Abstract
The role of the actin-based cytoskeleton in the internalization process of Burkholderia multivorans by well-differentiated human airway epithelia was investigated by immunohistology and confocal microscopy. Our data suggest that an intact actin cytoskeleton is required for biofilm formation but not single cell entry or paracytosis.
Burkholderia cepacia has emerged as a serious respiratory pathogen in cystic fibrosis patients (4). The clinical course of B. cepacia infection is variable. However, 20% of patients succumb to the cepacia syndrome, which is characterized by acute necrotizing pneumonia, bacteremia, and death (5). It was recently reported that different species of the B. cepacia complex use multiple pathways to invade well-differentiated human airway epithelia, including those via biofilm formation, single cell entry, and/or paracytosis (6). In addition, it was shown that invasion by Burkholderia multivorans J-1 and B. cepacia BC-7 (genomovar III, cblA+) was associated with rearrangement of the host actin filament network.
In the present study, we investigated in detail the contribution of the actin-based cytoskeleton to B. multivorans J-1 infection of human airway epithelia. First, we compared the structures of filamentous actin in control epithelial cells, epithelial cells treated with cytochalasin D, and cells exposed to B. multivorans. Well-differentiated human airway epithelia, grown under air-liquid interface conditions (6), were incubated either with vehicle (bronchial epithelial culture [BEC] medium [6]) or cytochalasin D (10 μM; Sigma Chemical, St. Louis, Mo.) for 60 min or with ∼5 × 107 CFU of B. multivorans J-1 in 50 μl of BEC medium for 24 h. After fixation with 4% paraformaldehyde, the actin cytoskeleton was labeled with BODIPY-phalloidin at a 1:25 dilution for 30 min at 25°C, and B. multivorans was stained with B. cepacia antiserum (1:250 dilution), followed by a fluorescein isothiocyanate-labeled secondary antibody (6), and the cultures were examined by confocal microscopy as recently described (6).
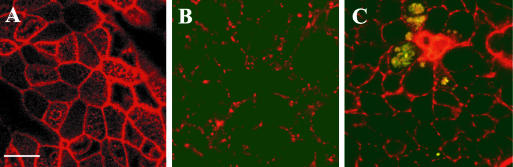
Figure 1A shows the intact actin filament pattern of a control culture. A similar pattern of the actin cytoskeleton disruption was observed in B. multivorans-exposed (Fig. 1C) and cytochalasin D-treated (noninfected; Fig. 1B) cultures, suggesting that B. multivorans promotes disruption of the actin filament network during epithelial infection.
FIG. 1.
Visualization of the structure of filamentous actin (red) in human airway epithelia. Confocal x-y scans from the apical epithelial domain from control airway epithelia (A), cytochalasin D-treated airway epithelia (B), and B. multivorans-infected airway epithelia (C). Note that colocalization of green bacteria within red epithelial cells is seen as yellow-stained areas. Bar, 10 μm.
Next, we investigated whether cytochalasin D affected invasion of airway epithelia by B. multivorans. Well-differentiated human airway epithelia were incubated with either 10 μM cytochalasin D or vehicle for 60 min, exposed to ∼5 × 105 CFU of B. multivorans J-1 in 50 μl of BEC medium, incubated at 37°C for 24 or 36 h, fixed with 4% paraformaldehyde, and paraffin embedded. Sections of B. multivorans-infected and noninfected cultures were incubated with the B. cepacia antiserum, followed by the biotinylated secondary antibody and the substrate diaminobenzidine tetrahydrochloride, counterstained with light green, and examined by light microscopy (6).
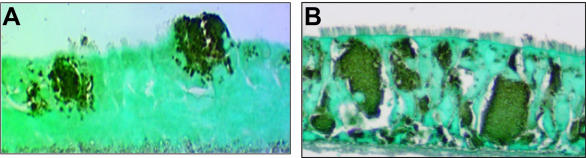
The control (non-cytochalasin D-treated) B. multivorans-infected cultures did not exhibit intra- and intercellular bacteria at 24 h (data not shown), but at 36 h biofilms were observed, and some areas of the tissue sections were invaded by intra- and intercellular bacteria (Fig. 2A). Cytochalasin D changed the morphology of cultured human airway epithelia, causing cell retraction but not cell detachment. However, this treatment did not inhibit B. multivorans invasion as described for other bacterial species in cultured epithelial cells (2, 3, 7). Instead, at 24 and 36 h the entire epithelium, including the basal cell layer, was heavily invaded by B. multivorans as demonstrated by a robust staining of high numbers of intra- and intercellular bacteria (Fig. 2B, showing a 36-h-incubated culture). These data suggest that B. multivorans can use actin-independent entry mechanisms. Indeed, single cell entry and translocation via the paracellular shunt are facilitated in the presence of actin disruption.
FIG. 2.
Immunostaining of B. multivorans in control (A) and cytochalasin D-pretreated (B) airway epithelial cultures. (A) Note multiple invasion pathways employed by this strain: biofilm formation, single cell entry, and paracytosis. (B) No biofilm formation but high numbers of intra- and intercellular bacteria in cytochalasin D-treated airway epithelia.
Interestingly, Coyne et al. (1) observed a decrease in transepithelial resistance (Rt) in primary human airway epithelial cells due to the modulatory effect of cytochalasin D on the permeability of tight junctions. Thus, the increase in epithelial paracellular permeability in cytochalasin D-treated cultures might allow access of B. multivorans to the intercellular compartment. Wells et al. (7) described cytochalasin D-induced enhancement of bacterial internalization in polarized enterocytes and concluded that this increase may be due to the exposure of the enterocyte lateral surfaces. At this point, we are not able to determine whether B. multivorans entered airway epithelial cells via the apical or the lateral surface of airway epithelial cells in our model system. After entry, intracellular replication may account for the large masses of intracellular bacteria observed in cytochalasin D-treated cultures.
Interestingly, we did not observe biofilms closely associated with the apical surface of airway epithelia in cytochalasin D-treated cultures for up to 36 h, while in non-cytochalasin D-treated cultures biofilms were formed (Fig. 2). One possible explanation for this finding is that the accelerated invasion by single cell entry and paracytosis reduced the number of bacteria remaining in the mucus layer to numbers insufficient for biofilm formation. Alternatively, biofilm formation may depend on an intact actin filament network.
To further explore these observations, we designed an experiment to observe invasion by B. multivorans of living airway epithelia. For this purpose, we labeled the bacteria and airway epithelia with BCECF and SNARF-1 (Molecular Probes, Eugene, Oreg.), respectively. For fluorescent labeling of live bacteria, bacteria were grown in 5 ml of BEC medium to an optical density of 0.25, pelleted, and then resuspended in phosphate-buffered saline (PBS) containing 5 μM acetoxymethylester of BCECF. After 1 h of incubation at 37°C, the bacteria were pelleted and resuspended in 100 μl of BEC medium, and 50 μl (∼2 × 108 CFU/50 μl) was applied to the apical cell surface of airway epithelial cultures. For labeling airway epithelia, the culture medium in the basolateral compartment of the T-COL supports was replaced by PBS containing 5 μM acetoxymethylester of SNARF-1. After 2 to 3 h of incubation at 37°C, PBS was replaced by fresh BEC medium. Airway epithelial cultures were then infected on the apical epithelial surface with fluorescently labeled bacteria for a time period that allowed cytochalasin D to reduce Rt (6 h).
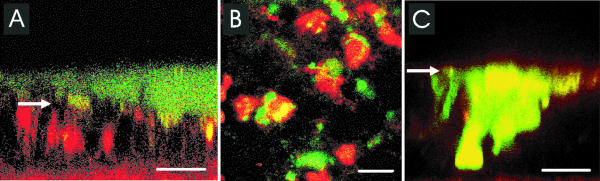
When control airway epithelial cultures were infected with this high inoculum of B. multivorans J-1 for 6 h, we found that the bacteria formed a biofilm closely associated with the apical epithelial surface (Fig. 3A and B). Moreover, yellow-stained areas within red-labeled cells were detected when a macrocolony was formed, suggesting that some bacteria also entered live epithelial cells. In cytochalasin D-treated cultures, no biofilm-mediated invasion was observed, but intra- and intercellular masses of bacteria were noted (Fig. 3C), confirming immunohistologic studies.
FIG. 3.
Invasion of airway epithelium (red) by live B. multivorans J-1 (green) observed by confocal microscopy. (A) Confocal x-z scan of airway epithelium. (B) Confocal x-y scan of the same culture. (C) Confocal x-z scan of cytochalasin D-treated airway epithelium. Colocalization of live bacteria within live epithelial cells is seen as yellow-stained areas. Bars, 10 μm. Arrows indicate apical cell surface of airway epithelia.
These data led us to conclude that single cell entry and paracytosis do not appear to require intact actin filaments (cytochalasin D insensitive). We speculate that no biofilm was formed because the density of bacteria remaining in the mucus layer upon cytochalasin D treatment was reduced due to epithelial penetration. Further studies have to be performed to identify molecules associated with the different invasion pathways used by B. multivorans. A better understanding of invasion mechanisms should assist in developing novel therapeutic approaches to prevent pulmonary sepsis in cystic fibrosis patients.
Acknowledgments
We thank Tracy Eldred and Kim Burns, UNC Histology Core, for immunohistology of infected airway epithelial cell cultures and Scott Randell for technical assistance in the preparation of well-differentiated human airway epithelial cultures.
Editor: D. L. Burns
REFERENCES
- 1.Coyne, C. B., M. M. Kelly, R. C. Boucher, and L. G. Johnson. 2000. Enhanced epithelial gene transfer by modulation of tight junctions with sodium caprate. Am. J. Respir. Cell Mol. Biol. 23:602-609. [DOI] [PubMed] [Google Scholar]
- 2.Dramsi, S., and P. Cossart. 1998. Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 14:137-166. [DOI] [PubMed] [Google Scholar]
- 3.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenesis revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govan, J. R., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 5.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 6.Schwab, U., M. Leigh, R. C. Ribeiro, J. Yankaskas, K. Burns, P. Gilligan, P. Sokol, and R. Boucher. 2002. Patterns of epithelial cell invasion by different species of the Burkholderia cepacia complex in well-differentiated human airway epithelia. Infect. Immun. 70:4547-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells, C. L., E. M. van de Westerlo, R. P. Jechorek, H. M. Haines, and S. L. Erlandsen. 1998. Cytochalasin-induced actin disruption of polarized enterocytes can augment internalization of bacteria. Infect. Immun. 66:2410-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]