Abstract
Streptococcus pneumoniae is becoming increasingly antibiotic resistant worldwide, and thus new antimicrobials are badly needed. We report the use of Cpl-1, the lytic enzyme of a pneumococcal bacteriophage, as an intravenous therapy for pneumococcal bacteremia in a mouse model. A 2,000-μg dose of Cpl-1 reduced pneumococcal titers from a median of log10 4.70 CFU/ml to undetectable levels (<log10 2.00 CFU/ml) within 15 min. This dose given 1 h after intravenous infection led to 100% survival at 48 h, compared to the 20% survival of buffer-treated controls. In advanced bacteremia, treatment with two doses at 5 and 10 h still resulted in significantly longer survival (P < 0.0001) and a hazard ratio of 0.29 (95% confidence interval, 0.04 to 0.35). The enzyme is immunogenic, but the treatment efficacy was not significantly diminished after previous intravenous exposure of mice and hyperimmune rabbit serum did not neutralize the activity. Cpl-1 is also very effective as a topical nasal treatment against colonization by S. pneumoniae. In vitro, the enzyme is active against many serotypes of S. pneumoniae, independent of their penicillin resistance, and it is very specific for this species. Bacteriophage enzymes are unusual but extremely effective antimicrobials and represent a new weapon against infections with resistant bacteria.
Streptococcus pneumoniae is a leading cause of acute otitis media, meningitis, pneumonia, and sepsis, and pneumococci are increasingly resistant to antibiotics. In the United States, for example, the level of penicillin resistance was 26% and the level of erythromycin resistance 20% in 1999 (11). Immunization against infection shows promise and may slow resistance trends to some extent, since several of the most resistant serotypes are affected (1, 2, 6, 19). Nevertheless, the efficacy of immunization against colonization is limited, and there is suspicion that there could be replacement with nonvaccine serotypes and fear that resistance may outpace vaccination (4, 8, 11). Phage lytic enzymes are highly evolved proteins that were designed over millions of years for a single purpose, to kill the host bacterium, and they may be used to circumvent many of these problems.
Previously, the effectiveness of topical application of the pneumococcal phage enzyme Pal (an N-acetyl-muramoyl-l-alanine amidase) for specific elimination of mucosal colonization by S. pneumoniae was described (10). Another enzyme, Cpl-1, a muramidase, has been described, but this enzyme has not been tested as an antimicrobial therapy (7, 9, 15). These two enzymes have a common binding domain, which binds to choline and accounts for the pneumococcal specificity (7, 17). We describe here for the first time the use of a phage lytic enzyme for control of bacteremia through intravenous administration. We describe the pharmacokinetics of Cpl-1 in blood, show that antibodies to Cpl-1 are not neutralizing, report that this enzyme is stable under storage conditions, and finally show that Cpl-1 does not appear to cause visible harm in mice after repeated administration of high doses.
MATERIALS AND METHODS
Production and purification of Cpl-1 and definition of activity.
Construction of DH5α(pJML6) and production and purification of Cpl-1 have been described elsewhere (9). Cpl-1 was suspended in 50 mM phosphate buffer with 1 mM EDTA and 1 mM dithiothreitol (pH 7.0) (buffer). Endotoxin was removed from Cpl-1 to ≤35 endotoxin units (EU)/ml with Acticlean Etox (Sterogene Bioseparations, Carlsbad, Calif.), and the remaining endotoxin was quantified by the Limulus amebocyte lysate assay (QCL-1000; Cambrex-BioWhittaker, Walkersville, Md.).
One unit of Cpl-1 activity is defined as the reciprocal of the dilution that decreases the optical density of a reaction mixture containing enzyme and log-phase S. pneumoniae from around 0.5 by one-half within 15 min (9, 10) compared to the optical density of a control containing only enzyme buffer. Generally, 1 U of enzyme activity corresponds to ∼1 μg of protein, as measured by the Bradford assay (Bio-Rad, Hercules, Calif.).
Intravenous killing efficacy.
All animal experiments were carried out according to federal and institutional guidelines. Four- to six-week-old female C3H/HeJ mice (The Jackson Laboratory, Bar Harbor, Maine) were infected through the tail vein with 3 × 107 CFU of log-phase S. pneumoniae serotype 14 (strain DCC 1490, penicillin susceptible) and were treated with either 2,000 μg of Cpl-1 (n = 9) or the same volume (100 μl) of buffer (n = 8) intravenously 10 h postinfection. Blood samples were obtained before and 15 and 120 min after treatment, and the bacterial titers were determined by serial dilution and plating on blood agar. The presence of S. pneumoniae in alpha-hemolytic colonies was confirmed by using optochin disks. Differences between the titer at zero time and the titer at 15 min were analyzed with the Mann-Whitney test. Endotoxin-resistant C3H/HeJ mice were used in this experiment because the enzyme preparation used contained some endotoxin.
Intravenous half-life of Cpl-1.
A single intravenous bolus containing 1.6 mg of Cpl-1 was injected into six BALB/c mice (Charles River Laboratories, Wilmington, Mass.), and blood was collected after 5, 10, and15 min from three animals and after 30, 60, and 120 min from the other three animals. Plasma enzyme concentrations were measured by spot densitometry by using a Western blot method and standard Cpl-1 concentrations. A one-phase exponential decay curve was fitted to calculate the half-life.
Mouse survival with Cpl-1.
Four- to six-week-old female BALB/c mice were infected intravenously with 3 × 108 CFU of log-phase S. pneumoniae serotype 14. This infectious dose was higher than the dose used previously, since BALB/c mice are somewhat more resistant to pneumococcal infection. The mice were first treated 1 h after infection with either 2,000 μg of Cpl-1 (n = 10) or the same volume (100 μl) of buffer (n = 10) intravenously, and death or survival was recorded until the endpoint at 48 h. The blood and spleens of euthanized survivors were tested for the presence of S. pneumoniae. In a second experiment mice were treated 5 and 10 h postinfection with 2,000 μg of Cpl-1 (n = 14) or the same volume of buffer (n = 14) and observed until they died. Survival curves were created by the Kaplan-Meier method, and the differences between the curves were analyzed by the log-rank test. The median survival time and a hazard ratio were calculated.
Eradication of pneumococci.
Four- to six-week-old BALB/c mice were colonized as described previously (10, 20) and were treated nasally and orally after 42 h with either 1,000 μg of Cpl-1 (n = 10) or buffer (n = 10). The difference between the groups was analyzed by the Mann-Whitney test.
In vitro killing assays and specificity.
The killing spectrum of Cpl-1 was assessed as previously described for the bacteriophage enzyme (amidase) Pal by using 15 clinical strains of S. pneumoniae, AR620 (serotype 1), DCC1714 (serotype 3), GB2092 (serotype 4), AR314 (serotype 5), DCC1850 (serotype 6), DCC1335 (serotype 9V, clonal type Sp9-3), GB2163 (serotype 10), DCC1811 (serotype 11), DCC1490 (serotype 14), DCC1494 (serotype 14, clonal type Sp14-3), DCC1476 (serotype 15), GB2017 (serotype 18), DCC1355 (serotype 19), DCC1420 (serotype 23F, clonal type Sp23-1), and DCC1808 (serotype 24). We also tested Cpl-1 against three pneumococcal mutants and their common ancestor, D39 (serotype 2). R36A is a capsule-free strain derived after numerous passages from D39, R6 was derived from R36A, and Lyt 4-4 was derived from R6 and has a point mutation in the gene for the major autolysin LytA, which renders it nonfunctional. All pneumococcal strains were obtained from A. Tomasz. We also included seven oral streptococcal species and Staphylococcus aureus (10).
Toxicity assays.
Four- to six-week-old BALB/c mice were injected intravenously three times with 1,000, 2,000, or 4,000 μg of of Cpl-1 (in a 100-μl bolus each time) at 4-h intervals (n = 2 for each dose). Two mice received three weekly nasal administrations of 1,000 μg of Cpl-1 in 50 μl (n = 2). The weight, aspect, and behavior were examined daily for 1 week and weekly thereafter for a total of 4 weeks.
Stability.
Enzyme activity was measured after exposure for 30 min to temperatures up to 65°C, for 21 days to 37°C, and for >6 months to 4°C. The activity was tested as described above at a pH range of 2 to 13 in a universal buffer (40 mM phosphoric acid and 40 mM boric acid with the pH adjusted with NaOH), in 75 to 600 mM NaCl, in 0 to 12.3 mM MgCl2, and in 0 to 34 mM CaCl2. Enzyme activity was also verified after lyophilization and resuspension in water after more than 16 months of storage at 4°C.
Enzyme activity with rabbit hyperimmune serum.
A 20-μl solution containing 2,000 μg of Cpl-1 or buffer (control) was preincubated for 10 min or 1 h with 180 μl of rabbit serum from the same animal before and after immunization. Then 150-μl portions of the incubated enzyme samples were each mixed with 150 μl of a pneumococcal suspension, and samples were taken at zero time and after 1, 2, 3, 4, 6, and 10 min for titer determinations. The rabbit antibody titer was defined as the reciprocal of the dilution that gave an absorption at 405 nm of 1.0 after a 30-min reaction with the chromogenic substrate in an enzyme-linked immunosorbent assay.
Enzyme efficacy after previous exposure to Cpl-1.
Eight- to ten-week- old BALB/c mice from the intravenous toxicity assay described above (n = 6) and naïve control mice of the same age (n = 6) were challenged intravenously with S. pneumoniae as described above and treated 10 h later with 200 μg of Cpl-1 intravenously. Blood titers were determined before treatment and 15 min after treatment. The difference between the groups was analyzed by the Mann-Whitney test.
Antibody production after previous exposure to Cpl-1.
Serum from the 12 mice described above, 6 of which were previously exposed to Cpl-1, was tested by using an enzyme-linked immunosorbent assay for immunoglobulin G against Cpl-1. The cutoff was defined as the average for the unexposed control mice plus three standard deviations. The antibody titer was defined as described above.
RESULTS
Bacteremia model.
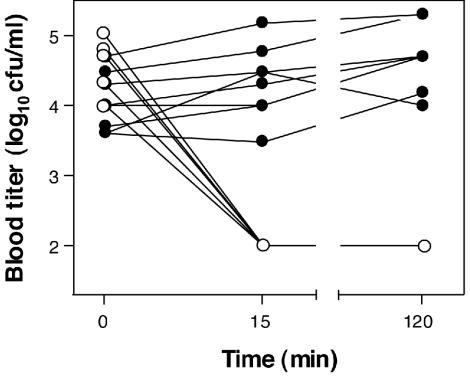
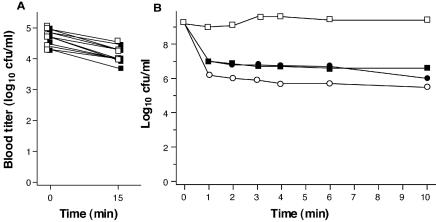
To test the bactericidal activity of Cpl-1 in the bloodstream of mice, C3H/HeJ mice (n = 17) were infected intravenously with a penicillin-susceptible S. pneumoniae serotype 14 strain and given either 2,000 μg of Cpl-1 (n = 9) or buffer (n = 8) as a single intravenous bolus 10 h later. Within 15 min or less Cpl-1 reduced the titers from a median of log10 4.70 CFU/ml (range, log10 4.00 to 5.00 CFU/ml) to ≤ log10 2.0 CFU/ml (detection limit), where they remained for at least 2 h (Fig. 1). The pneumococcal titers in buffer-treated mice, however, increased from log10 4.00 CFU/ml (range, log10 3.60 to 4.70 CFU/ml) to 4.39 CFU/ml (range, log10 3.48 to 5.18 CFU/ml) and log10 4.70 CFU/ml (range, log10 4.00 to 5.30 CFU/ml) at 15 min and 2 h, respectively (P < 0.0001 for the comparison of the titers for the groups at 15 min).
FIG. 1.
Bactericidal activity of Cpl-1 in the bloodstream of mice. In mice receiving a single bolus containing 2,000 μg of Cpl-1 (○), the bacterial blood titers dropped rapidly below the detection limit (log10 2.0 CFU/ml) and remained undetectable at 120 min, in contrast to the titers in mice that received only buffer (•).
We evaluated the pharmacokinetics of Cpl-1 in the plasma of mice after injection of a single bolus by Western blotting and spot densitometry. The results revealed a half-life of 20.5 min.
Survival.
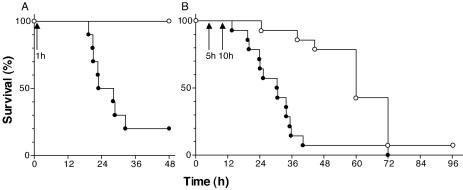
We next investigated whether the rapid intravenous killing of S. pneumoniae by Cpl-1 had a beneficial effect on the survival of the mice. We infected BALB/c mice (n = 20) intravenously with serotype 14 S. pneumoniae as described above and treated them 1 h later with a single bolus containing 2,000 μg of Cpl-1 (n = 10) or buffer (n = 10) by the same route. All animals treated with Cpl-1 survived until the endpoint at 48 h, whereas the median survival time of the buffer-treated mice was only 25.56 h and barely 20% of the latter mice survived for 48 h (P = 0.0003) (Fig. 2A). Blood and organ cultures of the euthanized surviving mice showed that only one Cpl-1-treated animal was totally free of infection at 48 h, and treatment with Cpl-1 may only have delayed death. To examine the effect of Cpl-1 treatment in advanced bacteremia, we treated mice in a second experiment with two doses of either 2,000 μg of Cpl-1 (n = 14) or buffer (n = 14) at 5 and 10 h postinfection and observed the animals for a longer period. The Cpl-1-treated mice had a median survival time of 60 h, whereas the buffer-treated animals again survived for only a median of 30.75 h, similar to the results of the first survival experiment. The survival curves were again significantly different (P < 0.0001), and the hazard ratio for the Cpl-1 treatment was 0.29 (Fig. 2B). At 96 h, one animal in the Cpl-1-treated group was still alive.
FIG. 2.
Survival of bacteremic mice after Cpl-1 treatment. (A) Treatment of mice with a single bolus containing 2,000 μg of Cpl-1 (○) (n = 10) at 1 h after infection (arrow) led to 100% survival at 48 h, compared to the significantly lower level of survival of buffer-treated mice (•) (20%; n = 10), which had a median survival time of 25.56 h. (B) Treatment with two doses of Cpl-1 (○) (n = 14) at 5 and 10 h after infection (arrows) significantly extended survival compared to the survival after two administrations of only buffer (•) (n = 14). The hazard ratio for the Cpl-1 treatment was 0.29.
Mucosal colonization model.
Because pneumococci normally colonize the nasopharynx, we determined whether Cpl-1 is able to eradicate or reduce this colonization. We found that Cpl-1 is as efficient as the previously described other pneumococcal phage enzyme, Pal, in eliminating experimental nasopharyngeal colonization in mice (10). Five hours after intranasal and oral treatment with 1,000 μg of Cpl-1 (n = 10), we recovered no pneumococci in a 10-μl sample of the nasal wash (detection limit); at this time the buffer-treated animals (n = 10) were still colonized with a median of log10 2.82 CFU/10-μl nasal wash (range, log10 2.30 to 3.70 CFU/10-μl nasal wash) (P < 0.0001).
In vitro killing.
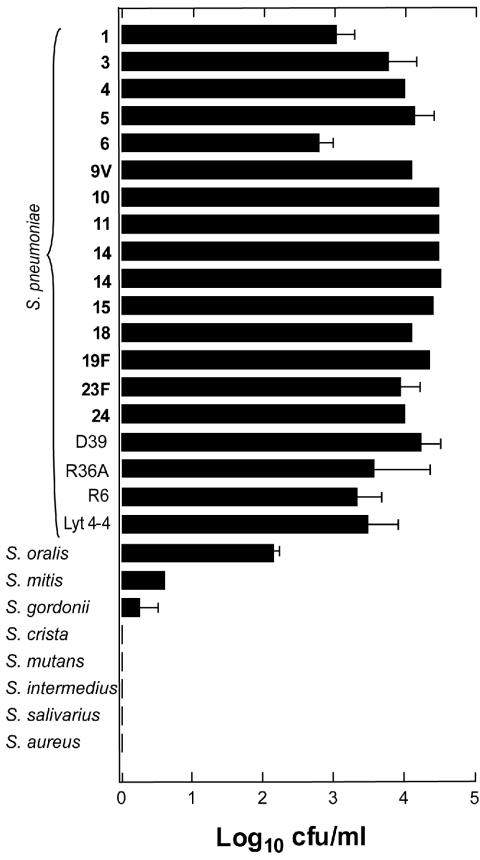
The antimicrobial spectrum of Cpl-1 was tested in vitro. In only 30 s, 100 μg of Cpl-1 per ml reduced a 108-CFU suspension of 19 strains of S. pneumoniae by 2.8 to 4.5 log10; this suspension included representatives of the 10 most frequent serogroups, three highly penicillin-resistant globally spread clones (Sp9-3, Sp14-3, and Sp23-1), mutants that have a nonfunctional major autolysin (Lyt 4-4) and no capsule (R36A and R6), and the common ancestor of these mutants (strain D39). The killing was specific for pneumococci, and there was lysis of no other gram-positive bacteria tested except Streptococcus oralis and Streptococcus mitis, whose levels were reduced by 2.1 and 0.6 log10, respectively (Fig. 3). Comparatively minor lysis of these organisms was observed previously with the other pneumococcal phage enzyme, Pal, and this can be explained by the choline contents of the cell walls of these two species, which are genetically very similar to S. pneumoniae. The pneumococcal mutants showed susceptibility identical to that of the clinical strains.
FIG. 3.
Activity spectrum of Cpl-1 against pneumococci and various gram-positive organisms. Treatment with 100 μg of Cpl-1 per ml reduced a 108-CFU suspension of 15 clinical strains of S. pneumoniae (capsular serotypes are indicated by boldface type; see Materials and Methods for strain designations) and three mutants and their ancestor (D39, R36A, R6, and Lyt 4-4) by 2.8 to 4.5 log10. Of the other tested species, only Streptococcus oralis, Streptococcus mitis, and Streptococcus gordonii lysed noticeably, but they lysed to a lesser extent than S. pneumoniae.
Toxicity and stability.
Toxicity assays performed with repeated intravenous and topical nasal administration of large amounts of Cpl-1 (see Materials and Methods for doses) revealed no signs of toxicity, as assessed by observing the weight, aspect, and behavior of the treated mice for 4 weeks.
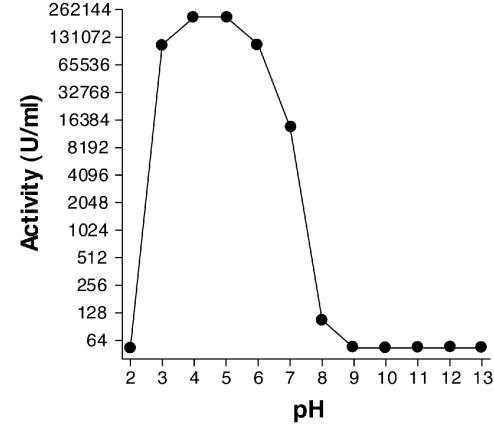
Cpl-1 was stable for more than 6 months at 4°C, for at least 3 weeks at 37°C, and for at least 30 min at 45°C. The activity was not affected by calcium concentrations between 0 and 34 mM (10 times the physiological concentration) or by magnesium concentrations between 0 and 12.3 mM (10 times the physiological concentration). However, treatment with NaCl at a concentration at or above 300 mM (two times the physiological concentration) resulted in a rapid decrease in the activity. Complete activity of the protein could be restored after lyophilization and storage at 4°C for more than 16 months. The pH optimum was between 4 and 5, and there was a complete loss of activity at pH values above 8 and below 3; the latter effect was due to precipitation (Fig. 4).
FIG. 4.
pH profile of Cpl-1 activity. Activity was tested in a universal buffer with a pH range of 2 to 13. The enzyme exhibited the highest activity at pH 4 and 5. At pH 7 it exhibited approximately 10% of the maximal lytic activity, and at pH 8 only 1% of the maximal lytic activity was found.
Immunogenicity.
Because phage lytic enzymes are immunogenic proteins, intravenous use of these enzymes is likely to induce an immune response that could interfere with the catalytic activity of the enzyme. To address this possibility, rabbit hyperimmune serum raised against Cpl-1 was tested to determine its effect on enzymatic bacterial lysis. Two thousand micrograms of Cpl-1 decreased the viable bacterial count in a pneumococcal solution from log10 9.2 to 6.2 CFU/ml within 1 min in nonimmunized serum. However, when the preparation was preincubated for 10 or 60 min in hyperimmune serum (titer, 10,000), the bacterial titer decreased slightly less, to log10 7.0 CFU/ml. All titers decreased another 0.5 log10 CFU/ml by 10 min (Fig. 5B). This shows that highly immune serum decreases or slows, but does not completely block, killing of S. pneumoniae by Cpl-1. To test the relevance of this in vivo, mice that had received three intravenous doses of the enzyme 4 weeks earlier (n = 6) and had tested positive for immunoglobulin G against Cpl-1 in five of six cases with titers of about 1:10 and naïve control mice (n = 6) were challenged with pneumococci intravenously and then treated with 200 μg of Cpl-1 after 10 h. This treatment reduced the bacteremic titer of previously exposed mice by a median of log10 0.40 CFU/ml (range, log10 0.30 to 0.70 CFU/ml) and the bacteremic titer of naïve mice by log10 0.57 CFU/ml (range, log10 0.37 to 0.74 CFU/ml) (Fig. 5A). These results were not significantly different, supporting the hyperimmune data.
FIG. 5.
Activity of Cpl-1 in previously exposed mice and in hyperimmune rabbit serum. (A) In vivo, 15 min after a single bolus containing 200 μg of Cpl-1, the pneumococcal blood titer of mice that had received three doses of 1,000, 2,000, or 4,000 μg of Cpl-1 4 weeks earlier (□) (n = 6) dropped by a median of log10 0.40 CFU/ml and the titer of unexposed mice (▪) (n = 6) decreased by log10 0.57 CFU/ml (the difference was not significant). (B) In vitro, the viable count of a pneumococcal suspension exposed to 2,000 μg of Cpl-1 in undiluted preimmune rabbit serum (○) exhibited a rapid 3-log10 CFU/ml reduction within 1 min, and the count continued to decrease more slowly up to 10 min. In hyperimmune serum after 10 min of preincubation (•) and 1 h of preincubation (▪) the killing was less efficient by 0.8 log10 but the lytic activity was not neutralized. □, control reaction with buffer in nonimmune serum.
DISCUSSION
Purified recombinant bacteriophage lytic enzymes are completely original agents for the control and prevention of diseases caused by gram-positive bacteria (12, 16). In this study we extended our previous studies and demonstrated that the muramidase Cpl-1 is not only very efficient as a topical application to clear pneumococci from the nasopharyngeal mucosa but also quickly kills S. pneumoniae in the bloodstream and significantly extends survival of bacteremic mice. Although bacteremic mice treated with Cpl-1 survived significantly longer than control animals survived, they eventually succumbed to the infection, indicating that one or two doses of the enzyme were not sufficient for complete eradication of pneumococci. The relatively short half-life of Cpl-1 in the blood of mice (20.5 min) likely contributed to this result. However, this short half-life is not unusual for foreign proteins and is similar to that found for streptokinase, a thrombolytic enzyme used for treatment of acute myocardial infarction (3). Nevertheless, after administration of a single bolus of Cpl-1, the bacterial titers in the blood of all animals tested dropped by ≥99% and remained there for at least 2 h. Such results suggest that phage enzymes may effectively eradicate pneumococci or reduce the level to a level at which the host immune system is able to take over, if the enzymes are administered by constant intravenous infusion for an established period. The fact that we found that Cpl-1 was safe when it was administered in high doses supports this hypothesis.
We found that we could achieve adequate killing with Cpl-1 in blood despite the fact that the physiological pH (pH 7.4) is at the upper limit of the range in which Cpl-1 is active. We have observed an activity maximum at a low pH for other bacteriophage enzymes (data not shown). This may confer an advantage in situations where acidic and anaerobic conditions prevail, such as in biofilms or pus, conditions in which a number of antibiotics are compromised (5).
While recurrent pneumococcal bacteremia is relatively rare (14), it would be beneficial if systemic administration of Cpl-1 could be performed repeatedly. Because they are proteins, phage enzymes are immunogenic, thus raising the possibility that enzyme-specific antibodies produced during treatment may reduce or completely block the enzyme's catalytic activity, making subsequent administration useless or perhaps dangerous (13, 18). The fact that hyperimmune rabbit serum did not neutralize Cpl-1 but only modestly inhibited Cpl-1 in vitro and the fact that Cpl-1 is equally active in previously exposed and unexposed mice are reassuring, but ultimately human testing will be required to be certain that repeated administration is feasible. Since several pneumococcal phage enzymes are known and provided that they all prove to be efficient, one could imagine a switch to another enzyme in case one loses potency due to an adverse immune reaction.
The temperature stability and long-term stability of Cpl-1, along with our ability to recombinantly produce large quantities of the molecule, are encouraging. Furthermore, it has previously been shown that a combination of two pneumococcus-specific lytic enzymes with different catalytic activities acts synergistically (9). This, combined with our finding that resistance to phage enzymes has not been found despite extensive experimental efforts (10, 16), adds additional support to the proposal that these enzymes could be used as a new form of anti-infective therapy. We believe that our findings warrant research into further therapeutic uses of this novel technology in order to advance its application in the clinical setting, particularly in an age of increased resistance to conventional antibiotics.
Acknowledgments
This study was supported by a grant from the Defense Advanced Research Projects Agency (DARPA) (to V.A.F.). J.M.L. was supported by fellowships from the Roche Research Foundation and the Swiss Foundation for Medical-Biological Scholarships, Switzerland.
Editor: J. N. Weiser
REFERENCES
- 1.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 2.Black, S. B., H. R. Shinefield, S. Ling, J. Hansen, B. Fireman, D. Spring, J. Noyes, E. Lewis, P. Ray, J. Lee, and J. Hackell. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810-815. [DOI] [PubMed] [Google Scholar]
- 3.Brucato, F. H., and S. V. Pizzo. 1990. Catabolism of streptokinase and polyethylene glycol-streptokinase: evidence for transport of intact forms through the biliary system in the mouse. Blood 76:73-79. [PubMed] [Google Scholar]
- 4.Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. [DOI] [PubMed] [Google Scholar]
- 5.Dunne, W. M., Jr. 2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fireman, B., S. B. Black, H. R. Shinefield, J. Lee, E. Lewis, and P. Ray. 2003. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr. Infect. Dis. J. 22:10-16. [DOI] [PubMed] [Google Scholar]
- 7.Garcia, J. L., E. Garcia, A. Arraras, P. Garcia, C. Ronda, and R. Lopez. 1987. Cloning, purification, and biochemical characterization of the pneumococcal bacteriophage Cp-1 lysin. J. Virol. 61:2573-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshman, R., C. Murdoch, G. Race, R. Burkinshaw, L. Shaw, and A. Finn. 2003. Pneumococcal nasopharyngeal carriage in children following heptavalent pneumococcal conjugate vaccination in infancy. Arch. Dis. Child. 88:211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeffler, J. M., and V. A. Fischetti. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 47:375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeffler, J. M., D. Nelson, and V. A. Fischetti. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170-2172. [DOI] [PubMed] [Google Scholar]
- 11.McCormick, A. W., C. G. Whitney, M. M. Farley, R. Lynfield, L. H. Harrison, N. M. Bennett, W. Schaffner, A. Reingold, J. Hadler, P. Cieslak, M. H. Samore, and M. Lipsitch. 2003. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat. Med. 10:10. [DOI] [PubMed] [Google Scholar]
- 12.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson, J. B., T. K. Nilsson, J. H. Jansson, K. Boman, S. Soderberg, and U. Naslund. 2002. The effect of streptokinase neutralizing antibodies on fibrinolytic activity and reperfusion following streptokinase treatment in acute myocardial infarction. J. Intern. Med. 252:405-411. [DOI] [PubMed] [Google Scholar]
- 14.Orlicek, S. L., H. G. Herrod, R. J. Leggiadro, G. Luedtke, and B. K. English. 1997. Repeated invasive pneumococcal infections in young children without apparent underlying immunodeficiency. J. Pediatr. 130:284-288. [DOI] [PubMed] [Google Scholar]
- 15.Sanz, J. M., and J. L. Garcia. 1990. Structural studies of the lysozyme coded by the pneumococcal phage Cp-1. Conformational changes induced by choline. Eur. J. Biochem. 187:409-416. [DOI] [PubMed] [Google Scholar]
- 16.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and killsBacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan, M. M., J. L. Garcia, R. Lopez, and P. Garcia. 1997. The lytic enzyme of the pneumococcal phage Dp-1: a chimeric lysin of intergeneric origin. Mol. Microbiol. 25:717-725. [DOI] [PubMed] [Google Scholar]
- 18.Squire, I. B., W. Lawley, S. Fletcher, E. Holme, W. S. Hillis, C. Hewitt, and K. L. Woods. 1999. Humoral and cellular immune responses up to 7.5 years after administration of streptokinase for acute myocardial infarction. Eur. Heart J. 20:1245-1252. [DOI] [PubMed] [Google Scholar]
- 19.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 20.Wu, H. Y., A. Virolainen, B. Mathews, J. King, M. W. Russell, and D. E. Briles. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb. Pathog. 23:127-137. [DOI] [PubMed] [Google Scholar]