Abstract
Normal pigmentation depends on the uniform distribution of melanin-containing vesicles, the melanosomes, in the epidermis. Griscelli syndrome (GS) is a rare autosomal recessive disease, characterized by an immune deficiency and a partial albinism that has been ascribed to an abnormal melanosome distribution. GS maps to 15q21 and was first associated with mutations in the myosin-V gene. However, it was demonstrated recently that GS can also be caused by a mutation in the Rab27a gene. These observations prompted us to investigate the role of Rab27a in melanosome transport. Using immunofluorescence and immunoelectron microscopy studies, we show that in normal melanocytes Rab27a colocalizes with melanosomes. In melanocytes isolated from a patient with GS, we show an abnormal melanosome distribution and a lack of Rab27a expression. Finally, reexpression of Rab27a in GS melanocytes restored melanosome transport to dendrite tips, leading to a phenotypic reversion of the diseased cells. These results identify Rab27a as a key component of vesicle transport machinery in melanocytes.
Keywords: melanocytes, melanosomes, transport, Rab27a, Griscelli syndrome
Introduction
Normal skin and hair pigmentation requires the synthesis of melanin pigment by melanocytes in specialized intracellular vesicles called melanosomes. Melanosomes are transported to dendrite tips of melanocytes and are transferred to surrounding keratinocytes (Jimbow and Sugiyama 1998). These events ensure a uniform pigmentation and allow melanin to play its vital photoprotective role against the noxious effects of UV light. Little is known about the molecular mechanisms of melanosome distribution in melanocytes. However, important notions emerged from recent studies of the dilute mouse and its human counterpart, the Griscelli syndrome (GS).
Dilute mice have a coat color dilution attributed to a clumping of melanin granules in their hair and an abnormal concentration of melanosomes in the cell body of melanocytes (Russell 1971; Silvers 1979b). It was demonstrated that dilute mutant phenotypes are caused by mutations in the myosin-V gene (MYOVA) (Mercer et al. 1991).
GS is a rare autosomal recessive disease consisting of a partial albinism of hair and skin, an immune deficiency with episodes of hemophagocytic lymphohistiocytosis (Griscelli et al. 1978; Klein et al. 1994). The immune deficiency in GS is poorly characterized, but a defect in cell-mediated cytotoxicity has been found consistently (Klein et al. 1994). The pigmentary dilution in GS consists of a striking silvery-metallic hair sheen and mild cutaneous depigmentation (Haraldsson et al. 1991; Mancini et al. 1998). Light microscopy examination of hair in GS shows a typical pattern of uneven accumulation of large pigment granules in the hair shaft instead of the homogeneous distribution of small pigment granules in normal hair. Fontana-Masson staining of melanin in skin sections of patients with GS reveals large hyperpigmented melanocytes contrasting with poorly pigmented adjacent keratinocytes, instead of the homogeneous distribution of melanin granules observed in melanocytes and surrounding keratinocytes in a normal epidermis. Electron microscopy of skin sections of GS patients shows that the cytoplasm of melanocytes is filled with numerous melanosomes, whereas adjacent keratinocytes are virtually devoid of these pigmented organelles (Griscelli et al. 1978; Kanitakis et al. 1991; Klein et al. 1994; Mancini et al. 1998). Recently, GS was mapped to 15q21, the region where MYOVA is located. Further, MYOVA mutations were identified in several GS patients (Pastural et al. 1997), pointing to MYOVA as the first gene involved in GS.
Myosin-V is an actin-based molecular motor involved in intracellular vesicle transport (Cheney et al. 1993; Mermall et al. 1998). In melanocytes, myosin-V binds to melanosomes and participates in their transport to dendrites (Provance et al. 1996; Nascimento et al. 1997; Wei et al. 1997; Wu et al. 1997; Lambert et al. 1998). More precisely, it seems that myosin-V captures melanosomes in subcortical actin bundles at the periphery of dendritic processes (Wu et al. 1998). Taken together, these observations suggest that the pigmentary dilution observed in GS is due to a defective acto-myosin–dependent docking of melanosomes at dendrite tips of melanocytes, resulting in a biased distribution of these organelles.
Interestingly, in several GS patients no mutation was found in MYOVA, suggesting the existence of a second GS gene also located in 15q21 (Pastural et al. 2000). Indeed, it was demonstrated very recently that GS can also be caused by a mutation in the Rab27a gene (RAB27A; Menasché et al. 2000). It should be noted that Rab GTPases have been involved previously in the control of intracellular vesicle trafficking by regulating the interactions of vesicles with the cytoskeleton and molecular motors (Echard et al. 1998; Nielsen et al. 1999). Taken together, these observations lead us to investigate the role of Rab27a in melanosome transport in melanocytes.
Materials and Methods
Patient
The patient was a 3-mo-old Turkish boy born from consanguineous parents. He was referred to the hospital for hemophagocytic lymphohistiocytosis. Silvery hair and white eyelashes were noted on admittance. The diagnosis of GS was confirmed by microscopic examination of the hair showing typical melanin clumps in the hair shaft.
Cell Culture
After informed consent, a 4-mm punch biopsy was taken from the patient. Melanocyte cultures were established as described previously (Aberdam et al. 1993). Control cultures of normal melanocytes from the foreskin of a normal infant were established simultaneously. Cultures up to the fourth passage were used for the following experiments.
Immunofluorescence Study
Cells were fixed for 20 min in 3% paraformaldehyde, incubated for 10 min in 50 mM NH4Cl, and permeabilized for 2 min with 0.1% Triton X-100. Cells were then labeled with the following antibodies: a polyclonal antimyosin-V antibody (Cheney et al. 1993) at 1:100 dilution, a monoclonal anti-Rab27a antibody raised against amino acids 45–211 (Transduction Laboratories) at 1:100 dilution, a polyclonal antibody against the COOH terminus of the murine tyrosinase-related protein 1 (TRP-1) both at 1:100 dilution (Jimenez et al. 1989). The secondary antibodies were an FITC-conjugated goat anti–rabbit antibody and a Texas red–conjugated goat anti–mouse antibody (Molecular Probes) at 1:1,000 dilution. Cells were mounted on glass slides and viewed either with an Axiophot fluorescent microscope (ZEISS) or a TCS SP confocal microscope (Leica).
Electron Microscopy
Melanocytes were fixed in wells with 2% glutaraldehyde, postfixed in osmium tetroxide, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Philips CM12 electron microscope.
Immunoelectron Microscopy
Immunoelectron microscopy was performed basically as described previously (Buscà et al. 1996). Ultrathin sections of melanocytes embedded in Lowicril K4M were labeled with the antibody to Rab27a at 1:200 dilution and gold-labeled protein A (15-nm particles), or with the antibody to TRP-1 at 1:200 dilution and gold-labeled protein A (5-nm particles). For double immunolocalization, after Rab27a immunogold labeling the singly labeled sections were put successively through PBS, 1% glutaraldehyde in PBS (10 mn), PBS, PBS/0.02% glycine, and PBS/1% BSA, before starting the anti–TRP-1 incubation (Slot et al. 1991). This procedure prevents any binding of 5-nm gold particle–labeled protein A to Rab27a antibody. The sections were contrasted with 0.3% uranyl acetate in methyl cellulose for 10 min on ice. Grids were examined with a Hitachi 600AB electron microscope.
Western Blotting
Cells were scraped and homogenized in 1% Triton X-100 in Tris buffer (50 mM, pH 7.6) containing 150 mM NaCl, 1 mM PMSF, 10 μg/ml aprotinin, and 5 μg/ml leupeptin. Solubilized protein (40 μg) was separated by SDS-PAGE (6 and 12.5% acrylamide gels) and transferred to nitrocellulose membranes (Hybond+; Amersham Pharmacia Biotech). Membranes were probed with the following antibodies: a polyclonal antimyosin-V antibody (Cheney et al. 1993) at 1:1,000 dilution; monoclonal anti-Rab8 and anti-Rab27a antibodies (Transduction Laboratories) at 1:1,000 and 1:250 dilution, respectively; a polyclonal antibody against the COOH terminus of the murine TRP-1 at 1:1,000 dilution (Jimenez et al. 1989); and a monoclonal antibody against tubulin (Amersham Pharmacia Biotech) at 1:2,000 dilution. The secondary antibodies were a peroxidase-conjugated goat anti–mouse antibody and a peroxidase-conjugated swine anti–rabbit antibody (Dako) at 1:4,000 dilution. The antigen–antibody complex was detected with the ECL kit (Amersham Pharmacia Biotech).
Construction of Expression Plasmids and Transfection
A cDNA encoding the RAB27A product was isolated by reverse transcription of human fibroblast RNA and amplified by PCR using the following primers: P1, 5′-GAAAATCATAACAAGCGGTTCTCTACCC-3′, and P2, 5′-GCCATGTATCAATCATAGAGAAGATCCC-3′. The PCR product (793 bp) was cloned into the pcDNA3 expression vector (Invitrogen) downstream from the cytomegalovirus promoter. A clone that was found to be 100% identical to the published sequence was used for the transfection experiments. Green fluorescent protein (GFP)-Rab7, kindly provided by A. Galmiche (INSERM U452, Nice, France), was obtained by fusing Rab7 to the COOH terminus of the enhanced GFP in the EcoRI site of pEGFP-C1 (CLONTECH Laboratories, Inc.). Melanocytes from the patient with GS were plated on glass coverslips and transfected with Transfast® (Promega) as recommended by the manufacturer. 24 h after transfection, cells were fixed and labeled with anti-Rab27a and anti–TRP-1 antibodies.
Results
Rab27a Colocalizes with Melanosomes and Myosin-V in Normal Human Melanocytes
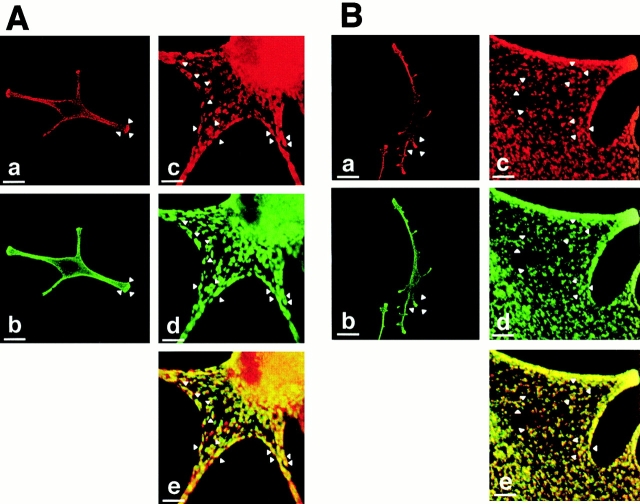
First, we performed immunofluorescence studies to visualize Rab27a expression and localization. Normal human melanocytes were labeled with a monoclonal antibody to Rab27a and with a polyclonal antibody to TRP-1, an integral membrane protein of melanosomes (Fig. 1 A). Rab27a was strongly expressed throughout the cell with a granular pattern (Fig. 1 A, a). Interestingly, Rab27a accumulated at the extremity of the dendrites, as observed for TRP-1 (Fig. 1 A, b). When viewed at higher magnification, confocal microscopy images showed that the antibody to Rab27a labeled individual vesicular structures within the cell cytoplasm (Fig. 1 A, c). Most of these vesicular structures were also labeled by the antibody to TRP-1 (Fig. 1 A, d) and appeared in yellow on the merged image (Fig. 1 A, e), indicating that Rab27a colocalizes with TRP-1. Then, human melanocytes were labeled with both antibodies to Rab27a and myosin-V, a molecular motor involved in melanosome transport that was previously reported to be associated with melanosomes (Nascimento et al. 1997). Rab27a and myosin-V showed a similar expression pattern in normal human melanocytes with an accumulation at dendrite tips (Fig. 1 B, a and b). Higher magnification confocal microscopy images showed that the antibody to myosin-V (Fig. 1 B, d) labeled vesicular structures that were also labeled by the anti-Rab27a antibody (Fig. 1 B, c). These structures appeared in yellow on the merged image (Fig. 1 B, e). Analysis of numerous cells indicates that there is no Rab27 labeling at the plasma membrane. The labeling at the edge of the cell instead reflects the accumulation of melanosomes near the plasma membrane. Thus, Rab27a colocalizes with both TRP-1 and myosin-V, indicating that Rab27a is associated with melanosomes. This was confirmed by electron microscopy after immunogold labeling of Rab27a and TRP-1. Indeed, labeling with the antibody to Rab27a showed that gold particles were associated with dense cytoplasmic structures (Fig. 1 C, a) that were identified as melanosomes by double labeling with both antibodies to TRP-1 (5-nm gold particles) and Rab27a (15-nm gold particles) (Fig. 1 C, b–d). These results, demonstrating that Rab27a is associated with melanosomes, strengthen the case for a role of Rab27a in melanosome transport.
Figure 1.
Rab27a colocalizes with melanosomes and myosin-V in normal human melanocytes. (A) Immunofluorescence microscopy of normal human melanocytes labeled with a monoclonal antibody to Rab27a (a) and with a polyclonal antibody to TRP-1 (b). Confocal immunofluorescence microscopy of normal human melanocytes labeled with a monoclonal antibody to Rab27a (c) and with a polyclonal antibody to TRP-1 (d). Image overlay (e). Arrowheads indicate the colocalization of Rab27a and melanosomes. (B) Immunofluorescence microscopy of normal human melanocytes labeled with a monoclonal antibody to Rab27a (a) and with a polyclonal antibody to myosin-V (b). Confocal microscopy images of normal human melanocytes labeled with a monoclonal antibody to Rab27a (c) and a polyclonal antibody to myosin-V (d). Image overlay (e). Arrowheads indicate the colocalization of Rab27a and myosin-V. (C) Immunogold labeling of normal human melanocytes with the anti-Rab27a antibody (15-nm gold particles) (a). Immunogold labeling of normal human melanocytes with the anti-Rab27a antibody (15-nm gold particles) and the anti–TRP-1 antibody (5-nm gold particles) (b, c, and d). Black arrows indicate Rab27a and white arrows indicate TRP-1. Bars: (A, a and b) 30 μm; (A, c–e) 3 μm; (B, a and b) 30 μm; (B, c–e) 3 μm; (C, a) 0.6 μm; (C, b–d) 0.2 μm.
Melanosome Distribution Is Markedly Impaired in GS Melanocytes
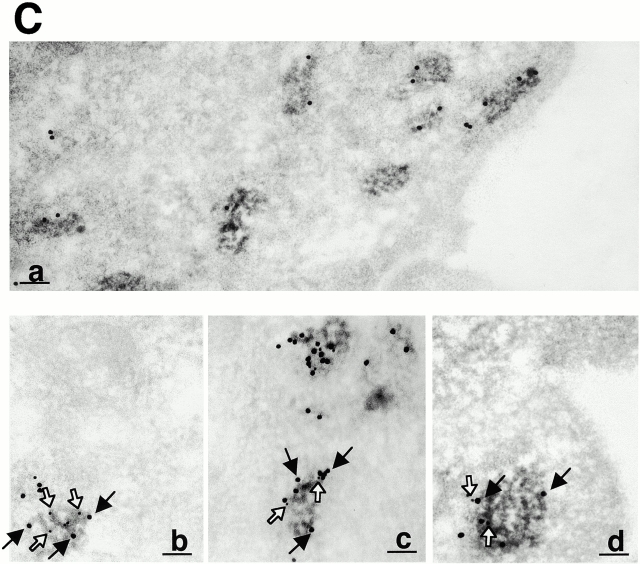
To verify this hypothesis we established melanocyte cultures from a skin biopsy of a patient with typical features of GS. Phase–contrast analysis of normal melanocytes showed an even distribution of pigment throughout the cell, with a marked accumulation at dendrite tips (Fig. 2 A, a). However, in all GS melanocytes, despite the presence of normal-looking dendrites, pigment accumulated massively in the perinuclear region (Fig. 2 A, b). Immunofluorescence studies with an anti–TRP-1 antibody confirmed the biased distribution of melanosomes in GS melanocytes and evidenced the absence of accumulation of melanosomes at their dendrite tips (Fig. 2 B, c and d). Western blotting of whole cell extracts showed that this difference was not caused by a reduction in the total expression of TRP-1 (not shown). Electron microscopy showed a reduction of melanosome number in the dendrites of GS melanocytes. However, the structure and maturation of these melanosomes were not different from those in normal cells (Fig. 2 C, e and f).
Figure 2.
Melanosome distribution is markedly impaired in GS melanocytes. (A) Phase–contrast microscopy images of a normal (a) and GS (b) melanocyte. (B) Confocal immunofluorescence microscopy images of a normal (c) and GS (d) melanocyte labeled for melanosomes with an anti–TRP-1 antibody. (C) Electron microscopy images of a dendritic extension of a normal (e) and GS (f) melanocyte. Note that despite the reduction of melanosome number, both partially (white arrowheads) and fully pigmented (black arrowheads) melanosomes are found in the dendrite of the GS melanocyte. Bars: (A) 25 μm; (B) 5 μm; (C) 1 μm.
Absence of Rab27a Expression and Normal Expression of Myosin-V in GS Melanocytes
Next, in GS melanocytes, we studied the expression of myosin-V, the actin-dependent molecular motor implicated in melanosome transport. Western blotting of whole cell extracts with a specific antibody against the head domain of myosin-V showed a band at the expected size of 190 kD. The expression level of myosin-V was similar in GS melanocytes and in normal melanocytes (Fig. 3 B). The immunofluorescence study showed that the expression pattern of myosin-V was identical in GS melanocytes and in normal melanocytes (Fig. 3 A, a and b). Unlike myosin-V, the immunofluorescence study failed to detect Rab27a in GS melanocytes (Fig. 3 A, c and d). Western blotting of whole cell extracts with a monoclonal anti-Rab27a antibody, raised against amino acids 45–211, detected a band at 27 kD in normal melanocytes that was absent in GS melanocytes. On the other hand, the expression of Rab8, another Rab GTPase, was identical in normal and GS melanocytes (Fig. 3 B). These results can be explained either by a deletion of the epitope detected by this anti-Rab27a antibody, or by a lack of Rab27a expression in GS melanocytes. However, by using three other antibodies directed against Rab27a, which detected a band at 27 kD in normal melanocytes, we failed to detect Rab27a in GS melanocytes either by immunofluorescence or by Western blotting (not shown). Therefore, we conclude that Rab27a is not expressed in the melanocytes of this patient. Taken together, the results show that these GS melanocytes have an abnormal distribution of melanosomes and a normal expression of myosin-V, but no expression of Rab27a.
Figure 3.
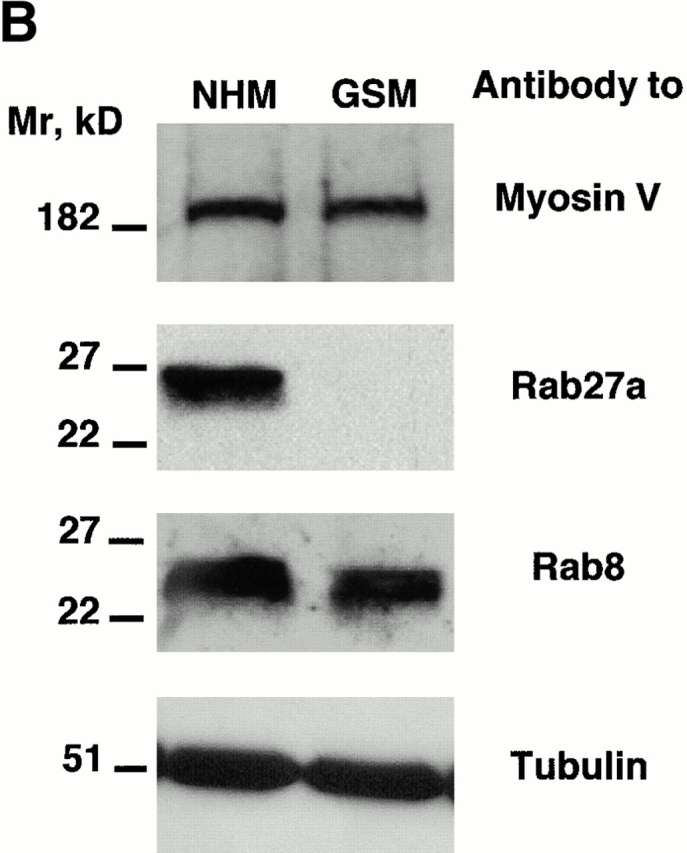
Absence of Rab27a expression in GS melanocytes. (A) Immunofluorescence labeling of myosin-V in normal (a) and GS (b) melanocytes, and of Rab27a in normal (c) and GS (d) melanocytes. For Rab27a labeling, Hoechst staining was used to visualize the nuclei. (B) Western blot analysis. Membranes were blotted with a polyclonal antimyosin-V antibody (top). Membranes were blotted first with a monoclonal anti-Rab27a antibody (middle). After stripping, membranes were reblotted with a monoclonal anti-Rab8 antibody. Membranes were blotted with a monoclonal antitubulin antibody to control the equal amount of protein in each lane (bottom). Bars, 30 μm.
Reexpression of Rab27a Restores Melanosome Transport to Dendrite Tips in GS Melanocytes
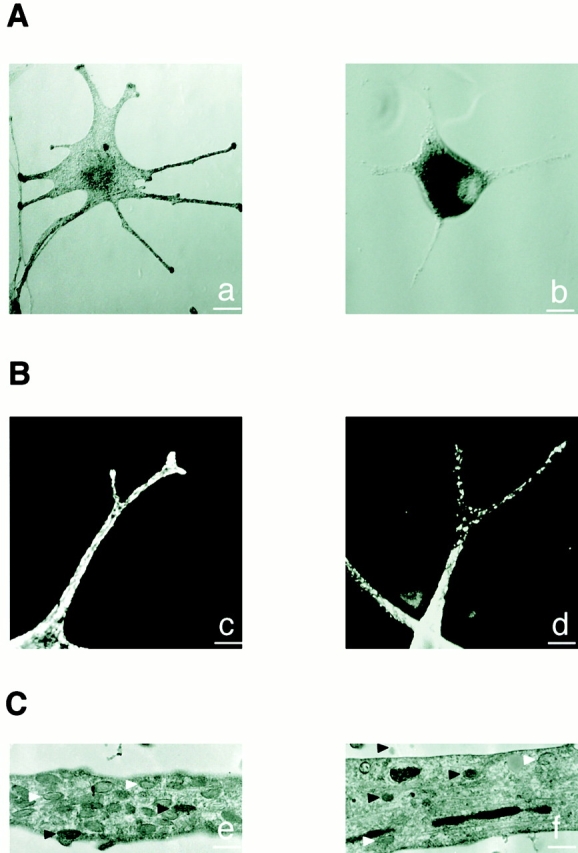
Finally, we transfected a plasmid encoding the normal Rab27a cDNA in GS melanocytes. In cells reexpressing Rab27a, immunofluorescence with an anti-Rab27a antibody showed a granular pattern and an accumulation at dendrite tips, contrasting with the lack of Rab27a expression in surrounding cells (Fig. 4 A, a and b). In all the cells reexpressing Rab27a, we also observed an accumulation of melanosomes at dendrite tips that could not be observed in GS melanocytes lacking Rab27a expression (Fig. 4 A, c–f), or in GS melanocytes transfected with GFP-tagged Rab7 (Fig. 4 B). In addition, double immunofluorescence labeling for Rab27a and melanosomes showed the same type of colocalization in transfected GS melanocytes (Fig. 4 A, g and h) as in normal melanocytes (Fig. 1 A). Thus, reexpression of Rab27a in GS melanocytes specifically restored melanosome transport to dendrite tips.
Figure 4.
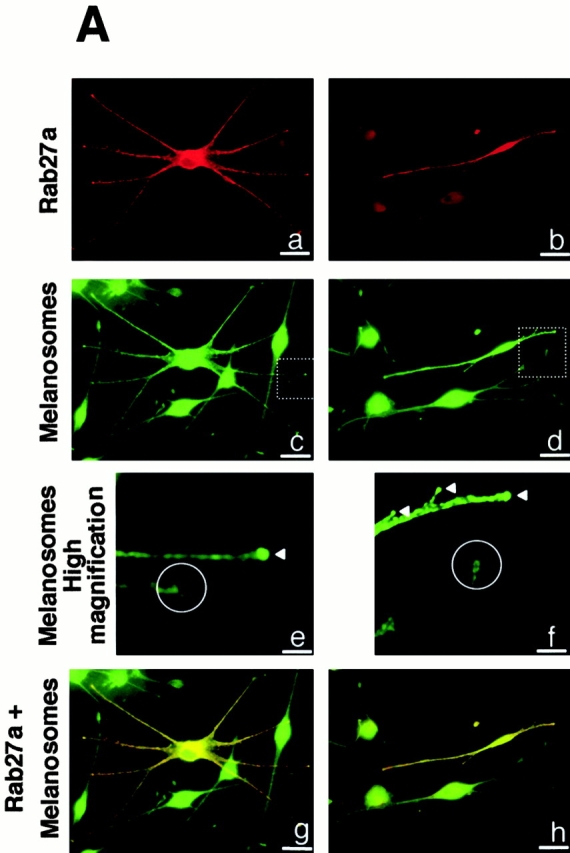
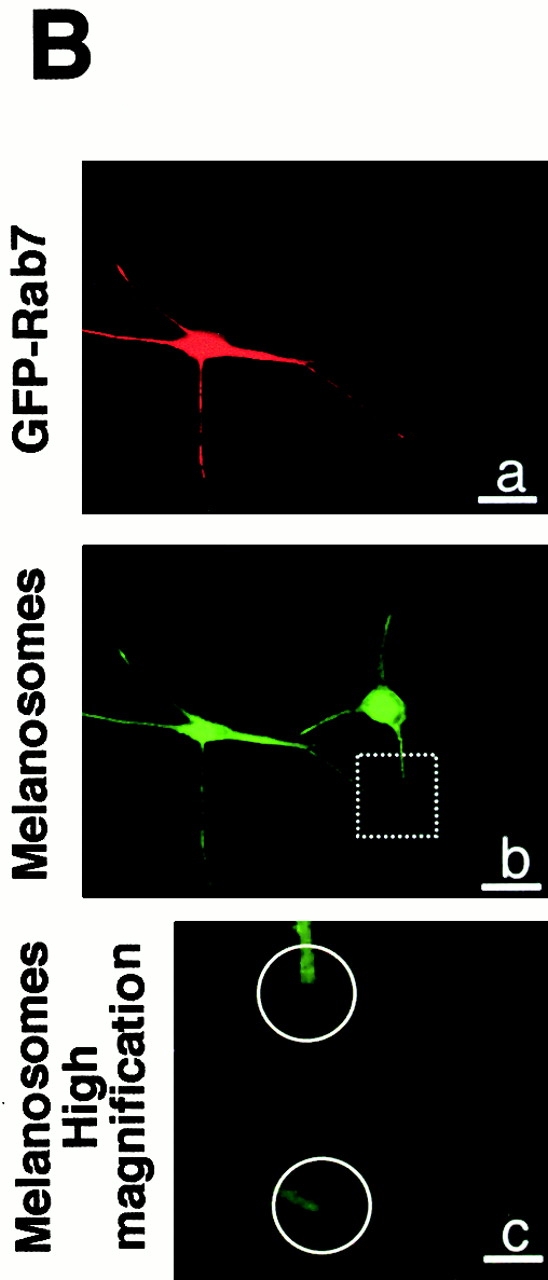
Reexpression of Rab27a restores melanosome transport to dendrite tips in GS melanocytes. Immunofluorescence labeling of two representative fields of GS melanocytes transfected with the normal Rab27a cDNA. The cells were labeled with a monoclonal antibody to Rab27a (a and b) and the melanosomes with a polyclonal antibody to TRP1 (c–f). Higher magnification of dendrite tips (e and f) clearly shows the accumulation of melanosomes at dendrite tips of transfected cells (white arrowheads) contrasting with the vacuity of dendrite extremities in untransfected GS melanocytes (white circles). Image overlay (g and h) shows the colocalization of transfected Rab27a with melanosomes. (B) Representative field of GS melanocytes transfected with GFP-Rab7 cDNA (shown in red by artificial color) (a). The cells were labeled for melanosomes with a polyclonal antibody to TRP-1 (b). Higher magnification of dendrite tips (c) clearly shows the absence of melanosome accumulation at dendrite tips in both untransfected and transfected melanocytes (b and c). Bars: (A, a–d, g, and h) 30 μm; (A, e and f) 8 μm; (B, a and b) 30 μm; (B, c) 8 μm.
Discussion
In this report, we show that in normal melanocytes Rab27a colocalizes with melanosomes and myosin-V. In melanocytes from a GS patient, we observed a lack of Rab27a expression that coincides with the absence of melanosome accumulation at the dendrite tips. Sequencing of Rab27a cDNA from this patient revealed a deletion of five nucleotides in the terminal exon. This deletion leads to a frameshift, a conversion Q172N, and the appearance of a premature termination codon in 173 (Menasché et al. 2000). At the protein level, the termination codon in 173 implies that 49 COOH-terminal amino acids are deleted in the mutant form of Rab27a. This deletion involves the hypervariable domain of Rab GTPases that contains a geranylgeranylation motif (CXC) and structural elements that determine the association of Rab proteins with their specific target vesicles. In choroideremia, impairment of Rab27a geranylgeranylation results in a decreased expression of the protein (Seabra et al. 1993). Thus, the mistargeting of the mutated Rab27a could lead to an increased degradation of the protein in GS melanocytes. However, we cannot rule out the possibility that the premature termination codon results in Rab27a messenger destabilization. The role of Rab27a in melanosome transport was clearly demonstrated by the ectopic reexpression of Rab27a in GS melanocytes, which leads to a normal accumulation of melanosomes at the dendrite tips and to a phenotypic reversion of diseased cells. In conclusion, we show that Rab27a is essential for melanosome transport to dendrite tips in human melanocytes, and that the absence of Rab27a causes a pigmentary dilution observed in GS patients.
In addition, these results raise interesting prospects concerning the role of Rab27a. Indeed, in the mouse, mutations at three different loci, dilute, ashen, and leaden lead to a very similar coat color pigmentary dilution (Silvers 1979a,Silvers 1979b). Dilute encodes for the mouse homologue of myosin-V (Mercer et al. 1991). The protein encoded by ashen and leaden has not yet been identified. Noteworthy, ashen maps to mouse chromosome 9 (9.41 cM), very close to dilute (9.42 cM), in a region orthologous to the human 15q21 region (Moore et al. 1988). Considering the recent localization of human RAB27A in 15q21(Tolmachova et al. 1999) and the implication of Rab27a in melanosome transport in human melanocytes, RAB27A is an attractive candidate gene for ashen. (Since the submission of this work, it was reported that the ashen locus corresponds to the murine RAB27A gene [Wilson et al. 2000].)
Mutations at dilute and ashen locus cause the same anomalies in melanocytes, and both mutations are corrected by the murine dilute suppressor gene dsu (Moore et al. 1988). In humans, defective expression of both myosin-V and Rab27a leads to pigmentary dilution and defective melanosome transport. These observations strongly suggest that myosin-V and Rab27a function in the same pathway and raise the possibility that Rab27a could interact with myosin-V. In agreement with this hypothesis, an association of Myo2p, a yeast myosin-V, with Sec4p, a vesicle-associated Rab protein, has been described recently (Schott et al. 1999).
Finally, since immune deficiency is the other hallmark of GS, Rab27a is expected to play an important role in the human immune system. Considering the alteration of cell-mediated toxicity observed in GS (Griscelli et al. 1978; Klein et al. 1994; Dufourcq-Lagelouse et al. 1999) and the implication of Rab27a in vesicle transport in melanocytes, it is tempting to speculate that Rab27a could be involved in the transport of cytotoxic granules.
Acknowledgments
We thank Dr. Mark Mooseker for the generous gift of the antimyosin-V antibody; Dr. Patrice Boquet and Dr. Jean-Philippe Lacour for careful reading of the manuscript; Anne Doye, Sophie Pagnotta, and Anne Spadafora for excellent technical assistance; and Aurore Grima and Christine Ordonez for preparation of artwork.
This work was supported by grants from Association pour la Recherche contre le Cancer (ARC 5209), Ligue contre le Cancer, and Centre de Recherches et d'Investigations Epidermiques et Sensorielles. Philippe Bahadoran is supported by a Poste d'Accueil grant from INSERM.
Footnotes
Abbreviations used in this paper: GFP, green fluorescent protein; GS, Griscelli syndrome; TRP-1, tyrosinase-related protein 1.
References
- Aberdam E., Romero C., Ortonne J.P. Repeated UVB irradiations do not have the same potential to promote stimulation of melanogenesis in cultured normal human melanocytes. J. Cell Sci. 1993;106:1015–1022. doi: 10.1242/jcs.106.4.1015. [DOI] [PubMed] [Google Scholar]
- Buscà R., Martinez M., Vilella E., Pognonec P., Deeb S., Auwerw J., Reina M., Vilaro S. The mutation Gly142-->Glu in human lipoprotein lipase produces a missorted protein that is diverted to lysosomes. J. Biol. Chem. 1996;271:2139–2146. doi: 10.1074/jbc.271.4.2139. [DOI] [PubMed] [Google Scholar]
- Cheney R.E., O'Shea M.K., Heuser J.E., Coelho M.V., Wolenski J.S., Espreafico E.M., Forscher P., Larson R.E., Mooseker M.S. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Dufourcq-Lagelouse R., Pastural E., Barrat F.J., Feldmann J., Le Deist F., Fischer A., De Saint Basile G. Genetic basis of hemophagocytic lymphohistiocytosis syndrome. Int. J. Mol. Med. 1999;4:127–133. doi: 10.3892/ijmm.4.2.127. [DOI] [PubMed] [Google Scholar]
- Echard A., Jollivet F., Martinez O., Lacapere J.J., Rousselet A., Janoueix-Lerosey I., Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- Griscelli C., Durandy A., Guy-Grand D., Daguillard F., Herzog C., Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am. J. Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- Haraldsson A., Weemaes C.M., Bakkeren J.A., Happle R. Griscelli disease with cerebral involvement. Eur. J. Pediatr. 1991;150:419–422. doi: 10.1007/BF02093723. [DOI] [PubMed] [Google Scholar]
- Jimbow K., Sugiyama S. Melanosomal translocation and transfer. In: Nordlund J.J., Boissy R.E., Hearing V.J., King R.A., Ortonne J.P., editors. The Pigmentary SystemPhysiology and Physiopathology. Oxford University Press; New York: 1998. pp. 107–114. [Google Scholar]
- Jimenez M., Maloy W.L., Hearing V.J. Specific identification of an authentic clone for mammalian tyrosinase. J. Biol. Chem. 1989;264:3397–3403. [PubMed] [Google Scholar]
- Kanitakis J., Cambazard F., Roca-Miralles M., Souillet G., Philippe N. Griscelli-Prunieras disease (partial albinism with immunodeficiency) Eur. J. Dermatol. 1991;1:206–213. [Google Scholar]
- Klein C., Philippe N., Le Deist F., Fraitag S., Prost C., Durandy A., Fischer A., Griscelli C. Partial albinism with immunodeficiency (Griscelli syndrome) J. Pediatr. 1994;125:886–895. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- Lambert J., Onderwater J., Vander Haeghen Y., Vancoillie G., Koerten H.K., Mommaas A.M., Naeyaert J.M. Myosin V colocalizes with melanosomes and subcortical actin bundles not associated with stress fibers in human epidermal melanocytes. J. Invest. Dermatol. 1998;111:835–840. doi: 10.1046/j.1523-1747.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- Mancini A.J., Chan L.S., Paller A.S. Partial albinism with immunodeficiencyGriscelli syndrome: report of a case and review of the literature. J. Am. Acad. Dermatol. 1998;38:295–300. doi: 10.1016/s0190-9622(98)70568-7. [DOI] [PubMed] [Google Scholar]
- Menasché G., Pastural E., Feldmann J., Certain S., Ersoy F., Dupuis S., Wulffraat N., Bianchi D., Fischer A., Le Deist F., de Saint-Basile G. Mutations in RAB27A cause Griscelli syndrome associated with hemophagocytic syndrome. Nat. Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- Mercer J.A., Seperack P.K., Strobel M.C., Copeland N.G., Jenkins N.A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature. 1991;349:709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Mermall V., Post P.L., Mooseker M.S. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science. 1998;279:527–533. doi: 10.1126/science.279.5350.527. [DOI] [PubMed] [Google Scholar]
- Moore K.J., Swing D.A., Rinchik E.M., Mucenski M.L., Buchberg A.M., Copeland N.G., Jenkins N.A. The murine dilute suppressor gene dsu suppresses the coat-color phenotype of three pigment mutations that alter melanocyte morphology, d, ash and ln. Genetics. 1988;119:933–941. doi: 10.1093/genetics/119.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento A.A., Amaral R.G., Bizario J.C., Larson R.E., Espreafico E.M. Subcellular localization of myosin-V in the B16 melanoma cells, a wild-type cell line for the dilute gene. Mol. Biol. Cell. 1997;8:1971–1988. doi: 10.1091/mbc.8.10.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E., Severin F., Backer J., Hyman A., Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Pastural E., Barrat F.J., Dufourcq-Lagelouse R., Certain S., Sanal O., Jabado N., Seger R., Griscelli C., Fischer A., de Saint Basile G. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat. Genet. 1997;16:289–292. doi: 10.1038/ng0797-289. [DOI] [PubMed] [Google Scholar]
- Pastural E., Ersoy F., Yalman N., Wulfraat N., Grillo E., Ozkinay F., Tezcan I., Gediköglu G., Philippe N., Fischer A., de Saint-Basile G. Two genes at the same 15q21 locus are responsible for Griscelli disease. Genomics. 2000;63:299–306. doi: 10.1006/geno.1999.6081. [DOI] [PubMed] [Google Scholar]
- Provance D.W., Jr., Wei M., Ipe V., Mercer J.A. Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc. Natl. Acad. Sci. USA. 1996;93:14554–14558. doi: 10.1073/pnas.93.25.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L.B. Definition of functional units in a small chromosomal segment of the mouse and its use in interpreting the nature of radiation-induced mutations. Mutat. Res. 1971;11:107–123. doi: 10.1016/0027-5107(71)90036-4. [DOI] [PubMed] [Google Scholar]
- Schott D., Ho J., Pruyne D., Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra M.C., Brown M.S., Goldstein J.L. Retinal degeneration in choroideremiadeficiency of rab geranylgeranyl transferase. Science. 1993;259:377–381. doi: 10.1126/science.8380507. [DOI] [PubMed] [Google Scholar]
- Silvers W.K. Beige, silver, greying with age, and other determinants Silvers W.K. The Coat Colors of Mice 1979. 125 138 Springer-Verlag; New York: a [Google Scholar]
- Silvers W.K. Dilute and leaden, the p-locus, ruby-eye, and ruby-eye-2 Silvers W.K. The Coat Colors of Mice 1979. 83 104 Springer-Verlag; New York: b [Google Scholar]
- Slot J.W., Geuze H.J., Gigengack S., Lienhard G.E., James D.E. Immunolocalization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 1991;141:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmachova T., Ramalho J.S., Anant J.S., Schultz R.A., Huxley C.M., Seabra M.C. Cloning, mapping and characterization of the human RAB27A gene. Gene. 1999;239:109–116. doi: 10.1016/s0378-1119(99)00371-6. [DOI] [PubMed] [Google Scholar]
- Wei Q., Wu X., Hammer J.A., III. The predominant defect in dilute melanocytes is in melanosome distribution and not cell shape, supporting a role for myosin V in melanosome transport. J. Muscle Res. Cell Motil. 1997;18:517–527. doi: 10.1023/a:1018659117569. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Yip R., Swing D.A., O'Sullivan N., Zhang Y., Novak E.K., Swank R.T., Russell L.B., Copeland N.G., Jenkins N.A. A mutation in Rab27a causes the vesicle transport observed in ashen mice. Proc. Natl. Acad. Sci. USA. 2000;97:7933–7938. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Bowers B., Wei Q., Kocher B., Hammer J.A., III. Myosin V associates with melanosomes in mouse melanocytesevidence that myosin V is an organelle motor. J. Cell Sci. 1997;110:847–859. doi: 10.1242/jcs.110.7.847. [DOI] [PubMed] [Google Scholar]
- Wu X., Bowers B., Rao K., Wei Q., Hammer J.A., III. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests aparadigm for myosin V function In vivo. J. Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]