Abstract
Infection of inbred mouse strains with Leishmania major is a well characterized model for analysis of T helper (Th)1 and Th2 cell development in vivo. In this study, to address the role of costimulatory molecules CD27, CD30, 4-1BB, and OX40, which belong to the tumor necrosis factor receptor superfamily, in the development of Th1 and Th2 cells in vivo, we administered monoclonal antibody (mAb) against their ligands, CD70, CD30 ligand (L), 4-1BBL, and OX40L, to mice infected with L. major. Whereas anti-CD70, anti-CD30L, and anti–4-1BBL mAb exhibited no effect in either susceptible BALB/c or resistant C57BL/6 mice, the administration of anti-OX40L mAb abrogated progressive disease in BALB/c mice. Flow cytometric analysis indicated that OX40 was expressed on CD4+ T cells and OX40L was expressed on CD11c+ dendritic cells in the popliteal lymph nodes of L. major–infected BALB/c mice. In vitro stimulation of these CD4+ T cells showed that anti-OX40L mAb treatment resulted in substantially reduced production of Th2 cytokines. Moreover, this change in cytokine levels was associated with reduced levels of anti–L. major immunoglobulin (Ig)G1 and serum IgE. These results indicate that anti-OX40L mAb abrogated progressive leishmaniasis in BALB/c mice by suppressing the development of Th2 responses, substantiating a critical role of OX40–OX40L interaction in Th2 development in vivo.
Keywords: OX40/OX40 ligand, experimental leishmaniasis, Th1/Th2 differentiation, costimulation, TNF/TNF receptor family
Introduction
Two functionally distinct CD4+ T cell subsets, designated Th1 and Th2, have been implicated in differential host responses to infectious diseases. Th1 cells produce IFN-γ and IL-2 and promote cellular immune responses and inflammatory reactions. Th2 cells produce IL-4, IL-5, and IL-6 and promote humoral immune responses and allergic reactions. Some cytokines play a definitive role in the development of Th1 and Th2; IL-12 induces Th1, whereas IL-4 induces Th2 1. In addition to these cytokines, the development of Th1 and Th2 appears to be influenced by costimulatory molecules expressed on APCs. It has been shown that CD80 and CD86, two ligands for CD28 on T cells, may differentially regulate Th1 and Th2 development 2. Some members of the TNFR superfamily, including CD27, CD30, 4-1BB, and OX40, have been shown to transmit a costimulatory signal for T cell proliferation and cytokine production like CD28 3 4. CD27 has been reported to enhance IL-2 production by human CD4+ T cells 5. CD30 is preferentially expressed on Th2 cells and reported to promote Th2 development in vitro 6. 4-1BB has been reported to promote Th1 cell responses by enhancing IL-2 and IFN-γ production but suppressing IL-4 production 7. Moreover, it has been reported that OX40 costimulation promoted the differentiation of naive CD4+ T cells into Th2 cells producing IL-4 in vitro 8 9. These in vitro observations raise the possibility that these costimulatory molecules may be involved in the development of Th1 and Th2 in vivo.
Experimental infection with the intracellular parasite Leishmania major is a well characterized model for analysis of Th1 and Th2 development in vivo 10. C57BL/6 mice are resistant to L. major infection in association with development of Th1 cells that produce IFN-γ. The critical role of IFN-γ in controlling L. major infection has been established by results showing that genetically resistant mice with disrupted IFN-γ or IFN-γR genes failed to resolve the lesions 11 12. In contrast, susceptible BALB/c mice develop progressive lesions in association with development of Th2 cells that produce IL-4. Studies using neutralizing anti–IL-4 antibodies or mice with a disrupted IL-4 gene have defined a critical role of IL-4 in mediating the differentiation of Th2 in vivo and the failure to control L. major infection 13 14. Using this well characterized system, we address the role of CD27, CD30, 4-1BB, and OX40 costimulation in Th1 and Th2 development in vivo by administrating blocking antibodies against their ligand to L. major–infected C57BL/6 or BALB/c mice. Notably, anti-OX40L mAb abrogated progressive disease in susceptible BALB/c mice in association with reduced production of Th2 cytokines by draining LN T cells and reduced levels of anti–L. major IgG1 and serum IgE. We found OX40 expression on CD4+ T cells and OX40L expression on CD11c+ dendritic cells (DCs) in popliteal LNs of L. major–infected mice. These findings substantiate a critical role of OX40–OX40L interaction in the development of Th2 cells in vivo, possibly through T cell–DC interaction in the draining LNs.
Materials and Methods
Animals and Antibodies.
Female BALB/c and C57BL/6 mice were purchased from Charles River Japan, Inc. The animals were 6–7 wk old at the beginning of all experiments. Anti–mouse OX40L (RM134L, rat IgG2b/κ), anti–mouse CD70 (FR70, rat IgG2b/κ), and anti–mouse CD30L (RM153, rat IgG2b/κ) mAbs were prepared as previously described 15 16 17. Control rat IgG was purchased from Sigma Chemical Co. An anti–mouse 4-1BBL mAb was also generated in our laboratory (detailed characterization of this mAb will be described elsewhere). In brief, an SD rat was immunized with mouse B lymphoma 2PK-3 cells. The splenocytes were fused with murine myeloma P3U1 cells as described 18. After HAT selection, one hybridoma producing mAb TKS-1 (rat IgG2a/κ) was identified by its strong reactivity with 4-1BBL–transfected cells but not with mock-transfected cells. The TKS-1 mAb inhibited binding of soluble 4-1BB to and T cell costimulatory activity of mouse 4-1BBL transfectants in vitro.
Parasites and Infection of Mice.
L. major (MHOM/SU/73/5ASKH) was provided by Drs. H. Ishikawa and K. Himeno (University of Tokushima School of Medicine, Tokushima, Japan). Parasite infectivity was maintained by in vivo passage in BALB/c mice. For experimental infection, the parasites were collected from footpad legions and expanded in Schnider's medium (GIBCO BRL) supplemented with 20% FCS at 25°C. Stationary phage promastigotes were harvested, washed with PBS, and used for infection and antigen preparation. Groups of six mice were infected with 5 × 106 promastigotes in the right hind footpad. In each group, mice were injected intraperitoneally with 300 μg of anti-CD70, anti-CD30L, anti–4-1BBL, or anti-OX40L mAb or control rat IgG at the time of infection and subsequently twice per week until the end of experiments. Progression of leishmaniasis was assessed by weekly measuring of the swelling of the infected right footpad compared with the uninfected left footpad. To prepare soluble leishmanial antigens (SLAs) for ELISA, 109 promastigotes were subjected to three cycles of freezing and thawing and homogenized. Protein concentration of SLA was determined using the Bio-Rad Protein Assay reagent (Bio-Rad Labs.).
Flow Cytometric Analysis.
Popliteal LNs were digested with collagenase (Wako Pure Chemical Industries, Ltd.), and cell suspension was collected and used for detection of OX40L on CD11b+ or B220+ cells or OX40 on CD4+ T cells. To enrich DCs, low density cells were isolated by a BSA gradient (Sigma Chemical Co.) according to the procedure described by Crowley et al. 19 and used for detection of OX40L, CD70, 4-1BBL, or CD30L on CD11c+ cells. Cell surface staining was performed as previously described 15. In brief, 106 cells were first preincubated with anti-CD16/32 mAb (FcBlock; PharMingen) and then incubated with biotinylated control rat IgG, anti-OX40L (RM134L), anti-CD70 (FR70), anti–4-1BBL (TKS-1), anti-CD30L (RM153), or anti-OX40 mAb (MRC OX86) (reference 20). After washing with PBS, the cells were incubated with FITC-labeled anti-CD11b, anti-B220, anti-CD11c, or anti-CD4 mAb (PharMingen) and PE-labeled streptavidin (PharMingen). After washing with PBS, the stained cells (live-gated on the basis of forward and side scatter profiles and propidium iodide exclusion) were analyzed on a FACScan™ (Becton Dickinson).
In Vitro Stimulation of CD4+ T Cells.
CD4+ T cells were purified from popliteal LNs at 50 d after the L. major infection by passage through a nylon fiber column (Wako Pure Chemical Industries, Ltd.) and treatment with a mixture of hybridoma supernatants (anti-MHC class II, M5/114; anti-CD8, 3.155; and anti-B220, RA3-3A1) and low-tox rabbit complement (Cedarlane Labs., Ltd.). CD4+ T cells (>95% pure) were pooled from three mice in each group and stimulated with 50 ng/ml of PMA and 500 ng/ml of ionomycin (both from Sigma Chemical Co.) at 5 × 105 cells/ml in 96-well round-bottomed plates containing 200 μl of RPMI 1640 medium supplemented with 10% FCS, 10 mM Hepes, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mg/ml penicillin and streptomycin, and 50 μM 2-ME. For estimating proliferative responses, the cultures were pulsed with 0.5 μCi/well of [3H]thymidine (DuPont-NEN) for the last 7 h. Incorporated radioactivity was measured as previously described 15. To determine the production of cytokines, cell-free supernatants were collected at 24–72 h and assayed for IL-2, IL-4, IL-5, IL-6, IL-10, and IFN-γ by ELISA using OptEIA kits (PharMingen) and IL-13 by using Quantikine mouse IL-13 kit (R & D Systems, Inc.) according to the manufacturer's instructions.
Measurement of Serum Ig by ELISA.
SLAs (10 μg/ml) were coated onto 96-well CovaLink NH plates (Nunc, Inc.). After blocking with 1% BSA and 0.05% Tween 20 in PBS, L. major–specific IgG isotypes were determined by incubating serially diluted serum samples for 2 h at 37°C. After washing with 0.05% Tween 20 in PBS, wells were incubated with biotin-conjugated isotype-specific mAbs, including anti–mouse IgG1 (Serotec) or anti–mouse IgG2a, IgG2b, or IgG3 (PharMingen), washed, and then developed with Vectastain ABC kit (Vector Labs., Inc.) and o-phenylendiamine (Wako Pure Chemical Industries, Ltd.). After terminating the reaction with 2N H2SO4, OD at 490/595 nm was measured on a microplate reader (Bio-Rad Labs., Inc.). Total serum IgG was quantitated by sandwich ELISA by using goat anti–mouse IgG (Zymed Labs.) and biotin-conjugated anti–mouse IgG (Vector Labs., Inc.) as described above. Total serum IgE was quantitated by IgE-specific sandwich ELISA as previously described 21.
Results
Effect of Anti-CD70, -CD30L, –4-1BBL, and -OX40L mAbs on Murine Leishmaniasis.
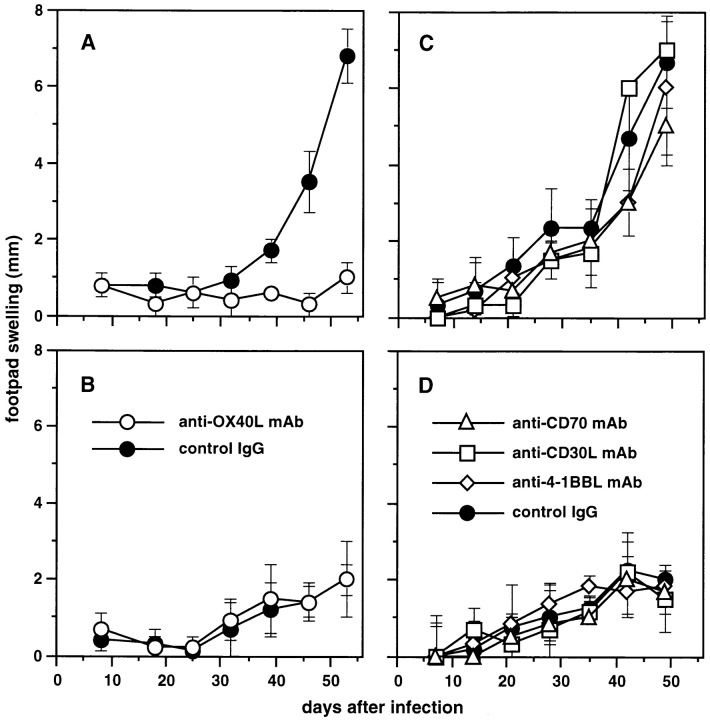
To determine the contribution of CD70, CD30L, 4-1BBL, and OX40L to murine leishmaniasis, susceptible BALB/c and resistant C57BL/6 mice were administrated with neutralizing anti-CD70, -CD30L, –4-1BBL, or -OX40L mAb or control rat IgG at the time of L. major infection and subsequently twice per week until the end of experiments. As shown in Fig. 1A and Fig. C, control IgG–treated BALB/c mice developed progressive lesions manifested by footpad swelling over a 50-d period after L. major infection, whereas C57BL/6 mice treated with control IgG developed only small lesions over that period (Fig. 1B and Fig. D). The administration of anti-OX40L mAb markedly reduced the footpad swelling in susceptible BALB/c mice (Fig. 1 A) but did not influence the resistance of C57BL/6 mice (Fig. 1 B). In contrast, the administration of anti-CD70, -CD30L, or –4-1BBL mAb exhibited no apparent effect in either susceptible BALB/c or resistant C57BL/6 mice (Fig. 1C and Fig. D). These results indicated that OX40L plays a unique role in the development of susceptible phenotype in BALB/c mice but not in the development of resistant phenotype in C57BL/6 mice.
Figure 1.
Effect of anti-OX40L, -CD70, -CD30L, and –4-1BBL mAb on the course of L. major infection. BALB/c mice (A and C) or C57BL/6 mice (B and D) were infected with 5 × 106 stationary phase promastigotes subcutaneously in the hind footpad. Mice were treated with 300 μg of anti-OX40L mAb (○, A and B), anti-CD70 (▵), anti-CD30L (□), or anti–4-1BBL (⋄) mAb (C and D) or control IgG (•, A–D) intraperitoneally at the time of infection and subsequently twice per week until the end of experiments. Net footpad swelling was measured by subtracting the thickness of the uninfected footpad from that of the infected footpad. Results are expressed as mean ± SD of six mice in each group. Similar results were obtained in two independent experiments.
Expression of OX40 and OX40L on Popliteal LN Cells from L. major–infected BALB/c Mice.
Infection with L. major induces the expansion and differentiation of parasite-specific CD4+ T cells that recognize L. major antigens presented by MHC class II molecules on the surfaces of DCs or macrophages (Mφ) in the draining LNs 10 22. To determine whether CD4+ T cells express OX40 and DCs and/or Mφ express OX40L, we examined the expression of OX40 and OX40L on popliteal LN cells from L. major–infected BALB/c mice. As shown in Fig. 2 A, OX40 was expressed on a substantial part of CD4+ T cells. On the other hand, the expression of OX40L was found on CD11c+ DCs (Fig. 2 C) but not on CD11b+ Mφ or B220+ B cells (Fig. 2 B). CD11c+ DCs also expressed CD70 and CD30L but not 4-1BBL (Fig. 2 C). These results suggested that the administration of anti-OX40L mAb ameliorated the leishmaniasis in BALB/c mice, possibly by interrupting the OX40–OX40L-mediated T cell–DC interaction in the draining LN.
Figure 2.
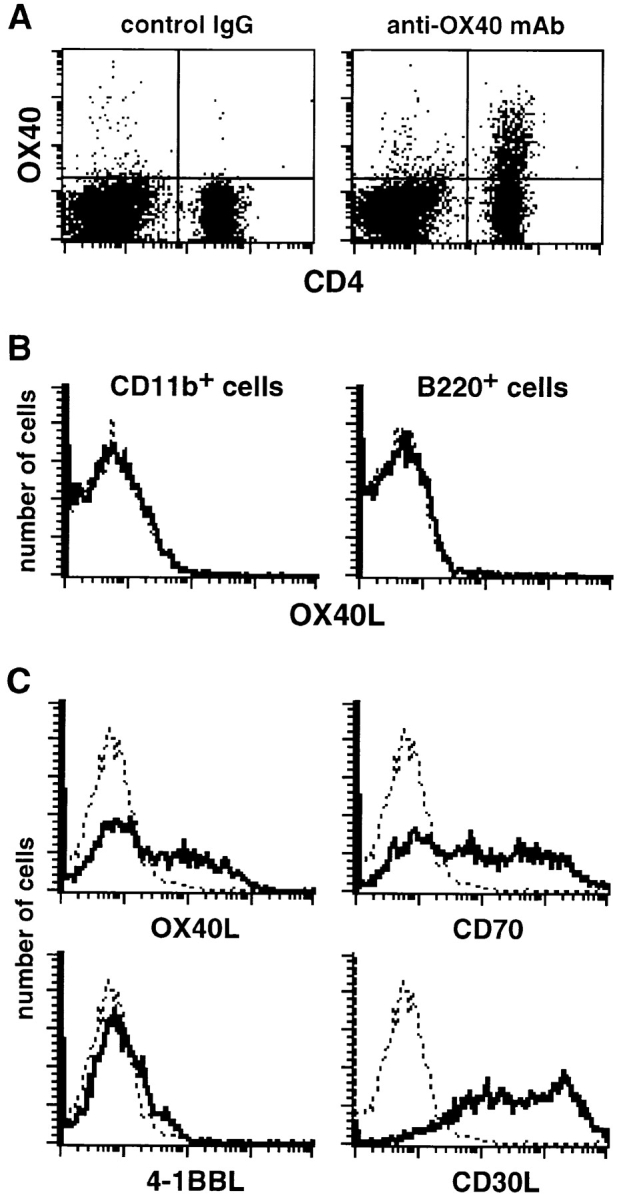
Expression of OX40 and OX40L on popliteal LN cells from L. major–infected BALB/c mice at day 40 after infection. (A) OX40 is expressed on CD4+ T cells. Popliteal LN cells proximal to the footpad lesions of L. major–infected BALB/c mice were double stained with FITC-labeled anti-CD4 mAb and biotinylated anti-OX40 mAb or control IgG followed by PE-labeled streptavidin. (B) OX40L is not expressed on CD11b+ Mφ and B220+ B cells. Popliteal LN cells were double stained with FITC-labeled anti-CD11b or anti-B220 mAb and biotinylated anti-OX40L mAb or control IgG followed by PE-labeled streptavidin. The histograms show staining of electronically gated CD11b+ or B220+ cells. Bold line shows staining with anti-OX40L mAb, and dotted line shows background staining with control IgG. (C) Expression of OX40L, CD70, 4-1BBL, and CD30L on CD11c+ DCs. Low density cells from popliteal LNs were double stained with FITC-labeled anti-CD11c mAb and biotinylated anti-OX40L, CD70, 4-1BBL, or CD30L mAb or control IgG followed by PE-labeled streptavidin. The histograms show staining of electronically gated CD11c+ cells. Bold line shows staining with mAb against the indicated molecules, and dotted line shows background staining with control IgG.
Functional Phenotype of CD4+ T Cells in Anti-OX40L–treated BALB/c Mice.
We next examined the functional phenotype of CD4+ T cells in the BALB/c mice that were rendered resistant to L. major by treatment with anti-OX40L mAb. CD4+ T cells were isolated from the draining popliteal LNs and stimulated with PMA and ionomycin, and then proliferative response and cytokine production in the culture supernatants was assessed. As shown in Fig. 3 A, proliferative responses of CD4+ T cells were almost comparable between the anti-OX40L–treated mice and the control IgG–treated mice. However, the production of Th1 cytokines IL-2 and IFN-γ was slightly increased in the anti-OX40L–treated mice as compared with the control IgG–treated mice (Fig. 3B and Fig. C). In contrast, the production of Th2 cytokines IL-4, IL-10, and IL-13 was substantially reduced in the anti-OX40L–treated mice as compared with the control IgG–treated mice (Fig. 3D–F). The mean suppression of IL-4, IL-10, and IL-13 was 56.8 ± 3.2% at 48 h, 39.2 ± 3.4% at 72 h, and 53.0 ± 4.9% at 72 h in three experiments, respectively. Other Th2 cytokines, IL-5 and IL-6, were also measured but not detectable in these experiments (data not shown). These results indicated that anti-OX40L mAb treatment suppressed the development of Th2 cells in L. major–infected BALB/c mice.
Figure 3.
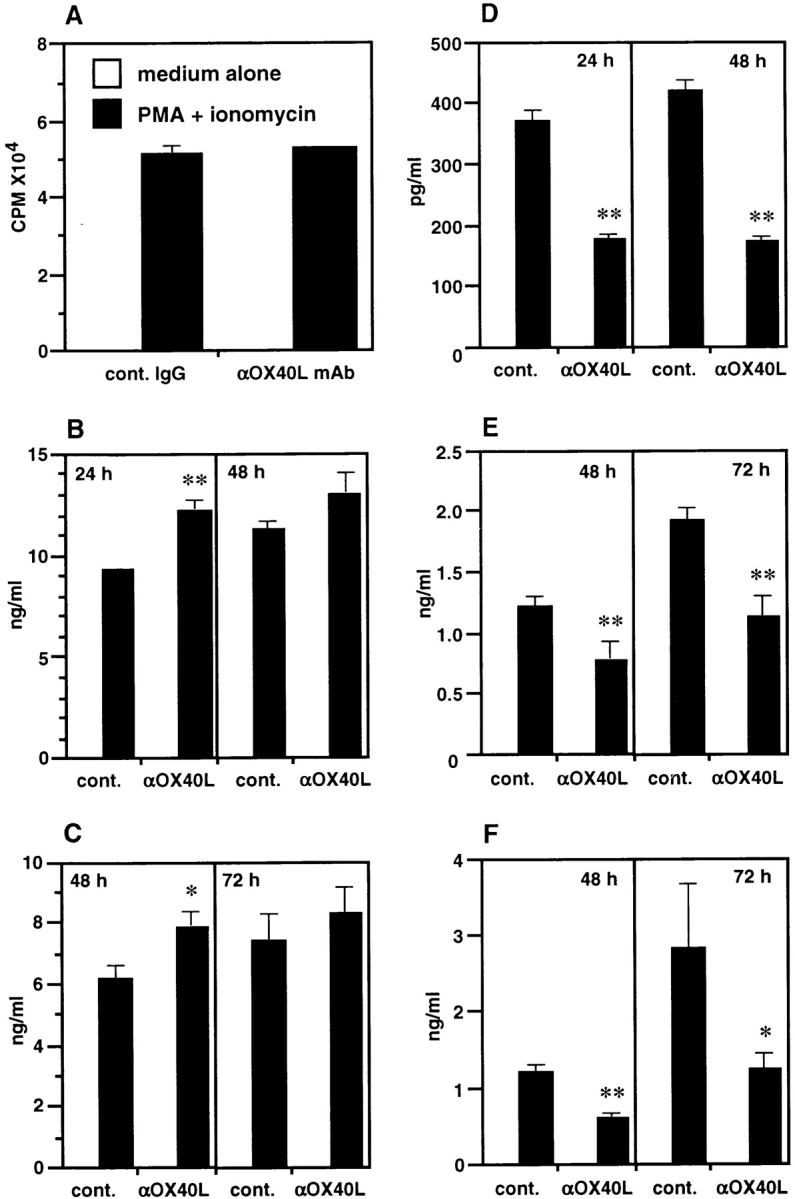
Functional phenotype of CD4+ T cells in anti-OX40L–treated BALB/c mice. CD4+ T cells were purified from popliteal LNs of L. major–infected BALB/c mice that were treated with anti-OX40L mAb or control IgG 50 d after infection and stimulated with PMA and ionomycin for 24–72 h. (A) Proliferative response at 48 h was assessed by pulsing the cultures with 0.5 μCi/well of [3H]thymidine for the last 7 h. Production of IL-2 (B), IFN-γ (C), IL-4 (D), IL-10 (E), and IL-13 (F) in culture supernatants at the indicated periods was measured by ELISA. Results are expressed as mean ± SD of triplicate cultures. Significant differences between control IgG and anti-OX40L mAb treatment are indicated by asterisks (*P < 0.05; **P < 0.01). Similar results were obtained in three independent experiments.
Humoral Responses in Anti-OX40L–treated BALB/c Mice.
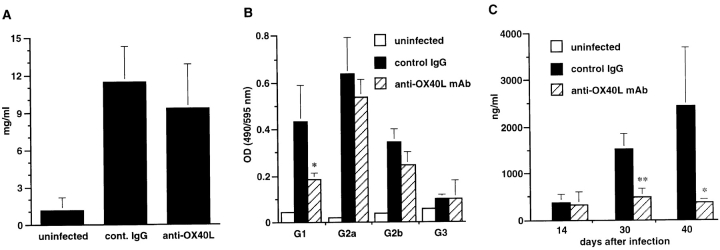
We also examined the total IgG, L. major–specific IgG, and total IgE levels in the sera of infected mice. As shown in Fig. 4 A, total IgG levels were slightly decreased in anti-OX40L–treated mice as compared with control IgG–treated mice, but the difference was not significant. In contrast, levels of anti–L. major IgG1 were significantly reduced in anti-OX40L–treated mice as compared with control IgG–treated mice, whereas the levels of IgG2a, IgG2b, and IgG3 were comparable (Fig. 4 B). Serum IgE levels in the anti-OX40L–treated mice were also substantially reduced as compared with those in the control IgG–treated mice (Fig. 4 C). These results are consistent with the reduced production of IL-4 and IL-13, which promote IgG1 and IgE production, by CD4+ T cells shown in Fig. 3 and further substantiate that anti-OX40L mAb treatment suppressed the Th2 development in L. major–infected BALB/c mice.
Figure 4.
Effect of anti-OX40L mAb treatment on serum IgG Ab and IgE in L. major–infected BALB/c mice. (A) Serum total IgG 40 d after infection was measured by sandwich ELISA. Results are expressed as mean ± SD of six mice in each group. (B) L. major–specific IgG Ab isotypes were determined with serum 40 d after infection against SLAs by isotype-specific ELISA. Data represent OD obtained at a 500-fold dilution of serum and are expressed as mean ± SD of six mice in each group. (C) Serum IgE at 14, 30, and 40 d after infection was measured by IgE-specific sandwich ELISA. Results are expressed as mean ± SD of six mice in each group. Significant differences between control IgG and anti-OX40L mAb treatment are indicated by asterisks (*P < 0.05; **P < 0.01). Similar results were obtained in two independent experiments.
Discussion
Recently, it has been reported that OX40–OX40L-mediated costimulation promotes the development of Th2 cells in vitro 8 9. In this study, we found that the blockade of OX40L ameliorates progressive leishmaniasis in BALB/c mice (Fig. 1 A). In vitro stimulation of CD4+ T cells from L. major–infected BALB/c mice showed that anti-OX40L mAb treatment markedly reduced the production of Th2 cytokines, including IL-4, IL-10, and IL-13, as compared with control IgG treatment (Fig. 3). We also observed that anti-OX40L mAb treatment substantially reduced anti–L. major IgG1 and serum IgE, which are promoted by Th2 cells (Fig. 4). Taken together, these results suggest that anti-OX40L mAb treatment ameliorated progressive leishmaniasis in BALB/c mice by suppressing Th2 development, substantiating a critical role of OX40–OX40L in the development of Th2 cells in vivo. However, it should be noted that the resistance conferred by anti-OX40L treatment in L. major–infected BALB/c mice was not permanent, and the disease progressed if treatment was stopped at day 60 after the infection (data not shown). Moreover, a short course of anti-OX40L treatment for 1 or 2 wk after the infection was not effective at preventing disease progression (data not shown). This seems to be due to persistent infection of L. major in anti-OX40L–treated BALB/c mice, which induces Th2 development persistently. In fact, we observed a small number of parasites that persisted in the infected footpads of the anti-OX40L–treated mice even at day 60 after the infection (data not shown). Therefore, anti-OX40L treatment did inhibit Th2 development, but this did not necessarily result in a dramatic shift to protective Th1 responses, as indicated by only modest increase in IFN-γ production (Fig. 3 C).
Some in vivo studies have demonstrated that CD28–CD86-mediated costimulation may be critical for Th2 development 2 23. However, subsequent studies using CD28-deficient BALB/c mice indicated that CD28 was not an absolute requirement for Th2 development after L. major infection 24. This also suggested that some compensatory pathway may exist that promotes Th2 development in the absence of CD28. In this respect, it is noteworthy that the blockade of OX40L suppressed Th2 development in the presence of CD28 in L. major–infected BALB/c mice. Moreover, we recently demonstrated that OX40L can provide a CD28-independent costimulatory signal to T cells 15. Therefore, the OX40–OX40L pathway may play a dominant role over the CD28–CD86 pathway toward promoting Th2 development in vivo.
In contrast to the unique effect of anti-OX40L mAb, the administration of anti-CD70, anti-CD30L, or anti–4-1BBL mAb exhibited no apparent effect in either susceptible BALB/c or resistant C57BL/6 mice (Fig. 1). We confirmed that rat IgG levels in the sera of mAb-treated mice were maintained over 10 μg/ml during the whole course of the experiments, which was sufficient to block T cell–costimulatory activity of each ligand in vitro (data not shown). This suggests that the dose of mAbs was sufficient to block each ligand in vivo. Our present results did not provide positive evidence for a unique and essential role of CD70, CD30L, and 4-1BBL in progressive leishmaniasis in BALB/c mice or protection in C57BL/6 mice, but we could not exclude the possible contribution of these molecules to the Th2 or Th1 development in these mice. It remains possible that these molecules or CD28/B7 may play a redundant role. Further studies are now underway by using CD28-deficient mice and combinations of anti-CD70, CD30L, and 4-1BBL mAbs.
It has been reported that L. major amastigotes, but not promastigotes, efficiently entered DCs and increased the expression of MHC class I and II, CD40, CD80, and CD86, and IL-12 production, whereas infection of Mφ with amastigotes or promastigotes did not lead to changes in surface antigen expression or cytokine production 25. Consistently, we could not detect OX40L expression on CD11b+ Mφ in popliteal LNs of L. major –infected BALB/c mice (Fig. 2). We also could not detect OX40L on CD11b+ Mφ from peritoneal exudate cells when infected with promastigotes in vitro or stimulated with LPS, IFN-γ, anti-CD40, or their combination (data not shown). Therefore, OX40L appears not to be expressed on Mφ under these conditions. In contrast, we found OX40L expression on CD11c+ DCs in popliteal LNs of L. major –infected BALB/c mice (Fig. 2). It has been reported that OX40L was expressed on CD40-stimulated human and mouse DCs 26 27. We also observed OX40L expression on CD40-stimulated CD11c+ DCs purified from normal mouse spleens (data not shown). Therefore, the OX40–OX40L pathway may play an important role for Th2 development through T cell–DC rather than T cell–Mφ interaction. This notion is consistent with the previous observation that the blockade of OX40L on DCs inhibited Th2 development in vitro 8. It has recently been shown that DCs contain two functionally distinct subsets that promote Th1 or Th2 development 28. Further studies are now underway to characterize the possibly differential expression and function of OX40L on these DC subpopulations.
Some recent studies indicated that the blockade of OX40–OX40L interaction by administration of OX40–Ig ameliorated experimental allergic encephalomyelitis (EAE) and experimental colitis, which are Th1-mediated inflammatory diseases 29 30. We also observed that anti-OX40L mAb treatment ameliorated actively induced or adoptive transferred EAE, which appeared to result from an antiinflammatory effect of anti-OX40L mAb to inhibit the recruitment of OX40+ activated T cells to inflammatory sites rather than the inhibition of Th1 development (Nohara, C., H. Akiba, H. Yagita, and K. Okumura, manuscript in preparation). In contrast, our present study demonstrated that the anti-OX40L mAb treatment abrogated the progressive leishmaniasis in susceptible BALB/c mice by selectively suppressing Th2 responses, suggesting a critical role of OX40L in Th2 development in vivo. Therefore, an intervention in the OX40–OX40L pathway of costimulation might be a novel clinical strategy for the treatment of human leishmaniasis and other Th2-associated diseases such as atopy and asthma.
Acknowledgments
We thank Drs. H. Ishikawa and K. Himeno for parasites, Dr. H. Miyajima for the IgE ELISA system, and Drs. H. Oshima, O. Shimozato, and T. Kodama for mAbs.
This work was supported in part by grants from the Science and Technology Agency, the Ministry of Education, Science and Culture, and the Ministry of Health of Japan.
References
- O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- Kuchroo V.K., Das M.P., Brown J.A., Ranger A.M., Zamvil S.S., Sobel R.A., Weiner H.L., Nabavi N., Glimcher L.H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathwaysapplication to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Farrah T., Goodwin R.G. The TNF receptor superfamily of cellular and viral proteinsactivation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Gruss H.J., Dower S.K. Tumor necrosis factor ligand superfamilyinvolvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. [PubMed] [Google Scholar]
- Sugita K., Torimoto Y., Nojima Y., Daley J.F., Schlossman S.F., Morimoto C. The 1A4 molecule (CD27) is involved in T cell activation. J. Immunol. 1991;147:1477–1483. [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Daniel C.K., D'Elios M.M., Zancuoghi G., Vinante F., Pizzolo G., Romagnani S. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995;9:81–86. [PubMed] [Google Scholar]
- Kim Y.J., Kim S.H., Mantel P., Kwon B.S. Human 4-1BB regulates CD28 co-stimulation to promote Th1 cell responses. Eur. J. Immunol. 1998;28:881–890. doi: 10.1002/(SICI)1521-4141(199803)28:03<881::AID-IMMU881>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Yang L.P., Uchiyama T., Tanaka Y., Baum P., Sergerie M., Hermann P., Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4 (+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- Flynn S., Toellner K.M., Raykundalia C., Goodall M., Lane P. CD4 T cell cytokine differentiationthe B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner S.L., Locksley R.M. The regulation of immunity to Leishmania major . Annu. Rev. Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Wang Z.E., Reiner S.L., Zheng S., Dalton D.K., Locksley R.M. CD4+ effector cells default to the Th2 pathway in interferon γ–deficient mice infected with Leishmania major . J. Exp. Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swihart K., Fruth U., Messmer N., Hug K., Behin R., Huang S., Del Giudice G., Aguet M., Louis J.A. Mice from a genetically resistant background lacking the interferon γ receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1–type CD4+ T cell response. J. Exp. Med. 1995;181:961–971. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R.L. IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- Kopf M., Brombacher F., Kohler G., Kienzle G., Widmann K.H., Lefrang K., Humborg C., Ledermann B., Solbach W. IL-4–deficient Balb/c mice resist infection with Leishmania major . J. Exp. Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba H., Oshima H., Takeda K., Atsuta M., Nakano H., Nakajima A., Nohara C., Yagita H., Okumura K. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J. Immunol. 1999;162:7058–7066. [PubMed] [Google Scholar]
- Oshima H., Nakano H., Nohara C., Kobata T., Nakajima A., Jenkins N.A., Gilbert D.J., Copeland N.G., Muto T., Yagita H. Characterization of murine CD70 by molecular cloning and mAb. Int. Immunol. 1998;10:517–526. doi: 10.1093/intimm/10.4.517. [DOI] [PubMed] [Google Scholar]
- Shimozato O., Takeda K., Yagita H., Okumura K. Expression of CD30 ligand (CD153) on murine activated T cells. Biochem. Biophys. Res. Commun. 1999;256:519–526. doi: 10.1006/bbrc.1999.0336. [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Kawasaki A., Ebata T., Ohmoto H., Ikeda S., Inoue S., Yoshino K., Okumura K., Yagita H. Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M., Inaba K., Witmer-Pack M., Steinman R.M. The cell surface of mouse dendritic cellsFACS analyses of dendritic cells from different tissues including thymus. Cell. Immunol. 1989;118:108–125. doi: 10.1016/0008-8749(89)90361-4. [DOI] [PubMed] [Google Scholar]
- Al-Shamkhani A., Birkeland M.L., Puklavec M., Brown M.H., James W., Barclay A.N. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur. J. Immunol. 1996;26:1695–1699. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- Azuma M., Hirano T., Miyajima H., Watanabe N., Yagita H., Enomoto S., Furusawa S., Ovary Z., Kinashi T., Honjo T. Regulation of murine IgE production in SJA/9 and nude mice. Potentiation of IgE production by recombinant interleukin 4. J. Immunol. 1987;139:2538–2544. [PubMed] [Google Scholar]
- Will A., Blank C., Rollinghoff M., Moll H. Murine epidermal Langerhans cells are potent stimulators of an antigen-specific T cell response to Leishmania major, the cause of cutaneous leishmaniasis. Eur. J. Immunol. 1992;22:1341–1347. doi: 10.1002/eji.1830220603. [DOI] [PubMed] [Google Scholar]
- Brown J.A., Titus R.G., Nabavi N., Glimcher L.H. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J. Infect. Dis. 1996;174:1303–1308. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- Brown D.R., Green J.M., Moskowitz N.H., Davis M., Thompson C.B., Reiner S.L. Limited role of CD28-mediated signals in T helper subset differentiation. J. Exp. Med. 1996;184:803–810. doi: 10.1084/jem.184.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stebut E., Belkaid Y., Jakob T., Sacks D.L., Udey M.C. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cellsimplications for the initiation of anti-Leishmania immunity. J. Exp. Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Tanaka Y., Tozawa H., Takahashi Y., Maliszewski C., Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Brocker T., Gulbranson-Judge A., Flynn S., Riedinger M., Raykundalia C., Lane P. CD4 T cell traffic controlin vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur. J. Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A.D., Wegmann K.W., Funatake C., Whitham R.H. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J. Immunol. 1999;162:1818–1826. [PubMed] [Google Scholar]
- Higgins L.M., McDonald S.A., Whittle N., Crockett N., Shields J.G., MacDonald T.T. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interactionamelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J. Immunol. 1999;162:486–493. [PubMed] [Google Scholar]