Abstract
Yersinia enterocolitica synthesizes N-acyl-l-homoserine lactone (AHL) signal molecules via the LuxR-LuxI homologues YenR-YenI. In this study we checked two prototypes of mouse-virulent Y. enterocolitica serotype O8 strains WA-314 and 8081 for AHL production in vitro and in vivo (mouse infection model). We used thin-layer chromatography in combination with the Escherichia coli AHL biosensor to identify the AHL species produced. We detected only OHHL [N-(3-oxohexanoyl)-l-homoserine lactone] and not HHL (N-hexanoyl-l-homoserine lactone) produced by Y. enterocolitica O8 in culture supernatant or infected mouse tissue. This is the first report demonstrating AHL production by yersiniae during infection.
Quorum sensing is employed by both gram-negative and gram-positive bacteria to regulate a variety of diverse functions in response to the density of the population. In many bacterial pathogens quorum-sensing systems control expression of virulence factors. In the case of gram-negative bacteria the most commonly used signal molecules appear to be N-acylhomoserine lactones (AHLs), which are usually synthesized by enzymes belonging to the LuxI family of proteins. At low population densities, the microorganisms produce a basal level of AHL via the activity of the LuxI homologue. As the cell density increases, the diffusible AHL signal molecules accumulate in the growth medium. On reaching a critical threshold concentration, the AHL binds to its cognate LuxR-type receptor protein, which in turn leads to the induction-repression of target genes (1, 3, 8, 12).
The genus Yersinia comprises three human-pathogenic species: Y. pestis, the “plague bacillus,” and the enteropathogenic Y. enterocolitica and Y. pseudotuberculosis. The Y. enterocolitica strains of biogroup IB are highly pathogenic for mice and thus are commonly used (in particular serotype O8 strains WA-314 and 8081) for the mouse intestinal infection model. After oral inoculation of mice, the pathogen invades the Peyer's patches of the small intestine and subsequently disseminates into spleen, liver, and lungs (2). The luxI-luxR homologues of Y. enterocolitica 10460 (serotype 1), yenI-yenR, have been cloned and sequenced (11). At the level of amino acids, YenI of this strain is 98.6% homologous to YenI of Y. enterocolitica 8081(http://www.sanger.ac.uk/Projects/Y_enterocolitica/). It wasshown that YenI directs the synthesis of N-hexanoyl-l-homoserine lactone (HHL) and N-(3-oxohexanoyl)-l-homoserine lactone (OHHL). Mutation in yenI abolished HHL and OHHL synthesis but did not affect the production or secretion of Yersinia outer proteins (Yops) encoded by the virulence plasmid pYV of Y. enterocolitica serotype O9 (nonvirulent for mice). Since no studies have been performed with Yersinia to elucidate the role of AHL in virulence, we initiated in vitro and in vivo quorum-sensing studies with the well-characterized, mouse-virulent Y. enterocolitica WA-314 and 8081 (serotype O8, biotype 1B), carrying the virulence plasmid pYVO8 (5). Previously, a report was published on an E. coli AHL biosensor strain which allows the detection of AHLs in sputum samples of cystic fibrosis patients colonized by Pseudomonas aeruginosa and Burkholderia cepacia (7). From these results we were encouraged to apply the same techniques for detection of AHLs produced by the mouse-virulent Y. enterocolitica strain WA-314 during growth in nutrient broth and during experimental infection of mice. To determine if the route of infection influences the distribution of AHLs within the mouse body, the animals were infected intraperitoneally (i.p.), intravenously (i.v.), or perorally (p.o.). In this report we describe for the first time the detection of OHHL from different tissues of the mouse which were infected with Y. enterocolitica. Strikingly, only OHHL, not HHL, could be detected in vitro as well as in vivo in homogenates of various mouse organs with the aid of a lux-based AHL biosensor.
AHL detection for strains WA-314 and 8081 after growth in Luria-Bertani (LB) broth.
Throup et al. (11) did not study the Y. enterocolitica prototype strains of serotype O8; thus, we decided to extract homoserine lactones from in vitro-grown cultures of WA-314 and 8081 strains as described previously (4). Extraction of culture supernatant (50 ml, corresponding to 5 × 1010 bacteria) yielded 500 μl of AHL concentrate in ethyl acetate. Appropriate dilutions of the extracts were loaded on a thin-layer chromatography (TLC) plate (RP-18 F254S; 20 by 20 cm; Merck) and were run in a moisture chamber containing a mixture of 60% methanol and 40% distilled water for 6 h (9). After the plate was dried, it was overlaid with 200 ml of 0.6% soft LB agar seeded with 20 ml of a logarithmically grown culture of the lux-based AHL biosensor strain E. coli [pSB403], which is able to respond to a range of different AHLs by luciferase production (e.g., BHL, HHL, OHHL, and ODHL) (13). The incubation was carried out overnight in a moisture chamber at 30°C, and AHLs were detected via autoradiography.
Throup et al. (11) showed that Y. enterocolitica produces OHHL and HHL. Our TLC analysis confirmed the presence of OHHL of serotype O8 strains in liquid culture (Fig. 1). However, we were unable to detect HHL production in both strains, for reasons yet to be found. Strains WA-314 and 8081 might produce only small amounts of HHL, which are not detectable with our sensor strain. In addition, strain 8081 produces only about 1/10 the amount of OHHL produced by strain WA-314 (Fig. 1). The reason for this also remains unknown. We also determined the minimum number of yersiniae producing detectable AHL amounts by the TLC assay used. Performing serial dilutions and running the dilution mixtures on a TLC plate, we found that a minimum number of about 107 in vitro-grown yersiniae produced OHHL amounts sufficient to be detected. This amount is equal to less than 30 fg of OHHL (Fig. 2, lanes 4 to 9).
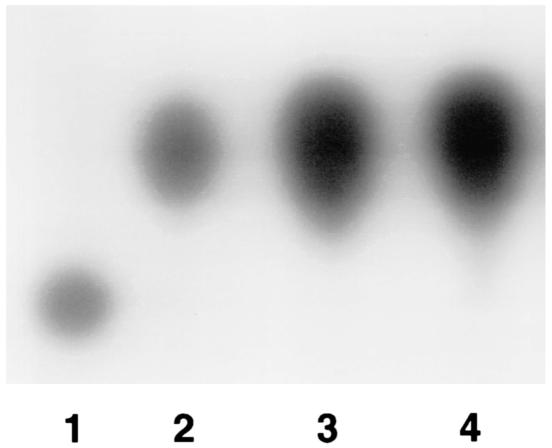
FIG. 1.
TLC analysis of in vitro-grown cultures of WA-314 and 8081. Lanes 1 and 2, synthetic HHL and OHHL, respectively; lane 3, supernatant of WA-314 corresponding to 4 × 107 bacteria; lane 4, supernatant of 8081 corresponding to 2.5 × 108 bacteria.
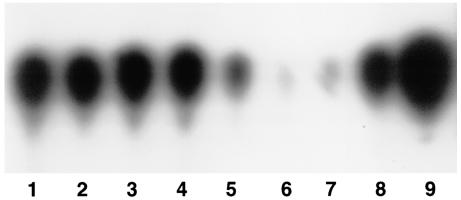
FIG. 2.
TLC analysis of control experiments to determine extraction efficiency in vivo. Lanes 1 to 3, intestinal lavage fluid, liver homogenate, and spleen homogenate, respectively, each seeded with supernatant of WA-314 (5 × 107 bacteria); lanes 4 to 6, supernatant of WA-314 corresponding to 5 × 107, 2.5 × 107, and 1.25 × 107 bacteria, respectively; lanes 7 to 9, synthetic OHHL at 30, 80, and 160 fg, respectively.
Extraction and detection of homoserine lactones in mice.
Female BALB/c mice (6 to 8 weeks old) were infected (groups of five) with strain WA-314 via p.o., i.v., or i.p. routes. For i.p. challenge, BALB/c mice were infected with 107 CFU of WA-314, previously grown in LB broth for 3 h at 27°C. For i.v. and p.o. inoculation, 5 × 105 and 5 × 109 CFU of yersiniae were used, respectively. The doses were set relatively high to ensure a quick onset of infection. Three days after infection, the mice were sacrificed. A peritoneal and an intestinal lavage were performed with 4 ml of ice-cold sterile 0.9% NaCl solution; the Peyer's patches of the small intestine were dissected, and the liver and spleen were recovered. After homogenization of the organs in phosphate-buffered saline (100 mg of spleen tissue in 2 ml and 400 mg of liver tissue in 4 ml), aliquots were taken for determination of bacterial numbers by plating appropriate dilutions on LB agar and counting them (6). An aliquot from each sample was taken for further analysis (see below). The homogenates of each of the organs were combined, ice-cold sterile 0.9% NaCl solution was added to 50 ml, and an aliquot was again taken for determination of bacterial numbers. The tissue homogenates (without cell lysis) and the peritoneal and the intestinal lavage fluids were centrifuged at 6,000 × g for 30 min (4°C), and the supernatants were frozen at −20°C until extraction of the AHLs. For control experiments we extracted AHLs from freshly removed and homogenized organs and compared these with homogenized organs which had been stored at 4°C for 6 h before extraction, to rule out the possibility that AHLs were produced after the organs were removed from the mice (results not shown).
The extraction of the AHLs was performed as described previously (4), with the following modifications. Tissue homogenates were extracted twice with 50 ml of dichloromethane, and after evaporation, the residual material was dissolved in 100 μl of ethyl acetate. The total extracts were loaded in 10 10-μl steps with drying steps in between (to increase the AHL concentration) on a TLC plate as described above. In addition, the samples collected from the supernatant of each organ were also used for the AHL bioassay, with use of the same E. coli biosensor strain. Fifty microliters of the supernatant was mixed with 50 μl of a logarithmically grown biosensor strain in a white microtiter well plate and was incubated overnight at 30°C. Luminescence was detected for each well for 10 s using a highly sensitive microtiter well plate reader, MicroLumatPlus from Berthold (Bad Wildbad, Germany). All infection experiments were repeated twice with similar results.
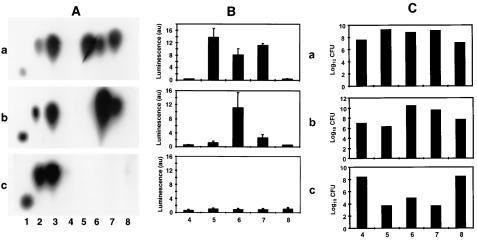
TLC analysis (Fig. 3A) showed that OHHL but not HHL was present in only those organs from which 108 or more Y. enterocolitica bacteria were isolated (Fig. 3C). The AHL bioassay (Fig. 3B) appears to be more sensitive than the TLC analysis, as shown in Fig. 3B, bottom panel. The reason for the discrepancy between the in vitro and in vivo detection limit (107 versus 108 bacterial cells) remains to be elucidated. Several reasons are plausible: (i) the yersiniae produce less OHHL in the mouse tissue than in liquid culture, (ii) OHHL enters the circulation and thus disappears from infected tissue by subsequent excretion, and (iii) OHHL accumulates in cells of infected tissue and thus resists extraction. The regulation of AHL production in Y. enterocolitica in liquid culture medium appears to be quite similar (apart from the differing amounts) to the in vivo situation, when the pathogen is growing to microcolonies or abscesses within the tissue. The route of infection (Fig. 3A) did not influence the production and distribution of the OHHL, since the amount of OHHL isolated in vivo was similar with respect to the bacterial numbers in the respective organs and in vitro (Fig. 2 and 3C). Our data show a close correlation between the number of bacteria present in the infected organ and the amount of OHHL extracted from it (Fig. 3A and C).
FIG. 3.
Detection of AHLs produced in vitro and in vivo by TLC analysis (A) and by the luxR-based biosensor E. coli [pSB403] (in 100,000 arbitrary units) (B). (A) Extracts of homogenates (lanes 6 to 8) and lavage fluids (lanes 4 and 5) of organs of five BALB/c mice infected with WA-314 via the i.p. (a), i.v. (b), and p.o. (c) routes after 3 days. Lanes 1 and 2, synthetic HHL and OHHL standards, respectively; lane 3, in vitro culture of WA-314; lane 4, intestinal lavage fluid; lane 5, peritoneal lavage fluid; lanes 6 to 8, homogenates of liver, spleen, and Peyer's patches, respectively. (B) AHLs of samples shown in panel A detected by the biosensor E. coli [pSB403]. (C) Bacterial count (in log10 CFU) corresponding to the 50-ml extraction volume of samples shown in panel B.
To investigate whether the organ homogenates have an influence on the extraction efficiency of AHLs, we performed control experiments. To this end, organs of five uninfected mice were homogenized as described above. The homogenates as well as intestinal and intraperitoneal lavage fluids from these noninfected animals were spiked each with 25 ml of supernatant from an overnight culture of WA-314. Following 2 h of incubation on ice, AHLs were extracted with dichloromethane and the AHL concentrations were compared to the one of 25 ml of pure culture supernatant. As shown in Fig. 2 the amounts of OHHL recovered from the complex samples were virtually indistinguishable from the one present in the pure culture supernatant (lanes 1 to 3 in comparison to lane 4), suggesting that the assay used allows a quantitative detection of OHHL in tissue homogenates and lavage samples.
In summary, by using TLC in combination with the E. coli AHL biosensor, we have demonstrated that the mouse-virulent Y. enterocolitica strains WA-314 and 8081 (serotype O8) produce OHHL but not detectable amounts of HHL, as has been reported for environmental strains (11). Since the two YenI proteins are highly homologous, it is unlikely that differences in the proteins account for the differences in AHL production. Whether quorum sensing in Y. enterocolitica plays a role in regulation of virulence genes or whether OHHL contributes directly to pathogenicity as an immunomodulator as has been reported for P. aeruginosa will be investigated with a YenI mutant (10).
Acknowledgments
C.A.J. and A.B. are supported by the Graduiertenkolleg “Infektion und Immunität” (GRK303/3) of the Deutsche Forschungsgemeinschaft.
We thank Gottfried Wilharm for the sequence analysis.
Editor: B. B. Finlay
REFERENCES
- 1.Atkinson, S., J. P. Throup, G. S. Stewart, and P. Williams. 1999. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol. Microbiol. 33:1267-1277. [DOI] [PubMed] [Google Scholar]
- 2.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberl, L. 1999. N-Acyl homoserine lactone-mediated gene regulation in gram-negative bacteria. Syst. Appl. Microbiol. 22:493-506. [DOI] [PubMed] [Google Scholar]
- 4.Geisenberger, O., M. Givskov, K. Riedel, N. Hoiby, B. Tummler, and L. Eberl. 2000. Production of N-acyl-l-homoserine lactones by P. aeruginosa isolates from chronic lung infections associated with cystic fibrosis. FEMS Microbiol. Lett. 184:273-278. [DOI] [PubMed] [Google Scholar]
- 5.Heesemann, J. 1987. Chromosomal-encoded siderophores are required for mouse virulence of enteropathogenic Yersinia species. FEMS Microbiol. Lett. 48:229-233. [Google Scholar]
- 6.Jacobi, C. A., S. Gregor, A. Rakin, and J. Heesemann. 2001. Expression analysis of the yersiniabactin receptor gene fyuA and the heme receptor hemR of Yersinia enterocolitica in vitro and in vivo using the reporter genes for green fluorescent protein and luciferase. Infect. Immun. 69:7772-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton, B., H. C. Rodgers, M. Camara, A. J. Knox, P. Williams, and A. Hardman. 2002. Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol. Lett. 207:1-7. [DOI] [PubMed] [Google Scholar]
- 8.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 9.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Throup, J. P., M. Camara, G. S. Briggs, M. K. Winson, S. R. Chhabra, B. W. Bycroft, P. Williams, and G. S. Stewart. 1995. Characterisation of the yenI/yenR locus from Yersinia enterocolitica mediating the synthesis of two N-acylhomoserine lactone signal molecules. Mol. Microbiol. 17:345-356. [DOI] [PubMed] [Google Scholar]
- 12.Williams, P., M. Camara, A. Hardman, S. Swift, D. Milton, V. J. Hope, K. Winzer, B. Middleton, D. I. Pritchard, and B. W. Bycroft. 2000. Quorum sensing and the population-dependent control of virulence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:667-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winson, M. K., S. Swift, L. Fish, J. P. Throup, F. Jorgensen, S. R. Chhabra, B. W. Bycroft, P. Williams, and G. S. Stewart. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 163:185-192. [DOI] [PubMed] [Google Scholar]