Abstract
OX40 expressed on activated T cells is known to be an important costimulatory molecule on T cell activation in vitro. However, the in vivo functional significance of the interaction between OX40 and its ligand, OX40L, is still unclear. To investigate the role of OX40L during in vivo immune responses, we generated OX40L-deficient mice and a blocking anti-OX40L monoclonal antibody, MGP34. OX40L expression was demonstrated on splenic B cells after CD40 and anti-immunoglobulin (Ig)M stimulation, while only CD40 ligation was capable of inducing OX40L on dendritic cells. OX40L-deficient and MGP34-treated mice engendered apparent suppression of the recall reaction of T cells primed with both protein antigens and alloantigens and a significant reduction in keyhole limpet hemocyanin–specific IgG production. The impaired T cell priming was also accompanied by a concomitant reduction of both T helper type 1 (Th1) and Th2 cytokines. Furthermore, antigen-presenting cells (APCs) derived from the mutant mice revealed an impaired intrinsic APC function, demonstrating the importance of OX40L in both the priming and effector phases of T cell activation. Collectively, these results provide convincing evidence that OX40L, expressed on APCs, plays a critical role in antigen-specific T cell responses in vivo.
Keywords: OX40 ligand, antigen-presenting cell function, T cell priming, OX40 ligand mutant mice, cytokine
Introduction
OX40, a membrane glycoprotein of the TNF receptor family originally identified as an activated T cell marker in the rat 1 2 3, has also been demonstrated on activated T cells in humans and mice 4 5 6. Recently, further investigations have revealed the presence of OX40-expressing T cells at inflammatory sites of various diseases 7 8 9 10 11 12 13 14. OX40 ligand (OX40L), a type II membrane protein, was identified as the mouse homologue of human gp34, which we molecularly cloned as a target molecule for a transacting transcriptional activator, Tax, of the human T cell lymphotropic virus type I 15. Expression of OX40L, initially described on human T cell lymphotropic virus type I–infected T cells 15, has been found on murine B cells 16 17, human endothelial cell lines 18, dendritic cells 19 20, and recently among a population of APCs in the central nervous system during the course of clinically apparent experimental allergic encephalomyelitis (EAE) 14.
Initiation of antigen-specific T cell responses requires an antigen presented by MHC molecules and costimulatory signals provided by APCs 21 22. Furthermore, OX40–OX40L has recently been reported to be implicated in the additional costimulatory signals in vitro 4 5 23 24 25. Intervention of the OX40–OX40L system has produced interesting findings in immune modulation. Cross-linking of OX40 with an anti-OX40 Ab generated a costimulatory signal in CD4+ T cells 1 4 5 23. An anti-OX40 polyclonal Ab that blocked the interaction between OX40 and OX40L, and a soluble (s)OX40-Ig fusion protein that led to an enhanced proliferation and Ig production of B cells have been able to highlight the significance of OX40L in the terminal differentiation of activated B cells 16 17. Similarly, ligation of OX40L expressed on human dendritic cells reportedly enhanced dendritic cell differentiation in vitro 19, thus correlating with an antigen-presenting function as a consequence of OX40L ligation.
Weinberg et al. previously reported the expression of OX40 on autoreactive T cells in rats with EAE, and that administration of an OX40 immunotoxin resulted in an amelioration of this disease 7. Moreover, an sOX40 fusion protein, antagonist for OX40L, has also been described not only to suppress ongoing EAE 14 and semiallogeneic graft-versus-host disease 12, but also to ameliorate ongoing colitis in murine models of inflammatory bowel disease 13. These observations shed further light on the requirement of the costimulatory function offered by the OX40–OX40L system in the regulation of immune disorders.
Our report shows that OX40 and OX40L are an indispensable pair in in vivo T cell priming as demonstrated by lack of functional OX40L expression on APCs, through the use of OX40L-deficient mice and an mAb antagonistic to OX40L.
Materials and Methods
Generation of OX40L-deficient Mice.
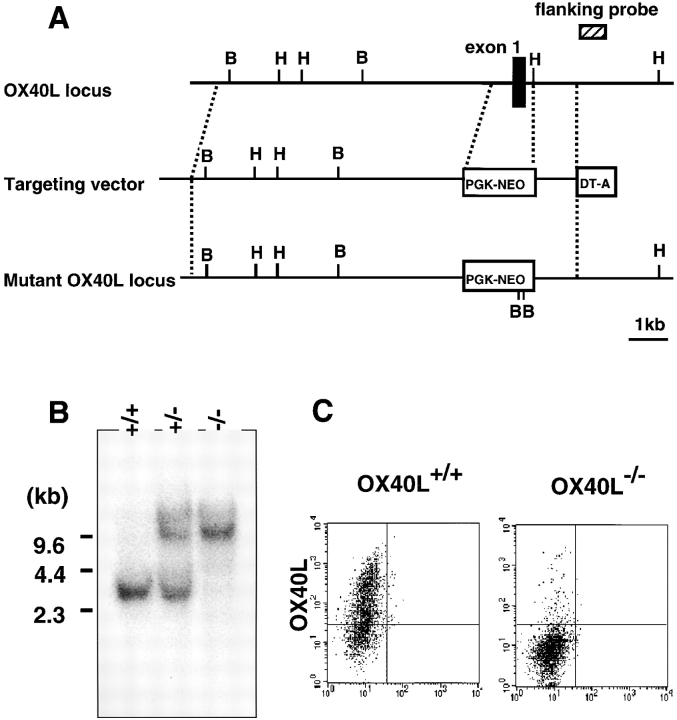
A phage clone containing an ∼13-kb DNA fragment, including exon 1 of the OX40L gene, was isolated from a mouse 129/Sv genomic library (Stratagene) by using the OX40L cDNA as a probe. The vector was constructed by replacement of the first exon with a neomycin resistance cassette (PGKNeo 26; see Fig. 1 A). The gene for the diphtheria toxin A subunit (DT-A) was incorporated into the 3′ end of the vector. The vector was linearized with NotI and electroporated into J1 embryonic stem (ES) cells. G418-resistant ES cell colonies were picked up, expanded as described previously 27, and screened for homologous recombination by Southern blotting using a flanking probe depicted in Fig. 1 A. Three ES clones were identified as heterozygous for OX40L disruption and injected into blastocysts derived from C57BL/6 Cr mice. Chimeric mice were bred against C57BL/6 Cr female mice, and the generated F1 mice heterozygous for the mutation were intercrossed to generate F2 offspring. The offspring were analyzed for the mutated OX40L allele by Southern blotting. The OX40L-deficient mice were backcrossed at least six times to C57BL/6 Cr mice for in vivo immunological analyses.
Figure 1.
Creation of OX40L-deficient mice. (A) Structure of the wild-type and mutant OX40L alleles. The targeting construct was designed to replace the first exon (black box) with a PGKneo gene cassette. The location of the probe for hybridization, the 0.8-kb PvuII-StuI fragment, is shown (hatched box). (B) Southern blot analysis of offspring of the germline chimera. Tail DNA harvested from wild-type, OX40L+/−, and OX40L−/− mice was digested with HindIII (the location of which is depicted as H in A), electrophoresed, and probed with the radiolabeled probe. The mutant and wild-type alleles gave 10.0- and 3.0-kb hybridizing bands, respectively. (C) FACS® analysis of surface expression of OX40L. Splenic B cells from wild-type and OX40L−/− mice were stimulated with anti-CD40 plus anti-IgM, and then stained with anti–mouse OX40L mAb, MGP34.
Preparation of MGP34, an mAb Specific for Mouse OX40L.
BW5147, a mouse T cell hybridoma cell line, was stably transfected with pSRmgp34 expression plasmid carrying the SRα promoter followed by mouse OX40L cDNA, yielding BW-gp34. TART-1, a rat T cell line, was stably transfected with pBCMGSneo-mgp34 expression plasmid carrying the CMV promoter followed by mouse OX40L cDNA, yielding TART-gp34. Cells used were cultured in RPMI 1640 medium containing 10% FCS. Wistar rats were intraperitoneally immunized four times at weekly intervals with 108 TART-gp34 cells. The sensitized spleen cells were fused with a mouse myeloma cell line, SP2/0-Ag14, and hybridoma clones were obtained as described previously 28. Culture supernatants of the hybridomas were screened by a radioimmunoassay with 125I-labeled anti–rat sheep Ig (IM1320; Amersham Pharmacia Biotech) using the transfectant cell lines. One hybridoma producing mAb specific for mouse OX40L was positively screened and named MGP34.
Surface Labeling and Immunoprecipitation.
Cell surface proteins of BW5147 and BW-gp34 cells were biotinylated using biotin–CNHS-ester (Boehringer Mannheim). The cells (107) were solubilized in 1 ml of buffer (25 mM Tris-HCl, pH 7.5, 140 mM NaCl, 1 mM EDTA, 2 mM PMSF, 0.1% aprotinin, and 1% NP-40). Protein A–Sepharose was pretreated with anti–rat IgG and then conjugated with the MGP34 mAb. The cell lysates were incubated with the protein A–Sepharose for 2 h at 4°C. Immunoprecipitates bound to the Sepharose were separated by SDS-PAGE with a 12.5% polyacrylamide gel and transferred to polyvinylidene difluoride filters (Millipore). The filters were probed with streptavidin–horseradish peroxidase and visualized by the ECL detection system (Amersham Pharmacia Biotech).
mAbs.
MGP34 (rat IgG2c) specific for mouse OX40L established here was conjugated to NHS-LC-biotin (Pierce Chemical Co.). Anti-CD4 (L3T4), B220 (RA3-6B2), anti-CD11c (HL3), anti-CD40 (HM40-3), and anti–mouse IgM (R6-60.2) dye-linked mAbs for immunofluorescence studies were all purchased from PharMingen. Anti–human Fc-FITC was purchased from Cappel. The specificity and optimal titer of the mAbs were determined before use.
Immunofluorescence Staining.
Preincubation with normal rat serum was carried out to prevent nonspecific association of labeled mAbs, including binding to the Fc receptor. The cells were incubated for 30 min at 4°C with biotin-conjugated MGP34 in combination with anti-IgM–FITC plus B220-PE for B cells or anti-CD11c–FITC plus 33D1-PE for dendritic cells. The cells labeled with the biotinylated mAb were visualized by streptavidin-allophycocyanin (PharMingen), and subjected to flow cytometric analysis. For negative control of OX40L, preincubation of the cells with unlabeled MGP34 was performed to abolish any specific staining by the biotinylated Abs. The mAb-treated cells were analyzed with a FACSCalibur™ flow cytometer (Becton Dickinson).
Preparation and Activation of Dendritic Cells.
The enriched dendritic cells were prepared as described 29. In brief, collagenase-treated spleen cells (Collagenase type IV; Sigma Chemical Co.) in RPMI 1640 were suspended in 1.077 g/ml Nycodenz (Nycoprep A; Nycomed) and centrifuged at 600 g for 20 min, and the low density fraction was collected and washed several times. The recovered cells were suspended and incubated in RPMI 1640 containing 10% FCS for 2 h at 37°C to remove nonadherent cells, and the remaining adherent cells were incubated overnight at 37°C in the medium plus mouse recombinant GM-CSF (5 ng/ml) and IL-4 (2 ng/ml) (PeproTech). After cultivation, the nonadherent cells harvested contained >60% CD11c+33D1+ dendritic cells. The enriched cells were cultured in the presence of anti-CD40 mAb (HM40-3, 5 μg/ml) plus goat anti–hamster Ab (5 μg/ml; Caltag) in RPMI 1640 including 10% FCS at 37°C for the indicated times.
In Vivo T Cell Priming and Recall Response Stimulated with Protein Antigens.
6-wk-old female C57BL/6 mice, OX40L-deficient mice, or wild-type littermates (n = 4 per each group) were immunized with 50 μg of KLH, hen egg lysozyme (HEL), or OVA, together with CFA in a total volume of 50 μl into each hind footpad. The C57BL/6 mice were injected intraperitoneally with 500 μg of MGP34 or rat IgG (Cappel) at days 0, 3, and 6 of immunization. On day 9 after immunization, inguinal lymph node cells on the antigen-immunized side were extracted. The lymph node cells (105) were added into each well of a 96-well plate and incubated with the indicated dose of the proteins at 37°C for 3 d. Alternatively, CD4+ T cells (5 × 104) purified from the lymph node were restimulated with KLH in the presence of APCs (2.5 × 105) in the same manner. The CD4+ T cells used were purified from the extracted lymph node by using mouse anti-CD4 Dynabeads® (Dynal) and further separated from the beads using DETACHaBEAD® (Dynal). Purity was confirmed to be >98% by flow cytometer. The APCs used were isolated from the spleens of 6-wk-old female C57BL/6 mice, OX40L-deficient mice, or wild-type littermates and irradiated (3,000 rad). The cells cultured were assayed for [3H]thymidine uptake and cytokine production in response to KLH in vitro as described previously 28 30 31. Culture supernatants were collected at 48 h for IL-2, or at 96 h for IL-5, IL-10, and IFN-γ, and were subjected to ELISA to measure the cytokine production.
In Vivo Production of KLH-specific Ab.
80 μg of KLH was used to immunize the OX40L-deficient and wild-type mice as described above, except that the mice were immunized again with 50 μg of KLH by the same method 4 wk after priming to elicit the secondary Ab response. For the primary Ab determination, serum was collected on day 7 for IgM and day 14 for the IgGs to examine the concentration of anti–KLH-specific Abs. For secondary Ab production, serum was collected on day 5 after the second immunization.
Assay for Anti–KLH-specific Ig Production.
Each well of an ELISA microtiter plate was coated with 10 μg/ml of KLH in carbonate buffer, pH 9.0, by overnight incubation at 4°C. Plates were washed and subsequently blocked with 1% BSA in PBS for 1 h at 37°C. Murine sera from OX40L-deficient or wild-type mice, diluted in PBS containing 1% BSA (from 1:100 to 1:100,000), were added to the wells and incubated for 2 h at room temperature. The plates were then washed, and bound Abs were detected by incubation with goat anti–murine IgM, IgG1, IgG2a, IgG2b, or IgG3 conjugated to alkaline phosphatase (1:200; Southern Biotechnology Associates). After an additional 1-h incubation, color reactions were performed using alkaline phosphatase substrate (Sigma Chemical Co.) in a diethanolamine buffer. The reaction was stopped by 3 M NaOH, and the OD readings at 405 nm from the dilution (1:300, 1:900, 1:2,700, or 1:8,100 for IgM and IgG3, IgG2a, IgG2b, or IgG1, respectively) were evaluated. This was demonstrated to be well within the linear part of the titration curve.
CTL Induction and Cytotoxicity Assay.
To induce a CTL response, OX40L-deficient, wild-type, MGP34-treated, or control IgG–treated C57BL/6 mice (n = 4 per group), as recipients, were immunized intraperitoneally with 5 × 107 irradiated splenocytes from BALB/c mice. In the Ab-treated groups, the mice were then administered intraperitoneally with 500 μg MGP34 or a control Ab on days 0, 2, and 4. On day 5, spleen cells were extracted and prepared as effector cells in 51Cr-release assays. BALB/c splenocytes stimulated with ConA (10 μg/ml) were used as target cells as described previously 32. After incubating the 51Cr-labeled target cells with the effector cells, radioactivities of the supernatants were determined with a γ-counter. Results were expressed as follows: % specific lysis = [(release in test – spontaneous release)/(maximum release – spontaneous release)] × 100.
Results
Generation and Characterization of OX40L-deficient Mice.
Using gene targeting and ES cell technology, the OX40L mutation was created by inserting the neomycin resistance gene cassette into the first exon, and was introduced into ES cells through homologous recombination as illustrated in Fig. 1 A. Homologous recombination events were genotyped by Southern blot analysis. Three ES clones carrying the mutant allele were isolated to generate chimeric mice. Chimeric mice derived from two of the three ES clones were found to transmit the mutant allele to their offspring. Wild-type, homozygous mutant, and heterozygous mutant genotypes were determined by Southern blot analyses of DNA from the progeny obtained by interbreeding of heterozygous mice (Fig. 1 B). 10.0- and 3.0-kb bands were detected as mutated and wild-type alleles, respectively (Fig. 1 B), by a flanking probe depicted in Fig. 1 A. To examine the expression of OX40L, flow cytometric analysis with splenic B cells from wild-type and homozygous mice was carried out. Since expression of OX40L on all freshly isolated lymphocyte populations in wild-type mice was undetectable, B cells from both wild-type and mutant mice were selected and stimulated with anti-CD40 plus anti-IgM Ab for 3 d to induce OX40L expression. Activated B cells from the wild-type mice had strong expression of OX40L, whereas no expression was observed on the B cells from the homozygous mice (Fig. 1 C), indicating that the mutation of the OX40L allele resulted in lack of OX40L expression on the cell surfaces. OX40L knockout mice had been backcrossed onto the C57BL/6 Cr background up to at least six generations before all immunological analyses.
Establishment of MGP34, Antagonistic mAb Specific for OX40L.
MGP34 was prepared by immunization with TART-1 transfectant cells expressing mouse OX40L. Specificity of MGP34 was determined by staining of BW-gp34 cells, stably transfected with mouse OX40L, and their parental BW5147 cells. MGP34 reacted to BW-gp34, but not to BW5147 cells (Fig. 2 A), demonstrating the specificity of the mAb for OX40L. MGP34 detected a 34-kD band in the immunoprecipitates from biotinylated BW-gp34 cells (Fig. 2 B), indicating that the mouse OX40L protein expressed on the cell surface is a 34-kD molecule similar to human OX40L in molecular mass 33.
Figure 2.
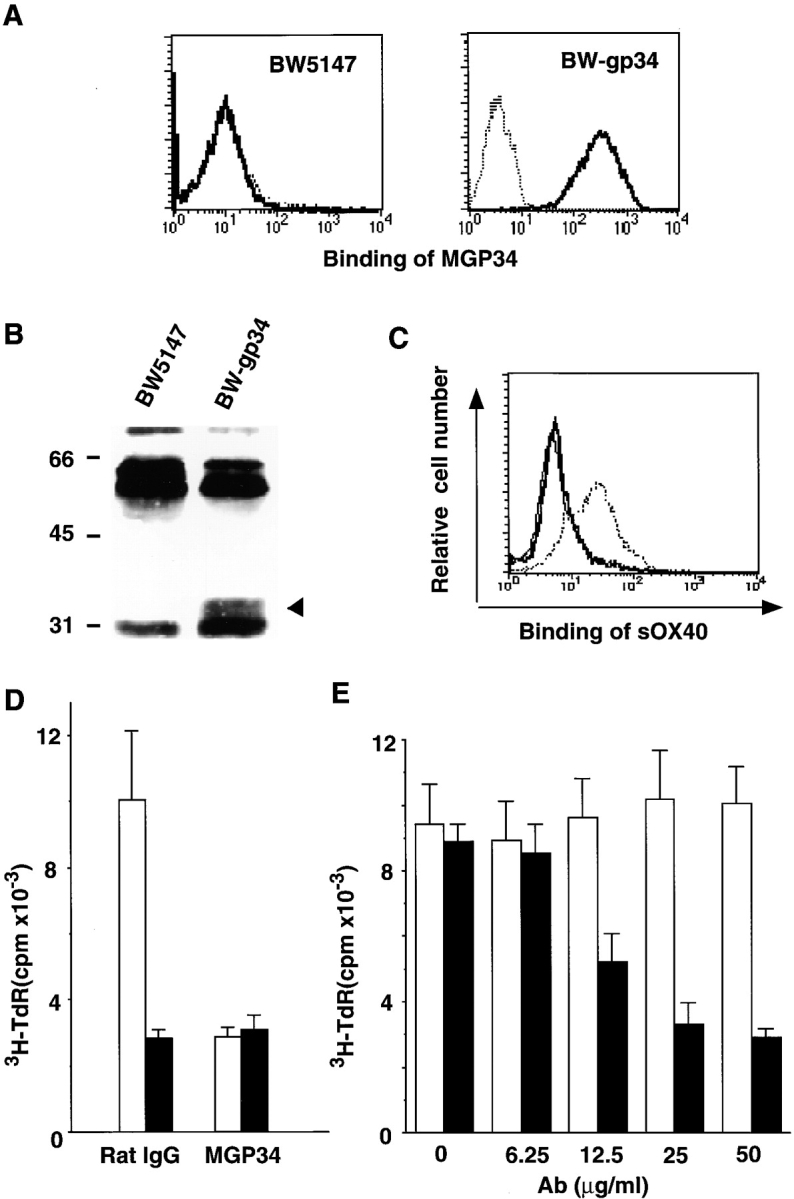
Binding specificity and blocking potential of MGP34. (A) Specificity of MGP34 for OX40L. BW5147 parental cells (left) and BW5147 cells transfected with mouse OX40L, BW-gp34 (right), were stained with MGP34 and analyzed by flow cytometry. Dotted lines represent control stainings conducted in the presence of excess unlabeled MGP34; solid lines represent MGP34-specific staining. (B) Immunoprecipitation of mouse OX40L with MGP34. BW5147 and BW-gp34 cells were surface-labeled with biotin, lysed, and immunoprecipitated with MGP34. Arrowhead, a 34-kD precipitate. Numbers on the left indicate standard molecular sizes. (C) Competitive binding of sOX40-Fc and MGP34 to OX40L. BW-gp34 cells were preincubated with MGP34 or control IgG, and incubated with sOX40-Fc at 4°C for 30 min. The binding of the sOX40-Fc (provided by Dr. P. Baum) was visualized by anti–human Fc-FITC. Solid thick or dotted line represents sOX40-Fc binding in the presence of MGP34 or the control Ab, respectively. Solid thin line represents background staining with anti–human Fc-FITC alone. (D) Blocking effect of MGP34 on the OX40-dependent proliferation of T cells. Purified splenic T cells (105/well) were assayed for their proliferation induced by immobilized anti-CD3 in the presence of irradiated BW5147 (black bars) or BW-gp34 cells (5 × 104; white bars). The cells were cultured for 72 h in the presence of 10 μg/ml of MGP34 or the control Ab, and assayed for thymidine incorporation. (E) The inhibitory effect of MGP34 is dose dependent. Purified splenic T cells (105/well) were cocultured with irradiated BW-gp34 cells (5 × 104/well) in the presence of anti-CD3 at the indicated concentrations of MGP34 (black bars) or control IgG (white bars). The proliferative response was assessed at 72 h as described above.
To examine the effect of MGP34 on the OX40–OX40L interaction, competitive binding assays were performed between a human sOX40-Fc fusion protein (provided by Dr. P. Baum, Immunex Research and Development Corp., Seattle, WA) and MGP34 to BW-gp34 cells. sOX40–Fc apparently bound to BW-gp34 cells in the presence of the control mAb, whereas the binding was completely eliminated in the presence of MGP34 (Fig. 2 C), clearly indicating an effective blocking potential of MGP34 towards the binding between OX40 and OX40L.
The OX40L–OX40 system is known to be implicated in costimulatory signal transduction in activated T cells 1 4 5 23 24 25. To assess the inhibitory effect of MGP34 on OX40-dependent T cell proliferation, T cells purified from spleens were stimulated with anti-CD3 plus irradiated BW-gp34 cells or anti-CD3 plus irradiated BW5147 parental cells, in the presence of either MGP34 or a control mAb, and then assayed for their [3H]thymidine uptake. Stimulation of T cells with BW-gp34 cells induced a significant increase in [3H]thymidine uptake compared with the BW5147 parental cells in the presence of the control IgG. However, the enhancement in T cell proliferation by OX40L was completely suppressed by MGP34 (Fig. 2 D), and in a dose-dependent manner (Fig. 2 E). These results indicate that MGP34 has an inhibitory effect towards T cell proliferation in response to OX40L in vitro.
Expression Kinetics of OX40L on T, B, and Dendritic Cells.
Although accumulating evidence currently demonstrates the expression of OX40L on T, B, and dendritic cells in humans and mice 15 16 19 20 25, the expression kinetics of OX40L is still unclear. Therefore, we carried out a detailed analysis of OX40L expression by immunostaining with MGP34. Thymocytes and splenic lymphocytes freshly isolated from 5-wk-old C57BL/6 male mice failed to express OX40L (data not shown). Splenic T and B cells were stimulated with anti-CD3 and anti-CD40 plus anti-IgM Ab, respectively. Little expression of OX40L was observed on both CD4+ and CD8+ T cells stimulated with anti-CD3 (data not shown). However, in the B220+ IgM+ B cell population, stimulation with anti-CD40 plus anti-IgM Ab was able to induce expression of OX40L 2 d after the stimulation (Fig. 3), and the expression persisted for up to 7 d. Stimulation with either anti-CD40 or anti-IgM Ab did not induce any expression of OX40L (data not shown). CD11c+33D1+ dendritic cells freshly isolated from spleen cells expressed little, if any, OX40L; however, the OX40L expression was significantly induced 1 d after stimulation with anti-CD40 (Fig. 3), but not by LPS (data not shown), and was sustained up until at least day 7. These results suggest that CD40 signaling is indispensable for OX40L expression on both B and dendritic cells.
Figure 3.
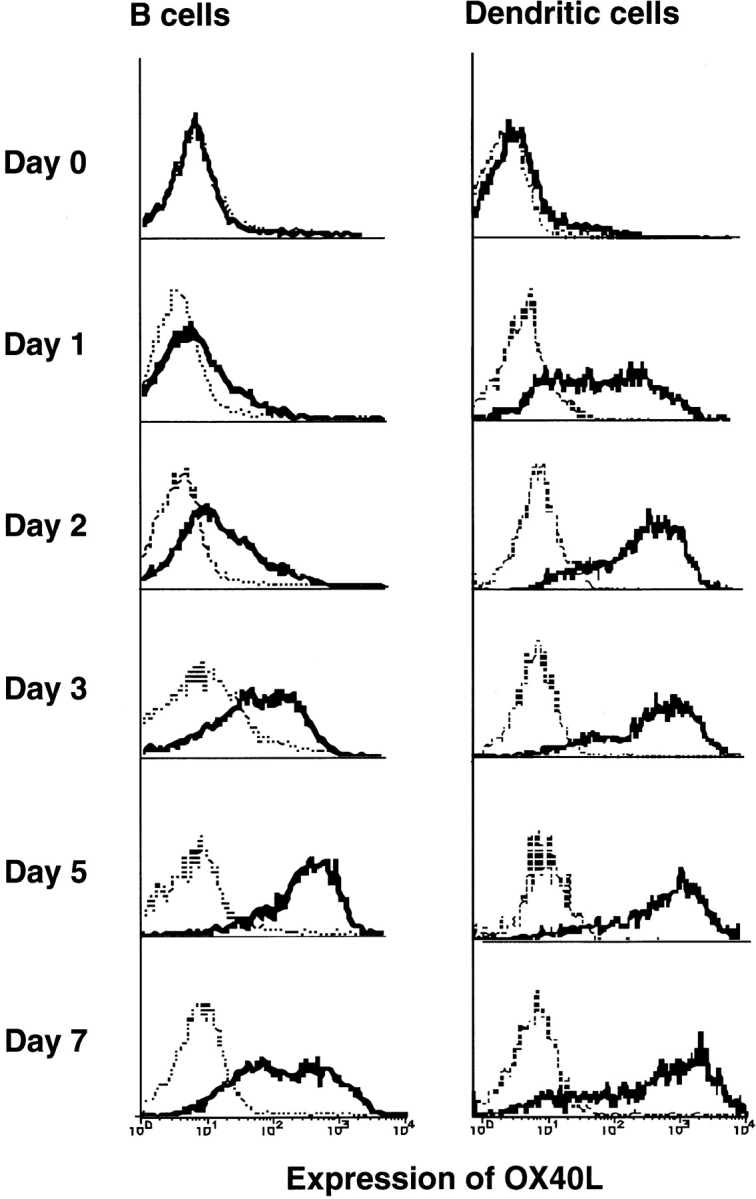
Kinetics of OX40L expression on APCs. Splenic B cells were cultured in the presence of anti-CD40 plus anti-IgM for the indicated time periods. The cells were stained with anti-IgM–FITC, B220-PE, and biotinylated MGP34 followed by allophycocyanin-labeled streptavidin. B220+ B cells during the culture were examined for expression of OX40L. Enriched splenic dendritic cells were cultured in the presence of anti-CD40 plus anti–hamster Ab for the indicated time. The cells were stained with anti-CD11c–FITC, 33D1-PE, and biotinylated MGP34 followed by allophycocyanin-labeled streptavidin. CD11c+33D1+ dendritic cells during the culture were examined for expression of OX40L. Dotted lines represent control stainings conducted in the presence of excess unlabeled MGP34; solid lines represent MGP34-specific staining.
Phenotypes of Lymphocytes and Dendritic Cells in OX40L-deficient Mice.
Flow cytometric analyses of thymocytes and splenocytes were performed to examine whether an absence of OX40L expression affects the development of lymphocytes and dendritic cells. OX40L-deficient mice did not display any apparent abnormality in the formation of lymphoid organ structures, in the numbers of T and B cells, and in the phenotypes of these cells as assessed by CD4 and CD8, or B220 and IgM expression on thymocytes and splenic lymphocytes (data not shown), respectively. No obvious difference in the ratio of CD11c+33D1+ dendritic cells was observed between OX40L-deficient and wild-type spleens (data not shown). These results suggest that OX40L is not crucial in the early development of T, B, and dendritic cells.
To gain further insight into the role played by OX40L in APC functions, we examined the B and dendritic cells of OX40L-deficient mice in their expression of several molecules known to be involved in APC functions such as MHC class II antigen, CD40, CD80, and CD86. Although a previous report demonstrated that cross-linking of OX40L increased the expression level of these costimulatory molecules on human dendritic cells 19, similar basal levels of expression of these molecules were observed on unstimulated B and dendritic cells in both OX40L-deficient and wild-type mice (data not shown). Since CD40-stimulated B and dendritic cells are considered to be functional APCs, we next examined the expression patterns of these activated APC markers on B and dendritic cells upon stimulation with anti-CD40. All of these molecules were expressed among both CD40-stimulated B and dendritic cells derived from wild-type and OX40L-deficient mice (data not shown), suggesting that the absence of OX40L does not affect the CD40-induced expression of such molecules on APCs.
Proliferative responses of splenic T and B cells obtained from unimmunized wild-type or OX40L-deficient mice were next evaluated. Equal levels of proliferation were observed in both wild-type and mutant T cells in response to various T cell stimuli (anti-CD3, anti-CD28 plus anti-CD3, ConA, or PMA plus ionomycin). Similarly, in response to B cell stimuli such as LPS, anti-IgM, anti-CD40, or a combination of anti-IgM plus anti-CD40, B cell proliferative responses were indistinguishable in both populations (data not shown). These results suggest that the intrinsic potential of T and B cells to respond to antigens is preserved in OX40L-deficient mice.
Impairment of T Cell Priming and Cytokine Production in OX40L-deficient Mice and MGP34-treated Mice.
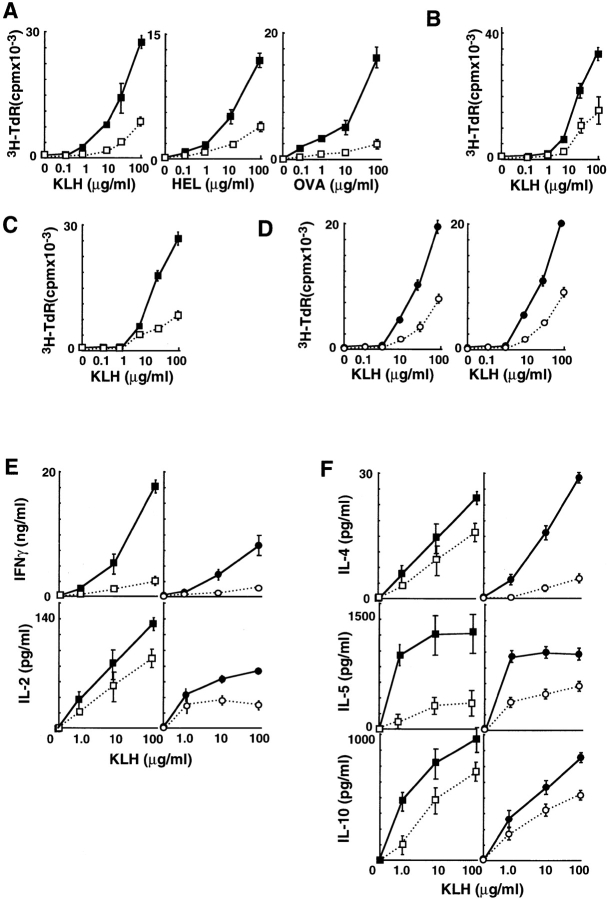
To examine the in vivo effect of OX40L on antigen-specific T cell activation, we immunized OX40L-deficient or wild-type mice with KLH and tested their in vitro T cell recall proliferative response. A significantly reduced response was observed in OX40L-deficient mice, and the antigen dose–response curve was shifted by several orders of magnitude (Fig. 4 A). Similarly reduced responses were seen with two other protein antigens, hen egg lysozyme (HEL) and OVA, in OX40L-deficient mice (Fig. 4 A). These results suggest that OX40L plays an important role in antigen-specific T cell priming. The reduced response in OX40L-deficient mice may be due to a defect at the level of the APCs, since the intrinsic potential of OX40L-deficient T cells in response to antigens is preserved (data not shown) and since OX40L expression was restricted to activated APCs but not T cells.
Figure 4.
Impaired T cell priming and cytokine production in OX40L-deficient mice and MGP34-treated mice. (A) OX40L-deficient mice have impaired recall proliferative responses to protein antigens. OX40L-deficient (□) or wild-type (▪) mice (four per group) were immunized with KLH, HEL, or OVA in the hind footpads. 9 d after immunization, draining lymph nodes were extracted and subjected to an in vitro challenge of the various protein antigens. After culturing for 3 d, their [3H]thymidine uptake was measured. (B) Impairment of recall proliferative response of the CD4+ T cells of OX40L-deficient mice. Purified CD4+ T cells from the draining lymph nodes of the OX40L-deficient (□) or wild-type mice (▪) were assayed for their recall proliferation in response to KLH in the presence of APCs from wild-type mice. (C) Defective APC function in OX40L-deficient mice. Purified CD4+ T cells from the draining lymph nodes from wild-type mice primed with KLH were assayed for their recall proliferation in response to KLH in the presence of OX40L-deficient (□) or wild-type (▪) irradiated spleen cells used as APCs. (D) MGP34-treated mice have similarly impaired lymph node recall proliferation to KLH. C57BL/6 mice (four per group) were immunized in the hind footpads with KLH in CFA. On days 0, 3, and the 6, mice received 500 μg anti-OX40L (○) or rat IgG (•) intraperitoneally. The recall proliferative responses of the draining lymph nodes (left) were tested in the same manner as described above. Purified CD4+ T cells from the draining lymph nodes of the MGP34-treated (○) or control IgG–treated (•) mice were assayed for their recall proliferation in response to KLH in the presence of APCs from C57BL/6 mice (right). (E and F) Absence of OX40L or MGP34 treatment inhibits recall cytokine production by lymph nodes in response to KLH. Production of Th1 (E) and Th2 (F) cytokines by the draining lymph nodes of the mice in the four groups (□, ▪, ○, •) immunized with KLH and further subjected to an in vitro challenge with KLH were measured.
Next, we asked whether the dysfunction of OX40L-deficient APCs causing the reduced reaction in the T cells occurs during the initial in vivo T cell priming phase, during the secondary in vitro reaction, or possibly during both. Recall proliferative assays were performed using several configurations consisting of purified T cells and APCs derived from both OX40L-deficient and wild-type mice. Initially, KLH-primed CD4+ T cells purified from OX40L-deficient or wild-type mice were examined for their recall reaction in the presence of the wild-type APCs. The response of OX40L-deficient CD4+ T cells was less than half of that observed among wild-type CD4+ T cells (Fig. 4 B), indicating a key role for OX40L in antigen-specific T cell priming in vivo. Next, to evaluate the in vitro APC function during secondary T cell activation, KLH-primed CD4+ T cells from wild-type mice were incubated with APCs isolated from OX40L-deficient or wild-type spleen, and their recall proliferation was assayed. Interestingly, CD4+ T cell proliferation upon stimulation with the OX40L-deficient APCs was significantly reduced compared with wild-type APCs (Fig. 4 C). These results revealed the intrinsic dysfunction of OX40L-deficient APCs in vitro. Taking the data together, OX40L is shown to be essential to fully activate antigen-specific T cell responses through OX40L-expressing APCs during both the priming and effector phases of T cell activation.
To rule out the possibility that the impairment of APC function could be caused by a mismatch of MHCs between T cells and APCs as a result of an insufficient backcross, we also examined whether C57BL/6 mice treated with MGP34, a blocking mAb for OX40L function, affects the in vivo T cell priming. C57BL/6 mice were immunized with KLH in a similar manner to the above experiment using OX40L-deficient mice and inoculated with either MGP34 or a control rat IgG at days 0, 2, 4, and 6 after immunization. In vitro proliferative recall responses of their lymph node cells were subsequently determined. Lymph node cells derived from the MGP34-administered mice showed suppressed recall reaction to KLH (Fig. 4 D) similar to that observed with the OX40L-deficient mice. Furthermore, we examined the in vitro recall reaction of the CD4+ T cells purified from the KLH-primed lymph nodes of MGP34-treated mice in the presence of C57BL/6 APCs. We observed that the MGP34-treated mice had a significant reduction in CD4+ T cell recall reaction compared with the control mice (Fig. 4 D), confirming the critical involvement of OX40L in antigen-specific T cell priming in vivo.
Cytokine Production in the Culture Supernatants Was Also Assayed by ELISA.
IFN-γ production by both the OX40L-deficient and MGP34-treated lymph nodes was dramatically reduced (Fig. 4 E). The production levels of IL-2, IL-4, IL-5, and IL-10 were also significantly lower than those of the control groups (Fig. 4E and Fig. F), suggesting that the OX40–OX40L system critically contributes to both the Th1 and Th2 responses.
Impairment of Antigen-specific Ab Production in OX40L-deficient Mice.
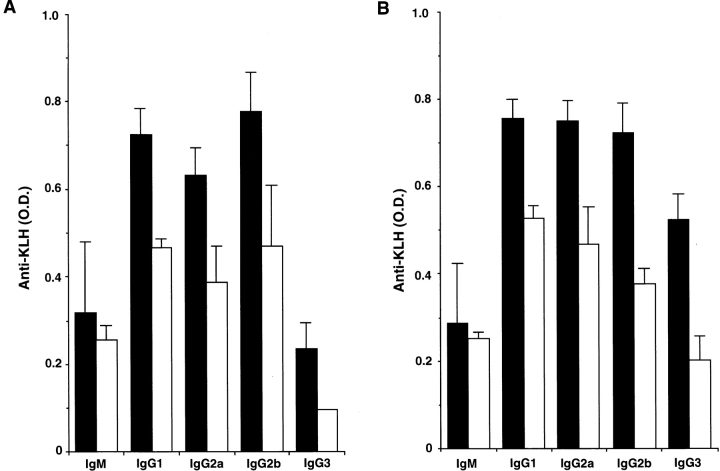
We next investigated the role of OX40L during the course of primary and secondary humoral immune responses against KLH, a well-known T cell–dependent antigen. Sera from OX40L-deficient mice clearly exhibited reduced KLH-specific IgG Ab production in both primary and secondary humoral responses, among all murine IgG subclasses (Fig. 5). No significant difference in both primary and secondary IgM production was observed between wild-type and OX40L-deficient mice (Fig. 5). Similarly, in vivo administration of MGP34 mAb also inhibited the primary IgG, and not IgM, response to KLH (data not shown). In contrast, OX40L-deficient mice demonstrated normal IgM and IgG production specific for trinitrophenol (TNP) in both primary and secondary responses to TNP-LPS, a T cell–independent antigen (data not shown), indicating the importance of the OX40–OX40L interaction in T cell–dependent humoral responses.
Figure 5.
Impaired Ab production to KLH in OX40L-deficient mice. (A) Primary Ab responses to KLH in wild-type (black bars) and OX40L-deficient (white bars) mice were evaluated. After KLH immunization, serum was collected on day 7 for IgM and day 14 for the IgGs to examine the concentration of anti–KLH-specific Abs. (B) Secondary Ab responses to KLH in wild-type (black bars) and OX40L-deficient (white bars) mice were evaluated. Serum was collected on day 5 after the second immunization.
Alloantigen-specific CTL Response in OX40L-deficient and MGP34-treated Mice.
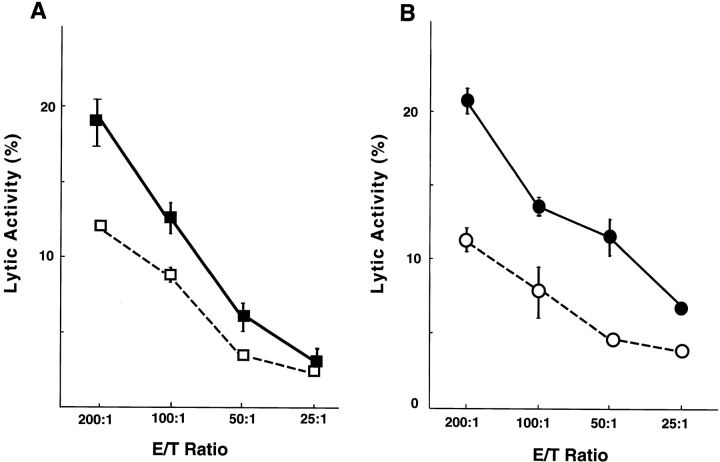
We examined the effect of OX40L on the induction of alloantigen-specific CTLs. Wild-type, OX40L-deficient, MGP34-treated, and control IgG–treated C57BL/6 mice used as recipients were immunized with splenocytes derived from BALB/c mice. On day 5 after immunization, splenocytes from the recipient mice were used as effector cells in the cytotoxicity assay. Allospecific CTLs were induced in the control mice, whereas induction of such CTLs was significantly inhibited in both OX40L-deficient and MGP34-treated mice, suggesting an important involvement of OX40L in alloantigen-specific CTL response (Fig. 6A and Fig. B).
Figure 6.
Suppression of CTL induction in OX40L-deficient and MGP34-treated mice. (A) OX40L-deficient (□) or wild-type (▪) mice (H-2b) were immunized intraperitoneally with splenocytes of BALB/c (H-2d) mice. On day 5, CTL activities of their splenocytes were tested with BALB/c target cells. Data shown are representative of four experiments. (B) C57BL/6 (H-2b) mice were immunized intraperitoneally with splenocytes of BALB/c (H-2d) mice. The mice were treated with 500 μg MGP34 (○) or rat IgG (•) on days 0, 2, and 4 after immunization. On day 5, CTL activities of their splenocytes were tested with BALB/c target cells. Data shown are representative of four experiments.
Discussion
Recently, OX40 has been highlighted as a new costimulatory molecule capable of activating T cells; however, the expression and function of its ligand OX40L are still unclear, particularly during in vivo immune responses. To elucidate the in vivo significance of OX40L, we produced OX40L-deficient mice and an antagonistic mAb specific for OX40L, MGP34. Using the two experimental tools, we revealed that OX40L expressed on APCs plays a key role on antigen-specific T cell initiation in vivo.
Several in vitro studies have demonstrated that OX40 stimulation enhances IL-4 expression and promotes T cell differentiation towards Th2 cells 34 35. In contrast to these studies, it has also been reported that in vivo administration of an OX40-IgG fusion protein, antagonistic for OX40, ameliorates Th1-associated diseases inflammatory bowel disease and EAE, and reduces transcripts for Th1 cytokines such as TNF-α, IL-1, IL-12, and IFN-γ in the affected tissues 13 14. These results suggest that OX40–OX40L interactions participate in enhancing both Th1 and Th2 responses during antigen-specific CD4+ T cell activation. This study clearly demonstrated that the lack of OX40–OX40L interaction inhibits both of these responses during in vivo T cell priming. Since suppression of the Th1 response usually induces an increase in Th2 responses and vise versa, reduction of both Th1 and Th2 cytokine production in OX40L-deficient mice may reflect either hyporesponsiveness or anergy in the T cell response. A consequence of OX40 ligation on activated T cells is to increase their survival, as demonstrated by a recent publication 36. To examine whether OX40L is involved in the survival of primed memory T cells, wild-type and OX40L-deficient mice were stimulated with staphylococcal enterotoxin A and LPS to induce survival and expansion of Vβ3+ T cells 36 37. Interestingly, the survival of these T cells is not significantly reduced in OX40L-deficient mice compared with wild-type mice (Murata, K., N. Ishii, L.C. Ndhlovu, and K. Sugamura, unpublished data). The increased survival of Vβ3+ T cells by constitutive ligation of OX40 36 might not necessarily mean that OX40L deficiency induces apoptosis of the T cells. To fully explain the possible mechanism of hyporesponsiveness of the T cells in OX40-deficient mice, further examination will be necessary.
Further analysis of OX40L-deficient mice demonstrated impairment of both primary and secondary Ab production to KLH, a T cell–dependent antigen (Fig. 5A and Fig. B), as well as suppression of the alloantigen-specific CTL induction (Fig. 6 A) compared with wild-type mice. The suppressed Ab production to KLH is similar to a previous report showing that blockade of OX40–OX40L interaction inhibited production of all of the IgG subclasses, but not IgM 17. Since KLH is known to be a T cell–dependent antigen on Ab production, the impairment of Ab responses observed may also be due to suppressed T cell priming. However, our data here cannot exclude the possibility that OX40L signaling in B cells may be involved in Ab production or B cell differentiation. Indeed, a previous report demonstrated that ligation of OX40L led to an enhanced proliferation and Ig production of B cells 16. Furthermore, the suppressed alloantigen-specific CTL induction among OX40L-deficient mice may be a consequence of impaired CD4+ T cell help for CD8 responses. However, a direct role of OX40–OX40L interaction on CD8+ T cells could exist, since OX40 has been reported to be expressed on activated CD8+ T cells in mice 3 5 but not in rats.
OX40 expressed on activated T cells has been reported to be an effector molecule for costimulatory signal transduction upon interaction with OX40L on APCs in immune responses 24. OX40L expression was previously detected on activated B and dendritic cells 19 20 25. Since the sensitivity in detection of OX40L expression with soluble OX40 is at least 10 times lower than that with MGP34 mAb (compare Fig. 2A and Fig. C), MGP34 mAb is useful as a sensitive assay in determining the expression kinetics of OX40L. Using MGP34 mAb, we found that CD40-stimulated B and dendritic cells express OX40L within 2 d of stimulation and are able to sustain this expression beyond day 7. Since expression of CD40L, as a CD40 stimulant, is immediately induced on T cells after antigen stimulation, and since CD40 is constitutively expressed on APCs independent of antigen stimulation 38 39, our findings indicate that OX40L expression is presumably induced by CD40 stimulation 2 d after antigen challenge. On the other hand, OX40 expression on CD4+ T cells has been reported to peak 48 h after TCR stimulation 24, which coincides with the onset of OX40L expression on CD40-stimulated APCs. This sequential expression of CD40–CD40L and OX40–OX40L suggests that the latter participates in the later phase of in vivo T cell priming.
Furthermore, we monitored the expression of CD80, CD86, and MHC class II molecules, known to be upregulated on B and dendritic cells after CD40 stimulation 14 25 40. Normal upregulation of the molecules was observed in OX40L-deficient APCs (data not shown), indicating that the OX40L deficiency may be responsible for the defective APC function in our study. As only partial suppression of recall response was observed, an OX40–OX40L-independent system may come into play. Indeed, the synergistic potential of the CD28–CD80–CD86 and OX40–OX40L systems in T cell activation has been suggested by the findings that in vitro T cell activation was markedly suppressed by a combination of both OX40L and CD28 blockade, rather than either alone 14 25. Other costimulatory systems such as CD28–CD80–CD86 may cooperatively work with the OX40–OX40L system in antigen-specific T cell priming.
Since CD40 signaling is known to be upstream in the pathway for nuclear factor κB activation 41, our findings depicting the requirement of CD40 stimulation in the induction of OX40L expression are consistent with our previous report showing the downstream upregulation of the promoter activity of OX40L by nuclear factor κB activation 42.
Engagement of CD40 on APCs is well known to be an essential event for the differentiation of APCs to become functional in the priming of antigen-specific T cells 30 43. Impairment of in vivo T cell priming in response to protein antigens in CD40L-deficient mice 30 appears to correlate well with our data here with OX40L-deficient mice. In addition, we demonstrated that CD40 signaling is a critical trigger for inducing OX40L expression on APCs, and that CD40 signaling, required for proliferation of B cells and differentiation of APCs, is intact in OX40L-deficient mice as demonstrated by normal expression of known CD40-induced markers (data not shown). These results highlight the close relationship between CD40 stimulation and OX40L expression during the priming of T cells. We hypothesize that the impairment of T cell priming in CD40- or CD40L-deficient mice may be partially due to a failure of expression of OX40L on APCs. This plausible interplay of the two systems in the subsequent development of an effective immune response remains to be examined.
OX40 has been reported to be expressed on autoreactive T cells in several autoimmune disorders, including in patients with rheumatoid arthritis and graft-versus-host disease, and in the mouse model of multiple sclerosis, EAE 7 8 11 12 13 14, suggesting that the OX40–OX40L system may be involved in autoimmune reactions. In vivo treatment with an sOX40 that blocks the interaction of OX40L with OX40 had a therapeutic effect during EAE 14. Weinberg et al. 14 also demonstrated that OX40L is expressed on a CD11b+ macrophage/microglia cell population in the central nervous system only during EAE onset, and that this population probably acts as APCs to activate the T cells causing EAE. In this context, our findings using MGP34, antagonistic for OX40, also encourage us to examine the clinical usefulness of the blocking Ab in various autoimmune disorders.
Acknowledgments
We thank Dr. P. Baum for providing us with the human sOX40-Fc.
This work was supported in part by Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Corporation, and a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Abbreviations used in this paper: EAE, experimental autoimmune encephalomyelitis; ES, embryonic stem; HEL, hen egg lysozyme; s, soluble.
References
- Paterson D.J., Jefferies W.A., Green J.R., Brandon M.R., Corthesy P., Puklavec M., Williams A.F. Antigens of activated rat T lymphocytes including a molecule of 50,000 M r detected only on CD4 positive T blasts. Mol. Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Mallett S., Fossum S., Barclay A.N. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes—a molecule related to nerve growth factor receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shamkhani A., Birkeland M.L., Puklavec M., Brown M.H., James W., Barclay A.N. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur. J. Immunol. 1996;26:1695–1699. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- Calderhead D.M., Buhlmann J.E., Van der Eetwegh A.J.M., Claasen E., Noelle R.J., Fell H.P. Cloning of mouse Ox40a T cell activation marker that may mediate T-B interactions. J. Immunol. 1993;151:5261–5271. [PubMed] [Google Scholar]
- Baum P.R., Gayle R.B., Ramsdell F., Srinivasan S., Sorensen R.A., Watson M.L., Seldin M.F., Baker E., Sutherland G.R., Clifford K.N. Molecular characterization of murine and human OX-40/OX-40 ligand systemsidentification of a human OX-40 ligand as the HTLV-1-regulated protein gp34. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimura N., Takasawa N., Tanaka Y., Nakamura M., Sugamura K. Induction of OX40, a receptor of gp34, on T cells by trans-acting transcriptional activator, Tax, of human T-cell leukemia virus type I. Jpn. J. Cancer Res. 1996;87:227–231. doi: 10.1111/j.1349-7006.1996.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A.D., Wallin J.J., Jones R.E., Sullivan T.J., Bourdette D.N., Vandenbark A.A., Offner H. Target organ-specific up-regulation of the MRC OX-40 marker and selective production of Th1 lymphokine mRNA by encephalitogenic T helper cells isolated from the spinal cord of rats with experimental autoimmune encephalomyelitis. J. Immunol. 1994;152:4712–4721. [PubMed] [Google Scholar]
- Weinberg A.D., Bourdette D.N., Sullivan T.J., Lemon M., Wallin J.J., Maziarz R., Davey M., Palida F., Godfrey W., Engleman E. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat. Med. 1996;2:183–189. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Imura A., Hori T., Uchiyama T., Imamura S. Localization of OX40/gp34 in inflammatory skin diseasesa clue to elucidate the interaction between activated T cells and endothelial cells in infiltration. Arch. Dermatol. Res. 1997;289:653–656. doi: 10.1007/s004030050255. [DOI] [PubMed] [Google Scholar]
- Vetto J.T., Lumm S., Morrism A., Sicotte M., Davis J., Lemon M., Weinberg A.D. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am. J. Surg. 1997;174:258–265. doi: 10.1016/s0002-9610(97)00139-6. [DOI] [PubMed] [Google Scholar]
- Tittle T.V., Weinberg A.D., Steinkeler C.N., Maziarz R.T. Expression of the T-cell activation antigen, OX-40, identifies alloreactive T cells in acute graft-versus-host disease. Blood. 1997;89:4652–4658. [PubMed] [Google Scholar]
- Stüber E., Von Freier A., Marinescu D., Folsch U.R. Involvement of OX40-OX40L interactions in the intestinal manifestations of the murine acute graft-versus-host disease. Gastroenterology. 1998;115:1205–1215. doi: 10.1016/s0016-5085(98)70092-7. [DOI] [PubMed] [Google Scholar]
- Higgins L.M., McDonald S.A., Whittle N., Crockett N., Shields J.G., MacDonald T.T. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interactionamelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J. Immunol. 1999;162:486–493. [PubMed] [Google Scholar]
- Weinberg A.D., Wegmann K.W., Funatake C., Whitham R.H. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J. Immunol. 1999;162:1818–1826. [PubMed] [Google Scholar]
- Miura S., Ohtani K., Numata N., Niki M., Ohbo K., Ina Y., Gojobori T., Tanaka Y., Tozawa H., Nakamura M., Sugamura K. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax. Mol. Cell. Biol. 1991;11:1313–1325. doi: 10.1128/mcb.11.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber E., Neurath M., Calderhead D., Fell H.P., Strober W. Crosslinking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Stüber E., Strober W. The T cell–B cell interaction via OX40–OX40L is necessary for the T cell–dependent humoral immune response. J. Exp. Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura A., Hori T., Imada K., Ishikawa T., Tanaka Y., Maeda M., Imamura S., Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J. Exp. Med. 1996;183:2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Tanaka Y., Tozawa H., Takahashi Y., Maliszewiski C., Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Brocker T., Gulbranson-Judge A., Flynn S., Riedinger M., Raykundalia C., Lane P. CD4 T cell traffic controlin vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur. J. Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Jr., Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Lenschow D.J., Walunas T.L., Bluestone J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Godfrey W.R., Fagnoni F.F., Harara M.A., Buck D., Engleman E.E. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J. Exp. Med. 1994;180:757–762. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I., Weinberg A.D., Lemon M., Croft M. OX-40 liganda potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- Akiba H., Oshima H., Takeda K., Atsuta M., Nakano H., Nakajima A., Nohara C., Yagita H., Okumura K. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J. Immunol. 1999;162:7058–7066. [PubMed] [Google Scholar]
- Niki M., Okada H., Takano H., Kuno J., Tani K., Hibino H., Asano S., Ito Y., Satake M., Noda T. Hematopoiesis in the fetal liver is impaired by targeted mutagenesis of a gene encoding a non-DNA binding subunit of the transcription factor, polyomavirus enhancer binding protein 2/core binding factor. Proc. Natl. Acad. Sci. USA. 1997;94:5697–5702. doi: 10.1073/pnas.94.11.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai S., Kawano H., Yudate T., Nishi M., Kuno J., Nagata A., Jishage K., Hamada H., Fujii H., Kawamura K. The POU domain transcription factor Brn-2 is required for the determination of specific neuronal lineages in the hypothalamus of the mouse. Genes Dev. 1995;9:3109–3121. doi: 10.1101/gad.9.24.3109. [DOI] [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J. Exp. Med. 1989;169:1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D., Shortman K. Dendritic cell subtypes in mouse lymphoid organs. J. Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- Grewal I.S., Jianchao X., Flavell R.A. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- Nagata K., Tanaka K., Ogawa K., Kemmotsu K., Imai T., Yoshie O., Abe H., Tada K., Nakamura M., Sugamura K., Takano S. Selective expression of a novel surface molecule by human Th2 cells in vivo. J. Immunol. 1999;162:1278–1286. [PubMed] [Google Scholar]
- Yasuda K., Nemoto T., Ohashi Y., Satomi S., Murata K., Ishii N., Takeshita T., Sugamura K. Prolongation of allograft survival by administration of mAb specific for the three subunits of IL-2 receptor. Int. Immunol. 1998;10:561–567. doi: 10.1093/intimm/10.5.561. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Inoi T., Tozawa H., Yamamoto N., Hinuma Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I (HTLV-I) Int. J. Cancer. 1985;36:549–555. doi: 10.1002/ijc.2910360506. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Yang L.P., Uchiyama T., Tanaka Y., Baum P., Sergerie M., Hermann P., Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4+ T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- Flynn S., Toellner K.M., Raykundalia C., Goodall M., Lane P. CD4 T cell cytokine differentiationthe B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A.D., Vella A.T., Croft M. OX-40life beyond the effector T cell stage. Semin. Immunol. 1998;10:471–480. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- Vella A.T., McCormack J.E., Linsley P.S., Kappler J.W., Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Foy T.M., Aruffo A., Bajorath J., Buhlmann J.E., Noelle R.J. Immune regulation by CD40 and its ligand gp39. Annu. Rev. Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- Grewal I.S., Flavell R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Van Gool S.W., Vandenberghe P., de Boer M., Ceuppens J.L. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol. Rev. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Rothe M., Sarma V., Dixit V.M., Goeddel D.V. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science. 1994;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- Ohtani K., Tsujimoto A., Tsukahara T., Numata N., Miura S., Sugamura K., Nakamura M. Molecular mechanisms of promoter regulation of the gp34 gene that is trans-activated by an oncoprotein tax of the human T cell leukemia virus type I. J. Biol. Chem. 1998;273:14119–14129. doi: 10.1074/jbc.273.23.14119. [DOI] [PubMed] [Google Scholar]
- Essen D., Kikutani H., Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]