Abstract
Neurofibromatosis type 1 (NF1) is a common autosomal-dominant disorder characterized by cutaneous neurofibromas infiltrated with large numbers of mast cells, melanocyte hyperplasia, and a predisposition to develop malignant neoplasms. NF1 encodes a GTPase activating protein (GAP) for Ras. Consistent with Knudson's “two hit” model of tumor suppressor genes, leukemias and malignant solid tumors in NF1 patients frequently demonstrate somatic loss of the normal NF1 allele. However, the phenotypic and biochemical consequences of heterozygous inactivation of Nf1 are largely unknown. Recently neurofibromin, the protein encoded by NF1, was shown to negatively regulate Ras activity in Nf1−/− murine myeloid hematopoietic cells in vitro through the c-kit receptor tyrosine kinase (dominant white spotting, W). Since the W and Nf1 locus appear to function along a common developmental pathway, we generated mice with mutations at both loci to examine potential interactions in vivo. Here, we show that haploinsufficiency at Nf1 perturbs cell fates in mast cells in vivo, and partially rescues coat color and mast cell defects in W41 mice. Haploinsufficiency at Nf1 also increased mast cell proliferation, survival, and colony formation in response to Steel factor, the ligand for c-kit. Furthermore, haploinsufficiency was associated with enhanced Ras–mitogen-activated protein kinase activity, a major downstream effector of Ras, via wild-type and mutant (W41) c-kit receptors. These observations identify a novel interaction between c-kit and neurofibromin in vivo, and offer experimental evidence that haploinsufficiency of Nf1 alters both cellular and biochemical phenotypes in two cell lineages that are affected in individuals with NF1. Collectively, these data support the emerging concept that heterozygous inactivation of tumor suppressor genes may have profound biological effects in multiple cell types.
Keywords: c-kit, mast cell, heterozygous, neurofibromatosis, tumor suppressor
Introduction
Neurofibromin, the protein encoded by neurofibromatosis type 1 (NF1), negatively regulates Ras output by accelerating the conversion of Ras-GTP to Ras-GDP 1 2. The murine c-kit receptor 3 and its ligand, Steel factor 4 5, are components of a signaling pathway that is essential for murine hematopoiesis, melanogenesis, and gametogenesis. These proteins are encoded by the dominant white spotting (W) and Steel (Sl) loci, respectively, and ligand binding to c-kit activates Ras in myeloid lineage cells (for a review, see reference 6). Children with NF1 are at a markedly increased risk of developing juvenile myelomonocytic leukemia (JMML 7). Genetic and biochemical analyses of these leukemias strongly support the hypothesis that NF1 functions as a tumor suppressor gene in immature myeloid cells by negatively regulating Ras output. Similarly, ∼10% of heterozygous Nf1+/− mice spontaneously develop a JMML-like myeloproliferative disorder (MPD) during the second year of life with loss of the wild-type Nf1 allele 8. In contrast to patients with NF1, Nf1+/− mice neither manifest pigmentary abnormalities nor develop neurofibromas 8. Although homozygous Nf1 knockout mice (Nf1−/−) die in utero around embryonic day (E)13.5 from complex cardiovascular defects 8 9, adoptive transfer of E13.5 Nf1−/− fetal liver hematopoietic stem cells into irradiated syngeneic recipients consistently induces the JMML-MPD 10. Interestingly, we have shown recently that murine Nf1−/− fetal liver cells form excessive numbers of myeloid progenitor colonies in methylcellulose cultures containing low concentrations of Steel factor. Mitogen-activated protein (MAP) kinase, a downstream target of Ras-GTP, is activated in unstimulated Nf1−/− myeloid lineage cells, and this kinase is hyperactivated in response to several hematopoietic growth factors, including GM-CSF and Steel factor 11. This observation and the involvement of myeloid, mast cell, and melanocyte lineages in some of the pathological complications of NF1 led us to generate mice with mutations at both W and Nf1 to examine potential genetic and biochemical interactions in vivo and in vitro.
Materials and Methods
Animals.
Nf1+/− mice were obtained from Dr. Tyler Jacks at the Massachusetts Institute of Technology (Cambridge, MA) in a C57BL/6.129 background, and were backcrossed for 13 generations into the C57BL/6 strain. C57BL/6+/+;W41/W41 mice were obtained from the Jackson Laboratory. These studies were conducted with a protocol approved by the Indiana University Laboratory Animal Research Center. The Nf1 allele was genotyped as described previously 11 12. The W41 genotyping was inferred from the characteristic mottled, white coat color in W41/W41mice, and a white abdominal spot on W41/+ mice. Multiple F0 founders were used to generate the F2 progeny used in these experiments. The crosses used to generate the four Nf1 and W genotypes used in these experiments are outlined below:
F0: Nf1+/−;+/+ × +/+;W41/W41
F1: Nf1+/−;W41/+ × +/+;W41/+
F2: Nf1+/−;W41/W41, +/+;W41/W41, Nf1+/−;+/+, +/+;+/+.
Analysis of Cutaneous Mast Cells.
1-cm sections of ears and dorsal skin were removed, fixed in buffered formalin, and processed in paraffin-embedded sections. Specimens were stained with hematoxylin-eosin to assess routine histology, and with Giemsa to identify mast cells. Some specimens were stained with Fontana-Masson to differentiate melanin-containing cells from mast cells. Cutaneous mast cells (Giemsa-positive, Fontana-Masson–negative) were quantitated in a blinded fashion by counting the distal 5 mm of ears or dorsal skin.
Peritoneal Lavages and Mast Cell Colony Assays.
Peritoneal cells from 8-wk-old mice were collected as described previously 13. 10-ml peritoneal lavages were then concentrated by centrifugation and stained with toluidine blue to quantify total number of mast cells per 10 ml lavage. To examine the proliferation of peritoneal cells for mast cell progenitors, mast cell colony assays were done in triplicate. In brief, 1 ml of culture mix containing 1 × 104 peritoneal cells, α-MEM, 1.2% methylcellulose (Terry Fox Laboratory), 30% fetal bovine serum (HyClone Laboratories), 1% deionized fraction V BSA (Sigma Chemical Co.), 10−4 M mercaptoethanol (Sigma Chemical Co.), 10 ng/ml of recombinant murine IL-3, and 100 ng/ml of recombinant murine Steel factor (PeproTech) was plated in each 35-mm suspension culture dish (Nalge Nunc International), then incubated at 37°C in a humidified atmosphere flush with 5% CO2 in air. Colonies were determined on day 14 of incubation by in situ observation using an inverted microscope. To assess the accuracy of in situ identification of the colonies, individual colonies were taken with an Eppendorf micropipette under direct microscope visualization, spread on glass slides using cytocentrifuge, and stained with May-Grunwald-Giemsa stain and Alcian blue/safranin stain for mast cells.
Bone Marrow Mast Cell Culture and Survival Assay.
Six lines from each of the four genotypes were generated and used for cell survival and proliferation assays. Bone marrow mast cells (BMMCs) were cultured as described, with minor modifications 14, and homogeneity of BMMCs was determined by Giemsa staining. Aliquots of cells were also stained with Alcian blue and safranin to confirm that they were mast cells. Furthermore, FACS® analysis revealed similar forward and side light scatter characteristics and the same percentage of c-kit+ cells in BMMCs of all four genotypes (data not shown). The mast cell survival assay was done as follows: BMMCs from each genotype were deprived of growth factors for 24 h, and 3 × 105 cells were plated in 24-well dishes in serum-free RPMI containing 1% glutamine and 100 ng/ml of recombinant murine Steel factor in a total volume of 1 ml. The number of surviving cells was determined by trypan blue exclusion at 48 h of culture in a 37°C, 5% CO2, humidified incubator.
Proliferation Assay.
Proliferation assays were performed as described previously 14. BMMCs were deprived of growth factors for 24 h, and 2 × 105 cells were plated in triplicate in 24-well dishes in 1 ml RPMI containing 1% glutamine, 10% fetal bovine serum, and 100 ng/ml recombinant murine Steel factor or no growth factors as indicated in a 37°C, 5% CO2, humidified incubator. After 1 and 3 d, viable cells were counted using a hemocytometer. Cell viability was determined by a trypan blue exclusion assay.
Analysis of p42 MAP Kinase Activation.
Activation of p42 MAP kinase was determined by depriving cells of growth factors for 24 h and stimulating them with 10 ng/ml of recombinant murine Steel factor for various amounts of time. Cells were collected and washed twice with cold PBS plus 1 mM sodium orthovandate, and then lysed in nonionic lysis buffer as described previously 15. The protein lysates were equalized for total protein concentration using a BCA assay (Pierce Chemical Co.), and equal loading of MAP kinases in these assays was confirmed by Western blot. p42 MAP kinase immunoprecipitations were carried out with an anti–extracellular signal–regulatory kinase (ERK)-2 (C-14) antibody (Santa Cruz Biotechnology). The p42 MAP kinase immune complex assay was performed as described, except that Elk-1 fusion protein (New England Biolabs) was used instead of myelin basic protein. The kinase reactions were resolved on 10% SDS-PAGE gels. Gels were dried and subjected to autoradiography. Densitometry of individual bands was conducted using NIH Image software.
Results
Haploinsufficiency at Nf1 Partially Restores the Coat Color Deficiency in W41 Mice.
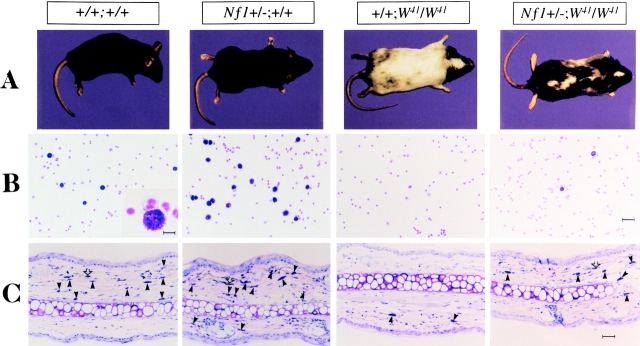
W mice display varying degrees of sterility, mast cell hypoplasia, anemia, and coat color deficiency correlating with the residual kinase activity of the mutant receptor 16 17. Mice homozygous for a point mutation in the cytoplasmic domain of the c-kit receptor (W41) have partial inactivation of the c-kit receptor tyrosine kinase, resulting in an abnormal mottled, white coat color 17. We crossed W41 and Nf1 mice and found that animals heterozygous at Nf1 and homozygous for the W41mutation (Nf1+/−;W41/W41) displayed a 60–70% restoration of coat color ( Fig. 1 A). This finding was consistent in >150 F2 progeny carrying mutations at both loci. Thus, haploinsufficiency at Nf1 partially corrects the aberrant pigmentation pattern of W41/W41 mice.
Figure 1.
Effect of haploinsufficiency of Nf1 on coat color and total numbers of cutaneous and peritoneal mast cells. (A) Coat color pattern of a representative mouse from each of the following genotypes: +/+;+/+, Nf1+/−;+/+, +/+;W41/W41, and Nf1+/−;W41/W41. Haploinsufficiency at Nf1 partially corrects the coat color deficiency in mice homozygous for the W41 allele in a C57BL/6 genetic background. (B) Representative cytospins from peritoneal lavages stained for mast cells from individual mice of the four Nf1 and W genotypes. Peritoneal cells were stained with toluidine blue to quantify the total number of mast cells per peritoneal lavage. A higher magnification of a representative mast cell is shown in the inset of the wild-type mouse (original magnification: ×200). Bar (inset) 10 μm. Bar (far right) 30 μm. (C) Representative ear biopsies stained for cutaneous mast cells from individual mice of the four Nf1 and W genotypes. Specimens were stained with hematoxylin-eosin to assess routine histology, and with Giemsa to identify mast cells. Ear biopsies were stained with Fontana-Masson to differentiate melanin-containing cells from mast cells. Cutaneous mast cells (Giemsa-positive, Fontana-Masson–negative) were quantitated in a blinded fashion by counting the distal 5 mm of ears. Black arrows indicate Giemsa-positive mast cells, and open arrows indicate Fontana-Masson melanin–containing cells. Bar, 35 μm.
Haploinsufficiency at Nf1 Increases Peritoneal and Cutaneous Mass Cell Numbers in Wild-Type and W41 Mice.
Mice homozygous for different mutant W alleles have reduced numbers of peritoneal and cutaneous mast cells 17 18. To investigate whether heterozygous inactivation of Nf1 could modulate the deficiency of this lineage, we compared numbers of peritoneal mast cells harvested from Nf1+/−;W41/W41 animals to those taken from singly mutant mice (+/+;W41/W41). We also compared peritoneal mast cell numbers in Nf1+/−;+/+ and wild-type mice. Cells isolated by peritoneal lavage were stained with toluidine blue to identify mast cells. Representative cytospins from individual mice from each of the four Nf1 and W genotypes are shown in Fig. 1 B. Peritoneal mast cell numbers in Nf1+/−;W 41 /W 41 mice were 40-fold higher than in +/+;W41/W41 littermates (Table ). Importantly, in addition to restoring peritoneal mast cell numbers to ∼10% of wild-type levels in W41/W41 mice, heterozygous inactivation of Nf1 significantly increased mast cell numbers in animals that were wild-type at the W locus (Table ). We performed similar experiments to determine if haploinsufficiency at Nf1 increased numbers of cutaneous mast cells in the mutant W41/W41 and wild-type backgrounds. Giemsa-stained ear biopsies from mice of the four W and Nf1 genotypes are shown in Fig. 1 C. Ear biopsies from Nf1+/−;W41/W41 mice showed a twofold increase in numbers of cutaneous mast cells compared with +/+;W41/W41 mice (Table ). Nf1+/−;+/+ mice also had a modest, though statistically insignificant, increase in cutaneous mast cell numbers compared with wild-type mice (Table ). Giemsa-stained biopsies obtained from a second site (dorsal skin) revealed similar differences in cutaneous mast cell numbers between the genotypes (data not shown). The increased numbers of peritoneal and cutaneous mast cells in Nf1+/− mice provide additional evidence that haploinsufficiency augments signaling through the c-kit receptor in vivo.
Table 1.
Effects of W and Nf1 Genotypes on Mast Cell Numbers and Mast Cell Colony Growth
| Genotype | ||||
|---|---|---|---|---|
| +/+ +/+ | Nf1+/− +/+ | +/+ W41/W41 | Nf1+/− W41/W41 | |
| No. of peritoneal mast cells (×102) | 780 ± 10 | 1,000 ± 14 | 2 ± 1 | 81 ± 17 |
| No. of cutaneous mast cells (mm2) | 25.6 ± 7 | 31 ± 2 | 6 ± 1 | 14 ± 2 |
| No. of mast cell colonies (×102) | 79 ± 6 | 117 ± 9 | 0 | 8 ± 0.8 |
Nos. of peritoneal and cutaneous mast cells were quantitated from peritoneal lavages and ear biopsies. Results represent mean numbers of mast cells ± SEM from six animals in each genotype. Peritoneal cells were cultured for the growth of mast cell colonies as described (reference 13), and results represent the mean number of mast cell colonies ± SEM of six independent experiments.
Haploinsufficiency at Nf1 Increases Colony Formation, Proliferation, and Survival of Mast Cells in Response to Steel Factor in W41 and Wild-Type Mice.
IL-3 and Steel factor support clonal mast cell colony formation from bone marrow and mature mast cells in vitro. To further characterize interactions between W and Nf1, we assessed the ability of peritoneal cells isolated from mice of all four genotypes to form mast cell colonies in methylcellulose cultures 13. No mast cell progenitor colonies formed in cultures of +/+;W41/W41 peritoneal cells (Table ). In contrast, cells from Nf1+/−;W41/W41 mice formed significant numbers of mast cell colonies in this assay. Heterozygous inactivation of Nf1 also significantly enhanced the growth of mast cell colonies in mice that were wild-type at the W locus (Table ).
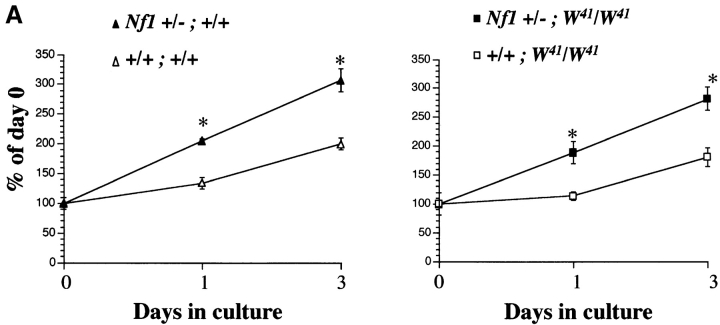
C-kit receptor activation in BMMCs mediates diverse biological responses, including proliferation. To assess whether haploinsufficiency at Nf1 augments the proliferative responses of a homogeneous population of mast cells to Steel factor, the growth kinetics of Nf1+/− BMMCs were compared with Nf1+/+ cells in mice with a normal or mutant (W41) c-kit receptor. BMMCs from mice of each genotype were cultured in 10% FCS without added growth factors for 24 h before the addition of Steel factor. Cell numbers were determined at the time Steel factor was added (day 0) and after 24 and 72 h in culture. Nf1+/− cells of both W genotypes showed greater proliferative responses to Steel factor after 24 and 72 h in culture than the corresponding Nf1+/+ populations ( Fig. 2 A). Using the same experimental design, thymidine incorporation assays gave similar results (data not shown). Thus, haploinsufficiency at Nf1 is associated with an increased proliferative response of wild-type and W41/W41 BMMCs in liquid cultures containing 10% FCS and exogenous Steel factor.
Figure 2.
Effect of haploinsufficiency of Nf1 and W on the survival and proliferation of BMMCs in response to Steel factor. (A) Proliferation of BMMCs from mice of the four Nf1 and W genotypes in response to recombinant murine Steel factor. After deprivation of growth factors for 24 h, 2 × 105 cells/ml were plated in triplicate in 24-well dishes in RPMI containing 1% glutamine, 10% fetal bovine serum, and 100 ng/ml of Steel factor in a total volume of 1 ml as described previously (reference 29). After 1 and 3 d, viable cells were counted using a hemocytometer and expressed as a percentage of input cells. *P < 0.05, Nf1+/−;W41/W41 vs. +/+;W41/W41 and Nf1+/−;+/+ vs. +/+;+/+ cells by Student's paired t test. (B) Percent survival of BMMCs of the four Nf1 and W genotypes. After deprivation of growth factors for 24 h, 3 × 105 cells of each genotype were plated in RPMI containing 1% BSA and 100 ng/ml of recombinant murine Steel factor. The number of surviving cells was determined by trypan blue exclusion and expressed as a percentage of input cells. *P < 0.05, Nf1+/−;W41/W41 vs. +/+;W41/W41 and Nf1 +/−;+/+ vs. +/+;+/+ cells by Student's paired t test.
Steel factor acts as both a mitogen and a survival factor for mast cells 5 17 19. Mast cells derived from different lines of W mutant mice display reduced or no survival when cultured in the presence of Steel factor alone, which correlates with residual receptor tyrosine kinase activity 17 19. Therefore, we examined the effect of heterozygous inactivation of Nf1 on the survival of BMMCs isolated from wild-type and W41/W41 mice ( Fig. 2 B). Nf1+/− BMMCs of both W genotypes demonstrated increased survival compared with Nf1+/+ cells after 48 h of culture in serum-free media in response to exogenous Steel factor. Since this assay has been shown to correlate with c-kit receptor tyrosine kinase activity 19, we quantitated surface expression of c-kit on the four W and Nf1 genotypes by fluorescence cytometry to ensure that these differences in cell survival were not explained by variable levels of receptor expression. No differences were observed (data not shown).
Haploinsufficiency at Nf1 Increases MAP Kinase Activity in Response to Steel Factor in Wild-Type and W41 Mice.
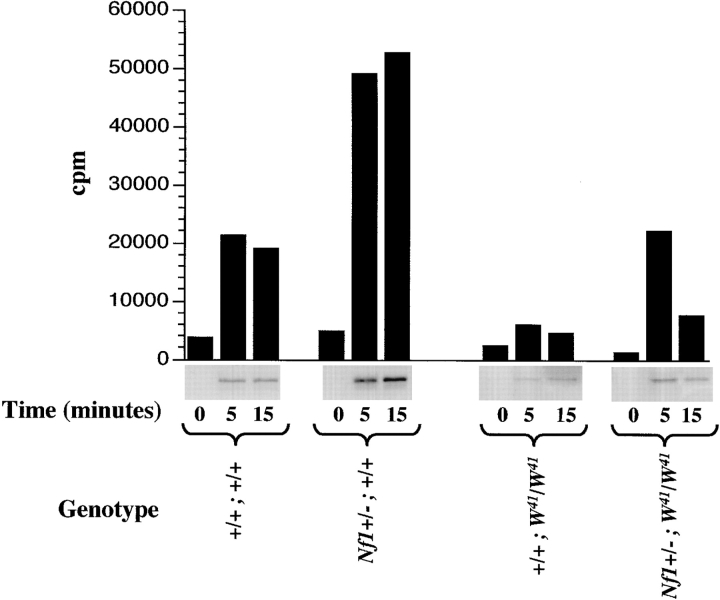
The data presented above provide evidence that haploinsufficiency of Nf1 augments the proliferation and survival of wild-type and W41/W41 mast cells in vitro and in vivo. Partial restoration of normal coat color in Nf1+/−;W 41 /W 41 mice indicates that this effect is not restricted to hematopoietic cells, but also includes melanocytes. The Ras-MAP kinase pathway is an important downstream target of c-kit receptor activation, and neurofibromin negatively regulates Ras signaling by functioning as a GTPase activating protein (GAP) for Ras 20 21. The phenotypic data presented above suggest a model whereby heterozygous inactivation of Nf1 enhances c-kit–induced Ras output by reducing neurofibromin levels (and GAP activity for Ras) in susceptible cell lineages. To test this hypothesis, we stimulated primary BMMCs with Steel factor, and measured p42 MAP kinase. MAP kinase protein levels were similar in BMMCs of all genotype combinations (data not shown). Nf1+/−;W41/W41 BMMCs demonstrated a fivefold greater increase in MAP kinase activity from baseline 5 min after the addition of Steel factor relative to +/+;W41/W41 BMMCs ( Fig. 3). Indeed, haploinsufficiency of Nf1 restored the ability of the mutant W41 c-kit receptor to activate MAP kinase to wild-type levels at the 5-min time point ( Fig. 3). Similarly, Nf1 +/−;+/+ BMMCs had a twofold greater increase in MAP kinase activity from baseline compared with wild-type mast cells that was sustained at both tested time points ( Fig. 3). These biochemical data indicate that the phenotypic effects in Nf1+/− mast cells correlate with enhanced signaling through a major downstream effector of Ras-GTP.
Figure 3.
Analysis of p42 MAP kinase activity from BMMCs stimulated with Steel factor in the four Nf1 and W genotypes. Activation of p42 MAP kinase was determined by depriving cells of growth factors for 24 h, followed by stimulation with 10 ng/ml of Steel factor for 5 and 15 min. Autoradiography and quantitative densitometry of the phosphorylation of Elk-1 fusion protein by MAP kinase from lysates obtained from Steel factor–stimulated BMMCs are shown. Data represent one of three independent experiments. Similar results were obtained in two other experiments.
Discussion
In these experiments, we present genetic, cellular, and biochemical data demonstrating that neurofibromin negatively regulates signaling through the c-kit receptor tyrosine kinase in a haploinsufficient state. Dermal cafe au lait macules, learning disabilities, and the development of multiple cutaneous neurofibromas are major nonmalignant pathologic complications of NF1. Although the finding of constitutional heterozygosity (LOH) in some neurofibromas supports the “two hit” tumor suppressor model 22 23, many of these lesions retain the normal NF1 allele. Other features of neurofibroma biology, including the very large numbers of lesions found in some patients, their self-limited growth, and the low propensity of these tumors to undergo malignant degeneration are also consistent with a possible dosage effect on cell growth. Neurofibromas are infiltrated with mast cells that have been hypothesized to promote growth by releasing mediators that act locally upon Schwann cells, endothelial cells, and fibroblasts 24. Treatment with mast cell stabilizers is associated with a reduction in pruritus in some patients with NF1, and represents the only known medical treatment that alters the growth of neurofibromas 24. Given the importance of Steel factor in regulating multiple mast cell functions, our data showing that haploinsufficiency at Nf1 alters mast cell numbers, survival, and Ras signaling in wild-type and W41/W41 mice in response to Steel factor implicate deregulated mast cell function as potentially important in neurofibroma formation.
In a recent study, Hemesath et al. 25 implicated the Micropthalmia (Mi) transcription factor as a direct target of MAP kinase activation in response to c-kit. The observation that haploinsufficiency at Nf1 effects a 60–70% rescue of the pigmentary defect seen in W41/W41 mice further supports the central role of this pathway in melanocyte development, and is consistent with the marked enhancement of Steel factor–induced MAP kinase activation that we observed in Nf1+/− mast cells. Although the molecular basis of cutaneous melanocyte hyperplasia in NF1 is poorly understood, our data suggest that melanocytes from Nf1+/− mice could offer an attractive system for discerning the role of Nf1 in examining the c-kit–MAP kinase–Mi pathway.
The relative contributions of homozygous NF1 inactivation and of haploinsufficiency to the pathologic complications of NF1 remain incompletely understood. Genetic analysis of malignant peripheral nerve sheath tumors (MPNSTs), pheochromocytomas, and myeloid leukemias from individuals with NF1 and Nf1+/− mice have demonstrated frequent somatic loss of the normal allele 8 26 27 28 29. Homozygous inactivation of NF1 in a human MPNST, in several leukemias, and in a variety of murine tumors provides formal proof that the gene functions as a tumor suppressor in a subset of cancers 8 30. Although these data confirm that tumorigenesis follows the Knudson paradigm 31 in some patients and in Nf1 mice, many tumors do not show loss of LOH, and it is uncertain if inactivation of both alleles is a prerequisite for tumor formation. In a recent study of heterozygous p53 knockout mice, some tumors retained a functional p53 allele 32. Tumor formation in p27 knockout mice is also associated with haploinsuffiency 33. The phenotypes that we have detected in Nf1+/− cells of two lineages suggest that haploinsufficiency may confer a growth advantage that could contribute to tumorigenesis by pathways that do not require inactivation of the normal allele. Full genetic and biochemical characterization of tumors from patients with NF1 and from Nf1+/− mice that retain heterozygosity is required to address this possibility. Finally, the finding that haploinsufficiency at Nf1 has dramatic phenotypic consequences in two cell lineages that are affected in NF1 patients has important therapeutic implications. In particular, if diseased cells retain a functional NF1 allele, increasing neurofibromin-specific GAP activity is an attractive strategy for preventing or treating the complications of NF1.
Acknowledgments
We are grateful to Dr. Tyler Jacks for generously providing Nf1 mice and for helpful discussions. We also thank our colleagues Drs. Hal Broxmeyer, Mervin Yoder, and Mary Dinauer for reading the manuscript.
This work was supported by a National Institutes of Health grant through the Pediatric Scientist Development Program to D.A. Ingram, by American Cancer Society grant DB80030 to K. Shannon and D.W. Clapp, by National Institutes of Health grant RO1CA73614 to K. Shannon, and by March of Dimes Birth Defects Foundation grant 6FY98-0219 and National Institutes of Health grant R29 (CA74177-01) to D.W. Clapp.
References
- Viskochil D., Buchberg A.M., Xu G., Cawthon R.M., Stevens J., Wolff R.K., Culver M., Carey J.C., Copeland N.G., Jenkins N.A. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–192 . doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- Wallace M.R., Marchuk D.A., Andersen L.B., Letcher R., Odeh H.M., Saulino A.M., Fountain J.W., Brereton A., Nicholson J., Mitchell A.L. Type 1 neurofibromatosis geneidentification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186 . doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Chabot B., Stephenson D.A., Chapman V.M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89 . doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Copeland N.G., Gilbert D.J., Cho B.C., Donovan P.J., Jenkins N.A., Cosman D., Anderson D., Lyman S.D., Williams D.E. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183 . doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- Zsebo K.M., Williams D.A., Geissier E.N., Broudy V.C., Martin F.H., Atkins H.L., Hsu R.-Y., Birkett N.C., Okino K.H., Murdock D.C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224 . doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- Broudy V.C. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364 . [PubMed] [Google Scholar]
- Side L., Taylor B., Cayouette M., Conner E., Thompson P., Luce M., Shannon K. Homozygous inactivation of the NF1 gene in bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders. N. Engl. J. Med. 1997;336:1713–1720 . doi: 10.1056/NEJM199706123362404. [DOI] [PubMed] [Google Scholar]
- Jacks T., Shih T.S., Schmitt E.M., Bronson R.T., Bernards A., Weinberg R.A. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1 . Nat. Genet. 1994;7:353–361 . doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- Brannan C.I., Perkins A.S., Vogel K.S., Ratner N., Nordlund M.L., Reid S.W., Buchberg A.M., Jenkins N.A., Parada L.F., Copeland N.G. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029 . doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- Largaespada D.A., Brannan C.I., Jenkins N.A., Copeland N.G. Nf1 deficiency causes Ras-mediated granulocyte/macrophage colony stimulating factor hypersensitivity and chronic myeloid leukaemia. Nat. Genet. 1996;12:137–143 . doi: 10.1038/ng0296-137. [DOI] [PubMed] [Google Scholar]
- Zhang Y.Y., Vik T.A., Ryder J.W., Srour E.F., Jacks T., Shannon K., Clapp D.W. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J. Exp. Med. 1998;187:1893–1902 . doi: 10.1084/jem.187.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag G., Clapp D.W., Shih S., Adler F., Zhang Y.Y., Thompson P., Lange B.J., Freedman M.H., McCormick F., Jacks T., Shannon K. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in hematopoietic cells. Nat. Genet. 1996;12:144–148 . doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc. Natl. Acad. Sci. USA. 1982;79:3843–3847 . doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serve H., Yee N.S., Stella G., Sepp-Lorenzino L., Tan J.C., Besmer P. Differential roles of P13-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:473–483 . doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag G., Adler F., elMasry N., McCabe P.C., Conner E., Jr., Thompson P., McCormick F., Shannon K. Biochemical characterization of a novel KRAS insertion mutation from a human leukemia. J. Biol. Chem. 1996;271:32491–32494 . doi: 10.1074/jbc.271.51.32491. [DOI] [PubMed] [Google Scholar]
- Nocka K., Tan J.C., Chiu E., Chu T.Y., Ray P., Traktman P., Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locusW 3 7, W v, W 41 and W . EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:1805–1813 . doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin R., Bernstein A. The white spotting and steel hereditary anemias of the mouse. In: Feig S., Freedman M., editors. Clinical Disorders and Experimental Models of Erythropoietic Failure. CRC Press; Boca Raton, FL : 1993. pp. 1257–1273. [Google Scholar]
- Tsujimura T., Koshimizu U., Katoh H., Isozaki K., Kanakura Y., Tono T., Adachi S., Kasugai T., Tei H., Nishimune Y. Mast cell number in the skin of heterozygotes reflects the molecular nature of c-kit mutation. Blood. 1993;81:2530–2538 . [PubMed] [Google Scholar]
- Paulson R.F., Vesely S., Siminovitch K.A., Bernstein A. Signalling by the W/Kit receptor tyrosine kinase is negatively regulated in vivo by the protein tyrosine phosphatase Shp1. Nat. Genet. 1996;13:309–315 . doi: 10.1038/ng0796-309. [DOI] [PubMed] [Google Scholar]
- Xu G.F., O'Connell P., Viskochil D., Cawthon R., Robertson M., Culver M., Dunn D., Stevens J., Gesteland R., White R., Weiss R. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608 . doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- Skuse G.R., Kosciolek B.A., Rowley P.T. Molecular genetic analysis of tumors in von Recklinghausen neurofibromatosisloss of heterozygosity for chromosome 17. Genes Chromosomes Cancer. 1989;1:36–41 . doi: 10.1002/gcc.2870010107. [DOI] [PubMed] [Google Scholar]
- Stark M., Assum G., Krone W. Single-cell PCR performed with neurofibroma Schwann cells reveals the presence of both alleles of the neurofibromatosis type 1 (NF1) gene. Hum. Genet. 1995;96:619–623 . doi: 10.1007/BF00197423. [DOI] [PubMed] [Google Scholar]
- Sawada S., Florell S., Purandare S.M., Ota M., Stephens K., Viskochil D. Identification of NF1 mutations in both alleles of dermal neurofibroma. Nat. Genet. 1996;14:110–112 . doi: 10.1038/ng0996-110. [DOI] [PubMed] [Google Scholar]
- Riccardi V.M. NeurofibromatosisPhenotype, Natural History and Pathogenesis 2nd ed 1992. Johns Hopkins University Press; Baltimore: pp. 498 pp [Google Scholar]
- Hemesath T.J., Price E.R., Takemoto C., Badalian T., Fisher D.E. MAP kinase links the transcription factor Microphthalmia to c-Kit signaling in melanocytes. Nature. 1998;391:298–301 . doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- Glover T.W., Stein C.K., Legius E., Andersen L.B., Brereton A., Johnson S. Molecular and cytogenetic analysis of tumors in von Recklinghausen neurofibromatosis. Genes Chromosomes Cancer. 1991;3:62–70 . doi: 10.1002/gcc.2870030111. [DOI] [PubMed] [Google Scholar]
- Menon A.G., Anderson K.M., Riccardi V.M., Chung R.Y., Whaley J.M., Yandell D.W., Farmer G.E., Freiman R.N., Lee J.K., Li F.P. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc. Natl. Acad. Sci. USA. 1990;87:5435–5439 . doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K.M., O'Connell P., Martin G.A., Paderanga D., Olson K., Dinndorf P., McCormick F. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. N. Engl. J. Med. 1994;330:597–601 . doi: 10.1056/NEJM199403033300903. [DOI] [PubMed] [Google Scholar]
- Xu W., Mulligan L.M., Ponder M.A., Liu L., Smith B.A., Mathew C.G., Ponder B.A. Loss of alleles in pheochromocytomas from patients with type 1 neurofibromatosis. Genes Chromosomes Cancer. 1992;4:337–342 . doi: 10.1002/gcc.2870040411. [DOI] [PubMed] [Google Scholar]
- Legius E., Marchuk D.A., Collins F.S., Glover T.W. Somatic deletion of the neurofibromatosis type 1 gene in a neurofibrosarcoma supports a tumour suppressor gene hypothesis. Nat. Genet. 1993;3:122–126 . doi: 10.1038/ng0293-122. [DOI] [PubMed] [Google Scholar]
- Knudson A.J. Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985;45:1437–1443 . [PubMed] [Google Scholar]
- Venkatachalam S., Shi Y.-P., Jones S.N., Vogel H., Bradley A., Pinkel D., Donehower L.A. Retention of wild-type p53 in tumors from p53 heterozygous micereduction of p53 dosage can promote cancer formation. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4657–4667 . doi: 10.1093/emboj/17.16.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fero M.L., Randel E., Gurley K.E., Roberts J.M., Kemp C.J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]