Abstract
To gain insights into the mechanisms by which soluble heat shock protein (hsp) fusions can elicit CD8+ cytotoxic T lymphocytes (CTLs) against the fusion partner, mycobacterial (Mycobacterium tuberculosis) hsp70 was dissected to ascertain whether a particular hsp domain is necessary, and knockout mice were used to determine whether the fusion protein's immunogenicity is dependent on CD4+ T lymphocytes. We found that the ability to elicit CD8+ CTLs depends on a discrete 200–amino acid protein domain, indicating that the fusion protein's immunogenicity for CD8+ T cells does not require coupled chaperone function or peptide binding. Further, we found that ovalbumin (OVA).hsp70 fusion protein elicited anti-OVA CD8+ CTLs about equally well in CD4 knockout and wild-type C57BL/6 mice, and also when the hsp70 was of murine (self) origin. The ability of hsp70 fusion proteins to elicit CD4-independent CTL responses suggests that hsp70 fusion proteins may be useful for immunological prophylaxis and therapy against disease in CD4+ T cell–deficient individuals.
Keywords: CD8+, CD4+, domain, knockout, vaccine
Introduction
There is now substantial evidence that heat shock proteins (hsps) isolated from tumors can be used as adjuvant-free antitumor vaccines in animals; hsp70 and the distantly related chaperones gp96 and calreticulin share this immunostimulatory activity 1 2 3 4 5 6. The fusion of large polypeptides (80–110 amino acids [aa] in length) to mycobacterial hsp70 (TBhsp70) creates potent immunogens that can elicit MHC class I–restricted, CD8+ CTL responses sufficient to mediate rejection of tumors expressing the fusion partner 7. The need for more effective immunological prophylaxis and therapy for cancer and infectious diseases caused by intracellular pathogens has spurred intense investigation of immunogens and immunization strategies aimed at eliciting effective CD8+ CTL responses. For example, CTL epitopes have been expressed in the context of Ty virus–like particles, or tailed with lipids 8. Antigenic peptides have been fused to toxins 9 10 11, cytokines 12 13, and proteins that cross cell membranes, such as HIV-1 Tat 14, and hsp70 7.
The means by which soluble hsp70 fusion proteins stimulate CD8+ CTL responses are unknown. Among the possible mechanisms are (a) strong hsp-specific CD4+ helper cell responses that enhance what might otherwise be a minimal response to the soluble proteins 15 16 17 18, and (b) the chaperone function of hsps that delivers the fusion protein to intracellular compartments of APCs for processing into short peptides and loading onto MHC class I 19 20. We demonstrate here that hsp70 fusion proteins can elicit CD8+ CTLs in the absence of CD4+ T lymphocytes and that this function resides in a 200-aa segment of TBhsp70, indicating that chaperone activity is not required.
Materials and Methods
Expression Vectors.
All constructs used to produce OVA.hsp70 fusion proteins were made in the bacterial expression plasmid pKS11h 7. Fusion constructs, consisting of OVA fused to the NH2 terminus of various segments of hsp70, were inserted downstream of the histidine tag sequence. A portion of OVA (aa 230–359) was amplified from pOV230 21 by PCR using upstream primer oQH025 and the downstream primer oQH027 (the sequences of these and other PCR primers are listed at the end of Materials and Methods). The OVA expression vector pQH07 was constructed by subcloning OVA into the NdeI and NheI sites of pKS11h. Full-length TBhsp70 and four truncated TBhsp70 segments I (aa 1–166), II (aa 161–370), III (aa 360–517), and IV (aa 510–625), were amplified from plasmid pY3111/8 (gift of W. Wu, StressGen Biotechnologies, Victoria, Canada). The upstream primer for the full-length TBhsp70 and segment I is oQH001, and the downstream primers are oJR061 and oQH011, respectively. The upstream primers for TBhsp70 II, III, and IV are oQH012, oQH014, and oQH016, respectively; the downstream primers are oQH013, oQH015, and oJR061, respectively. The plasmids pQH06, pQH08, pQH09, pQH10, and pQH11, which express OVA fused to TBhsp70, TBhsp70 I, II, III, and IV, respectively, were constructed by subcloning the full-length and truncated TBhsp70 PCR products into the BamHI and EcoRI sites of pQH07 (at the COOH terminus of OVA). Murine hsp70.1 coding sequence (mhsp70) was amplified from plasmid pmhsp70.1 by PCR using the upstream primer oJR102 and the downstream primer oJR103. Plasmid pQH12, expressing OVA. mhsp70 fusion protein, was created by subcloning mhsp70 into the BamHI and EcoRI sites of pQH07. All plasmids were verified by sequencing in both directions with double-stranded DNA templates.
Recombinant Protein Purification.
OVA, OVA.TBhsp70, OVA. TBhsp70 II, OVA.TBhsp70 III, and OVA.TBhsp70 IV were induced in Escherichia coli (BL21[DE3]pLysS) for 9 h at 25°C in the presence of 0.5–1 mM isopropyl thiogalactoside (IPTG), and were purified as soluble proteins. The mycobacterial segment I and murine hsp70 fusion proteins were induced in E. coli for 4 h at 37°C with 1 mM IPTG, purified from inclusion bodies, and then refolded as described previously 7 16. All proteins were purified using nitrilo-triacetic acid Ni+ column (Qiagen) and HiTrap® Q anion exchange chromatography (Amersham Pharmacia Biotech), as described previously 7 16. Purity was assessed using 4–20% gradient SDS-PAGE gels stained with Coomassie blue (Bio-Rad). All proteins were dialyzed against PBS and sterile filtered at 0.2 μM. Protein concentrations were measured by Lowry assay (Bio-Rad) and expressed in molar terms to allow simple comparison of proteins of differing molecular weights.
Mice and Immunizations.
6–8-wk-old female C57BL/6, CD4−/−, and β2-microglobulin (β2m)−/− mice were obtained from The Jackson Laboratory and Taconic Farms. Both knockout mice have C57BL/6 (H-2b) genetic backgrounds. Groups of three or four mice were injected intraperitoneally with 120 pmol of recombinant protein in PBS; a second injection was performed subcutaneously 2 wk later. The mice were killed 10 d after the boost, and splenocytes within groups were pooled 7.
Cell Line.
EG7-OVA cells were cultured as described previously 7.
CTL Assays.
CTL assays were performed as described 7. Results shown are representative of experiments repeated two to five times.
The PCR primer sequences were as follows: for oQH025, 5′-GCAGTACTCATATGATCCTGGAGCTTCCATTTGCCAGTGGGACAATG-3′; for oQH027, 5′-CTCCGACCTCACCTACGACGTTCGCAGAGACTTCTTAAAATTATCCGATG-CTAGACCTAGT-3′; for oQH001, 5′-ATAGTACTGGATCCATGGCTCGTGCGGTCGGGATCGACCTCGGG-3′; for oJR061, 5′-GGAATTCCTATCTAGTCACTTGCCCTCCCGGCCGTC-3′; for oQH011, 5′-GTCGACGAATTCATCATCAGATCGCGCTCTTCTCGCCCTTGTCGAG-3′; for oQH012, 5′-GTCGACGGATCCATGGAGAAGGAGCAGCGAATCCT-GGTCTTCGACTTG-3′; for oQH014, 5′-GTCGACGGATCCATGGTGAAAGACGTTCTGCTGCTTGATGTTACCCCG-3′; for oQH016, 5′-GTCGACGGATCCATGCGTAATCAAGCCGAGACATTGGTCTACCAGACG-3′; for oQH013, 5′-GTCGACGAATTCATCACGGGGTAACATCAAGCAGCAGAAC-GTCTTTCAC-3′; for oQH015, 5′-GTCGACGAATTCATCA-GACCAATGTCTCGGCTTGATTACGAACATCGGC-3′; for oJR102, 5′-TCTAGAGGATCCATGGCCAAGAACACGGCGATC-3′; and for oJR103, 5′-TCTAGAGAATTCCTAATCCACCTCCTCGATGGTGGGTCC-3′.
Results and Discussion
Our previous studies demonstrated that soluble, adjuvant-free TBhsp70 fusion proteins elicit substantial immune responses, including CD8+ CTLs, in mice 7 16. The basis for the effectiveness of hsp70 fusions is unclear, as most soluble proteins do not elicit significant CD8+ T cell responses (for reviews, see references 22 and 23). Although there is evidence that the hsp moiety of TBhsp fusion proteins acts as an effective carrier in the classic sense, enhancing B cell responses to chemically conjugated pneumococcal polysaccharides 18 and malarial polypeptide 15, carriers are not known to stimulate CTL production. We thought it more likely that hsp70 fusion proteins provide hsp70-specific cognate CD4+ T cell help to OVA-specific CD8+ CTLs by activating shared professional APCs, as suggested by many and demonstrated recently 24 25 26.
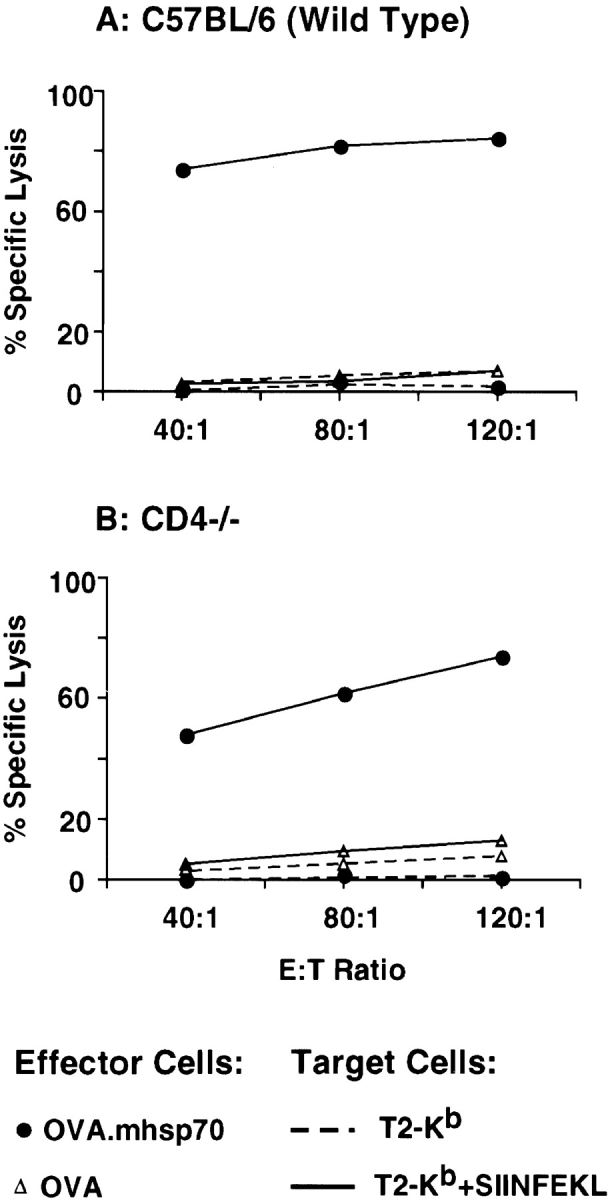
We tested this cognate help hypothesis using CD4-deficient (knockout) mice (CD4−/−). Wild-type C57BL/6, CD4−/−, and β2m−/− mice were each immunized with OVA or OVA.TBhsp70 fusion protein. As expected, immunization of wild-type mice with OVA.TBhsp70, but not OVA, generated CTLs specific for the immunodominant epitope of OVA (SIINFEKL; Fig. 1 A). The same results were obtained when the CD4−/− mice were immunized with OVA.TBhsp70 (Fig. 1 B). β2m−/− mice, which have very few CD8+ T cells, did not develop OVA-specific CTLs after immunization with OVA.TBhsp70 or with OVA alone (Fig. 1 C).
Figure 1.
OVA-specific CTLs elicited by immunization with OVA.TBhsp70 fusion protein without adjuvant. The splenocytes from mice immunized with OVA (▵) or OVA.TBhsp70 (▪) were incubated with irradiated EG7-OVA cells for 6 d in the absence of added cytokines, and then used as effector cells (E) in a standard 4-h cytotoxicity assay. The 51Cr-labeled target cells (T) were T2-Kb (dashed line) and T2-Kb pulsed with SIINFEKL peptide (solid line) at 33 μg/ml. (A) Splenocytes from wild-type C57BL/6 mice. (B) Splenocytes from CD4−/− mice. (C) Splenocytes from β2m−/− mice (this β2m−/− experiment was not repeated).
Previous efforts to determine whether CD4+ T cell help is necessary for generation of CD8+ CTLs have drawn differing conclusions. CD4 knockout mice exhibit a range of CD8+ CTL responses: CD4-dependent, weakly dependent, or independent. CTL responses to minor histocompatibility antigens 25 27 or to OVA loaded into spleen cells 28 are CD4 dependent. Some potent CD8+ T cell immunogens, including viruses 29 such as lymphocytic choriomeningitis virus 30 31 32 33, ectromelia virus 34, and some influenza virus subtypes 35, as well as allogeneic cells 36, elicit strong CD8+ T cell responses in wild-type and CD4−/− mice. The CD4 independence of the CTL response seen in Fig. 1 B does not exclude some role for CD4+ help in normal mice immunized with hsp70 fusion proteins. Although it is possible that CD4−/CD8− double-negative T cells provide weak residual help in CD4−/− mice, it is unlikely that such weak help is responsible for the anti-OVA CTLs seen in Fig. 1 B. The similarity of CD8+ CTL responses to OVA.TBhsp70 in CD4−/− and wild-type mice suggests that hsp70 fusion proteins are relatively potent CD8+ CTL immunogens. A similar result, showing that CD4+ T cells are not required for the CD8+ CTL response elicited by another mycobacterial heat shock fusion protein (hsp65 fused to a polypeptide containing an epitope for 2C CD8+ T cells), has also been obtained using CD4−/− mice (Cho, B., personal communication). In addition, the ability of a nonhomologous hsp, gp96, to elicit tumor rejection requires CD4+ T cells at tumor challenge, but not during priming with tumor-derived gp96 2.
It has been proposed that the immunostimulatory effects of certain hsp fusion proteins may be due to the bacterial origin of the hsp moiety 20. We examined this possibility by making OVA.hsp70 fusion proteins with the murine homologue of TBhsp70 37, here referred to as mhsp70. Immunization of wild-type C57BL/6 mice with OVA.mhsp70, but not OVA, elicited CTL responses equivalent to those generated by the TBhsp70 fusion protein (Fig. 2 A). The response to OVA.mhsp70 was also independent of CD4 (Fig. 2 B). Since a CD4+ T cell response to self (murine) hsp70 is unlikely, the effectiveness of the murine hsp70 fusion protein is in accord with the more direct evidence for CD4 independence obtained using CD4−/− mice (see above).
Figure 2.
Murine hsp70 fusion protein elicits CTL responses in wild-type and CD4−/− mice. Soluble OVA.mhsp70 (•) was injected without adjuvant into C57BL/6 mice (A) and CD4−/− mice (B).
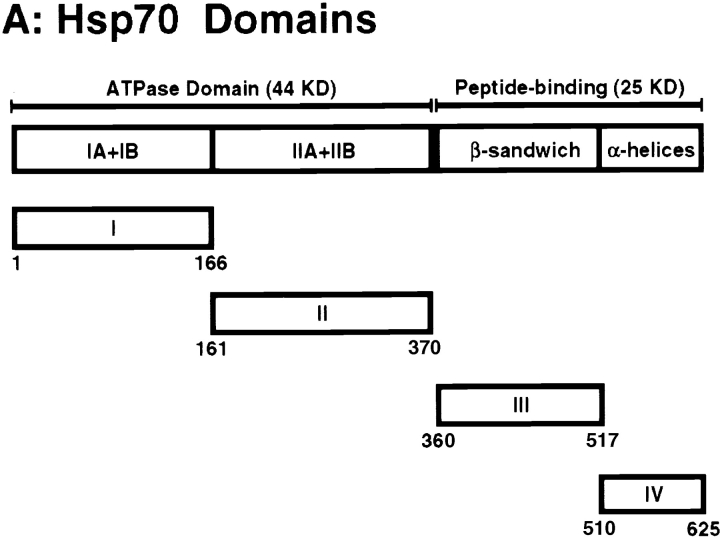
The ability of hsp fusion proteins to elicit CTLs against the fusion partner may be a consequence of the hsp moieties' chaperone activity, assuming that this activity is preserved in the fusion protein. To investigate this issue, we divided TBhsp70 into four linear segments and fused OVA to the NH2 terminus of each segment, creating OVA.TBhsp70s I–IV (Fig. 3 A). Each segment corresponds to a distinct structural domain of hsp70, as described by Flaherty et al. 38 and Zhu et al. 39. As shown in Fig. 3 A, the NH2-terminal ATP-binding domain was divided into its two structural lobes: I (aa 1–166) and II (aa 161–370). The COOH-terminal peptide-binding domain was divided into a β sandwich domain, III (aa 360–517), and an α helical domain, IV (aa 510–625).
Figure 3.
Production of OVA.TBhsp70 fusion proteins using TBhsp70 segments. (A) Functional and structural domains of TBhsp70 based on crystal structures of the ATPase domain of bovine hsp70 (reference 38) and the peptide-binding domain of E. coli DnaK (reference 39). The full-length TBhsp70 was separated into four segments, I, II, III, and IV. The full-length TBhsp70 and each segment were fused to the COOH terminus of OVA to make OVA.TBhsp70 fusion proteins. The numbers beneath each segment refer to the aa positions in TBhsp70. (B) Purification of OVA.TBhsp70 fusion proteins. Purity was assessed using SDS-PAGE and visualized with Coomassie blue. Each lane was loaded with ∼4 μg of protein. The numbers at left represent molecular weight markers (in kD).
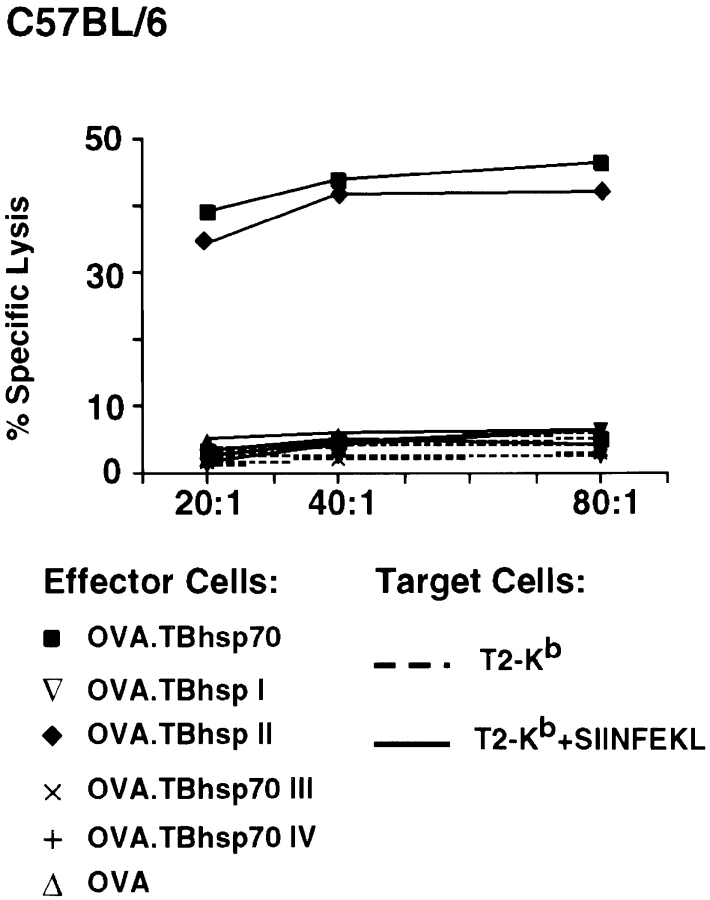
Six groups of three C57BL/6 mice were immunized with 120 pmol of OVA, OVA.TBhsp70, and OVA fused to segments I, II, III, and IV. CTL assays showed that splenocytes from mice immunized with OVA.TBhsp70 and OVA fused to segment II lysed T2-Kb cells in the presence, but not absence, of the OVA Kb epitope, SIINFEKL (Fig. 4). In contrast, cells from mice immunized with OVA and OVA fused to segments I, III, and IV were ineffective, even at an E/T ratio of 80:1. Levels of cytolysis obtained with splenocytes from mice immunized with OVA.TBhsp70 and OVA fused to segment II were indistinguishable (Fig. 4). These results show that half of the ATP-binding domain of TBhsp70 (aa 161–370) is sufficient to stimulate substantial production of anti-OVA CTL response in the absence of adjuvant.
Figure 4.
Examination of OVA-specific T cell responses in mice immunized with OVA fused to domains of TBhsp70. Splenocyte cultures from mice primed with OVA (▵), OVA.TBhsp70 (▪), OVA.TBhsp70 I (▿), II (♦), III (x), and IV (+) were used as effector cells in the cytotoxicity assay.
Since it is highly unlikely that segment II retains chaperone activity, we conclude that the ability of the fusion proteins to elicit CD8+ T cells does not depend on the hsp moieties' chaperone properties. How then can one account for the ability of these heat shock fusion proteins to act as CD8+ CTL immunogens? Our data would support a model in which hsp70 bypasses the need for CD4+ help by directly or indirectly activating or affecting the maturation state of APCs, such as dendritic cells, in a manner similar to some viruses 40. According to this model, hsp70 fusion proteins may activate a few CD8+ T cells to release immunostimulatory cytokines in draining LNs. These cytokines may, in turn, provide the help required to upregulate expression of costimulatory molecules on APCs in the LN, leading to further CD8+ T cell activation 40. Recent studies demonstrate that exposure of macrophages to bacterial and human hsp60 41 42, murine hsp70, and gp96 3 43 increases expression of adhesion molecules and cytokines. We are currently examining expression of costimulatory molecules, adhesion molecules, and cytokines by APCs after exposure to fusion proteins made with full-length hsp70 or segment II.
Whatever the underlying mechanism, the ability of hsp70 fusion proteins to elicit CTL responses in the absence of CD4+ cells suggests that hsp70 may be a useful vehicle for the development of prophylaxis and therapy of HIV-1 and its opportunistic infections. Infection by HIV and its cousin simian immunodeficiency virus (SIV) can lead to a substantial reduction in CD4+ T cells, thereby crippling the host's immune response to HIV and other pathogens. This loss of CD4+ cells is thought to impair the development and maintenance of CD8+ CTL responses 44. Recent studies conclude that strong HIV-specific CTL responses are required to keep HIV-1 infection in check and to destroy HIV-infected cells 45 46 47 48 49 50. It will thus be of interest to determine whether hsp70 fusion constructs can elicit anti-SIV CTL responses in SIV-infected macaques having low CD4+ T cell counts, and if similar effects are observed in HIV-infected humans.
Acknowledgments
We are most grateful to Carol McKinley for her generous and expert assistance with CTL assays. We thank Susan Byrne for her help with protein purification. We also thank Nir Hacohen and Jerry Nau for their many valuable discussions and insights.
This work was supported by National Institutes of Health grants AI44476 and AI44477 and by StressGen Biotechnologies.
Footnotes
Q. Huang and J.F.L. Richmond contributed equally to this work.
References
- Udono H., Srivastava P.K. Heat shock protein 70–associated peptides elicit specific cancer immunity. J. Exp. Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H., Levey D.L., Srivastava P.K. Cellular requirements for tumor-specific immunity elicited by heat shock proteinstumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc. Natl. Acad. Sci. USA. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto R., Srivastava P.K. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Blanchere N.E., Li Z., Chandawarkar R.Y., Suto R., Jaikaria N.S., Basu S., Udono H., Srivastava P.K. Heat shock protein–peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J. Exp. Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y., Peng P., Kang L., Daou M., Srivastava P.K. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Nair S., Wearsch P.A., Mitchell D.A., Wassenberg J.J., Gilboa E., Nicchitta C.V. Calreticulin displays in vivo peptide-binding activity and can elicit CTL responses against bound peptides. J. Immunol. 1999;162:6426–6432. [PubMed] [Google Scholar]
- Suzue K., Zhou X., Eisen H.N., Young R.A. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc. Natl. Acad. Sci. USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp C.E., Plebanski M., Gilbert S., Sinden R.E., Harris S., Frankel G., Dougan G., Hioe C., Nixon D., Paoletti E. Comparison of numerous delivery systems for the induction of cytotoxic T lymphocytes by immunization. Eur. J. Immunol. 1996;26:1951–1959. doi: 10.1002/eji.1830260841. [DOI] [PubMed] [Google Scholar]
- Ballard J.D., Collier R.J., Starnbach M.N. Anthrax toxin-mediated delivery of a cytotoxic T-cell epitope in vivo. Proc. Natl. Acad. Sci. USA. 1996;93:12531–12534. doi: 10.1073/pnas.93.22.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.A., Spellerberg M.B., Zhu D., Rice J., Sahota S.S., Thompsett A.R., Hamblin T.J., Radl J., Stevenson F.K. DNA vaccines with a single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat. Med. 1998;4:1281–1286. doi: 10.1038/3266. [DOI] [PubMed] [Google Scholar]
- Carbonetti N.H., Irish T.J., Chen C.H., O'Connell C.B., Hadley G.A., McNamara U., Tuskan R.G., Lewis G.K. Intracellular delivery of a cytolytic T-lymphocyte epitope peptide by pertussis toxin to major histocompatibility complex class I without involvement of the cytosolic class I antigen processing pathway. Infect. Immun. 1999;67:602–607. doi: 10.1128/iai.67.2.602-607.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker H.T., Umetsu D.T., De Kruyff R.H., Levy S. DNA vaccination with cytokine fusion constructs biases the immune response to ovalbumin. Vaccine. 1997;15:1687–1696. doi: 10.1016/s0264-410x(97)00088-1. [DOI] [PubMed] [Google Scholar]
- Kang B.Y., Lim Y.S., Chung S.W., Kim E.J., Hwang S.Y., Kim T.S. Antigen-specific cytotoxicity and cell number of adoptively transferred T cells are efficiently maintained in vivo by re-stimulation with an antigen/interleukin-2 fusion protein. Int. J. Cancer. 1999;82:569–573. doi: 10.1002/(sici)1097-0215(19990812)82:4<569::aid-ijc16>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kim D.T., Mitchell D.J., Brockstedt D.G., Fong L., Nolan G.P., Fathman C.G., Engleman E.G., Rothbard J.B. Introduction of soluble proteins into the MHC class I pathway by conjugation to an HIV tat peptide. J. Immunol. 1997;159:1666–1668. [PubMed] [Google Scholar]
- Barrios C., Lussow A.R., Van Embden J., Van der Zee R., Rappuoli R., Constantino P., Louis J.A., Lambert P.H., Del Giudice G. Mycobacterial heat-shock proteins as carrier molecules. IIThe use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guérin priming. Eur. J. Immunol. 1992;22:1365–1372. doi: 10.1002/eji.1830220606. [DOI] [PubMed] [Google Scholar]
- Suzue K., Young R.A. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J. Immunol. 1996;156:873–876. [PubMed] [Google Scholar]
- Horwitz M.S., Bradley L.M., Harbertson J., Krahl T., Lee J., Sarvetnick N. Diabetes induced by Coxsackie virusinitiation by bystander damage and not molecular mimicry. Nat. Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- Könen-Waisman S., Cohen A., Fridkin M., Cohen I.R. Self heat-shock protein (hsp60) peptide serves in a conjugate vaccine against a lethal pneumococcal infection. J. Infect. Dis. 1999;179:403–413. doi: 10.1086/314590. [DOI] [PubMed] [Google Scholar]
- Young R.A. Stress proteins and immunology. Annu. Rev. Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- Schild H., Arnold-Schild D., Lammert E., Rammensee H-G. Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr. Opin. Immunol. 1999;11:109–113. doi: 10.1016/s0952-7915(99)80019-3. [DOI] [PubMed] [Google Scholar]
- McReynolds L.A., Catterall J.F., O'Malley B.W. The ovalbumin genecloning of a complete ds-cDNA in a bacterial plasmid. Gene. 1977;2:217–231. [Google Scholar]
- Braciale T.J., Morrison L.A., Sweetser M.T., Sambrook J., Gething M.J., Braciale V.L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol. Rev. 1987;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Jondal M., Schirmbeck R., Reimann J. MHC class I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- Bennett S.R., Carbone F.R., Karamalis F., Flavell R.A., Miller J.F., Heath W.R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Ridge J.P., Di Rosa F., Matzinger P. A conditional dendritic cell can become a temporary bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Schoenberger S.P., Toes R.E., van der Voort E.I., Offringa R., Melief C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Guerder S., Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J. Exp. Med. 1992;176:553–564. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S.R., Carbone F.R., Karamalis F., Miller J.F., Heath W.R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Zinkernagel R.M., Oxenius A. Immune responses in the absence of costimulationviruses know the trick. J. Immunol. 1988;161:5791–5794. [PubMed] [Google Scholar]
- Leist T.P., Cobbold S.P., Waldmann H., Aguet M., Zinkernagel R.M. Functional analysis of T lymphocyte subsets in antiviral host defense. J. Immunol. 1987;138:2278–2281. [PubMed] [Google Scholar]
- Ahmed R., Butler L.D., Bhatti L. T4+ T helper cell function in vivodifferential requirements for induction of antiviral cytotoxic T cell and antibody responses. J. Virol. 1988;62:2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahemtulla A., Fung-Leung W.P., Schilham M.W., Kündig T.M., Sambhara S.R., Narendran A., Arabian A., Wakeham A., Paige C.J., Zinkernagel R.M. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- von Herrath M., Yokoyama M., Dockter J., Oldstone M.B., Whitton J.L. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R.M., Holmes K.L., Hügin A., Fredrickson T.N., Morse H.C. Induction of cytotoxic T cell responses in vivo in the absence of CD4 helper cells. Nature. 1987;328:77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Wu Y., Liu Y. Viral induction of co-stimulatory activity on antigen-presenting cells bypasses the need for CD4+ T-cell help in CD8+ T-cell responses. Curr. Biol. 1994;4:499–505. doi: 10.1016/s0960-9822(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Krieger N.R., D-P. Yin, Fathman C.G. CD4+ but not CD8+ cells are essential for allorejection. J. Exp. Med. 1996;184:2013–2018. doi: 10.1084/jem.184.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Calderwood S. Characterization and sequence of a mouse hsp70 gene and its expression in mouse cell lines. Gene. 1990;87:199–204. doi: 10.1016/0378-1119(90)90302-8. [DOI] [PubMed] [Google Scholar]
- Flaherty K.M., DeLuca-Flaherty C., McKay D.B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990;346:623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Zhu X., Zhao Z., Burkholder W.F., Gragerov A., Ogata C.M., Gottesman M.E., Hendrickson W.A. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedl C., Kopf M., Bachmann M.F. CD8+ T cells mediate CD40-independent maturation of dendritic cells in vivo. J. Exp. Med. 1999;189:1875–1883. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Syldath U., Bellmann K., Burkart V., Kolb H. Human 60-kDa heat-shock proteina danger signal to the innate immune system. J. Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- Kol A., Bourcier T., Lichtman A.H., Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophage. J. Clin. Invest. 1990;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breloer M., Fleischer B., von Bonin A. In vivo and in vitro activation of T cells after administration of Ag-negative heat shock proteins. J. Immunol. 1999;162:3141–3147. [PubMed] [Google Scholar]
- Kalams S.A., Buchbinder S.P., Rosenberg E.S., Billingsley J.M., Colbert D.S., Jones N.G., Shea A.K., Trocha A.K., Walker B.D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer T., Harrer E., Kalams S.A., Elbeik T., Staprans S.I., Feinberg M.B., Cao Y., Ho D.D., Yilma T., Caliendo A.M. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res. Hum. Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- Harrer T., Harrer E., Kalams S.A., Barbosa P., Trocha A., Johnson R.P., Elbeik T., Feinberg M.B., Buchbinder S.P., Walker B.D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J. Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- Yang O.O., Kalams S.A., Rosenzweig M., Trocha S., Jones N., Koziel M., Walker B.D., Johnson R.P. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang O.O., Kalams S.A., Trocha A., Cao H., Luster A., Johnson R.P., Walker B.D. Suppression of human immunodeficiency virus type 1 replication by CD8+ cellsevidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matano T., Shibata R., Siemon C., Connors M., Lane H.C., Martin M.A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner L., Yang O.O., Garcia-Zepeda E.A., Ge Y., Kalamas S.A., Walker B.D., Pasternack M.S., Luster A.D. Beta-chemokines are released from HIV-1-specific cytotoxic T cell granules complexed to proteoglycans. Nature. 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]