Apoptosis, or programmed cell death (PCD), is a physiological response that eliminates unwanted cells, an evolutionarily ancient process that is present in all multicellular organisms. Apoptosis is used during embryogenesis as a way to shape future adult forms (e.g., limbs, fingers) or to suppress vestigial embryonic structures (e.g., genital organ involution during mammalian sexual differentiation). By eliminating unwanted cells, PCD is an important tool that provides space for new cells during the process of cell renewal (41). In higher vertebrates, apoptosis is employed to eliminate self-reactive lymphocytes during differentiation and again at the termination of an immune response to remove antigen-specific lymphocytes (3). The advantage of apoptosis over necrosis is the lack of an inflammatory response after cell death. Indeed, apoptotic cells do not release their contents into the milieu but shrink and are phagocytosed by tissue macrophages or dendritic cells (42).
Apoptosis is a continually occurring, tightly regulated process that maintains the homeostatic cellular balance in a normal animal. PCD is relatively rarely directly observed in healthy animals. This is because apoptotic cells are potent triggers for phagocytosis and thus are rapidly removed from the environment. Nevertheless, recent studies have revealed high levels of host cell apoptosis associated with several protozoan infections, particularly among cells of the immune system. In this review we address how protozoan infection triggers apoptosis and consider the consequences of dysregulated PCD for the parasite and the host.
MOLECULAR PATHWAYS UNDERLYING PCD
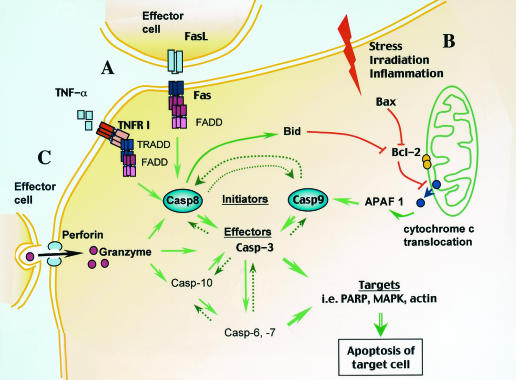
Apoptosis centers on proteolytic activation of a family of cysteine proteases known as caspases (Fig. 1). The caspase targets comprise a variety of proteins involved in cell survival, including DNA repair, cell cycle control, and nuclear and cytoskeletal assembly, and these enzymes also target downstream caspases in the cascade leading to apoptosis. The caspases are present as proenzymes in unstimulated cells. During activation, an N-terminal 3- to 24-kDa caspase prodomain is cleaved, and the remaining enzyme is further divided into a large subunit (17 to 21 kDa) and small subunit (10 to 13 kDa), which together form the active molecule. There are 14 known mammalian caspases. These molecules are divided into initiator and effector groups that act in cascade, with the former activating the latter by specific cleavage and the latter processing other cellular substrates. Caspase-8 (Casp-8) and Casp-9 activation cascades converge on Casp-3, which in turn activates the downstream effector caspases, Casp-6 and Casp-7 (49). Nevertheless, once triggered, the downstream effector caspases can activate initiator caspases, resulting in a potent positive feedback loop (60). Poly(ADP-ribose) polymerase, a 116-kDa nuclear protein involved in DNA repair, cell survival, and proliferation and differentiation mechanisms, is a major target substrate of caspases. Western blot detection of the signature p89 poly(ADP-ribose) polymerase fragment or other smaller fragments resulting from caspase cleavage is widely used to reveal apoptotic death in cells, tissues, and organs (14).
FIG. 1.
PCD induced through multiple pathways. After cleavage and activation (green arrows) of the initiator Casp-8 and -9, the caspase cascade leads to cell death through further activation of death effector molecules. (A) Receptor-mediated induction of apoptosis. Extracellular molecules (TNF-α and FasL) bind to TNFR family members (TNFR I and Fas, respectively). Recruitment of FADD to the receptors leads to Casp-8 activation, which in turn results in activation of Casp-3, -6, and -7. (B) Stress, irradiation, and inflammation act on mitochondria through intermediary proapoptotic Bcl-2 family members such as Bax, resulting in blocking of the antiapoptotic activity of Bcl-2 (red lines). As a result of the latter event, cytochrome c translocates into the cytoplasm and activates Casp-9 through APAF-1. Casp-8 may also trigger the mitochondrial PCD pathway through activation of Bid, which, like Bax, inhibits the antiapoptotic activity of Bcl-2. (C) Cytotoxic cells introduce granzyme molecules into the target cell in a process mediated by multimerization of the perforin molecule. Granzymes cleave various substrates, including caspases, resulting in cell death. The dashed arrows indicate secondary effects of activated caspases. PARP, poly(ADP-ribose) polymerase; MAPK, mitogen-activated protein kinase.
Initiator caspases are activated by two general mechanisms; the first involves ligation of cell surface receptors (in the case of Casp-8), and the second is a result of an internal, stress-mediated stimulus (in the case of Casp-9). Casp-8 is activated through a cell membrane receptor of the CD95 (Fas/Apo1) or CD120 (tumor necrosis factor [TNF] receptor) family which, after binding its relevant ligand (Fas or TNF-α), trimerizes and attracts the docking proteins Fas-associated death domain (FADD) and TNF receptor-associated death domain (TRADD) (Fig. 1A). The latter molecules recognize death domains contained within the receptor molecules themselves. FADD contains two death effector domains that directly bind and cleave Casp-8. TRADD lacks death effector domains but can activate Casp-8 by first binding FADD (65). Casp-8 has been shown to be essential for Fas-induced apoptosis (28) (Fig. 1A).
Casp-9 activation involves changes in the membrane potential of the mitochondria, resulting in cytochrome c translocation from the inner mitochondrial membrane to the cytoplasm (Fig. 1B). Loss of mitochondrial membrane potential can be induced by toxic agents, excess reactive oxygen and nitrogen intermediates, stress induced by UV or gamma irradiation, or growth factor deprivation. Cytoplasmic cytochrome c binds to apoptotic protease-activating factor 1 (APAF-1), resulting in a conformational change in the latter molecule. In presence of ATP, activated APAF-1 binds to procaspase 9, cleaving it to an active Casp-9 form (21). Furthermore, a change in the mitochondrial membrane potential and release of cytochrome c into the cytoplasm are tightly regulated by Bcl-2 family proteins that act by dimerization in the outer mitochondrial membrane. Some of these proteins are antiapoptotic (Bcl-2 and Bcl-XL), while others are proapoptotic (Bax and Bad), and the ultimate outcome eventually depends on the ratio of homodimers and heterodimers of each kind (2) (Fig. 1B).
Recently, a link between Casp-8 and Casp-9 activation was revealed by discovery of Bid, a small 23-kDa proapoptotic Bcl-2 family member. Upon activation of Casp-8 by an extracellular cell death signal, Bid is cleaved to a 15-kDa active fragment that translocates to the mitochondrial membrane. Through interaction with Bax, mitochondrial membrane depolarization and cytochrome c release occur, resulting in Casp-9 activation (38, 66, 69). Thus, Bid activation bypasses the need for mitochondrial stress factors in activation of Casp-9.
APOPTOSIS DURING THE IMMUNE RESPONSE
PCD is an important process during the normal immune response to infection. Cytolytic T lymphocytes employ perforin and granzyme molecules to directly trigger apoptosis in infected target cells (Fig. 1C). The mechanism involves perforin-dependent introduction of granzymes into the target cell cytoplasm. Granzyme B, the best-characterized granzyme, activates caspases, such as Casp-10 and -7 (24, 62) (Fig. 1C). In addition, granzyme B may directly cleave a variety of intranuclear factors, resulting in caspase-independent apoptosis. More recently, granzyme B has been shown to directly cleave Bid to a 14-kDa fragment. This peptide, while distinct from the 15-kDa Bid fragment generated by Casp-8, displays similar functions in that it translocates to the mitochondrial membrane, attracts Bax, and thus mediates cytochrome c release and Casp-9 activation (22).
Several intracellular protozoans elicit major histocompatibility complex class I-restricted CD8+ cytotoxic T-lymphocyte (CTL) activity; these protozoans include Toxoplasma gondii (19, 61), Plasmodium spp. (4, 5, 56), Trypanosoma cruzi (51), Leishmania major (11), and Encephalitozoon cuniculi (33). Studies with perforin knockout (KO) mice support the hypothesis that CTL activity plays a limited role in host protection against T. cruzi and T. gondii infection (13, 50). In the case of Toxoplasma, human CD4+ CTL clones have also been isolated (10, 54).
PCD also plays an important role in homeostasis of the immune system through antigen-induced apoptosis of mature T and B cells at the termination of an immune response. This process, termed activation-induced cell death (AICD), although well established as an essential component of the immune response, is not well understood and is likely to encompass several mechanisms. Strong antigenic stimulation has been reported to downregulate Bcl-2 and upregulate FasL, leaving cells sensitive to Fas-mediated death. Proximity to activated TNF-α-producing cells renders lymphocytes sensitive to TNF receptor I (TNFR-I)-induced apoptosis. AICD has also been demonstrated in vitro to be a response of mature T cells to T-cell receptor-CD3 triggering in the absence of costimulatory signals. Finally, activated T cells may upregulate CTLA-4 while simultaneously downregulating CD28. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is then capable of delivering proapoptotic signals after CD80/CD86 triggering, instead of the survival signals that normally accompany CD28-CD80/CD86 ligation (27). The total effect of these processes is to remove clonally expanded effector cells that are no longer needed once an infection has been resolved. Apoptosis is the mechanism that does this without the complications of an inflammatory response that occur during cell necrosis.
Direct evidence that AICD occurs during protozoan infection comes from studies of acute murine toxoplasmosis. In this disease, CD4+ T lymphocytes become unresponsive at the peak of infection, and AICD can be triggered by in vitro restimulation of CD4+ T lymphocytes from the animals (32). Similarly, splenocytes from T. cruzi-infected mice can be induced to undergo AICD in response to in vitro mitogenic stimulation (36). The mechanisms underlying AICD during these and other protozoan infections may involve TNF-α-TNFR I and Fas-FasL interactions (see below).
TNF-α-TNFR I PATHWAY
Clearance of cellular infiltrates, presumably by phagocytosis of apoptotic cells, has been linked to the TNF-α-TNFR I pathway during experimental infections with L. major and T. cruzi. Infection of TNFR I (p55) gene KO mice leads to development of nonhealing lesions at the site of infection, although the animals retain the ability to clear parasites (64). Footpad lesions in KO mice have a major infiltrate of lymphocytes along with focal epidermal necrosis and surface ulcerations. Similar results were obtained with wild-type (WT) mice by antibody (Ab) neutralization of TNF-α (29). In addition, cells isolated from L. major lesions in WT mice are hypersensitive to TNF-α-mediated apoptosis in vitro. Thus, in the L. major infection model, the TNFR I pathway triggers cell death in lymphocyte infiltrates at the site of infection, and this plays a role in resolving the lesion. Nevertheless, TNF-α−/− animals display a phenotype distinct from that of mice lacking TNFR I (67). Thus, TNF KO animals infected with L. major rapidly succumb to progressive visceral leishmaniasis. The results suggest either that TNF binds to additional receptors or that the course of infection varies depending on the L. major strain.
Infection of TNFR I KO mice with T. cruzi results in increased CD45+ cellular infiltrates in myocardiac tissue, suggesting that TNF-α also plays an important role in control of inflammation in the context of this parasite (7). Unlike L. major infection, the absence of signaling through TNFR I leads to uncontrolled parasitemia and decreased parasite killing in myocardiac fibroblasts after in vitro interleukin-1 (IL-1) and gamma interferon (IFN-γ) stimulation. However, WT and KO mice have comparable levels of apoptotic cells in the inflammatory lesions. This would not be predicted based upon the hypothesis that TNF-α-mediated apoptosis contributes to lesion resolution by triggering PCD. Possibly, the abnormally high parasitemia in TNFR I KO mice triggers other apoptotic pathways that do not occur during WT T. cruzi infection.
When T. gondii infection was studied, we and other workers found high levels of apoptotic death in the spleens of mice that experienced acute infection with high-virulence parasites, and analysis of infected TNFR I KO mice revealed partially reduced apoptosis (17, 18, 45). Because PCD was not totally eliminated in the absence of TNFR I, the results suggest that induction of PCD by high-virulence T. gondii involves activation of multiple pathways, including the TNF-α-TNFR I pathway.
FAS-FASL PATHWAY
The Fas-FasL pathway has no known function other than to mediate PCD. This contrasts with TNF-α-TNFRI signaling, which can have multiple effects in addition to PCD. Therefore, in vivo consequences of disrupting Fas-FasL signaling can be directly attributed to alteration of steady-state apoptosis.
Similar to findings obtained with TNFR I−/− mice, L. major infection of lpr and gld mutant mice (which lack functional Fas and FasL, respectively) resulted in lesions that failed to resolve (9, 25). The lack of functional Fas or FasL resulted in increased numbers of parasites despite normal or even greater expression of IFN-γ and nitric oxide, factors usually linked to resistance. When the Fas-FasL pathway was reconstituted in gld mice by administration of recombinant soluble FasL, the animals resolved their lesions and eliminated the parasites. These studies provide clear evidence that FasL-triggered apoptosis plays a role in resistance to protozoan infection. However, whether this process involves lysis of infected macrophages or prevention of uncontrolled inflammatory reactions at the site of the lesion is not clear.
In a recent study of human patients infected with the visceral parasite Leishmania donovani, elevated levels of soluble Fas and FasL were found in sera, and spleen cell aspirates contained similarly increased amounts of membrane Fas and FasL (15). Additionally, in vitro infection of macrophages with L. donovani resulted in increased membrane-bound Fas expression and release of soluble FasL. These results suggest that Fas-FasL interactions may be an important component in human leishmaniasis.
CD4+ T lymphocytes from T. cruzi-infected mice express high levels of Fas and FasL mRNA and are sensitive to T-cell receptor-CD3-induced AICD in vitro. PCD in these cells is dependent upon the Fas-FasL pathway, since cells from gld mice are not sensitive to AICD (37). In cocultures of infected macrophages and T cells, parasite replication increases as T cells undergo Fas-mediated AICD (53). Indeed, interaction of apoptotic cells with T. cruzi-infected macrophages has been shown to fuel parasite growth, and this has been shown to be dependent upon synthesis of transforming growth factor β, prostaglandins, and polyamines (16).
Nevertheless, during in vivo T. cruzi infection, loss of the Fas-FasL pathway results in increased parasitemia and elevated IL-4 and IL-10 production (37). Therefore, while the Fas-FasL pathway sensitizes CD4+ T cells to AICD and increased parasite replication in vitro, in an in vivo situation this pathway is required to maintain an intact Th1 response to T. cruzi, and disruption of the pathway leads to emergence of a Th2-dominant response and increased susceptibility.
The Fas-FasL pathway also has effects on B lymphocytes during T. cruzi infection. Up to 18% of splenic B cells from infected animals have been reported to undergo apoptotic death during acute murine T. cruzi infection (1). These cells display increased levels of both Fas and FasL, expression of which is positively correlated with the activation status of the cells. The Fas-FasL-bearing B cells also express membrane antiparasite immunoglobulin G. Apoptosis of the activated B cells could be prevented in vitro by addition of anti-FasL Ab and was associated with AICD through Fas-FasL B cell-B cell interactions in a process termed fratricide. Thus, apoptosis through the Fas-FasL pathway specifically targets T. cruzi-restricted immunoglobulin G-producing B cells in this model, resulting in weakened antiparasite humoral immunity.
T-cell apoptosis can also be restricted to the cells specific for parasite antigen. When ovalbumin- or parasite-specific T cells are adoptively transferred into Plasmodium yoellii-infected T-cell-deficient nude BALB/c mice only the P. yoelii-specific T cells undergo PCD (68). Parasite-specific T cells display increased Fas expression, which implicates the Fas-FasL pathway of apoptosis in this model. The results suggest that there is a parasite strategy of eliminating anti-Plasmodium T-cell effectors. Moreover, up to 60% of the spleen cells express Fas during the acute phase of infection with blood stage Plasmodium chabaudi chabaudi AS, showing in this case a much more generalized sensitivity to the parasite (23).
In humans infected with Plasmodium falciparum, high levels of apoptotic markers are found in peripheral blood mononuclear cells. The cells are sensitive to AICD induced in vitro, and the levels of serum-soluble FasL are elevated. The levels of FasL decrease after anti-Plasmodium chemotherapy (30). Thus, Fas-FasL-induced apoptosis may also be an important component of human malaria.
In the absence of NF-κB2, mice succumb to T. gondii infection and display increased splenic apoptosis and elevated expression of Fas. Thus, T. gondii may trigger Fas-FasL-dependent apoptosis, in a process under the negative control of NF-κB2 (6). During intraocular infection with a low-virulence T. gondii strain, expression of Fas and FasL is increased in the inflamed retina, as well as in the spleen. The presence of apoptotic cells in the eyes of infected WT mice is associated with pathology and is absent in gld and lpr infected mice. Nevertheless, Fas- and FasL-defective animals display increased inflammatory infiltrates and higher clinical scores. These results are reminiscent of the results obtained for L. major infection of TNFR KO mice, in which apoptosis is employed as a mechanism to remove unwanted inflammatory cells. After peroral Toxoplasma infection, susceptible mouse strains develop a lethal inflammatory gut pathology accompanied by Fas-dependent apoptosis of Peyer's patch T cells, which is probably the result of inflammatory cytokine overproduction (35). We found generalized apoptosis in the spleens of infected mice, a phenomenon partially dependent upon Fas-FasL interaction, as shown by the lack of apoptotic cells in gld infected animals (18).
Similar to Toxoplasma infection in the gut, infection with Cryptosporidium parvum elicits death of intestinal epithelia through a Fas-FasL-dependent pathway. Indeed, in vitro infected epithelial cells upregulate cell surface FasL, while increased FasL mRNA levels are detected in intestinal epithelia during in vivo infection (46). C. parvum-infected, CD4-depleted gld and lpr mice display reduced weight loss compared to CD4-depleted WT animals, which clearly implicates Fas-FasL-dependent apoptosis in the pathogenesis of C. parvum infection.
MITOCHONDRION-INDUCED APOPTOSIS
Mitochondrion-dependent apoptosis during infections with both T. gondii and Plasmodium berghei has been documented recently. During acute T. gondii infection, CD4+ and CD8+ T lymphocytes accumulating in the brain expressed increased FasL, Bad, and Bax mRNA levels (58). The latter two gene products are associated with mitochondrion-dependent apoptosis (Fig. 1B). During chronic infection, T-lymphocyte expression of proapoptotic molecules is downregulated, which is accompanied by increased levels of antiapoptotic Bcl-2 and Bcl-XL. When restimulated in vitro, T cells do not proliferate, but they upregulate expression of the cell cycle inhibitors p21Waf/cip1 and p27kip1. The data suggest that low-level apoptosis plays a role in gradually decreasing the size of the pool of intracerebral T cells during chronic infection. We also found increased expression of both Bax and Bcl-2 in the spleens of mice experiencing acute infection with high-virulence parasites, although it was difficult to correlate this with either a pro- or antiapoptotic outcome (unpublished observations).
In a recent study, mice experiencing lethal infection with P. berghei were similarly found to express increased levels of Bax, Bcl-2, p53, and cytoplasmic cytochrome c in the brain (34). Furthermore, mitochondria analyzed by electron microscopy displayed an abnormal morphology suggestive of late-stage degeneration. While it is not clear whether in this case mitochondrion-dependent PCD was triggered through intrinsic or extrinsic apoptotic cascades, dysfunction of this cellular organelle and the subsequent death of the cell may be important components of cerebral malaria.
INFLAMMATORY TRIGGERS OF APOPTOSIS
Several studies have linked production of proinflammatory mediators with PCD during T. gondii infection (17, 35, 45). Furthermore, apoptosis resulting from inflammatory cytokine overproduction is associated with high-virulence Toxoplasma strains but not with low-virulence Toxoplasma strains (17, 45). The widespread apoptosis associated with acute T. gondii infection is greatly reduced in mice lacking expression of either IFN-γ or IL-12 (18) or treated with anti-IFN-γ Abs (35). Similarly, treatment of T. cruzi-infected mice with either anti-IFN-γ Ab or anti-TNF-α Ab reduced apoptosis (39), while cells infected with Neospora caninum were sensitive to IFN-γ-mediated apoptosis (52). The proapoptotic effects observed were most probably the result of the ability of IFN-γ to upregulate expression of the Fas-FasL and TNF-α-TNFR I pathways (35). Interestingly, induction of apoptosis was correlated with STAT-1 activation during T. gondii infection (18).
Nitric oxide has also been implicated as an apoptotic trigger during T. gondii and T. cruzi infections. An absence of NO in gene-targeted or drug-treated mice reduces the levels of splenocyte apoptosis that normally occur during these infections (18, 39, 40, 55). The mechanism by which NO induces PCD is not clear, but it may involve the effects of peroxynitrite formation from NO and superoxide in mitochondria (43).
It is difficult to determine the biological significance of apoptosis driven by proinflammatory mediators such as IFN-γ, IL-12, NO, and TNF-α. Because these molecules are involved in resistance to microbial pathogens, their presence is often required for host survival. Thus, Ab depletion and gene KO experiments may well reveal a role for proinflammatory mediators in pathogen-triggered PCD. Nevertheless, in these situations host death occurs as a result of uncontrolled parasite replication and tissue damage.
PROTOZOAN MOLECULES THAT DIRECTLY TRIGGER APOPTOSIS
In many cases the cell death that accompanies protozoan infection results from the effects of host inflammatory mediators. However, several studies have pointed to factors produced by protozoan parasites that directly activate apoptotic pathways in target cells. Thus, Entamoeba histolytica was found to induce contact-dependent apoptosis in epithelial cell cultures. In this case apoptosis is Fas-FasL and TNFR I independent. The response is Casp-3 dependent, but interestingly it does not appear to require either Casp-8 or Casp-9 (26, 59). Host protein dephosphorylation, associated with phosphatase PTP1B activation, was recently reported in E. histolytica-infected Jurkat cell cultures and was found to be parasite contact dependent. Infected Jurkat cells undergo apoptosis, but they are rescued in the presence of phosphatase inhibitors (63). These results indicate that host protein dephosphorylation may be involved in Casp-3 activation leading to PCD.
T. cruzi induces strong but transient cell depletion in the thymus during acute Chagas' disease. The effect appears to be the result of parasite trans-sialidase (TS) shedding in the blood, resulting in direct apoptosis of the thymic nurse cell complex mediated by this virulence factor, as treatment with anti-TS Ab eliminates the effect (47). TS-induced T-cell depletion may therefore represent a T. cruzi strategy to avoid host immunity. Paradoxically, T. cruzi TS has an opposite, antiapoptotic effect on human Schwann cells, the principal target in the peripheral nervous system, apparently through a pathway dependent upon a phosphatidylinositol 3-kinase-Akt signaling cascade (8).
CONCLUSION: IS APOPTOSIS GOOD OR BAD?
It is clear that activation of PCD pathways contributes to the pathology of several protozoan infections (Table 1). Less clear is whether apoptosis is a beneficial or detrimental response in these situations. The correct answer is probably dependent upon the particular protozoan and even the particular model system. In many cases, it is difficult to determine whether the host or parasite (or neither) benefits from host cell death. This is because the same cytokines that trigger apoptosis also are necessary to promote antimicrobial effector functions. Removal of the cytokines, either by genetic inactivation or by Ab depletion, may eliminate parasite-induced PCD. Nevertheless, the result may be uncontrolled microbial proliferation, causing pathology in affected tissues and frequently host death.
TABLE 1.
Outcomes of apoptotic cell death during protozoan infections
| Protozoan | Mechanism | Reference(s) |
|---|---|---|
| Homeostatic outcomes | ||
| T. gondii | CTL | 10, 12, 19, 31, 54 |
| Bcl-2 family | 58 | |
| NO | 55 | |
| Plasmodium spp. | CTL | 4, 5, 56 |
| L. major | CTL | 11 |
| TNFRI | 29, 64 | |
| Fas-FasL | 9, 25 | |
| T. cruzi | CTL | 51 |
| TNFRI | 7 | |
| Fas-FasL | 37 | |
| Pathogenic outcomes | ||
| T. gondii | AICD | 32, 36 |
| Proinflammatory cytokines | 17, 45 | |
| Fas-FasL | 35 | |
| L. donovani | Fas-FasL | 15 |
| T. cruzi | AICD | 53 |
| NO | 39 | |
| Fas-FasL | 40 | |
| Parasite factor | 47 | |
| Plasmodium spp. | AICD | 68 |
| Fas-FasL | 23, 30 | |
| Bcl-2 family | 34 | |
| C. parvum | Fas-FasL | 46 |
| N. caninum | IFN-γ | 52 |
| E. histolytica | Parasite factor | 26, 59, 63 |
Studies with Leishmania provide a clear example of a situation in which PCD plays an important role in lesion resolution following control of infection. T. cruzi TS appears to provide an example of a proapoptotic parasite molecule whose purpose is to suppress host immunity and promote infection. The purpose of host cell death during T. gondii and Plasmodium spp. infections is more obscure. Here, apoptosis may be the accidental consequence of the inflammatory cytokine response to infection, a response otherwise needed to allow host survival. A more interesting possibility is the possibility that apoptosis is actively induced by T. gondii and other protozoans and may therefore promote infection by removing effectors of the immune system.
Not considered in this review is the other side of the coin, the possibility that some microbial pathogens suppress apoptosis. For example, macrophages infected with Toxoplasma are resistant to multiple inducers of apoptosis, and this is associated with decreased caspase activation and reduced poly(ADP-ribose) polymerase expression (20, 48). L. donovani-infected macrophages display increased viability in the absence of growth factors, and this is the result of soluble mediators released by infected cells (44). For protozoan pathogens such as these organisms, apoptosis during in vivo infection is the net result of both pro- and antiapoptotic effects on the host.
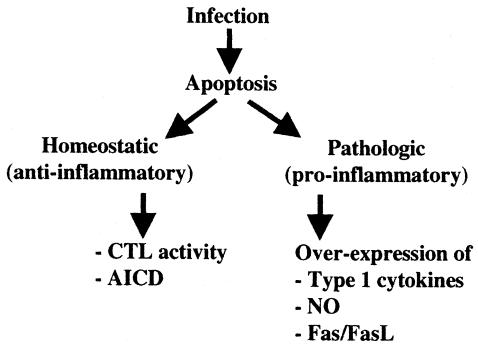
Induction of apoptosis during protozoan infection can be viewed from two angles (Fig. 2). First, it is a homeostatic mechanism that acts as a stabilizing factor in the host-parasite relationship. Both CTL activity and AICD contribute to this process, which, in general, decreases inflammatory responses by immunologically silent removal of infected and unwanted cells. The effect is to promote host survival, which also benefits the parasite, although CTL activity could potentially result in complete clearance of the pathogen. Second, apoptosis may be a destabilizing factor in the encounter between host and parasite. In this case, dysregulated PCD can be a component of a pathology resulting from inflammatory cytokine overproduction (Fig. 2). In this case, and like Shigella and Salmonella infections (57, 70), apoptosis becomes a proinflammatory phenomenon. The outcome of such inflammatory pathology could be detrimental to both the host and the parasite. It may also promote parasite spread by interfering with host defense, or alternatively, it may be a necessary cost incurred by the host during the process of controlling infection. Insights into the molecular pathogenesis of proapoptotic protozoans can be expected to lead to novel therapeutic intervention strategies to control microbial infections.
FIG. 2.
Apoptosis during protozoan infection. From the host perspective, CTL activity and AICD can be viewed as homeostatic, anti-inflammatory responses to protozoan parasite infection that control the spread of infection and resolve the immune response after resolution of the infection. Alternatively, overexpression of type 1 proinflammatory cytokines, nitric oxide, or Fas-FasL may lead to uncontrolled apoptosis, in this situation becoming a proinflammatory response. A switch from a homeostatic response to a pathological response may occur in the course of infection, as when type 1 cytokines act to limit infection but then cause tissue and cellular apoptotic damage.
Acknowledgments
We thank past and present members of the Denkers laboratory for insightful discussions and constructive criticism. We apologize to scientists whose work was not cited due to space limitations.
Our work on apoptosis was supported by NIH grants AI47888 and AI40540.
Editor: D. A. Portnoy
REFERENCES
- 1.Acosta Rodriguez, E. V., E. Zuniga, C. L. Montes, and A. Gruppi. 2003. Interleukin-4 biases differentiation of B cells from Trypanosoma cruzi-infected mice and restrains their fratricide: role of Fas ligand down-regulation and MHC class II-transactivator upregulation. J. Leukoc. Biol. 73:127-136. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. M., and S. Cory. 2001. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 26:61-66. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, S., A. Krueger, S. Kirchhoff, and P. H. Krammer. 2002. Regulation of T cell apoptosis during the immune response. Curr. Mol. Med. 2:257-272. [DOI] [PubMed] [Google Scholar]
- 4.Blum-Tirouvanziam, U., C. Servis, A. Habluetzel, D. Valmori, Y. Men, F. Esposito, L. Del Nero, N. Holmes, N. Fasel, and G. Corradin. 1995. Localization of HLA-A2.1-restricted T cell epitopes in the circumsporozoite protein of Plasmodium falciparum. J. Immunol. 154:3922-3931. [PubMed] [Google Scholar]
- 5.Bonelo, A., D. Valmori, F. Triponez, J. M. Tiercy, G. Mentha, J. Oberholzer, P. Champagne, J. F. Romero, F. Esposito, I. Nebie, C. Barbey, P. Romero, S. Herrera, G. Corradin, and J. A. Lopez. 2000. Generation and characterization of malaria-specific human CD8(+) lymphocyte clones: effect of natural polymorphism on T cell recognition and endogenous cognate antigen presentation by liver cells. Eur. J. Immunol. 30:3079-3088. [DOI] [PubMed] [Google Scholar]
- 6.Caamano, J., C. Tato, G. Cai, E. N. Villegas, K. Speirs, L. Craig, J. Alexander, and C. A. Hunter. 2000. Identification of a role for NF-κB2 in the regulation of apoptosis and in maintenance of T cell-mediated immunity to Toxoplasma gondii. J. Immunol. 165:5720-5728. [DOI] [PubMed] [Google Scholar]
- 7.Castanos-Velez, E., S. Maerlan, L. M. Osorio, F. Aberg, P. Biberfeld, A. Orn, and M. E. Rottenberg. 1998. Trypanosoma cruzi infection in tumor necrosis factor receptor p55-deficient mice. Infect. Immun. 66:2960-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuenkova, M. V., F. B. Furnari, W. K. Cavenee, and M. A. Pereira. 2001. Trypanosoma cruzi trans-sialidase: a potent and specific survival factor for human Schwann cells by means of phosphatidylinositol 3-kinase/AKT signaling. Proc. Natl. Acad. Sci. USA 98:9936-9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conceicao-Silva, F., M. Hahne, M. Schronter, J. Louis, and J. Tschopp. 1998. The resolution of lesions induced by Leishmania major requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur. J. Immunol. 28:237-245. [DOI] [PubMed] [Google Scholar]
- 10.Curiel, T. J., E. C. Krug, M. B. Purner, P. Poignard, and R. L. Berens. 1993. Cloned human CD4+ cytotoxic T lymphocytes specific for Toxoplasma gondii lyse tachyzoite-infected target cells. J. Immunol. 151:2024-2031. [PubMed] [Google Scholar]
- 11.da Conceicao-Silva, F., B. L. Perlaza, J. A. Louis, and P. Romero. 1994. Leishmania major infection in mice primes for specific major histocompatibility complex class I-restricted CD8+ cytotoxic T cell responses. Eur. J. Immunol. 24:2813-2817. [DOI] [PubMed] [Google Scholar]
- 12.Denkers, E. Y., T. Scharton-Kersten, R. T. Gazzinelli, G. Yap, H. Charest, and A. Sher. 1997. Cell-mediated immunity to Toxoplasma gondii: redundant and required mechanisms as revealed by studies in gene knockout mice, p. 167-181. In S. H. E. Kaufmann (ed.), Medical intelligence unit: host response to intracellular pathogens. R. G. Landes Company, Austin, Tex.
- 13.Denkers, E. Y., G. Yap, T. Scharton-Kersten, H. Charest, B. Butcher, P. Caspar, S. Heiny, and A. Sher. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 159:1903-1908. [PubMed] [Google Scholar]
- 14.Duriez, P. J., and G. M. Shah. 1997. Cleavage of poly(ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem. Cell Biol. 75:337-349. [PubMed] [Google Scholar]
- 15.Eidsmo, L., D. Wolday, N. Berhe, F. Sabri, I. Satti, A. M. El Hassan, S. Sundar, F. Chiodi, and H. Akuffo. 2002. Alteration in Fas and Fas ligand expression during human visceral leishmaniasis. Clin. Exp. Immunol. 130:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friere-de-Lima, C. G., D. O. Nascimento, M. B. P. Soares, P. T. Bozza, H. C. Castro-Faria-Neto, F. G. de Mello, G. A. DosReis, and M. F. Lopes. 2000. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403:199-203. [DOI] [PubMed] [Google Scholar]
- 17.Gavrilescu, L. C., and E. Y. Denkers. 2001. IFN-γ overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J. Immunol. 167:902-909. [DOI] [PubMed] [Google Scholar]
- 18.Gavrilescu, L. C., and E. Y. Denkers. 2003. Interleukin-12 p40- and FasL-dependent apoptotic pathways involving STAT-1 phosphorylation are triggered during infection with a virulent strain of Toxoplasma gondii. Infect. Immun. 71:2577-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazzinelli, R. T., E. Y. Denkers, F. T. Hakim, and A. Sher. 1993. Immunologic control of Toxoplasma gondii infection by CD8+ lymphocytes: a model for class I restricted recognition of intracellular parasites, p. 370-377. In M. Sitkovsky and P. Henkart (ed.), Cytotoxic cells: generation, triggering, effector functions, methods. Birkhauser Press, Boston, Mass.
- 20.Goebel, S., U. Gross, and C. G. K. Luder. 2001. Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J. Cell Sci. 114:3495-3505. [DOI] [PubMed] [Google Scholar]
- 21.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 22.Heibein, J. A., I. S. Goping, M. Barry, M. J. Pinkoski, G. C. Shore, D. R. Green, and R. C. Bleackley. 2000. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members Bid and Bax. J. Exp. Med. 192:1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helmby, H., G. Jonsson, and M. Troye-Blomberg. 2000. Cellular changes and apoptosis in the spleens and peripheral blood of mice infected with blood-stage Plasmodium chabaudi chabaudi AS. Infect. Immun. 68:1485-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heusel, J. W., R. L. Wesselschmidt, S. Shresta, J. H. Russell, and T. J. Ley. 1994. Cytotoxic lymophocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 76:977-987. [DOI] [PubMed] [Google Scholar]
- 25.Huang, F. P., D. Xu, E. O. Esfandiari, W. Sands, X. Q. Wei, and F. Y. Liew. 1998. Mice defective in Fas are highly susceptible to Leishmania major infection despite elevated IL-12 synthesis, strong Th1 responses, and enhanced nitric oxide production. J. Immunol. 160:4143-4147. [PubMed] [Google Scholar]
- 26.Huston, C. D., E. R. Houpt, B. J. Mann, C. S. Hahn, and W. A. J. Petri. 2000. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell. Microbiol. 2:617-625. [DOI] [PubMed] [Google Scholar]
- 27.Janssen, O., R. Sanzenbacher, and D. Kabelitz. 2000. Regulation of activation-induced cell death of mature T-lymphocyte populations. Cell Tissue Res. 301:85-99. [DOI] [PubMed] [Google Scholar]
- 28.Juo, P., C. J. Kuo, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8:1001-1008. [DOI] [PubMed] [Google Scholar]
- 29.Kanaly, S. T., M. Nashleanas, B. Hondowicz, and P. Scott. 1999. TNF receptor p55 is required for elimination of inflammatory cells following control of intracellular pathogens. J. Immunol. 163:3883-3889. [PubMed] [Google Scholar]
- 30.Kemp, K., B. D. Akanmori, V. Adabayeri, B. Q. Goka, J. A. Kurtzhals, C. Behr, and L. Hviid. 2002. Cytokine production and apoptosis among T cells from patients under treatment for Plasmodium falciparum malaria. Clin. Exp. Immunol. 127:151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan, I. A., and L. Casciotti. 1999. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J. Immunol. 163:4503-4509. [PubMed] [Google Scholar]
- 32.Khan, I. A., T. Matsuura, and L. H. Kasper. 1996. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int. Immunol. 8:887-896. [DOI] [PubMed] [Google Scholar]
- 33.Khan, I. A., J. D. Schwartzman, L. H. Kasper, and M. Moretto. 1999. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J. Immunol. 162:6086-6091. [PubMed] [Google Scholar]
- 34.Kumar, K. A., and P. P. Babu. 2002. Mitochondrial anomalies are associated with the induction of intrinsic cell death proteins BclII, Bax, cytochrome-c and p53 in mice brain during experimental fatal murine cerebral malaria. Neurosci. Lett. 329:319-323. [DOI] [PubMed] [Google Scholar]
- 35.Liesenfeld, O., J. C. Kosek, and Y. Suzuki. 1997. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect. Immun. 65:4682-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopes, M. F., V. F. Da Veiga, A. R. Santos, M. E. Fonseca, and G. A. DosReis. 1995. Activation-induced CD4+ T cell death by apoptosis in experimental Chagas' disease. J. Immunol. 154:744-752. [PubMed] [Google Scholar]
- 37.Lopes, M. F., M. P. Nunes, A. Henriques-Pons, N. Giese, H. C. Morse, W. F. Davidson, T. C. Araujo-Jorge, and G. A. DosReis. 1999. Increased susceptibility of Fas ligand-deficient gld mice to Trypanosoma cruzi infection due to a Th2-biased host immune response. Eur. J. Immunol. 29:81-89. [DOI] [PubMed] [Google Scholar]
- 38.Luo, X., I. Budihardjo, H. Zuo, C. Slaughter, and X. Wang. 1998. Bid, a BclII interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 39.Martins, G. A., M. A. Cardoso, J. C. Aliberti, and J. S. Silva. 1998. Nitric oxide-induced apoptotic cell death in the acute phase of Trypanosoma cruzi infection in mice. Immnuol. Lett. 63:113-120. [DOI] [PubMed] [Google Scholar]
- 40.Martins, G. A., L. Q. Vieira, F. Q. Cunha, and J. S. Silva. 1999. Gamma interferon modulates CD95 (Fas) and CD95 ligand (Fas-L) expression and nitric oxide-induced apoptosis during the acute phase of Trypanosoma cruzi infection: a possible role in immune response control. Infect. Immun. 67:3864-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier, P., A. Finch, and G. Evan. 2000. Apoptosis in development. Nature 407:796-801. [DOI] [PubMed] [Google Scholar]
- 42.Messmer, U. K., and J. Pfleischifter. 2000. New insights into the mechanisms for clearance of apoptotic cells. Bioessays 22:878-881. [DOI] [PubMed] [Google Scholar]
- 43.Moncada, S., and J. D. Erusalimsky. 2002. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 3:214-220. [DOI] [PubMed] [Google Scholar]
- 44.Moore, K. J., S. J. Turco, and G. Matlashewski. 1994. Leishmania donovani infection enhances macrophage viability in the absence of exogenous growth factor. J. Leukoc. Biol. 55:91-98. [DOI] [PubMed] [Google Scholar]
- 45.Mordue, D. G., F. Monroy, M. La Regina, C. A. Dinarello, and L. D. Sibley. 2001. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 167:4574-4584. [DOI] [PubMed] [Google Scholar]
- 46.Motta, I., M. Gissot, J. M. Kanellopoulos, and D. M. Ojcius. 2002. Absence of weight loss during Cryptosporidium infection in susceptible mice deficient in Fas-mediated apoptosis. Microbes Infect. 4:821-827. [DOI] [PubMed] [Google Scholar]
- 47.Mucci, J., A. Hidalgo, E. Mocetti, P. F. Argibay, M. S. Leguizamon, and O. Campetella. 2002. Thymocyte depleton in Trypanosoma cruzi infection is mediated by trans-sialidase-induced apoptosis on nurse cells complex. Proc. Natl. Acad. Sci. USA 99:3896-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nash, P. B., M. B. Purner, R. P. Leon, P. Clarke, R. C. Duke, and T. J. Curiel. 1998. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J. Immunol. 160:1824-1830. [PubMed] [Google Scholar]
- 49.Nicholson, D. W. 1999. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6:1028-1042. [DOI] [PubMed] [Google Scholar]
- 50.Nickell, S. P., and D. Sharma. 2000. Trypanosoma cruzi: roles for perforin-dependent and perforin-independent immune mechanisms in acute resistance. Exp. Parasitol. 94:207-216. [DOI] [PubMed] [Google Scholar]
- 51.Nickell, S. P., G. A. Stryker, and C. Arevalo. 1993. Isolation from Trypanosoma cruzi-infected mice of CD8+, MHC-restricted cytotoxic T cells that lyse parasite-infected target cells. J. Immunol. 150:1446-1457. [PubMed] [Google Scholar]
- 52.Nishikawa, Y., M. Mishima, H. Nagasawa, I. Igarishi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Interferon-gamma-induced apoptosis in host cells infected with Neospora caninum. Parasitology 123:25-31. [DOI] [PubMed] [Google Scholar]
- 53.Nunes, M. P., R. M. Andrade, M. F. Lopes, and G. A. DosReis. 1998. Activation-induced T cell death exacerbates Trypanosoma cruzi replication in macrophages cocultured with CD4+ T lymphocytes from infected hosts. J. Immunol. 160:1313-1319. [PubMed] [Google Scholar]
- 54.Purner, M. B., R. L. Berens, P. B. Nash, A. van Linden, E. Ross, C. Kruse, E. C. Krug, and T. J. Curiel. 1996. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect. Immun. 64:4330-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts, F., C. W. Roberts, D. J. Ferguson, and R. McLeod. 2000. Inhibition of nitric oxide production exacerbates chronic ocular toxoplasmosis. Parasite Immunol. 22:1-5. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigues, M. M., A. S. Cordey, G. Arreaza, G. Corradin, P. Romero, J. L. Maryanski, R. S. Nussenzweig, and F. Zavala. 1991. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3:579-585. [DOI] [PubMed] [Google Scholar]
- 57.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 58.Schluter, D., T. Meyer, L.-Y. Kwok, M. Montesinos-Rongen, S. Lutjen, A. Strack, M. L. Schmitz, and M. Deckert. 2002. Phenotype and regulation of persistent intracerebral T cells in murine Toxoplasma encephalitis. J. Immunol. 169:315-322. [DOI] [PubMed] [Google Scholar]
- 59.Seydel, K. B., and S. L. Stanley, Jr. 1998. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent pathway of apoptosis. Infect. Immun. 66:2980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated capsase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subauste, C. S., A. H. Koniaris, and J. S. Remington. 1991. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J. Immunol. 147:3955-3959. [PubMed] [Google Scholar]
- 62.Talanian, R. V., X. Yang, J. Turboy, P. Seth, T. Ghayur, A. Asiano, K. Orth, and C. J. Froelich. 1997. Granule-mediated killing: pathways for granzyme B-initiated apoptosis. J. Exp. Med. 186:1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teixeira, J. E., and B. J. Mann. 2002. Entamoeba histolytica-induced dephosphorylation in host cells. Infect. Immun. 70:1816-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vieira, L. Q., M. Goldschmidt, M. Nashleanas, K. Pfeffer, T. Mak, and P. Scott. 1996. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J. Immunol. 157:827-835. [PubMed] [Google Scholar]
- 65.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Koyalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331-367. [DOI] [PubMed] [Google Scholar]
- 66.Wang, K., X. M. Yin, D. T. Chao, C. L. Milliman, and S. J. Korsmeyer. 1996. BID: a novel BH3 domain-only death agonist. Genes Dev. 10:2859-2869. [DOI] [PubMed] [Google Scholar]
- 67.Wilhelm, P., U. Ritter, S. Labbow, N. Donhauser, M. Rollinghoff, C. Bogdan, and H. Korner. 2001. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J. Immunol. 166:4012-4019. [DOI] [PubMed] [Google Scholar]
- 68.Wipasa, J., H. Xu, A. Stowers, and M. F. Good. 2001. Apoptotic deletion of Th cells specific for the 19-kDa carboxyl-terminal fragment of merozoite surface protein 1 during malaria infection. J. Immunol. 167:3903-3909. [DOI] [PubMed] [Google Scholar]
- 69.Yin, X. 2000. Bid, a critical mediator for apoptosis induced by the activation of Fas/TNF-RI death receptors in hepatocytes. J. Mol. Med. 78:203-211. [DOI] [PubMed] [Google Scholar]
- 70.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5:201-204. [DOI] [PubMed] [Google Scholar]