Our survival depends on our ability to clonally expand rare CD4 lymphocytes and instruct them to help effector cells. Two very different types of CD4 T cell response are required for protective immunity. Immunity to intracellular infections like tuberculosis is dependent on priming and expanding inflammatory IFN-γ–expressing CD4 T cells that acquire the capability to migrate out into the tissue and activate macrophages to kill infected cells. In contrast, immunity to the exotoxin produced by diphtheria requires CD4 T cells to be primed to migrate into B follicles to foster germinal center (GC) development and the rapid production of high-affinity neutralizing antibodies. Although the outcomes of these two types of CD4 response are different, they have the same three components: (a) Identification and expansion of antigen-specific CD4 T cells; (b) instruction to secrete the appropriate cytokines; and (c) instruction to express chemokine and adhesion molecules that guide their migration to the appropriate location.
Recent evidence has highlighted the fact that dendritic cells (DCs) direct CD4 T cell fate during priming 1, and this has focused attention on costimulatory interactions at the initiation of CD4 immune responses. In addition to their potential to costimulate CD4 cells through CD28 ligands and IL-12, DCs have recently been shown 2 3 to express the ligand for the CD4 activation antigen, OX40. Both OX40 and its ligand are upregulated during CD4 T cell priming 4, and a model that illustrates the putative costimulatory events is shown in Fig. 1.
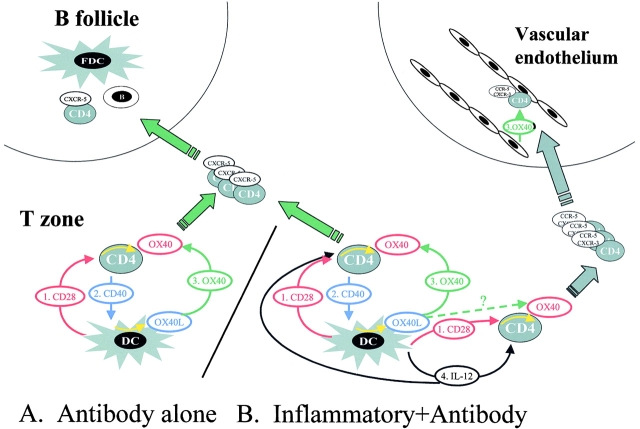
Figure 1.
Schematic representation of (A) immune responses evoked by protein antigens that do not promote IL-12 in DCs and (B) immune responses (e.g., bacteria) that do. (A) Antigen-activated CD4 T cells are initially costimulated through CD28 by CD86 expressed on DCs 1. This CD28 signal upregulates (yellow arrow indicates intracellular signals) OX40 (red color indicates CD28 dependence). Activated CD4 T cells express CD40L 2 and activate DCs (yellow arrow) to express OX40L (blue color indicates CD40L dependence). Synergistic costimulation through CD28 and OX40 leads to rapid expansion of CD4 T cells on the basis of their ability to recognize antigen presented by DCs (green color). These CD4 cells upregulate expression of the cytokine IL-4 and the chemokine CXCR-5, and CD4 T cells migrate to B follicles to foster GCs. (B) The situation is identical for inflammatory antigens, except that CD4 T cells are signaled by IL-12 (4; black color) in addition to CD28 1. These antigens generate CD4 cells that help both antibody and inflammatory responses, perhaps because IL-12 upregulates inflammatory chemokine receptors. Whether an individual CD4 T cells becomes committed to help B cells or inflammatory responses may depend on the strength of the signal through OX40L 3. This model predicts that high-affinity CD4 cells are selected to migrate to B follicles. If CD4 cells express inflammatory chemokines, they migrate into the bloodstream, where OX40L on vascular endothelium directs their migration into tissue 3.
Role of OX40 and CD28 in Priming CD4 Cells: Rapid Expansion in the T Zone.
The CD28 ligand, CD86, is constitutively expressed on mature DCs, so all antigen-specific CD4 T cells receive an initial CD28 signal. In contrast, expression of the T cell activation antigen OX40 on naive CD4 T cells is largely CD28 dependent, both in vivo and in vitro 5. Although others have suggested that OX40 expression is CD28 independent 6, these studies looked at total rather than naive CD4 T cell responses. This is critical, as activated T cells appear to secrete cytokines that can both promote the survival of naive CD4 T cells and upregulate OX40 (unpublished observations).
After activation, CD4 T cells reciprocally activate DCs through CD40. In vitro, CD40 ligand (CD40L) upregulates the expression of IL-12 in cultured DCs 7, but this may not happen in vivo for the following reason: CD4-dependent recognition of foreign proteins presented by DCs evokes a primary CD4 T cell cytokine response that is almost exclusively IL-4 and is associated with the production of IgG1 (Th2)- but not IgG2a (Th1)-specific antibodies 1. If IL-12 is produced by CD40L-activated DCs during responses to protein antigens, it is not sufficient to bias the CD4 response to Th1 cytokines and IgG2a antibody production. This contrasts with bacterial antigens that activate DCs to express IL-12 expression in vivo, evoke CD4 Th1 IFN-γ expression, and switch B cells to make IgG2a antibodies.
CD40-activated but not freshly isolated DCs do express the TNF family member OX40L in mice 3 and humans 2, and mRNA for OX40L and OX40 is upregulated in lymph nodes around the time of CD4 priming 4. In human tonsil, OX40 protein is most strongly expressed on CD4 T cell blasts in the T zone 8, which is compatible with activated CD4 T cells receiving OX40 signals when they are primed on DCs.
This suggests a sequential model of naive CD4 T cell activation with OX40 expression being dependent on an initial CD28 signal. Subsequent activation of DCs through CD40 by CD4 T cell CD40L in turn upregulates DC OX40L. OX40 and CD28 signals are highly synergistic for CD4 T cell proliferation and survival 9. In this issue, Murata et al. 10 report that OX40L-deficient mice have impaired CD4 T cell priming. In contrast, constitutive expression of OX40L on DCs 3 is associated with the opposite phenotype, with increased numbers of activated/memory CD4 T cells 6. It seems likely that during normal CD4 T cell priming, the combination of costimulation through CD28 and OX40 is responsible for the rapid expansion of antigen-specific CD4 blasts that is observed in the T zone, peaking around day 7 11 12.
Role of CD28 and OX40 in Directing CD4 T Cell Migration to B Follicles.
After immunization with soluble protein antigens, rapid expansion of antigen-specific cells in the T zone is accompanied by their selective migration to B follicles, and this coincides with the in vivo expression of CXCR-5 13. This chemokine receptor is responsible for localizing lymphocytes in B follicles 14, where the ligand is expressed 15. A key point concerning recall CD4 responses is that most primed CD4 cells are located in B follicles and GCs by day 10. Signaling through CD28 and OX40 upregulates mRNA for CXCR-5 in vitro 4, and CD28 signaling is required to generate the CXCR-5 subset of memory CD4 T cells in vivo 5. The lack of CXCR-5–positive CD4 T cells in CD28-deficient mice is directly correlated with their failure to form GCs 16. Whereas CD28 signaling is absolutely required for the generation of CD4 T cell help for GCs, an IL-12–dependent Th1 inflammatory CD4 immune response to the intracellular pathogen Leishmania is not CD28 dependent 17.
The migration of CXCR-5–positive CD4 cells into B follicles is mediated in part by CD28-dependent OX40 signals. Constitutive expression of OX40L on DCs leads to a CD28-dependent increase in the numbers of CXCR-5 CD4 cells 5, greatly increased numbers of CD4 T cells within B follicles, and large GCs 3. Preliminary analysis of OX40-deficient mice shows the opposite phenotype, with GC formation being reduced (unpublished observations). These data are consistent with two hypotheses that are not mutually exclusive: (a) OX40 signals, because they synergize with CD28 signals, are required for the optimal expansion of CD4 T cells that subsequently migrate to B follicles; and (b) CD28 signaling is permissive for a second OX40-independent costimulatory event that directs expression of CXCR-5–positive CD4 T cells to follicles (OX40-deficient mice can make GCs).
The data on OX40L-deficient mice in this issue are consistent with the latter hypothesis. Murata et al. 10 have looked at day 9 recall CD4 responses when the majority of primed CD4 T cells in normal mice are “helping” B cells in GCs. The in vitro CD4 recall response from T cells primed in OX40L-deficient mice hardly depends on the expression of OX40L upon restimulating APCs (Murata et al., compare Figure 6 A and Figure 6 B). In marked contrast, the CD4 memory response in OX40L-sufficient mice is highly OX40L dependent (Murata et al., Figure 6 C). This is compatible with OX40-dependent and -independent CD4 memory pools.
The accompanying paper on OX40 in this issue by Akiba et al. 18 also raises some interesting points in this regard. OX40 blockade promoted rather than inhibited an inflammatory CD4 response to the intracellular pathogen, Leishmania. In contrast, there was a selective deficit in Th2 B cell help for IgG1- and IgE-specific antibody production to Leishmania antigens, suggesting that CD4 B cell help was OX40L dependent.
CD28-dependent OX40 Signaling of CD4 Cells during DC Priming Biases Cytokine Production to Th2 but Promotes Migration of Both Th1 and Th2 Cells to B Follicles.
In vitro activation of naive CD4 T cells through CD28 and OX40 promotes expression of IL-4 4 19. Th2 CD4 cells support GC development more effectively than Th1 CD4 cells, although Th1 and Th2 cells are effective in combination 20. This point emphasizes how OX40L signals optimize B cell help. It is not clear how OX40 signals IL-4 production; it may simply cause preferential survival of cells that have started to express IL-4.
Direct evidence that OX40 signals facilitate Th2 development in vivo is seen in the paper by Akiba et al. 18, where OX40 blockade of Leishmania-infected BALB/c mice inhibits production of Th2-associated antibody isotypes IgG1 and IgE and favors inflammatory CD4 Th1 responses. The generation of protective Th1 inflammatory CD4 T cell responses in C57BL/6 mice is also independent of OX40 signaling. This is somewhat contradicted by Murata et al. 10, who show reduced production of the Th1 cytokine, IFN-γ, in OX40L-deficient mice. I think the confusion arises because Murata et al. fail to distinguish Th1 CD4 help for B cells and Th1 CD4 help for inflammatory responses. The Freund's complete adjuvant used by Murata et al. is a potent Th1 immunogen for antibody responses. By performing CD4 recall responses 9 d after immunization, when most antigen-specific CD4 cells are in B follicles, they are mostly looking at CD4 Th1 cells that have been primed to help B cells. Just as OX40L primes the migration of Th2 CD4 cells to follicles, I think the data are consistent with OX40L promoting Th1 B cell help. DC subsets that differ in their IL-12 expression and their capacity to foster Th1 antibody responses have recently been described 21 22. These papers suggest that Th1 and Th2 CD4 B cell help is primed by different DC subsets. However, Murata et al. 10 have not examined an inflammatory CD4 response, so on the available data they cannot conclude that OX40 signals promote inflammatory Th1 CD4 T cells.
Strong Synergy between CD28 and OX40 Signals May Be an Effective Mechanism for Selecting High-Affinity CD4 T Cells and Promoting Their Migration to B Follicles.
Unlike CD86 that is constitutively expressed on DCs, costimulation of CD4 T cells through OX40 depends on how effectively individual CD4 T cells solicit OX40L expression on DCs. I think it is plausible that high-affinity CD4 cells, by costimulating DCs more effectively through CD40, receive a more potent reciprocal OX40L signal than low-affinity CD4 cells. As CD28 and OX40 signals are synergistic, this would provide a mechanism to selectively expand high-affinity CD4 cells and favor their migration to B follicles. This model of costimulation explains experimental data that shows that high-affinity CD4 T cells are expanded in the T zone 23 and then selectively exported to B follicles to foster GCs 24.
Immune responses to most bacterial antigens elicit both CD4 help for antibody responses and inflammatory CD4 T cells. The model presented here predicts that high-affinity CD4 T cells, by virtue of their capacity to be more strongly signaled through OX40, are more likely to expand rapidly, secrete IL-4, and migrate to B follicles to foster GCs than are low-affinity CD4 cells. This argument might explain at least some of the data linking antigen dosage in transgenic CD4 T cells with differences in Th1 and Th2 commitment 25.
IL-12 Primes Inflammatory CD4 T Cells That Are Signaled to Migrate into Tissue via OX40L Signals from Vascular Endothelium.
The main argument presented above has been that CD28 and OX40 signals by DCs coordinate the selection, preferential but not exclusive Th2 differentiation, and migration of CD4 cells to B follicles to foster GC development. How can this explain why OX40 blockade attenuates inflammatory diseases like experimental allergic encephalitis (EAE; reference 26) and hapten-induced colitis 27? The clue is that OX40L is expressed on vascular endothelium 28. The critical role of IL-12 but not IFN-γ in the generation of inflammatory CD4 cells has recently been highlighted 29. Just as CD28 and OX40 program the expression of CXCR-5 that directs primed CD4 cell migration to B follicles, it seems likely that IL-12 induces expression of inflammatory chemokine receptors. Normally, activated inflammatory CD4 T cells migrate via the efferent lymphatics into the bloodstream under the influence of chemokines and adhere to blood vessels. It seems probable that the effects of OX40 blockade in EAE 26 and hapten-induced colitis 27 are because OX40 signals on endothelium promote inflammatory CD4 T cell migration into tissue 27. As OX40 expression (and therefore migration) is CD28 dependent, CD28-deficient mice are also not susceptible to EAE, although CD4 T cells are primed 30. This does not exclude a role for OX40 signals by DCs in expanding inflammatory CD4 T cells in combination with IL-12. However, it is clear from the data of Akiba et al. 18 that OX40 blockade facilitates rather than inhibits the development of a Th1 inflammatory CD4 response, whereas it clearly attenuates Th2 cell help for B cells. One final point is that OX40 blockade did not prevent CD4 T cells from controlling footpad infections with Leishmania in BALB/c mice. Perhaps this infection causes local inflammation that allows OX40-independent CD4 T cell migration into tissues.
New Experiments.
These new and interesting data on OX40 raise several points and suggest many new avenues of inquiry into the function of OX40. First, How does OX40 regulate CD4 inflammation? The role of OX40L expressed on vascular endothelium needs to be dissected from expression on DCs and other cells. This could be addressed using chimeric mice in which the bone marrow–derived cells are OX40L sufficient (normal) but the endothelial cells are OX40L deficient. The reverse experiment would test whether OX40L-deficient DCs could prime an inflammatory CD4 T cell response if OX40L was expressed on endothelium. These experiments would address whether OX40L plays a significant role in the priming of inflammatory CD4 T cells as distinct for modifying migration of inflammatory CD4 cells into tissues.
Second, Are there OX40-dependent and independent CD4 memory subsets? The experimental data of Murata et al. 10 indicate that this might be so. If confirmed, it would be very interesting to define the cytokines and chemokine receptors expressed by these CD4 T cell subsets.
Third, the synergy between OX40 and CD28 provides a potential mechanism for selecting CD4 T cells on the basis of their affinity. The CD4 T cell response to pigeon cytochrome C model is well characterized and would provide an excellent system in which to test this possible mechanism 11 23. It would be very interesting to establish whether the impaired CD4 T cell memory reported here in OX40L-deficient mice was linked with a failure to efficiently expand high-affinity CD4 T cells and export them to B follicles.
Conclusions.
In this commentary, I have cited evidence that CD28-dependent OX40 signals from DCs coordinate the selection, migration, and cytokine differentiation of both Th1 and Th2 CD4 T cell help for B cell GCs. Although OX40L signals bias cytokine production to Th2, this does not exclude OX40L, in combination with IL-12 priming, Th1 B cell help. I think the preferential involvement of these costimulatory molecules in the GC pathway is because there has been strong selection for rapid onset neutralizing antibody responses that are GC dependent. High-affinity CD4 cells by virtue of their capacity to activate and select B cell somatic variants are well suited to this role. Evidence for the priming of CD4 inflammatory cells by OX40 signals is less convincing. The role of OX40 signals in inflammatory CD4 responses may be restricted to directing their migration from vascular endothelium into tissues.
Acknowledgments
This work was supported by grants from the Wellcome Trust to P. Lane.
References
- Toellner K.M., Luther S.A., Sze D.M., Choy R.K., Taylor D.R., MacLennan I.C.M., Acha-Orbea H. Th1 and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J. Exp. Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Tanaka Y., Tozawa H., Takahashi Y., Maliszewski C., Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Brocker T., Gulbranson-Judge A., Flynn S., Riedinger M., Raykundalia C., Lane P. CD4 T cell traffic controlin vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells into B follicles. Eur. J. Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Flynn S., Toellner K.-M., Raykundalia C., Goodall M., Lane P. CD4 T cell cytokine differentiationthe B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.S.K., Gulbranson-Judge A., Flynn S., Brocker T., Raykundalia C., Goodhall M., Forster R., Lipp M., Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5–positive CD4 cells and germinal centers. J. Exp. Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba H., Oshima H., Takeda K., Atsuta M., Nakano H., Nakajima A., Nohara C., Yagita H., Okumura K. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J. Immunol. 1999;162:7058–7066. [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehman K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell costimulatory capacityT–T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkop H., Latza U., Himmelreich P., Stein H. Expression of the human OX40 (hOX40) antigen in normal and neoplastic tissues. Br. J. Haematol. 1995;91:927–931. doi: 10.1111/j.1365-2141.1995.tb05413.x. [DOI] [PubMed] [Google Scholar]
- Gramaglia I., Weinberg A.D., Lemon M., Croft M. Ox-40 liganda potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- Murata K., Ishii N., Takano H., Miura S., Ndhlovu L.C., Nose M., Noda T., Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 1999;191:365–374. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbranson-Judge A., MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome C. Eur. J. Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- Zheng B., Han S., Zhu Q., Goldsby R., Kelsoe G. Alternative pathways for the selection of antigen-specific peripheral T cells. Nature. 1996;384:263–266. doi: 10.1038/384263a0. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., McHeyzer-Williams L.J., Ngo V.N., McHeyzer-Williams M.G., Cyster J.G. In vivo–activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. A putative chemokine receptor, blr1, directs B-cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Ngo V.N., Ansel K.M., Ekland E.H., Cyster J.G., Williams L.T. A B-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma type receptor-1. Nature. 1998;391:799–802. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Lane P., Burdet C., Hubele S., Scheidegger D., Müller U., McConnell F., Kosco-Villbois M. B cell function in mice transgenic for mCTLA4-H gamma 1lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+ T cells. J. Exp. Med. 1994;179:819–830. doi: 10.1084/jem.179.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.R., Green J.M., Moskowitz N.H., Davis M., Thompson C.B., Reiner S.L. Limited role of CD28-mediated signals in T helper subset differentiation. J. Exp. Med. 1996;184:803–810. doi: 10.1084/jem.184.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba H., Miyahira Y., Atsuta M., Takeda K., Nohara C., Futagawa T., Matsuda H., Aoki T., Yagita H., Okumura K. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J. Exp. Med. 1999;191:375–380. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Yang L.P., Uchiyama T., Tanaka Y., Baum P., Sergerie M., Hermann P., Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- Secord E.A., Rizzo L.V., Barroso E.W.S., Umetsu D.T., Thorbecke G.J., DeKruyff R.H. Reconstitution of germinal center formation in nude mice with Th1 and Th2 clones. Cell. Immunol. 1996;174:173–179. doi: 10.1006/cimm.1996.0307. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8α1 and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams L.J., Panus J.F., Mikszta J.A., McHeyzer-Williams M.G. Evolution of antigen-specific T cell receptors in vivopreimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J. Exp. Med. 1999;189:1823–1838. doi: 10.1084/jem.189.11.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Han S.H., Kelsoe G. T helper cells in murine germinal centers are antigen-specific emigrants that downregulate Thy-1. J. Exp. Med. 1996;184:1083–1091. doi: 10.1084/jem.184.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant S., Pfeiffer C., Woodard A., Pasqualini T., Bottomly K. Extent of T-cell receptor ligation can determine the functional differentiation of naive CD4(+) T cells. J. Exp. Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A.D., Wegmann K.W., Funatake C., Whitham R.H. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J. Immunol. 1999;162:1818–1826. [PubMed] [Google Scholar]
- Higgins L.M., McDonald S.A., Whittle N., Crockett N., Shields J.G., MacDonald T.T. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interactionamelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J. Immunol. 1999;162:486–493. [PubMed] [Google Scholar]
- Imura A., Hori T., Imada K., Ishikawa T., Tanaka Y., Maeda M., Imamura S., Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J. Exp. Med. 1996;183:2185–2191. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B.M., Dwyer B.K., Shevach E.M. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J. Exp. Med. 1998;187:537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.T., Jabs C., Sobel R.A., Kuchroo V.K., Sharpe A.H. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J. Exp. Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]