Abstract
CD40 signaling in B cells and dendritic cells (DCs) is critical for the development of humoral and cell-mediated immunity, respectively. Nuclear factor κB (NF-κB)–inducing kinase (NIK) has been implicated as a central transducing kinase in CD40-dependent activation. Here, we show that although NIK is essential for B cell activation, it is dispensable for activation of DCs. Such data provide compelling evidence that different intermediary kinases are used by different cellular lineages to trigger NF-κB activation via CD40.
Keywords: CD40 signaling, nuclear factor κB–inducing kinase, dendritic cell, nuclear factor κB activation, alymphoplasia mice
Introduction
CD40 is a member of the TNFR family and plays a central role in the regulation of both humoral and cell-mediated immunity 1. Engagement of CD40 on B lymphocytes triggers the clonal expansion and differentiation of these cells and is an essential signal in the regulation of thymus-dependent humoral immunity 2 3 4. Furthermore, stimulation of APCs through CD40 promotes their differentiation and maturation into effective inducers of cell-mediated immunity, as manifested by enhanced production of cytokines and chemokines and expression of costimulatory molecules 5 6 7.
Although the functional significance of CD40–CD154 interactions in immunity has been studied extensively, the molecular components of the CD40 signal transduction cascade are still not thoroughly understood. One of the downstream events in CD40 signaling is activation of nuclear factor κB (NF-κB 8), a transcription factor that promotes expression of genes involved in immune and inflammatory responses. The CD40-proximal event in NF-κB activation is recruitment of adaptor proteins called TNFR-associated factors (TRAFs) to the CD40 receptor complex; five of the six known TRAFs (TRAF1, 2, 3, 5, and 6 9 10 11 12 13 14) associate with CD40 upon stimulation by its ligand, CD154 15. After recruitment to the receptor complex, one or more of the TRAFs activate NF-κB 10 11 16 via the IκB kinase (IKK) complex 17, a process that probably involves an intermediate kinase 18 19 20. The IKK complex then phosphorylates IκB, which triggers degradation of IκB via ubiquitin-mediated proteolysis (for a review, see reference 21).
Degradation of IκB releases NF-κB, and NF-κB then translocates to the nucleus and initiates transcription of genes involved in immune and inflammatory responses. Two serine/threonine kinases have been implicated as intermediary kinases between TRAF recruitment to TNFRs and activation of the IKK complex: NF-κB–inducing kinase (NIK) and mitogen-activated protein kinase/extracellular signal regulatory kinase kinase (MEKK1 [18–20]). However, most of the available data on the role of NIK and MEKK1 in NF-κB activation were derived from experiments using transfected cell lines.
Evidence that NIK is an important kinase in mediating TNFR family signal transduction in vivo has recently been deduced using alymphoplasia (aly) mice. aly mice are characterized by the absence of Peyer's patches and LNs, as well as by a loss of lymphoid organization in the spleen 22. Furthermore, aly mice have a severely reduced level of serum Ig, particularly IgA. This phenotype resembles the phenotype of the lymphotoxin (LT)βR 23 and LTα knockout mice 24. However, the aly mice have a more severe reduction in serum IgM levels than either the LTα or LTβR knockout mice. It has been demonstrated that the genetic lesion in the aly mouse is a point mutation that results in a single amino acid substitution in the COOH terminus of NIK, and that wild-type NIK expressed in transgenic (Tg) mice can restore a normal phenotype in these mice 25. The similarity between the phenotypes of the aly, LTα knockout, and LTβR knockout mice suggests that the aly mutation interferes with LTβR signal transduction, but an involvement of other signal transduction cascades through other TNFR family members is likely. The studies described here were undertaken to determine whether or not NIK has a direct role in the CD40 signal transduction cascade by analyzing the biological responses of B cells and DCs from aly mice to stimulation through CD40.
Materials and Methods
B Cell Activation Studies.
aly/aly and aly/+ mice were obtained (Clea Japan) and bred in the Dartmouth College Animal Resource Center. B cells were isolated from spleens of aly/aly and aly/+ mice, cultured in vitro, and assayed for their ability to proliferate, produce Ig, and upregulate cell surface markers in response to CD40 stimulation as described 26. Induction of proliferation by anti-CD40 (10 μg/ml FGK115 27), LPS (50 μg/ml), and anti-IgM (goat anti–mouse IgM) was measured by the incorporation of [3H]thymidine from 66 to 72 h after initation of culture. All cultures contained 10 ng/ml of IL-4. Induction of Ig secretion was performed using soluble, recombinant CD154 28 or LPS in combination with IL-4 (10 ng/ml) and IL-5 (10 ng/ml). Ig secretion was measured using an isotype-specific ELISA, as described 4. Expression of cell surface molecules on splenic B cells was measured by flow cytometry as described previously 15.
Phospho-IκBα Immunoblotting.
10 × 106 cells/ml were left unstimulated or stimulated with the optimal dose of CD8–CD154 (COS cell supernatant) or TNF-α (PeproTech) for the indicated times. After stimulation, cells were lysed in lysis buffer containing 1% IGEPAL, 50 mM Hepes (pH 7.4), 150 mM NaCl, 10 mM NaF, 0.4 mM EDTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM PMSF. Lysates were spun to remove nuclei and cell debris. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. Phosphorylation of IκBα was assessed by immunoblotting with a phospho-specific anti-IκBα antibody (New England Biolabs) according to the manufacturer's instructions. Goat anti–rabbit horseradish peroxidase (HRP; Vector Laboratories) was used to detect the bound phospho-IκBα antibodies, followed by incubation with Supersignal Chemiluminescence substrate (Pierce Chemical Co.).
DC Assays.
DCs were purified from the spleens of Flt3L-Ig–treated aly/+ or aly/aly mice by negative selection using magnetic beads, as described previously 29. DCs were cultured at 2 × 106 cells/ml in complete RPMI with GM-CSF/IL-4 (PeproTech), both at 10 ng/ml, with or without anti-CD40 (10 μg/ml). Culture supernatant was assayed for IL-12 on day 3 by commercial ELISA kit (PharMingen).
DCs purified as described above were pulsed with OVA peptide (323–339) for 90 min, washed extensively, and then plated with 105 OTII cells 30 at various DC densities as indicated. At 48 h, culture supernatants were assayed for the presence of IL-2 by commercial ELISA kit (PharMingen).
Results and Discussion
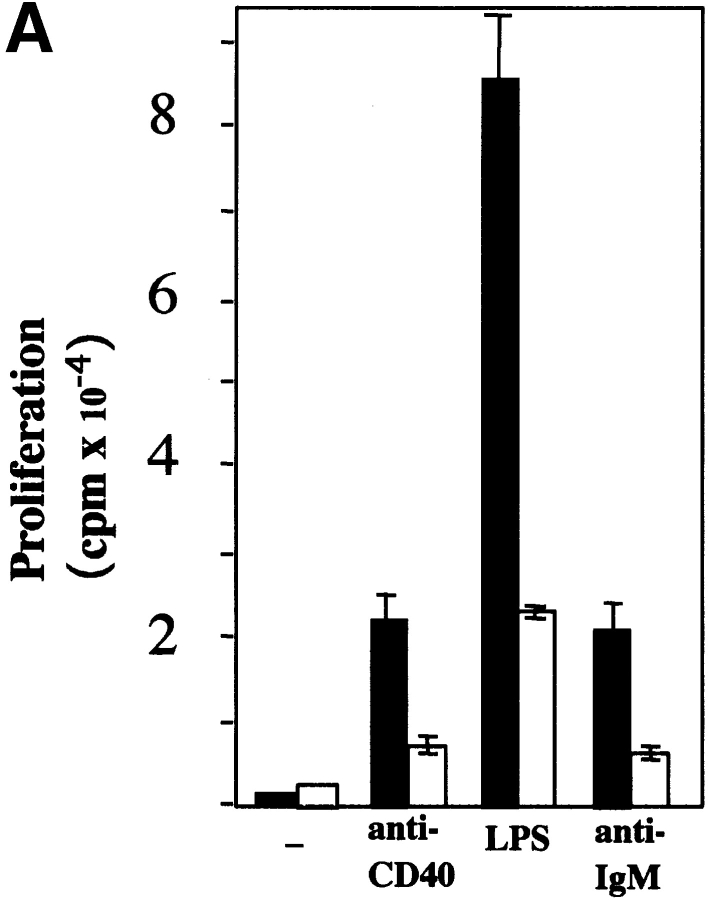
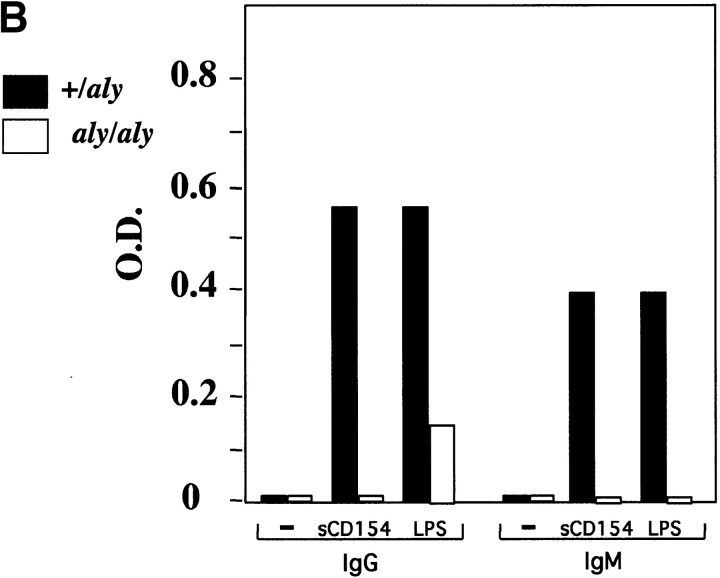
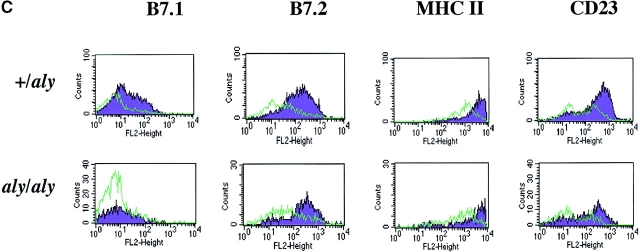
To investigate the function of NIK in CD40-mediated B cell activation, B cells from aly/+ and aly/aly mice were assessed for their ability to proliferate, produce Ig, and upregulate costimulatory molecules in response to soluble (s)CD154 and other polyclonal activators. B cells from aly/aly mice displayed a significant reduction in proliferative capacity in response to CD40, LPS, and anti-Ig activation relative to B cells from aly/+ mice (Fig. 1 A). Similarly, the capacity of B cells from aly/aly mice to produce IgM and IgG in response to sCD154 and LPS was also reduced (Fig. 1 B). In contrast to the diminished proliferation and Ig production observed in the aly/aly B cells, upregulation of several surface molecules (MHC class II, intercellular adhesion molecule 1 [ICAM-1], CD23, and B7.2) that are hallmarks of B cell activation in response to CD40 stimulation appeared intact. However, upregulation of one surface marker, B7.1, was impaired by the aly mutation (Fig. 1 C).
Figure 1.
Role of NIK in activation of B cells. aly/aly and aly/+ splenic B cells were cultured in vitro and assayed for their ability to proliferate (A), produce Ig (B), and upregulate cell surface markers (C) in response to CD40 stimulation. (A) Induction of proliferation by anti-CD40 (10 μg/ml), FGK115, LPS (50 μg/ml), and anti-IgM (goat anti–mouse IgM) was measured by the incorporation of [3H]thymidine from 66 to 72 h after initiation of culture. All cultures contained 10 ng/ml of IL-4. (B) Induction of Ig secretion was performed using sCD154 or LPS in combination with IL-4 (10 ng/ml) and IL-5 (10 ng/ml). Ig secretion was measured using an isotype-specific ELISA. (C) Expression of cell surface molecules on splenic B cells from aly/+ (top) or aly/aly (bottom) mice after culture with (purple histogram) or without (green outline histogram) sCD154 for 48 h was measured by flow cytometry.
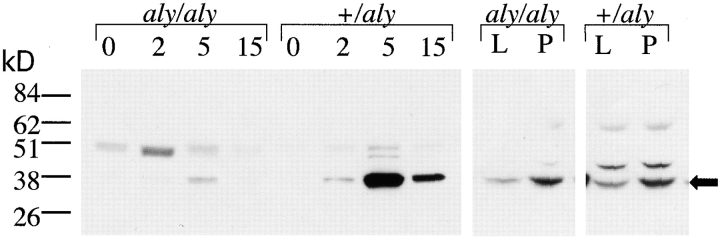
To further elucidate the molecular basis of the defects in biological responses of aly/aly B cells to CD40 stimulation, phosphorylation of IκBα in response to CD40 stimulation was analyzed as an indicator of NF-κB activation. B cells from spleens of aly/+ and aly/aly mice were stimulated in vitro with sCD154, LPS, or PMA plus ionomycin and assayed for phosphorylation of IκBα by Western blot analysis (Fig. 2). A significant reduction in CD40-stimulated phosphorylation of IκBα was observed in B cells from aly/aly mice relative to B cells from aly/+ animals. Interestingly, LPS-induced NF-κB activation was similar in B cells from aly/aly mice, even though the aly mutation did impact the biological responses to LPS. Finally, no difference in phosphorylation of IκBα was observed when B cells from aly/aly mice were pharmacologically activated with PMA plus ionomycin.
Figure 2.
Activation of NF-κB by NIK and aly NIK. Effect of aly NIK expression on IκBα phosphorylation in primary B cells. B cells from aly/aly and aly/+ mice were stimulated in vitro for 2, 5, or 15 min with sCD154, or for 5 min with LPS (50 μg/ml) or PMA (10 ng/ml) plus ionomycin (25 nM), and were assayed for phosphorylation of IκBα by Western blot with a phospho-specific anti-IκBα.
The direct effects of the aly NIK mutation on CD40-dependent B cell proliferation and Ig production indicate that NIK is an important intermediary of B cell activation. Since the aly NIK mutation did not have a noticeable effect on upregulation of several surface proteins, other signaling cascades such as c-Jun NH2-terminal kinase (JNK)- or tyrosine kinase–mediated pathways 31 32 may be necessary for complete B cell activation. Alternatively, the aly mutation may have left some aspects of NIK function intact.
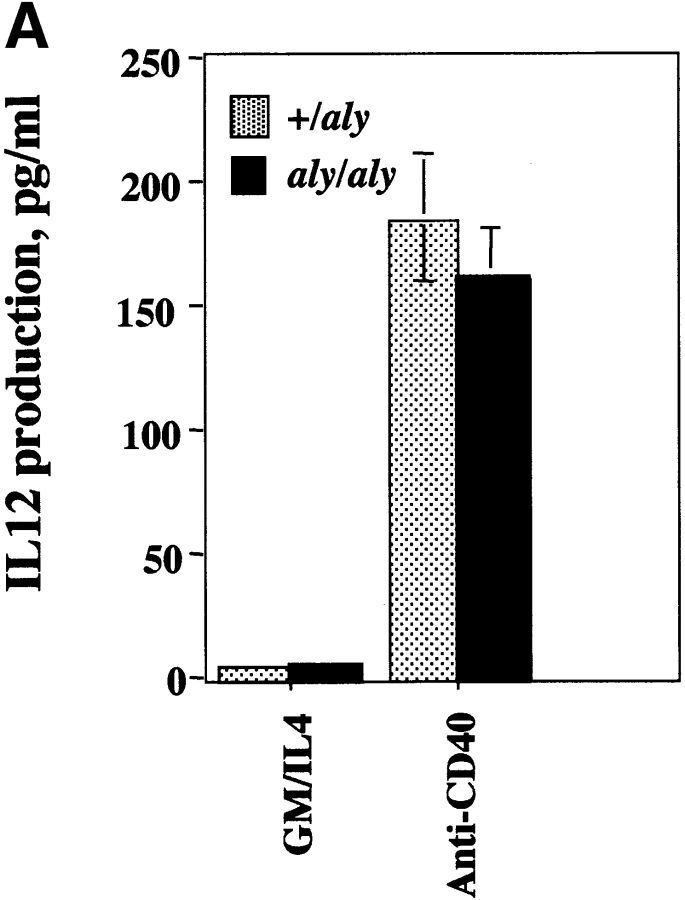
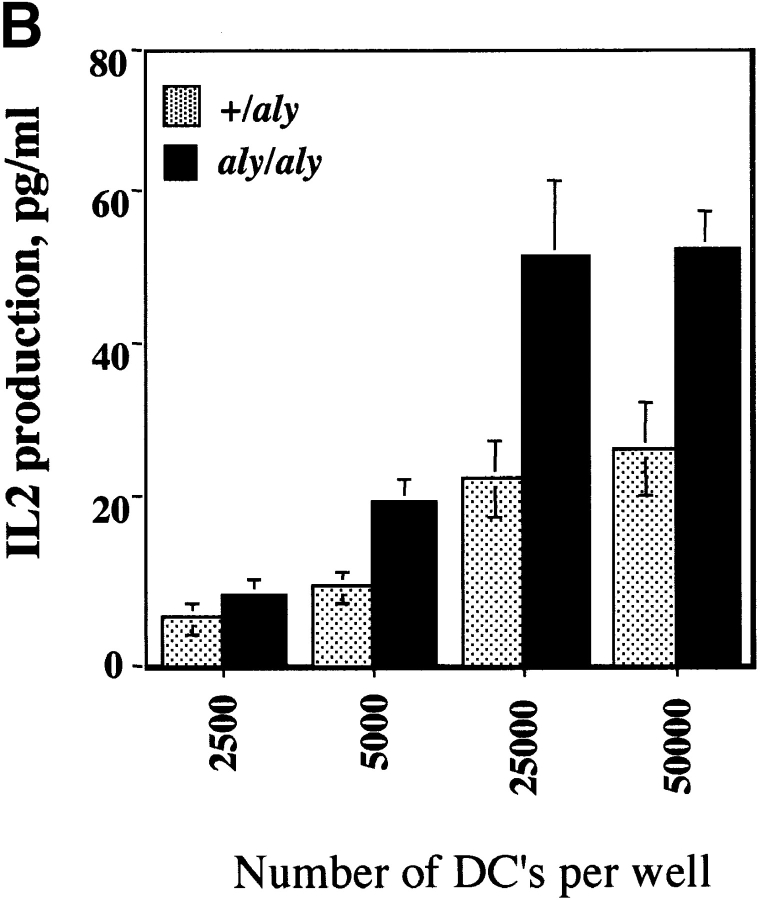
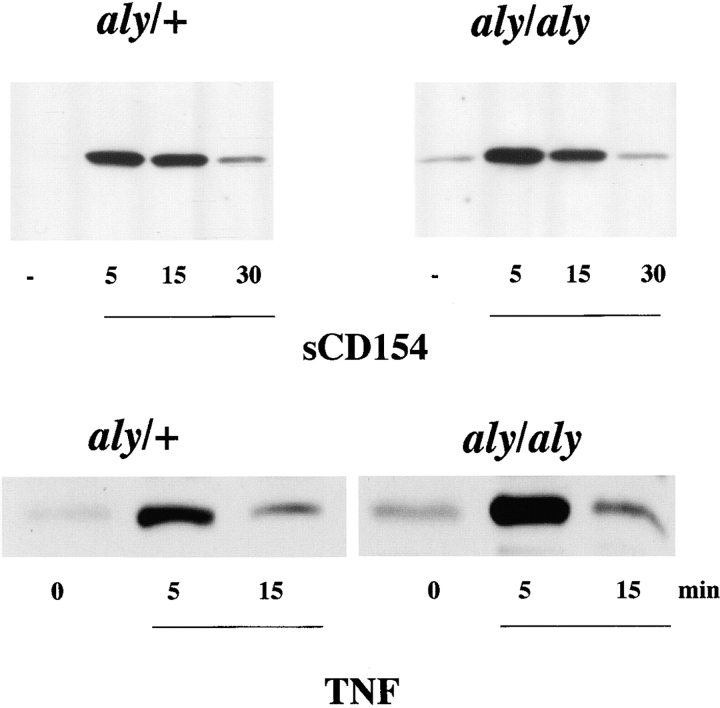
Given that the aly mutation exerted a severe biological phenotype in B cells, the impact of the aly mutation in another CD40-responsive cell type was evaluated. It is known that the APC capacity of DCs is influenced by CD40 ligation, as is the capacity of DCs to produce cytokines (IL-12 33) and chemokines (regulated on activation, normal T cell expressed and secreted [RANTES]) 34. To evaluate whether DC responses were impaired by the aly mutation, DCs from the spleens of aly/aly and aly/+ mice were assayed in vitro for their ability to produce IL-12 and present antigen in response to CD40 engagement. DCs from both aly/aly and aly/+ mice produced IL-12 at comparable levels in response to CD40 triggering (Fig. 3 A). To assess DC maturation, DCs from aly/aly mice and aly/+ mice were used as a source of APCs for Tg T cells that express a TCR that specifically recognizes a peptide (amino acids 323–339) from OVA. DCs were pulsed with OVA peptide and cultured with OVA-specific Tg T cells for 2 d, and release of IL-2 into the culture medium was measured by ELISA. DCs from aly/aly and aly/+ mice were equally effective at stimulating IL-2 release from Tg T cells (Fig. 3 B). Furthermore, the CD40-induced upregulation of B7.1, B7.2, ICAM-1, and MHC class II by aly/+ and aly/aly DCs was indistinguishable (data not shown). To evaluate if the CD40-stimulated NF-κB activation pathway in DCs is impaired as a result of the aly mutation, DCs from aly/aly and aly/+ were stimulated in vitro with sCD154 and assayed for phosphorylation of IκBα by Western blot analysis (Fig. 4). Phosphorylation of IκBα in response to sCD154 was indistinguishable in DCs from both aly/aly and aly/+ mice. Since TNF-α is a known inducer of NF-κB, and NIK has been implicated in TNFR signaling, we evaluated whether the activation of NF-κB by TNF-α in aly/aly DCs was impaired. As observed with CD40 activation, the activation of NF-κB by TNF-α was indistinguishable between the aly/aly and aly/+ DCs. These results indicate that NIK is not essential for CD40-induced IL-12 production or maturation or for NF-κB activation in DCs. It further shows that NIK is dispensable for activation of NF-κB in DCs by TNF-α.
Figure 3.
Responses of DCs in aly/aly and aly/+ mice. (A) The NIK mutation does not affect CD40-induced IL-12 production by DCs. aly/+ or aly/aly DCs were cultured at 2 × 106 cells/ml in cRPMI with GM-CSF/IL-4 (both at 10 ng/ml) with or without anti-CD40 (10 μg/ml). Culture supernatant was assayed for IL-12 on day 3 by ELISA. (B) The NIK mutation does not affect the antigen-presentation capacity of DCs. DCs were purified as in A and were pulsed with OVA peptide (323–339) for 90 min, washed extensively, and then plated with 105 OTII cells (OVA-specific Tg T cells) at various DC densities as indicated. At 48 h, culture supernatants were assayed for the presence of IL-2 by ELISA.
Figure 4.
Phosphorylation of IκBα in response to CD40 engagement is normal in DCs from aly/aly mice. DCs were stimulated in vitro with sCD154 or TNF-α as indicated, and were assayed for phosphorylation of IκBα by Western blot with a phospho-specific IκBα antibody.
Although experiments with transfected cell lines have suggested that NIK is involved in CD40 signaling, the data presented in this report demonstrate that NIK plays an essential role in vivo in CD40-dependent biological responses of B cells, but not in DCs. B cells from aly mice were largely unresponsive to CD40-induced proliferation, Ig production, and NF-κB activation. However, induced expression of several early activation molecules (e.g., CD23, B7.2) was not impaired by the aly mutation. In contrast, DCs from aly/aly mice were indistinguishable from aly/+ mice in their capacity to produce IL-12 in response to CD40. Furthermore, aly/aly-derived DCs were able to present antigen as efficiently as DCs from aly/+ mice. Direct assessment of NF-κB activation in aly-derived DCs demonstrated that NF-κB was intact. Hence, the function of NIK as a mediator of NF-κB activation via CD40 is lineage restricted, and it is likely that another mitogen-activated protein kinase (MAPK) is critical for NF-κB activation via CD40 in DCs. Since MEKK1 has also been shown to be an IKK, it is possible that DCs may utilize MEKK1 to trigger CD40-dependent responses.
Responses to LPS and anti-Ig were also significantly reduced, suggesting that NIK plays a role in these biological responses as well. The role of NIK as a kinase involved in signal transduction through non-TNFR family members has been suggested by studies showing that NIK may regulate CD28-induced IL-2 production 35. Hence, NIK may have multiple roles in the regulation of lymphocyte activities that are not directly related to its ability to associate with TRAFs.
Interestingly, the aly mutation that has been reported in mice bears a striking resemblance to a mutation observed in humans. Although most hyper-IgM (HIM) patients have a mutation in the CD154 gene, there is a cohort of HIM patients that have an autosomal recessive mutation that manifests as a defect in CD40 signaling 36 37. One such patient 38 was shown to have defective responses in B cell activation, but normal responses within the DC compartment in response to CD40 signaling. In addition, these patients do not exhibit an overt enhanced susceptibility to opportunistic infections, unlike HIM patients with mutations in the CD154 gene. It is possible that mutations in NIK may account for the selective loss of CD40-dependent immunity in the humoral, but not cellular, compartments of the immune system.
Acknowledgments
We are grateful to Satish Menon at DNAX Research Institute for generously providing the Flt3L-Ig used in these studies.
This work was supported by National Institutes of Health grants R01 AI42234 and R01 AI26296 to R.J. Noelle.
Footnotes
Address correspondence to Randolph J. Noelle, Department of Microbiology, Dartmouth Medical School, 1 Medical Center Dr., Lebanon, NH 03756. Phone: 603-650-7670; Fax: 603-650-6223; E-mail: rjn@dartmouth.edu
References
- Noelle R.J. CD40 and its ligand in cell-mediated immunity. Agents Actions Suppl. 1998;49:17–22. doi: 10.1007/978-3-0348-8857-8_4. [DOI] [PubMed] [Google Scholar]
- Xu J., Foy T.M., Laman J.D., Elliott E.A., Dunn J.J., Waldschmidt T.J., Elsemore J., Noelle R.J., Flavell R.A. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. The immune responses in CD40-deficient miceimpaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Foy T.M., Aruffo A., Ledbetter J.A., Noelle R.J. In vivo CD40–gp39 interactions are essential for thymus-dependent immunity. II. Prolonged in vivo suppression of primary and secondary humoral immune responses by an antibody targeted to the CD40 ligand, gp39. J. Exp. Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., van Kooten C., Durand I., Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey M.F., Barth R.J., Jr., Noelle R.J. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J. Leukoc. Biol. 1998;63:418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Dubois B., Fayette J., Burdin N., Briere F., Miossec P., Rissoan M.C., van Kooten C., Caux C. Functional CD40 antigen on B cells, dendritic cells and fibroblasts. Adv. Exp. Med. Biol. 1995;378:79–83. doi: 10.1007/978-1-4615-1971-3_16. [DOI] [PubMed] [Google Scholar]
- Berberich I., Shu G.L., Clark E.A. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J. Immunol. 1994;153:4357–4366. [PubMed] [Google Scholar]
- Hu H.M., O'Rourke K., Boguski M.S., Dixit V.M. A novel RING finger protein interacts with the cytoplasmic domain of CD40. J. Biol. Chem. 1994;269:30069–30072. [PubMed] [Google Scholar]
- Ishida T.K., Tojo T., Aoki T., Kobayashi N., Ohisho T., Watanabe T., Yamamoto T., Inoue J. TRAF5, a novel tumor necrosis factor-receptor associated factor family protein, mediates CD40 signaling. Proc. Natl. Acad. Sci. USA. 1996;93:9437–9441. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Mizushima S., Azuma S., Kobayashi N., Tojo T., Suzuki K., Aizawa S., Watanabe T., Mosialos G., Kieff E. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J. Biol. Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- Regnier C.H., Tomasetto C., Moog-Lutz C., Chenard M.P., Wendling C., Basset P., Rio M.C. Presence of a new conserved domain in CART1, a novel member of the tumor necrosis factor receptor-associated protein family, which is expressed in breast carcinoma. J. Biol. Chem. 1995;270:25715–25721. doi: 10.1074/jbc.270.43.25715. [DOI] [PubMed] [Google Scholar]
- Rothe M., Wong S.C., Henzel W.J., Goeddel D.V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- Song H.Y., Regnier C.H., Kirschning C.J., Goeddel D.V., Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascadesbifurcation of nuclear factor-kappaB and c-Jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc. Natl. Acad. Sci. USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne M.R., Robbins M., Hambor J.E., Mackey M.F., Kosaka Y., Nishimura T., Gigley J.P., Noelle R.J., Calderhead D.M. Assembly and regulation of the CD40 receptor complex in human B cells. J. Exp. Med. 1997;186:337–342. doi: 10.1084/jem.186.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M., Sarma V., Dixit V.M., Goeddel D.V. TRAF2-mediated activation of NF-kappaB by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- DiDonato J., Mercurio F., Rosette C., Wu-Li J., Suyang H., Ghosh S., Karin M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinin N.L., Boldin M.P., Kovalenko A.V., Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- Lee F.S., Peters R.T., Dang L.C., Maniatis T. MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta. Proc. Natl. Acad. Sci. USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F.S., Hagler J., Chen Z.J., Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- Zandi E., Karin M. Bridging the gapcomposition, regulation, and physiological function of the IkappaB kinase complex. Mol. Cell. Biol. 1999;19:4547–4551. doi: 10.1128/mcb.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M.J., Ghosh S. Signal transduction through NF-kappaB. Immunol. Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- Futterer A., Mink K., Luz A., Kosco-Vilbois M.H., Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- De Togni P., Goellner J., Ruddle N.H., Streeter P.R., Fick A., Mariathasan S., Smith S.C., Carlson R., Shornick L.P., Strauss-Schoenberger J. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Shinkura R., Kitada K., Matsuda F., Tashiro K., Ikuta K., Suzuki M., Kogishi K., Serikawa T., Honjo T. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappaB-inducing kinase. Nat. Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- Roy M., Aruffo A., Ledbetter J.A., Linsley P., Kehry M., Noelle R.J. Studies on the independence of gp39 and B7 expression and function during antigen-specific immune responses. Eur. J. Immunol. 1995;25:596–603. doi: 10.1002/eji.1830250243. [DOI] [PubMed] [Google Scholar]
- Buhlmann J.E., Gonzalez M., Ginther B., Panoskaltsis-Mortari A., Blazar B.R., Greiner D.L., Rossini A.A., Flavell R., Noelle R.J. Cutting edgesustained expansion of CD8+ T cells requires CD154 expression by Th cells in acute graft versus host disease. J. Immunol. 1999;162:4373–4376. [PubMed] [Google Scholar]
- Aruffo A., Farrington M., Hollenbaugh D., Li X., Milatovich A., Nonoyama S., Bajorath J., Grosmaire L.S., Stenkamp R., Neubauer M. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E., Brasel K., Teepe M., Roux E.R., Lyman S.D., Shortman K., McKenna H.J. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand–treated micemultiple dendritic cell subpopulations identified. J. Exp. Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Carbone F.R., Barnden M., Blanas E., Allison J., Heath W.R., Miller J.F. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J. Exp. Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata N., Patel H.R., Terada N., Aruffo A., Johnson G.L., Gelfand E.W. Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J. Biol. Chem. 1995;270:30823–30828. doi: 10.1074/jbc.270.51.30823. [DOI] [PubMed] [Google Scholar]
- Faris M., Gaskin F., Parsons J.T., Fu S.M. CD40 signaling pathwayanti-CD40 monoclonal antibody induces rapid dephosphorylation and phosphorylation of tyrosine-phosphorylated proteins including protein tyrosine kinase Lyn, Fyn, and Syk and the appearance of a 28-kD tyrosine phosphorylated protein. J. Exp. Med. 1994;179:1923–1931. doi: 10.1084/jem.179.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin 12 and enhances T cell stimulatory capacityT–T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Palermo B., Lenig D., Miettinen M., Matikainen S., Julkunen I., Forster R., Burgstahler R., Lipp M., Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999;29:1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lin X., Cunningham E.T., Jr., Mu Y., Geleziunas R., Greene W.C. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-kappaB acting through the NF-kappaB-inducing kinase and IkappaB kinases. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- Conley M.E., Larche M., Bonagura V.R., Lawton A.R., III, Buckley R.H., Fu S.M., Coustan-Smith E., Herrod H.G., Campana D. Hyper IgM syndrome associated with defective CD40-mediated B cell activation. J. Clin. Invest. 1994;94:1404–1409. doi: 10.1172/JCI117476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy A., Hivroz C., Mazerolles F., Schiff C., Bernard F., Jouanguy E., Revy P., DiSanto J.P., Gauchat J.F., Bonnefoy J.Y. Abnormal CD40-mediated activation pathway in B lymphocytes from patients with hyper-IgM syndrome and normal CD40 ligand expression. J. Immunol. 1997;158:2576–2584. [PubMed] [Google Scholar]
- Revy P., Geissmann F., Debre M., Fischer A., Durandy A. Normal CD40-mediated activation of monocytes and dendritic cells from patients with hyper-IgM syndrome due to a CD40 pathway defect in B cells. Eur. J. Immunol. 1998;28:3648–3654. doi: 10.1002/(SICI)1521-4141(199811)28:11<3648::AID-IMMU3648>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]