 and E47 are encoded by one gene, designated E2A, and arise through alternative splicing of the exon encoding the HLH domain 5 6. E12 and E47 bind to sequence motifs, termed E-boxes, that are found in the regulatory regions of numerous lineage-specific genes 5 6. In B lymphocytes, E47 homodimeric complexes are the predominate E-box binding species. However, in thymocytes, a portion of the E47 protein binds DNA as a complex with HeLa E-box binding protein (HEB), another HLH protein which is expressed at particularly high levels in developing T cells 7.
Several putative B and T lineage–specific E2A target genes have been described. For example, overexpression of either E12 or E47 in pre-T, macrophage, or pro-B cell lines leads to the induction of the B cell–specific genes λ5, V–pre-B, early B cell factor (EBF), and Pax-5 8 9 10. Additionally, recombination activating gene (RAG)-1 expression is induced upon ectopic expression of either E12 or E47 in a pre-T or macrophage cell line 9 10. Another study using enforced expression of Id3, a dominant negative inhibitor of E2A activity, in committed T cell progenitors has also suggested that E2A and/or HEB regulates RAG gene expression 11.
Specific functions of the E2A proteins have been most extensively studied in the context of B lineage development, which is completely blocked at a stage preceding the onset of immunoglobulin gene rearrangement in E2A-deficient mice 4 12. However, recent experiments have demonstrated that E2A activity is also critical for proper T lineage development. Specifically, E2A-deficient mice are lacking in certain populations of γ/δ T cells. This deficiency in distinct γ/δ T cell populations is due, in part, to perturbations in TCR-γ/δ V(D)J recombination 13. Within the α/β T cell lineage, development is partially blocked at the double-negative stage, before the initiation of TCR β chain gene rearrangement. This early developmental defect likely contributes to the significant decrease in total thymocyte numbers observed in E2A-deficient mice 7. In other studies, overexpression of the negative regulator, Id3, in progenitor thymocytes completely blocked T lineage development 14. We have shown previously that E2A-deficient thymi show increased percentages of mature SP T cells and decreased proportions of immature DP cells 7. These data raised the possibility that the E2A proteins are functionally important beyond the double-negative stage of thymocyte development. Here, we reveal a role for E2A in DP thymocyte maturation. In the absence of E47 activity, an increased proportion of DP thymocytes matures into CD4 and CD8 SP thymocytes. Interestingly, maturation of CD8 SP thymocytes in class I–restricted TCR transgenic mice is sensitive to the dosage of E47, whereas CD4+ thymocyte maturation in class II–restricted TCR transgenic mice is not. In addition, inhibition of E47 DNA binding activity in a DP T cell line by retroviral transduction of a dominant negative inhibitor promotes the maturation of these cells. Taken together, these data suggest that E47 activity contributes to the regulation of thymocyte positive selection.
Materials and Methods
Mouse Strains.
The E2A- and E47-deficient mice have been described previously 4 15. The β2-microglobulin (β2M) mice were purchased from The Jackson Laboratory. All mice were analyzed between 4 and 7 wk of age.
In Vitro Viability Analysis.
Total thymocytes from E47- or E2A-deficient mice and wild-type littermates were dissected into RPMI medium containing 10% fetal bovine serum and 50 μM 2-ME and supplemented with glutamine, penicillin, and streptomycin. The cells were stained with antibodies to CD4 and CD8 or with propidium iodide (to determine viability) before culture (time = 0). The cells were then placed in culture at 37°C plus 5% CO2 for 28 h before harvesting and staining with anti-CD4–PE and anti-CD8–FITC antibodies and propidium iodide to determine cell viability. The percentage of viable DP cells was determined by multiplying the number of viable cells at 28 h by the percentage of DP cells at 28 h and dividing this by the number of DP cells put into culture at time = 0.
Flow Cytometric Analysis.
Staining of the cells has been described previously 4. For two-color analysis, cells were stained with anti-CD4–PE (RM4-5; PharMingen), anti-CD8α–FITC (53-6.7; PharMingen), anti–TCR-α/β–FITC (H57-597; PharMingen), and anti-CD69–PE (H1.2F3; PharMingen). Hybridoma supernatant from the T3.70 line, provided by Dr. Ellen Robey (University of California, Berkeley, CA), was used for the Vα3 staining. For three-color analysis, cells were stained with anti-CD4–TriColor (CT-CD4; Caltag), anti-CD8α–PE (53-6.7; PharMingen), anti-CD69–FITC (H1.2F3), anti–heat stable antigen (HSA)–FITC (M1/69; PharMingen), and anti–TCR-α/β–FITC. Cells were analyzed on a FACScan™ (Becton Dickinson), and live cells were gated on the basis of forward and side scatter.
Bromodeoxyuridine Analysis of Thymocyte Development.
For the kinetic analysis of thymic development, mice were continuously exposed to the thymidine analogue bromodeoxyuridine (BrdU; 0.8 mg/ml) in their drinking water for the indicated times. Thymocytes were labeled with antibodies extracellularly as described above using biotinylated anti-CD4 or anti–TCR-α/β (PharMingen) and PE-labeled anti-CD8 or anti-CD69 (PharMingen). Cells were then fixed and stained with FITC-labeled anti-BrdU (Becton Dickinson) as described previously 16.
Retroviral Supernatant Production and T Lymphoma Transduction.
The 16610D9 cell line was derived from a thymoma that developed spontaneously in a p53-deficient mouse and was adapted to culture. The cells were subcloned to generate the 16610D9 line, which was then cultured at 37°C plus 5% CO2 in Optimem (GIBCO BRL) containing 10% fetal bovine serum and 50 μM 2-ME and supplemented with glutamine, penicillin, and streptomycin. The retroviral vector LZRSpBMN-linker-IRES-EGFP (S-003) was obtained from Hergen Spits (The Netherlands Cancer Institute, Amsterdam, The Netherlands). The mouse Id3 cDNA that was cloned into the polylinker was generated by PCR of an Id3 cDNA with the following primers: Id3 forward, 5′-CCGGAATTCATGGACTACAAGGACGACGATGACAAGGGATCCATGAA-GGCGCTGAGCCCGGTG; and Id3 reverse, 5′-CCGGAATTCTCAGTGGCAAAAGCTCCTC, to generate FLAG-tagged Id3. The PCR product was cloned into pBSK, digested out of pBSK with EcoRI, and cloned into the EcoRI site in S-003. The φNX-eco packaging line 17 was transfected with the retroviral constructs by calcium phosphate precipitation. The transfected cells were switched to 16610D9 culture medium 24 h after transfection, and supernatants were harvested after an additional 24 h. Transduction of the 16610D9 cells was performed as described previously 18. Cells were harvested 48 h after infection and analyzed by flow cytometry as described above using PE-conjugated antibodies (with the exception of CD4, which was TriColor conjugated), or lysed to make whole cell extracts (WCEs). To prepare WCE, cells were pelleted, washed once in cold 1× PBS, and the cell pellet was then frozen on dry ice for ∼15 min. The pellet was thawed, resuspended in 15 μl/106 cells cold buffer C (20 mM Hepes, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1 mM PMSF plus protease inhibitors) containing 1% NP-40, and vortexed vigorously for 2 min at 4°C. Debris was pelleted by spinning at high speed in a microfuge at 4°C, and the supernatant was removed as the WCE. Protein concentrations were determined using Bio-Rad's protein assay reagent as described by the manufacturer. Double-stranded DNA probes were end-labeled using T4 polynucleotide kinase and purified over a G25 Sepharose column. 15 μg of WCE was used in a gel shift assay as described previously 19. The sequence of the μE5 oligo probe is as follows: 5′-TCGAAGAACACCTGCAGCAGCT-3′.
Results
Absence of E2A Leads to Altered Thymocyte Maturation.
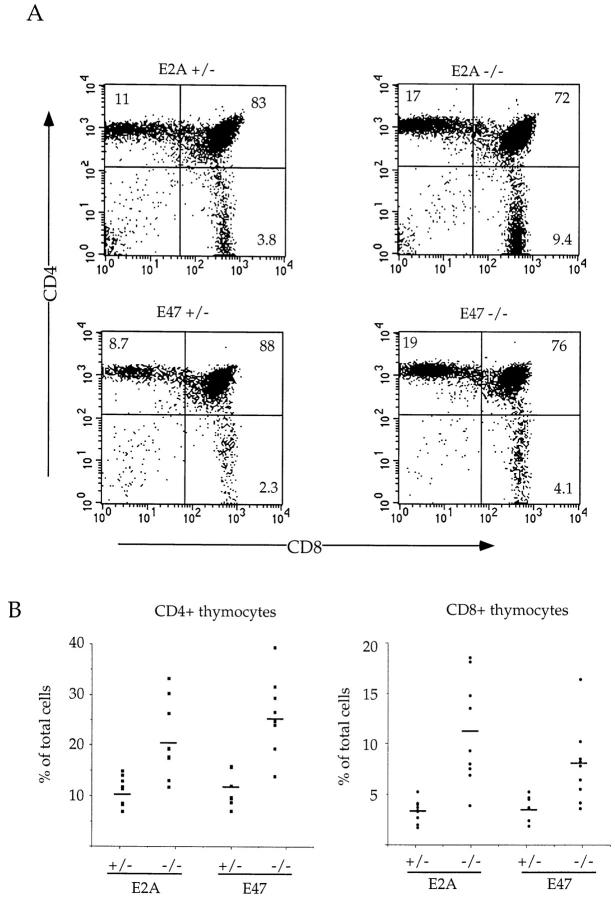
Previous studies have established that mutant mice lacking both E12 and E47 exhibit abnormalities in thymocyte development 7. To generate sufficient numbers of mice to further characterize the T cell phenotype, we analyzed E47-deficient mice, which have a significantly lower rate of postnatal lethality compared with the E2A−/− mice 15. However, we note that the E47-deficient mice also express reduced levels of the E12 protein compared with control littermates 15. We analyzed thymocytes derived from 4–6-wk-old E2A- or E47-deficient mice for the expression of CD4 and CD8 by flow cytometry. Wild-type and E47 or E2A heterozygous mice had virtually identical thymic profiles (data not shown). However, E2A- and E47-deficient thymi showed significant decreases in the percentage of DP thymocytes compared with their heterozygous littermates (Fig. 1 A). In contrast, the proportions of both the CD4 and CD8 SP populations were increased an average of two- to threefold in the E2A- and E47-deficient thymi (Fig. 1A and Fig. B).
Figure 1.
Increased percentage of SP thymocytes in E2A- and E47-deficient mice. (A) Two-color flow cytometric analysis of thymocytes from 4–6-wk-old E2A- and E47-deficient mice and heterozygous littermates. Thymocytes were analyzed by staining with anti-CD8α and anti-CD4. The numbers in each quadrant indicate the percentage of thymocytes in that population. (B) The percentage of mature CD4+ (left) or CD8+ (right) thymocytes is plotted for eight to nine individual E2A- and E47-deficient mice and heterozygous controls. The horizontal bars indicate the average percentage of CD4+ or CD8+ thymocytes for each genotype.
Within the thymus, an increase in the relative percentage of mature SP thymocytes can arise through several mechanisms. For example, either aberrant proliferation of the SP populations, increased frequency of apoptosis within the DP population, or increased maturation of the DP cells could explain the phenotypical changes observed in the E2A- and E47-deficient mice. To begin to address these possibilities, we analyzed thymi from E2A-deficient mice for incorporation of BrdU and survival in vitro. E2A-deficient mice and control littermates were injected with BrdU, and the thymic subpopulations were analyzed for BrdU incorporation 24 h after injection. We found no increase in the percentage of SP thymocytes from E2A-deficient mice staining positive for BrdU compared with the control littermates (data not shown). Thus, the increased percentage of SP cells is not a result of abnormal proliferation within this population. To determine whether the absence of E2A promotes DP cell death, we isolated total thymocytes from E47- or E2A-deficient mice and wild-type littermates and analyzed the viability of the cells after 28 h in culture. Whereas an average of 52% of the wild-type DP cells was alive after the culture period, only ∼17% of the E47- and E2A-deficient DP cells survived (Table , and data not shown). Similar percentages of wild-type or E2A-deficient CD4+ cells were alive after 28 h in culture, suggesting that the increase in cell death is specific to the DP population (Table ). Thus, DP thymocytes lacking E2A activity display decreased survival in vitro.
Table 1.
In Vitro Viability of DP and CD4+ Thymocytes from E2A-deficient Mice and Wild-type Littermates
| Percent DP | Percent CD4+ | |||
|---|---|---|---|---|
| Exp. | +/+ | −/− | +/+ | −/− |
| 1 | 44 | 15 | 74 | 79 |
| 2 | 56 | 14 | 85 | 82 |
| 3 | 55 | 23 | 92 | 100 |
| Ave. | 52 (± 6) | 17 (± 5) | 84 (± 9) | 87 (± 11) |
Total thymocytes from E2A-deficient mice or wild-type littermates were stained with antibodies against the CD4 and CD8 coreceptors and with propidium iodide before culture and after 28 h in culture. The percentage of viable DP or CD4+ cells for three independent experiments is shown. The average (Ave.) for the three experiments (Exp.) is indicated, with the SD in parentheses.
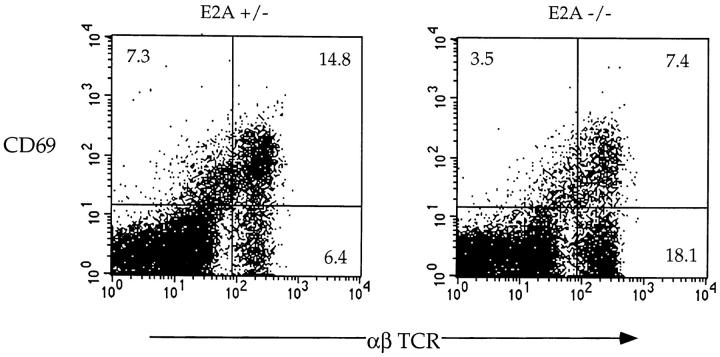
To address the possibility of an increase in DP thymocyte maturation, we further characterized the changes in the T cell populations by analyzing thymocytes from E2A- and E47-deficient mice for the expression of TCR-α/β and CD69. During normal thymocyte development, expression of both TCR-α/β and CD69 increases upon positive selection 20 21 22 23 24. However, whereas SP thymocytes retain high levels of TCR-α/β expression, CD69 levels are subsequently downregulated upon completion of maturation 21 24 25. Thus, TCRhiCD69+ cells represent those thymocytes in the process of positive selection, whereas TCRhi CD69− thymocytes have completed the maturation process. We find that thymocytes derived from E2A- or E47-deficient mice have a three- to fourfold increase in the proportion of TCRhiCD69− cells compared with their littermates (Fig. 2, and data not shown). The absolute number of mature TCRhiCD69− thymocytes is increased an average of 1.5–3-fold (data not shown). These data indicate that the absence of E47 leads to an increase in the proportion of SP cells that have completed thymocyte maturation, and suggest that E47 might have an additional role in regulating DP thymocyte differentiation.
Figure 2.
E47 deficiency leads to altered thymocyte maturation. Two-color flow cytometric analysis of TCR-α/β and CD69 expression on thymocytes from a 5-wk-old E2A-deficient mouse and a heterozygous littermate. The numbers in each quadrant indicate the percentage of thymocytes in that population.
Positive Selection in E47-deficient Mice Expressing Class I– and Class II–restricted TCR Transgenes.
To further explore whether the absence of E47 leads to an increase in thymocyte maturation, E47-deficient mice were crossed to AND TCR mice which express a class II–restricted TCR transgene. Maturation of CD4 SP thymocytes is strongly enhanced in H-2b mice expressing the AND TCR 26. Thymocytes derived from E47-deficient mice and wild-type or heterozygous littermates carrying the AND transgene were analyzed by flow cytometry for the expression of CD4 and CD8. As expected, E47+/+;AND mice contained a high fraction of mature CD4+ cells and a correspondingly lower fraction of immature DP thymocytes than nontransgenic thymi (Fig. 3, top panels). Thymic profiles from E47+/−;AND mice were not significantly different from the E47+/+;AND profiles (Fig. 3, top panels). However, the percentage of mature CD4 SP cells was significantly higher (87 vs. 73%) in thymocytes derived from E47−/−;AND mice, and the proportion of DP cells was significantly lower (10 vs. 22%; Fig. 3, top panels). Importantly, we observed a similar trend in the peripheral T cell populations. The ratio of CD4+ to CD8+ splenic T cells increased from ∼22 in the wild-type or heterozygous mice expressing the AND TCR to 55 in the E47−/−;AND mice (Fig. 3, middle panels). In the lymph nodes, the ratio of CD4+ to CD8+ cells increased from 11 in the E47+/+; AND mice to 69 in the E47−/−;AND mice (Fig. 3, bottom panels). The increase in total percentage of T cells in the E47-deficient lymph nodes (98 vs. 55%) is due to the complete absence of B cells in the E2A- and E47-deficient mice 4 15. Thus, there is a selective increase in only those SP cells expressing the relevant TCR, and this increase is reflected in the periphery. These data suggest that the absence of E47 can markedly enhance the positive selection of class II–restricted TCRs.
Figure 3.

Absence of E2A leads to an increased generation of mature SP cells in class II–restricted TCR transgenic mice. Flow cytometric analysis of 4–6-wk-old E47-deficient mice and littermate controls expressing the class II–restricted AND TCR transgene. Thymocytes (top panels), splenocytes (middle panels), and lymph node cells (bottom panels) were analyzed by staining with anti-CD4–PE and anti-CD8α–FITC. The numbers in each quadrant indicate the percentage of cells in that population. Genotypes of the mice are indicated above the thymic profiles. The numbers shown above the splenocyte and lymph node panels indicate the CD4/CD8 ratio within those populations.
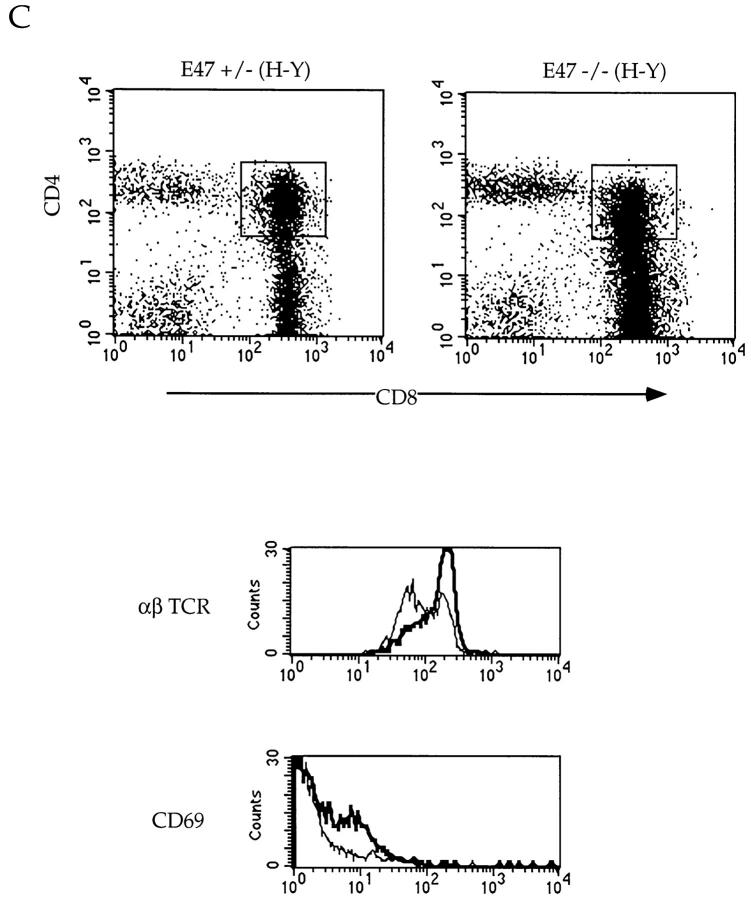
To determine whether the absence of E47 also promotes increased selection of class I–restricted TCRs, we analyzed E47-deficient mice expressing the H-Y TCR transgene, which specifies reactivity with the male H-Y antigen presented by the H-2Db class I molecule 27 28. Expression of the H-Y TCR transgene in females of the H-2b background leads to positive selection of CD8 SP thymocytes 27 28. In contrast, thymocytes in male mice expressing the H-Y TCR are deleted at the DP stage. Negative selection mediated by the H-Y TCR transgene was unaffected by the absence of E47 (data not shown). However, E47−/−; H-Y female mice displayed an increased percentage of mature CD8 SP thymocytes compared with the E47 heterozygous littermates (56.6 vs. 33.6%; Fig. 4 A). This phenotype was accompanied by a significant decrease in the proportion of immature DP thymocytes (29.6 vs. 46%; Fig. 4 A). Because of continued rearrangement of endogenous α chain genes in TCR transgenic mice, a fraction of the thymocytes will express a TCR composed of the transgenic β chain and an endogenous α chain 25 27 29. To analyze the proportion of cells expressing the transgenic TCR-α/β, we stained total thymocytes with the T3.70 antibody, which reacts specifically with the transgenic α chain molecule 30. Thymocytes isolated from E47−/−;H-Y mice contained almost twice the percentage of cells expressing high levels of both the transgenic α and β chains, consistent with a higher proportion of mature CD8 SP T cells (Fig. 4 A).
Figure 4.
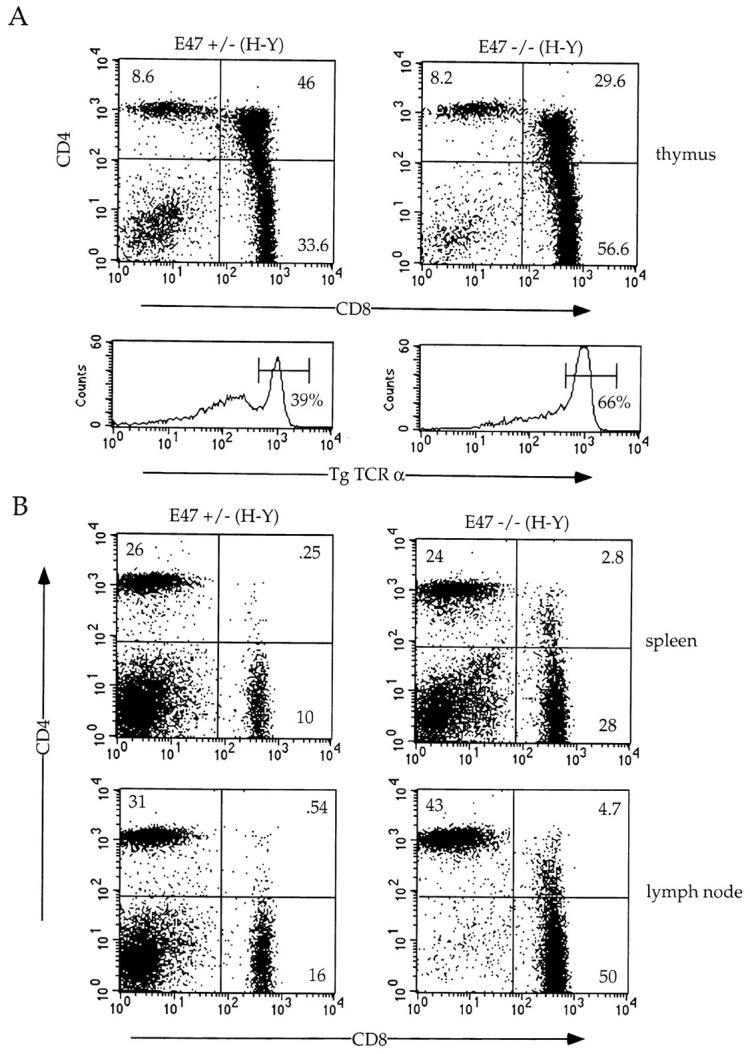
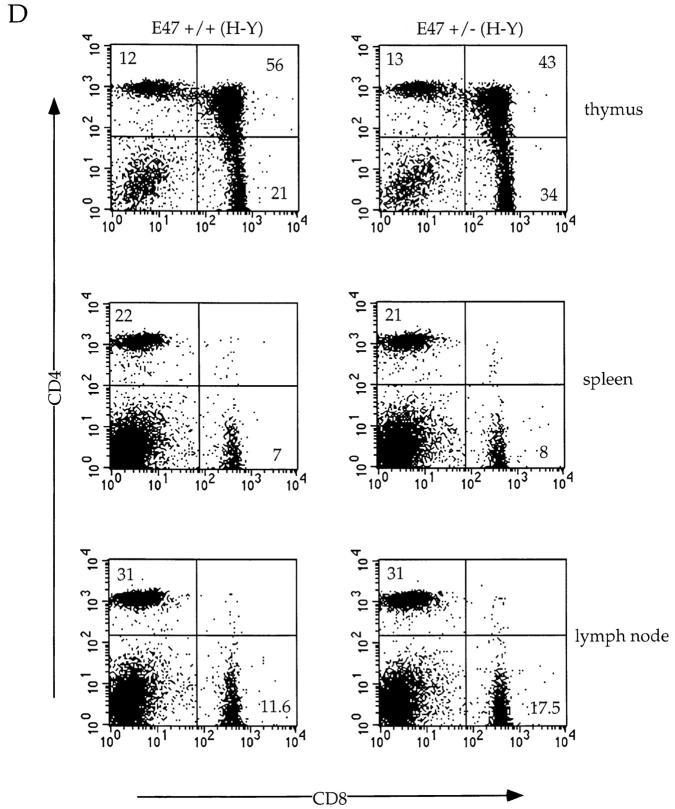
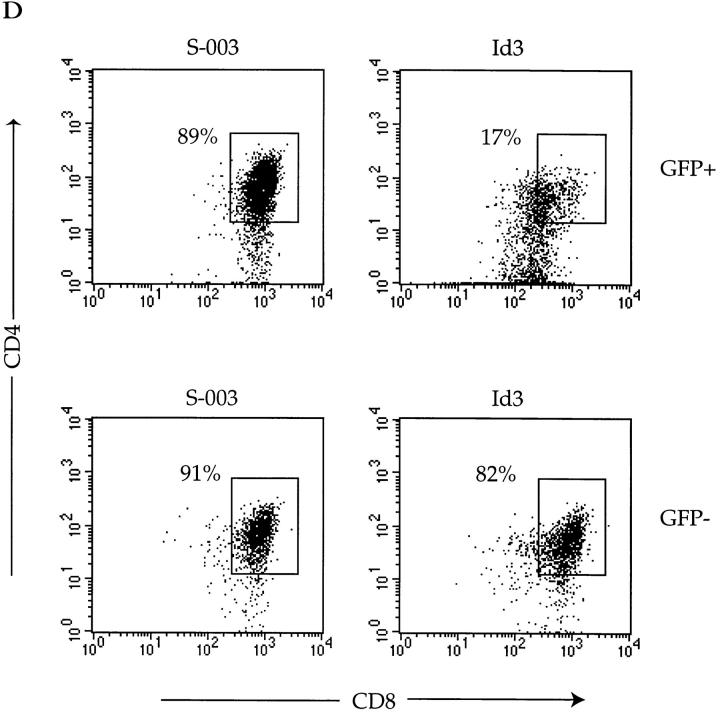
Altered maturation of thymocytes in class I–restricted TCR transgenic mice expressing decreased levels of E47. Flow cytometric analysis of 4–6-wk-old E47-deficient female mice and littermate controls expressing the class I–restricted H-Y TCR transgene. (A) Thymocytes from E47 knockout and heterozygous littermate females were stained with anti-CD4–PE and anti-CD8α–FITC or with the anti-Vα3 antibody, T3.70, which recognizes the transgenic α chain. The numbers in each quadrant indicate the percentage of cells in that population, and the percentage of cells staining brightly for the transgenic α chain is indicated in the histograms. (B) Splenocytes (top panels) and lymph node cells (bottom panels) from female H-Y transgenics of the indicated E47 genotype were analyzed by staining with anti-CD4–PE and anti-CD8α–FITC. The numbers in each quadrant indicate the percentage of cells in that population. (C) Thymocytes from an E47 heterozygous and knockout littermate expressing the H-Y TCR transgene were analyzed by three-color flow cytometric analysis for expression of CD4, CD8, and either TCR-α/β or CD69. The boxed regions in the CD4 versus CD8 plots indicate the analysis gates used to define the DP population. In the histogram plots, the thin lines indicate staining on the E47 heterozygous H-Y DP cells, and the thick lines indicate staining on the E47-deficient H-Y DP cells. The third color antibody used in each case is indicated. (D) Thymocytes (top panels), splenocytes (middle panels), and lymph node cells (bottom panels) from female H-Y transgenics that are wild-type or heterozygous for E47 were analyzed by staining with antibodies to CD4 and CD8. The numbers in each quadrant indicate the percentage of cells in that population.
The increased percentage of mature CD8+ T cells observed in the E47−/−;H-Y mice is also reflected in the periphery. The ratio of CD8+ to CD4+ T cells is increased from ∼0.4 in the E47+/−;H-Y spleen to 1.2 in the E47−/−;H-Y spleen (Fig. 4 B, top panels). A similar change in the CD8/CD4 ratio was observed in the lymph nodes (Fig. 4 B). Therefore, like the E47−/−;AND mice, the E47−/−;H-Y mice display an increase in only the percentage of cells expressing the relevant TCR, and the relative proportions of CD4 and CD8 lineage cells are altered in the peripheral lymphoid organs as well. These changes in peripheral CD8/CD4 ratios from E47-deficient/TCR transgenic mice would not be expected if the increase in proportion of thymic SP cells was entirely the result of increased death within the DP thymocyte population. In addition, a selective increase in only the population of cells expressing the relevant TCR is not consistent with a defect in emigration of mature SP thymocytes from the thymus.
Interestingly, in addition to the increase in CD8 SP cells, E47−/−;H-Y thymi contained elevated percentages of DP cells that expressed CD69 and high levels of TCR-α/β (Fig. 4 C). Thus, a higher proportion of the DP population is undergoing positive selection in the absence of E47. Taken together, these data suggest that the absence of E47 results in increased selection to both class I– and class II–restricted T cell receptors.
As described above, heterozygosity at the E47 locus does not perturb maturation of SP thymocytes expressing class II–restricted TCR transgenes. In contrast, we consistently observed increases in the proportion of CD8+ cells in female H-Y transgenic mice that were heterozygous for E47 compared with wild-type littermates (Fig. 4 D). In most cases, the percentage of CD8+ cells was increased in the periphery of E47+/−;H-Y mice as well (Fig. 4 D). Thus, maturation of CD8 SP cells in mice expressing the H-Y TCR transgene is influenced by the dosage of E47.
Absence of E2A Leads to the Appearance of DP Cells in Peripheral Lymphoid Organs of H-Y Transgenic Mice.
In addition to the increased production of CD8 SP cells, female E47−/−;H-Y mice displayed an aberrant population of T cells in the peripheral lymphoid organs that expressed high levels of CD8 and intermediate to high levels of CD4 (Fig. 4 A and Fig. 5 A). To examine whether the peripheral DP (PDP) population expressed markers characteristic of mature T lineage cells, we analyzed this population for the expression of TCR-α/β and HSA. The PDP cells expressed HSA at a level similar to normal SP thymocytes, and significantly lower than the level of expression on thymic DP cells (Fig. 5 B). However, the expression of HSA on the PDP population is significantly higher than the level observed on normal SP peripheral T cells (Fig. 5 B, right). TCR-α/β expression on the PDP cells was identical to that of the mature SP T cells, with all of the cells expressing uniformly high levels of TCR (Fig. 5 C). Taken together, the data suggest that the PDP cells in the E47−/−;H-Y mice are positively selected T lineage cells that fail to complete maturation before exiting to the periphery.
Figure 5.
Appearance of DP cells in the periphery of E47-deficient/ H-Y transgenic mice. 6-wk-old H-Y transgenic mice of the indicated E47 genotype were analyzed by three-color flow cytometry for the expression of CD4, CD8, and either HSA (B) or TCR-α/β (C). The CD4 versus CD8 profiles for the thymocytes (top panels) and splenocytes (bottom panels) are shown (A), and the numbers in each quadrant indicate the percentage of cells in that population. The boxed regions in the CD4 versus CD8 plots (a–e) indicate the analysis gates used to define the populations analyzed in B and C. In the histogram plots (B and C), the lettered arrows indicate the staining pattern for the corresponding boxed populations in A. To make comparisons clearer, the histograms were plotted in pairs to compare the peripheral DP population with the thymic DP population (left panels) and with the SP populations (right panels).
Absence of E47 Allows Inefficient Maturation of CD8 T Lineage Development in the Absence of Class I MHC.
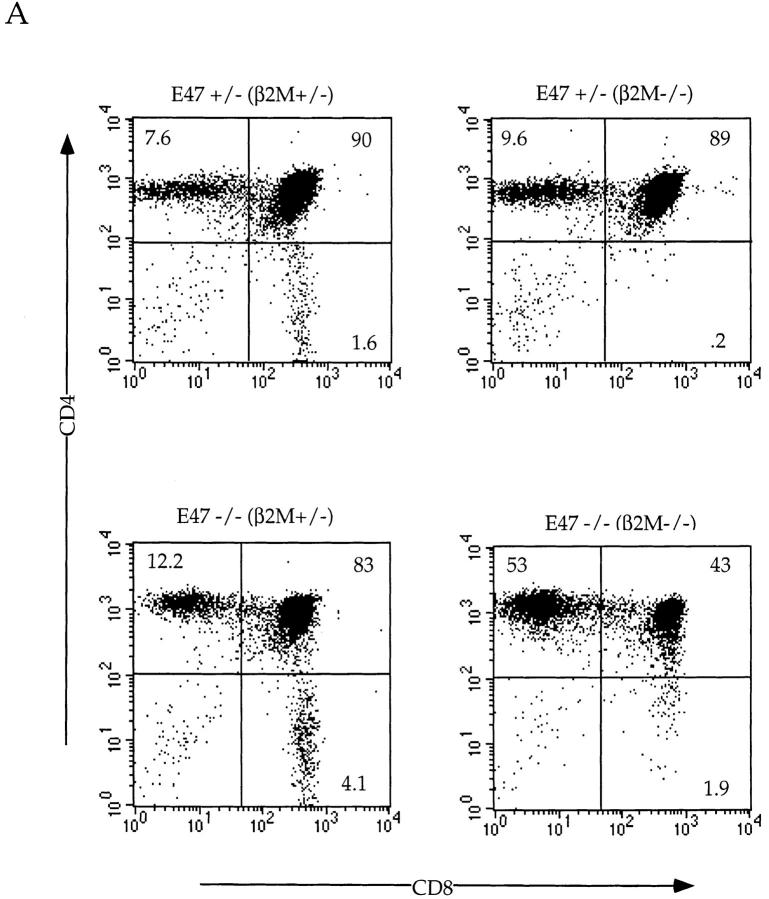
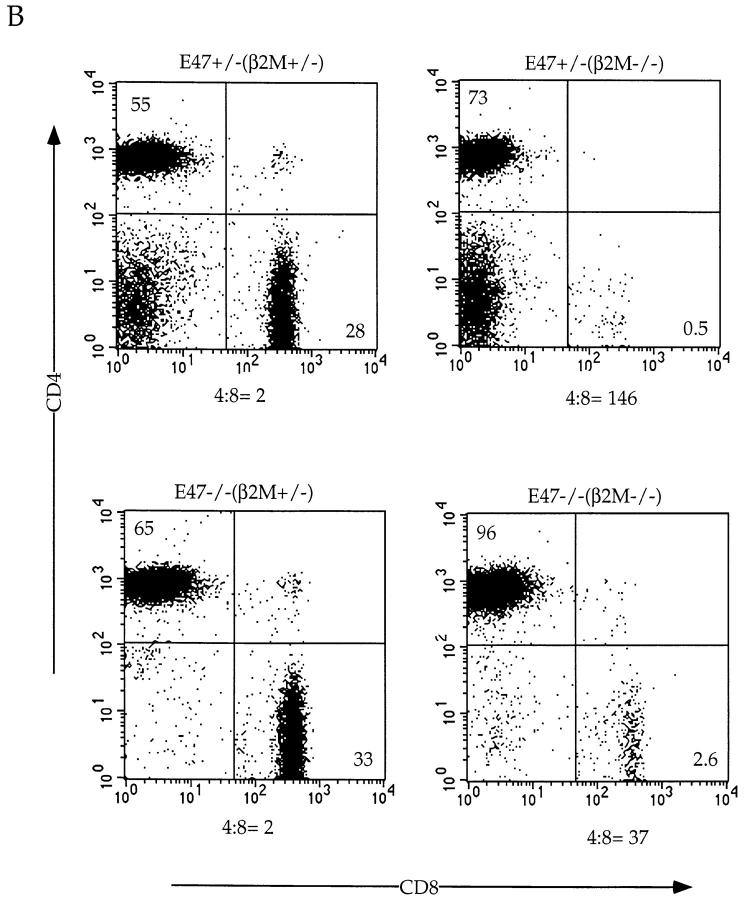
As described above, the absence of E47 promotes the development of SP thymocytes. To determine whether the absence of E47 allows the development of SP cells in the absence of an appropriate signal, we bred the E47-deficient mice to β2M–deficient mice, which virtually lack MHC class I expression and produce very few mature CD8 SP thymocytes 31 32. Although the E47 deficiency did not promote CD8 lineage development in the β2M-deficient background to wild-type levels, we consistently noted a small percentage of TCRhi CD8 SP cells in the E47−/−;β2M−/− thymi that were absent in the E47+/−;β2M−/− thymi (Fig. 6 A, and data not shown). Furthermore, E47−/−;β2M−/− mice displayed a higher proportion of TCRhi CD8 SP cells in the peripheral lymphoid organs compared with the control littermates (Fig. 6 B, and data not shown). The decreased CD4/CD8 ratio from 146 in the E47+/−;β2M−/− spleen to 37 in the E47−/−;β2M−/− spleen indicates that the absence of E47 allows for an increase in maturation of CD8+ cells even in the absence of the appropriate TCR–MHC interaction. Thus, the data suggest that the downregulation of E2A activity can promote thymocyte maturation.
Figure 6.
Development of CD8+ cells in the E47-deficient mice requires MHC class I expression. E47-deficient mice were bred onto the β2M-deficient background, and 4–6-wk-old littermates were analyzed for CD4, CD8, and TCR expression. Thymocytes (A) and splenocytes (B) from E47-deficient and heterozygous littermates on a β2M-deficient or heterozygous background were analyzed by staining with anti-CD8α and anti-CD4. The numbers in each quadrant indicate the percentage of thymocytes in that population. The CD4/CD8 ratios for the spleens are indicated under the FACS® plots in B.
Absence of E47 Leads to an Increased Production Rate of Mature SP Thymocytes.
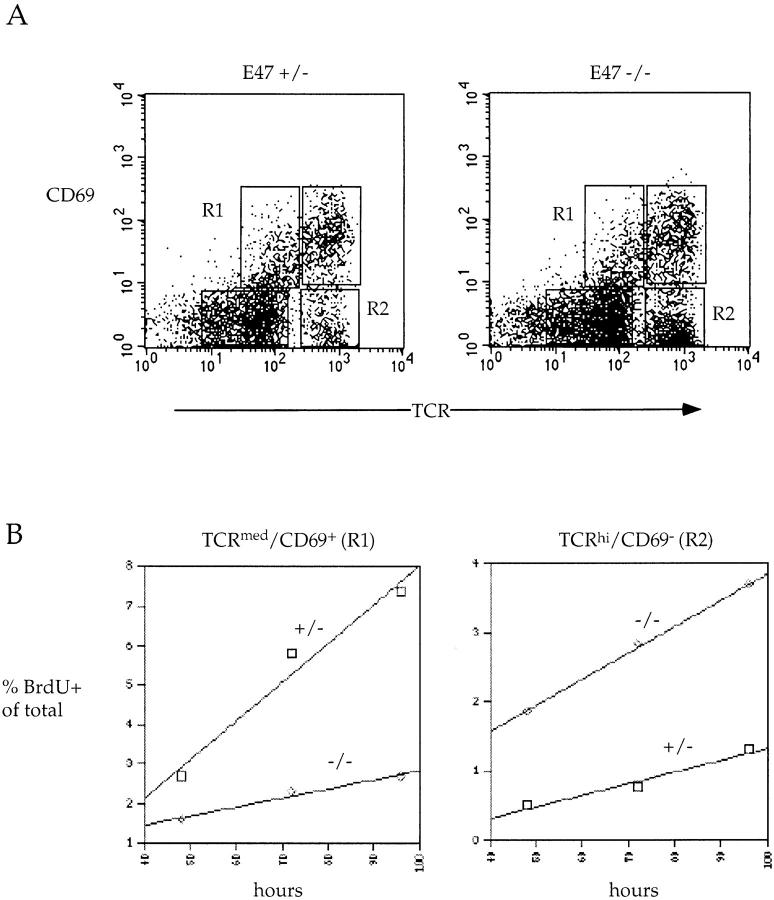
The steady state size of the thymic subpopulations is determined by the rate at which cells are generated, the rate at which they die, and the rate at which they emigrate from the thymus. To more directly assess the effect of an E47 deficiency on the production of mature SP T cells, we measured the kinetics of appearance of mature thymocytes using the thymidine analogue BrdU. We exposed E47-deficient mice and their heterozygous littermates to BrdU in their drinking water and then analyzed for the presence of BrdU and the expression of TCR-α/β and CD69 (Fig. 7A and Fig. B). Because cells expressing medium to high levels of TCR are not dividing, there is a lag in the appearance of BrdU in these populations. As described above, both the E47-deficient and control mice display this lag in labeling of mature thymocytes, demonstrating that the absence of E47 does not lead to the aberrant proliferation of the SP cells. Because the TCRmed-hi populations are nondividing, the rate of appearance of BrdU in these populations reflects the rate at which these cells are produced. In mice deficient for E47, the rate of appearance of BrdU-labeled TCRhiCD69− mature thymocytes is significantly enhanced, suggesting that the relative proportion of cells that develop into mature CD69− T cells is increased in the absence of E47 (Fig. 7 B). In contrast, E47-deficient mice display a decrease in the rate of appearance of BrdU in the TCRmed CD69+ population (Fig. 7 B). Taken together, these data suggest that the rate at which DP thymocytes mature into TCRhiCD69− SP T cells is enhanced in the absence of E47.
Figure 7.
Increased rate of appearance of mature thymocytes in mice deficient for E47. Mice were continuously exposed to the thymidine analogue BrdU in their drinking water for the indicated times and then analyzed for BrdU content and TCR-α/β and CD69 surface expression. (A) Representative TCR-α/β/CD69 FACS® plots for E47-deficient and heterozygous mice. (B) Rate of appearance of BrdU-labeled TCRmed CD69+ cells (left) and TCRhiCD69− cells (right). The percent BrdU-labeled cells of total cells is plotted against the time of continuous exposure to BrdU. The gates used to define the TCRmedCD69+ cells and TCRhiCD69− cells are shown in A as R1 and R2, respectively. The numbers plotted are the averages from two mice.
Inhibition of E47 Binding Activity Promotes T Cell Differentiation.
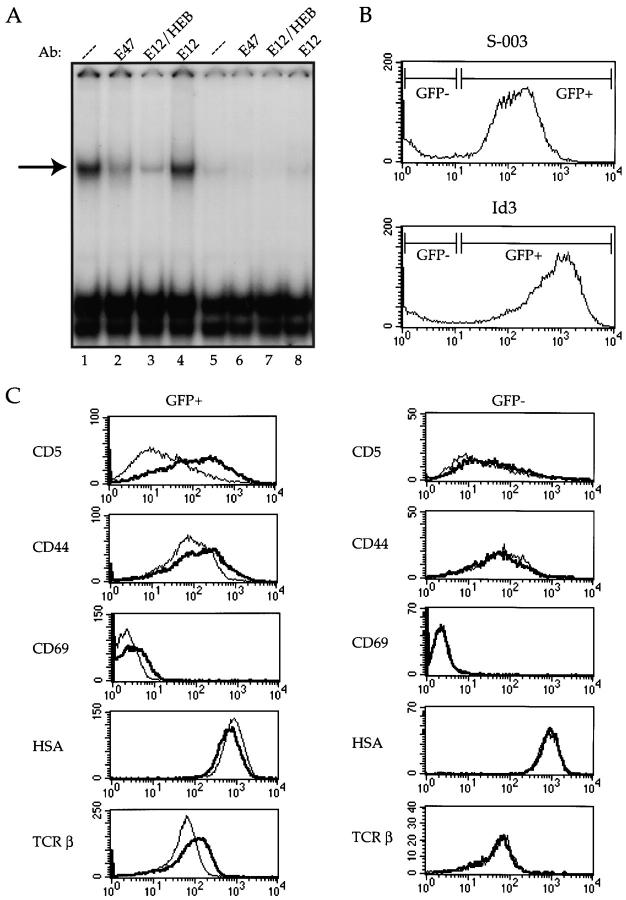
As shown in Table , E2A-deficient DP thymocytes show reduced survival in vitro. However, the data described above suggest that the absence of E47 also promotes thymocyte positive selection. To test directly whether lowering the activity of E47 promotes maturation, we used a retroviral transduction system to study the effects of inhibiting E2A activity in an immature DP T cell line. The mouse cDNA for Id3, a negative regulator of E2A binding activity, was cloned into the retroviral vector S-003 14. This vector allows translation of both Id3 and enhanced green fluorescent protein (EGFP) from one retroviral transcript. Thus, retroviral transductants can be identified by the expression of EGFP. The S-003/Id3 and S-003 empty vector constructs were transfected into the φNX-eco retroviral packaging line, and supernatants from these cells were used to transduce virus into the T cells. The 16610D9 T cell line used was derived from a thymoma that developed spontaneously in a p53-deficient mouse and was adapted to culture. This T cell line expresses characteristics typical of DP thymocytes, including high levels of HSA, intermediate levels of TCR, and low levels of CD5 and CD44 (Fig. 8 C). In addition, the 16610D9 cells express significant levels of E2A binding activity (Fig. 8 A).
Figure 8.
Inhibition of E47 activity in a DP T cell line promotes differentiation. The 16610D9 DP T cell line was retrovirally infected with Id3 or control (S-003) virus. 48 h after infection, the cells were harvested and WCE was prepared as described from half of the cells while the remainder of the cells was stained with the indicated antibodies and analyzed by flow cytometry. (A) E2A binding activity was analyzed by electrophoretic mobility shift assay using a labeled μE5 oligo probe. Lanes 1–4, S-003–infected cells; lanes 5–8, Id3-infected cells. Extracts were preincubated with mAbs (Ab) against E47 (lanes 2 and 6), E12/HEB (lanes 3 and 7), or E12 (lanes 4 and 8) before the addition of the probe. The arrow at left indicates the E47-containing complex. (B) EGFP expression on the S-003 (top) and Id3 (bottom) infected cells. The gates used to define the GFP+ and GFP− populations are indicated in the histograms. (C) Infected cells were stained with the indicated antibodies and analyzed by flow cytometry. The left panels show staining patterns on the GFP+ cells, and the right panels show staining patterns on the GFP− cells within the same infection. Thin line indicates staining on the S-003–infected cells, and thick line indicates staining on the Id3-infected cells. (D) CD4/CD8 staining on GFP+ (top panels) and GFP− (bottom panels) from control (S-003) and Id3-infected cells. The percentage of CD4/CD8 DP cells (boxed area) is indicated in each plot.
Id3 was transduced into the 16610D9 line and analyzed 48 h after infection for the expression of HSA, CD69, CD44, CD5, TCR β chain, and CD4 and CD8. The early stages of positive selection are marked by increased expression of CD69, CD5, and TCR 20 22 24 25. During the later stages of positive selection, CD44 is upregulated whereas HSA is downregulated 33. In addition, T cells undergoing the initial stages of positive selection downmodulate both CD4 and CD8 expression 34. At the time of analysis, ∼85% of both the control and Id3-infected cells were EGFP+ (Fig. 8 B). The ability of Id3 to inhibit E47 binding activity was assayed by electrophoretic mobility shift assay using an E-box probe. E47 and HEB binding activity was easily detectable in the control transduced cells, but dropped to 10–20% of that level in the Id3 transductants (Fig. 8 A). Thus, overexpression of Id3 is sufficient to inhibit E47 binding activity within the 16610D9 cells.
The infected cells were then analyzed by flow cytometry for the expression of the indicated markers. Strikingly, the Id3 transductants displayed altered expression of all the markers analyzed. In particular, expression of both TCR-β and CD5 increased dramatically in the cells lacking E47 binding activity, but was unchanged in the nontransduced cells (Fig. 8 C). In addition, the Id3 transductants clearly upregulated CD69 and CD44 expression, as evidenced by an increase in both the mean linear value of the histogram and the median linear value of the histograms (Fig. 8 C). On the other hand, HSA expression was marginally downregulated in the Id3-expressing cells (Fig. 8 C). Furthermore, we detected a dramatic downmodulation of CD4 and CD8 coreceptor expression in Id3-transduced cells (Fig. 8 E). Thus, these data clearly show that Id3-transduced T cells acquire phenotypic characteristics of thymocytes undergoing positive selection. Taken together, these data demonstrate that lowering the level of E47 activity promotes T cell differentiation in vitro and are consistent with a key role for E47 in promoting DP thymocyte maturation in vivo.
Discussion
Immature thymocytes have the ability to undergo two distinct fates. A DP thymocyte can be selected to mature into either a CD4+ or CD8+ T cell, or alternatively it may undergo cell death. Although a large number of signaling molecules have been implicated in thymic selection, their nuclear targets have remained largely unknown. Here, we show that the activity of one particular transcriptional regulator, E47, is important in regulating thymocyte positive selection. Our data indicate that the relative level of E47 can influence thymocyte selection to both the CD4 and CD8 lineages. In the absence of E47 activity, the rate of production of TCRhiCD69− thymocytes is enhanced, resulting in increased percentages of SP thymocytes. In a TCR transgenic background, a deficiency in E47 results in an increase in positive selection of only those T cells expressing the relevant TCR. In addition, inhibition of E47 activity within a DP T cell line induces the cells to differentiate. Based on these observations, we propose that the downregulation of E2A activity promotes thymocyte maturation.
A Role for E47 in Controlling Thymocyte Maturation.
Mice deficient for E2A display an increased percentage of mature SP thymocytes coupled with a decreased proportion of DP cells. Here we show that the E2A-deficient mice exhibit increases in DP cell death in vitro. Although it is likely that a decreased rate of DP survival could contribute to the observed phenotype, it is doubtful that the increase in proportion of SP cells is solely a result of increased death within the DP population. Our kinetic data demonstrate that the absence of E2A activity additionally promotes thymocyte maturation. Furthermore, E47-deficient TCR transgenic mice display a corresponding increase of SP T cells expressing only the relevant TCR in both the thymus and the peripheral lymphoid organs. The appearance of DP T cells expressing characteristics of positively selected cells in the periphery of E47−/−;H-Y mice also indicates that the absence of E2A affects DP thymocyte maturation. Moreover, our data show that CD8+ T cells have the ability to mature in the absence of class I, albeit with low efficiency, when E47 is lacking. Finally, inhibition of E47 activity within a DP T cell line alters the expression of markers that correlate with DP maturation. Taken together, these data demonstrate that a downregulation of E47 activity promotes thymocyte maturation and that a combination of increased DP cell death and enhanced maturation contributes significantly to the phenotype observed in the E47-deficient mice.
This raises the question of how E47 is involved in positive selection. TCR–MHC interactions that induce positive selection must have two important effects. These signals must rescue the cells from apoptosis and promote maturation. Recent data indicate that the restoration of E47 activity in E2A-deficient DP thymoma cell lines leads to programmed cell death 18. It is possible that the continued presence of E2A activity in DP thymocytes that have received a signal through the TCR contributes to the induction of apoptosis. Thus, only those cells that downregulate E2A activity survive. We note that the thymic phenotype of E47-deficient mice shows similarities to the bcl-2 transgenic mice, including a higher percentage of CD8 SP cells and an alteration in the CD4/CD8 ratio 7 35 36 37 38. Like bcl-2 expression, decreased E47 activity might prolong the survival of the DP thymocytes, thus leading to enhanced production of CD8+ cells. However, as mentioned above, DP thymocytes lacking E2A show an increase in cell death in vitro, whereas bcl-2 expression prolongs DP thymocyte survival 36 38. Additionally, expression of bcl-2 promotes CD8+ thymocyte maturation in MHC class I–deficient mice and in class II–restricted TCR transgenic mice, whereas the absence of E47 does not (35; Fig. 3 and Fig. 6). Finally, in contrast to the E47-deficient mice, the excess CD8+ thymocytes that develop in the bcl-2 transgenic mice do not survive in the periphery 35 36.
Recently, activation of the Notch signaling pathway has been shown to upregulate several markers that correlate with DP maturation. In particular, a constitutively active fragment of Notch was capable of upregulating bcl-2 expression in DP thymomas and rendering these cells resistant to glucocorticoid-induced apoptosis 39. Interestingly, like the E47-deficient mice, activated Notch1 transgenics have increased percentages of CD8+ thymocytes 40. However, a significant population of CD8+ thymocytes can develop in Notch1 transgenic mice lacking expression of class I MHC, whereas only a small CD8+ population can develop in class I–deficient mice lacking E47 40. Nonetheless, it is possible that Notch signaling regulates E2A activity. In fact, others have shown that activated Notch1 and Notch2 effectively inhibit E47 activity in transient transfection assays 41.
It is conceivable that the lack of E47 activity lowers the threshold of avidity required for positive selection. This model has been proposed to explain the phenotype of the activated Notch1 transgenic mice 39. For example, the absence of E47 might allow for the development of T cells that would normally die by neglect because of an insufficient affinity for MHC. It is thought that CD8 lineage commitment requires weaker signals from the TCR compared with the strength of the signal required for CD4 lineage commitment 42 43. If less stringent signals are required for commitment to the CD8 lineage, then a slight lowering of the threshold affinity of positive selection may result in an even greater increase in CD8+ cells compared with CD4+ cells. Such a model might explain the decrease in the CD4+/CD8+ thymocyte ratio in E2A-deficient mice and the finding that more CD8+ T cells develop in MHC class I–deficient mice that also lack E47 expression. Thus, the absence of E47 might allow for the development of some T cells that would normally die by neglect.
E47 Target Genes.
The data described above suggest that E47 activity is downregulated during the final stages of thymocyte maturation. A key step during the transition of a DP to SP thymocyte is the termination of TCR-α rearrangements. Previous studies have suggested a link between E2A activity and RAG expression, which is also downregulated in immature thymocytes upon interaction of the TCR with the appropriate MHC–peptide complex 44. For example, expression of both RAG-1 and RAG-2 can be activated in a pre-T cell line by the ectopic expression of E47 9. Additionally, E2A and HEB have been implicated in regulating RAG transcription in committed T lineage cells 11. Consistent with this idea, E2A-deficient thymocytes show decreased levels of both RAG-1 and RAG-2 transcripts (Bain, G., unpublished observations). However, RAG levels are not completely abolished, and TCR V(D)J recombination proceeds in E2A-deficient mice, presumably due to the presence of an HEB homodimeric complex detectable in E2A-deficient thymocyte extracts 7. We would like to suggest that an interaction of a TCR with the appropriate peptide–MHC complex results in the downregulation of total HLH activity, which leads to the abatement of RAG expression 44. We also note that lowering the DNA binding activity of E47 in a DP cell line leads to the activation of CD5, CD69, and TCR expression and the downmodulation of CD4, CD8, and HSA levels. Thus, our observations indicate that E47 directly or indirectly regulates the expression of a wide variety of genes that are characteristic of thymocyte maturation.
Acknowledgments
We thank Leslie Sharp and Drs. Dawne Page, Eben Massari, and Isaac Engel for critical reading of the manuscript. The H-Y–specific antibody was kindly provided by Dr. Ellen Robey. We are indebted to Drs. Jack Bui and Barbara Kee for generously providing mice.
This work was supported by a Blood and Haemoglobin Training Grant (G. Bain) and grants from the National Institutes of Health (C. Murre).
Footnotes
Abbreviations used in this paper: β2M, β2-microglobulin; BrdU, bromodeoxyuridine; DP, double-positive; EGFP, enhanced green fluorescent protein; HEB, HeLa E-box binding protein; HLH, helix-loop-helix; HSA, heat stable antigen; PDP, peripheral DP; RAG, recombination activating gene; SP, single-positive; WCE, whole cell extract.
References
- Jameson S., Bevan M. T-cell selection. Curr. Opin. Immunol. 1998;10:214–219. doi: 10.1016/s0952-7915(98)80251-3. [DOI] [PubMed] [Google Scholar]
- Kisielow P., von Boehmer H. Development and selection of T cellsfacts and puzzles. Adv. Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- Robey E., Fowlkes B. Selective events in T cell development. Annu. Rev. Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- Bain G., Robanus-Maandag E., Izon D., Amsen D., Kruisbeek A., Weintraub B., Krop I., Schlissel M., Feeney A., van Roon M. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P.S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P.S., Vaessin H., Caudy M., Jan L.Y., Jan Y.N., Cabrera C.V., Buskin J.N., Hauschka S.D., Lassar A.B., Weintraub H., Baltimore D. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Bain G., Engel I., Robanus-Maandag E., te Riele H., Voland J., Sharp L., Chun J., Huey B., Pinkel D., Murre C. E2A deficiency leads to abnormalities in αβ T cell development and to the rapid development of T cell lymphomas. Mol. Cell. Biol. 1997;17:4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigvardsson M., O'Riordan M., Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25–36. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- Schlissel M., Voronova A., Baltimore D. Helix loop helix transcription factor-E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev. 1991;5:1367–1376. doi: 10.1101/gad.5.8.1367. [DOI] [PubMed] [Google Scholar]
- Kee B., Murre C. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J. Exp. Med. 1998;188:699–713. doi: 10.1084/jem.188.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom B., Heemskerk M.H., Verschuren M.C., van Dongen J.J., Stegmann A.P., Bakker A.Q., Couwenberg F., Res P.C., Spits H. Disruption of αβ but not γδ T cell development by overexpression of the helix-loop-helix protein Id3 in committed T cell progenitors. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:2793–2802. doi: 10.1093/emboj/18.10.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Soriano P., Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Bain G., Romanow W.J., Albers K., Havran W.L., Murre C. Positive and negative regulation of V(D)J recombination by the E2A proteins. J. Exp. Med. 1999;189:289–300. doi: 10.1084/jem.189.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk M., Blom B., Nolan G., Stegmann A., Bakker A., Weijer K., Res P., Spits H. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J. Exp. Med. 1997;186:1597–1602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G., Robanus-Maandag E., te Riele H., Feeney A., Sheehy A., Schlissel M., Shinton S., Hardy R., Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- Tough D., Sprent J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella T., Nolan G. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Engel I., Murre C. Ectopic expression of E47 or E12 promotes the death of E2A-deficient lymphomas. Proc. Natl. Acad. Sci. USA. 1999;96:996–1001. doi: 10.1073/pnas.96.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G., Gruenwald S., Murre C. E2A and E2-2 are subunits of B-cell-specific E2-box DNA-binding proteins. Mol. Cell. Biol. 1993;13:3522–3529. doi: 10.1128/mcb.13.6.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi P., Pircher H., Buerki K., Zinkernagel R., Hengartner H. Distinct sequence of negative or positive selection implied by thymocyte T cell receptor densities. Nature. 1990;346:861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- Dutz J., Ong C., Marth J., Teh H.-S. Distinct differentiative stages of CD4+CD8+ thymocyte development defined by lack of coreceptor binding in positive selection. J. Immunol. 1995;154:2588–2599. [PubMed] [Google Scholar]
- Bendelac A., Matzinger P., Seder R.A., Paul W.E., Schwartz R.H. Activation events during thymic selection. J. Exp. Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swat W., Dessing M., von Boehmer H., Kisielow P. CD69 expression during selection and maturation of CD4+CD8+ thymocytes. Eur. J. Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- Yamashita I., Nagata T., Tada T., Nakayama T. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor-mediated positive selection. Int. Immunol. 1993;5:1139–1150. doi: 10.1093/intimm/5.9.1139. [DOI] [PubMed] [Google Scholar]
- Swat W., Dessing M., Baron A., Kisielow P., von Boehmer H. Phenotypic changes accompanying positive selection of CD4+ CD8+ thymocytes. Eur. J. Immunol. 1992;22:2367–2372. doi: 10.1002/eji.1830220928. [DOI] [PubMed] [Google Scholar]
- Kaye J., Hsu M.-L., Sauron M.-E., Jameson S.C., Gascoigne N.R.J., Hedrick S.M. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Bluthmann H., Staerz U., Steinmetz M., von Boehmer H. Tolerance in T cell receptor transgenic mice involves deletion of non-mature CD4+CD8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Teh H., Kisielow P., Scott B., Kishi H., Uematsu Y., Bluthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the αβ T cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Borgulya P., Kishi H., Uematsu Y., von Boehmer H. Exclusion and inclusion of α and β T cell receptor alleles. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- Teh H., Kishi H., Scott B., Borgulya P., von Boehmer H., Kisielow P. Early deletion and late positive selection of T cells expressing a male-specific receptor in T-cell receptor transgenic mice. Dev. Immunol. 1990;1:1–10. doi: 10.1155/1990/18208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N.E., Loring J.M., Raulet D.H., Jaenisch R. β2-microglobulin-deficient mice lack CD4−8+ cytolytic T cells. Nature. 1990;344:742–747. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- Koller B.H., Marrack P., Kappler J.W., Smithies O. Normal development of mice deficient in beta-2m, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Jameson S., Hogquist K., Bevan M. Positive selection of thymocytes. Annu. Rev. Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- Lucas B., Germain R.N. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+ thymocyte differentiation. Immunity. 1996;5:461–477. doi: 10.1016/s1074-7613(00)80502-6. [DOI] [PubMed] [Google Scholar]
- Linette G.P., Grusby M.J., Hedrick S.M., Hansen T.H., Glimcher L.H., Korsmeyer S.J. Bcl-2 is upregulated at the CD4+CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Sentman C.L., Shutter J.R., Hockenbery D., Kanagawa O., Korsmeyer S.J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Siegel R.M., Katsumata M., Miyashita T., Louie D.C., Greene M.I., Reed J.C. Inhibition of thymocyte apoptosis and negative antigenic selection in bcl-2 transgenic mice. Proc. Natl. Acad. Sci. USA. 1992;89:7003–7007. doi: 10.1073/pnas.89.15.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A.W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Deftos M.L., He Y.-W., Ojala E.W., Bevan M.J. Correlating notch signaling with thymocyte maturation. Immunity. 1998;9:777–786. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E., Chang D., Itano A., Cado D., Alexander H., Lans D., Weinmaster G., Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- Ordentlich P., Lin A., Shen C.-P., Blaumueller C., Matsuno K., Artavanis-Tsakonas S., Kadesch T. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol. Cell. Biol. 1998;18:2230–2239. doi: 10.1128/mcb.18.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matechak E.O., Killeen N., Hedrick S.M., Fowlkes B.J. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity. 1996;4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- Itano A., Salmon P., Kioussis D., Tolaini M., Corbella P., Robey E. The cytoplasmic domain of CD4 promotes the development of CD4 lineage T cells. J. Exp. Med. 1996;183:731–741. doi: 10.1084/jem.183.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turka L., Schatz D., Oettinger M., Chun J., Gorka C., Lee K., McCormack W., Thompson C. Thymocyte expression of RAG-1 and RAG-2termination by T cell receptor cross-linking. Science. 1991;253:778–781. doi: 10.1126/science.1831564. [DOI] [PubMed] [Google Scholar]