Abstract
The MC148 CC chemokine from the human poxvirus molluscum contagiosum (MCV) was probed in parallel with viral macrophage inflammatory protein (vMIP)-II encoded by human herpesvirus 8 (HHV8) in 16 classified human chemokine receptors. In competition binding using radiolabeled endogenous chemokines as well as radiolabeled MC148, MC148 bound with high affinity only to CCR8. In calcium mobilization assays, MC148 had no effect on its own on any of the chemokine receptors, but in a dose-dependent manner blocked the stimulatory effect of the endogenous I-309 chemokine on CCR8 without affecting chemokine-induced signaling of any other receptor. In contrast, vMIP-II acted as an antagonist on 10 of the 16 chemokine receptors, covering all four classes: XCR, CCR, CXCR, and CX3CR. In chemotaxis assays, MC148 specifically blocked the I-309–induced response but, for example, not stromal cell–derived factor 1α, monocyte chemoattractant protein 1, or interleukin 8–induced chemotaxis. We thus concluded that the two viruses choose two different ways to block the chemokine system: HHV8 encodes the broad-spectrum chemokine antagonist vMIP-II, whereas MCV encodes a highly selective CCR8 antagonist, MC148, conceivably to interfere with monocyte invasion and dendritic cell function. Because of its pharmacological selectivity, the MC148 protein could be a useful tool in the delineation of the role played by CCR8 and its endogenous ligand, I-309.
Keywords: monocytes, Kaposi sarcoma–associated herpesvirus, dendritic cells, chemokines, chemokine receptors
Introduction
The poxvirus molluscum contagiosum (MCV) causes small benign tumors in the skin of immunocompetent children and opportunistic infections in immunocompromised patients. MCV lesions are characterized by a surprising absence of surrounding inflammatory cell infiltrates 1 2 3. The genome of MCV contains several genes corresponding to human genes suggested to take part in immune evasion, such as a homologue of the MHC, a protein with identity to the human peroxidase, and finally an open reading frame (ORF), MC148R, which codes for a chemokine-like protein that, based on its sequence, was originally suggested to be a chemokine antagonist 4.
Chemokines are 70–80-amino acid proteins with well characterized three-dimensional structures usually stabilized by two disulfide bridges. They are involved in attracting and activating distinct leukocyte subsets, for example to inflammatory foci and specific tissues and microenvironments within tissues, as in a lymph node 5 6 7. The precise number of human chemokines is still unclear but is likely to be ∼45–55. They are divided into four families on the basis of the pattern of the conserved cysteine residues located near their NH2 terminus and forming disulfide bridges with cysteines located further COOH-terminally in the molecule. In the CC family, the two NH2-terminal cysteines are adjacent; in the CXC family, the two residues are separated by a single amino acid; and in the CX3C family, the separation is by three amino acids. The XC family, which as yet has only one member, has just one cysteine near the NH2 terminus. Chemokines exert their function through seven-transmembrane (7TM), G protein–coupled receptors, of which today nine are CC chemokine receptors, five are CXC chemokine receptors, one is a CX3C chemokine receptor, and one is an XC chemokine receptor. On the basis on their function, chemokine receptors can be classified in an “inflammatory group,” which is activated under pathological conditions, and a “housekeeping group,” taking care of re-circulation and maturation of lymphocytes in the unchallenged host 5.
In the last couple of years, it has become clear that several herpes- and poxviruses code for chemokine proteins and/or chemokine-like 7TM receptors 8 9 10 11 12. Viral 7TM receptors are encoded by CMV 13 14, human herpesvirus (HHV)6 15 16, HHV7 17, and HHV8 18. In addition to this, EBV as well as HHV6 and HHV7 have been shown to upregulate certain endogenous chemokine receptors in their target cells. Thus, EBV actively upregulates the expression of endogenous CCR7, previously called EBV-induced molecule (EBI) 1, as well as an orphant receptor, EBI 2, in infected B cell lines 19, whereas HHV6 and HHV7 actively upregulate CCR7 in infected CD4+ T cells 20. As CCR7 is believed to be a receptor that mediates migration of cells to lymph nodes, this could be a way that the virus obtains tissue targeting to secondary lymphoid tissues, i.e., by “telling the infected cell” where it would like to go by upregulating or expressing a suitable chemokine receptor. However, for most of the receptors encoded by the virus itself, a function has not yet been identified, except in the case of the ORF-74 receptor from HHV8, which apparently functions as a virally encoded oncogene due to its high constitutive signaling activity 18 21 22, and the US28 receptor from CMV, which appears to be involved in the cellular transfer of virus 23.
As for the receptors, only some of the virally encoded chemokine proteins have been characterized pharmacologically. Thus, CMV encodes an uncharacterized CXC chemokine as well as a newly characterized CXCR2 agonist 24 25, whereas HHV6 encodes a uncharacterized CC chemokine 15 26. Regarding HHV8, functions for two of the three chemokines encoded by the virus have been suggested. The first is viral macrophage inflammatory protein (vMIP)-II, which has been shown to be a surprisingly broad-spectrum antagonist for both CC and CXC chemokine receptors in vitro 27. In accordance with this finding, vMIP-II was shown to function as an efficient immunosuppressive agent in vivo, i.e., in a rat model for glomerulonephritis 28. Very recently, it was demonstrated that vMIP-I, also from HHV8, could act not as a blocker but as a selective agonist for CCR8 29 30. Interestingly, it has also been shown that vMIP-II, which otherwise is a broad-spectrum chemokine antagonist, in fact can act selectively as a chemoattractant for eosinophils 31.
In this study, we characterize the binding and function of the MCV-encoded chemokine MC148 in parallel with the broad-spectrum chemokine antagonist vMIP-II from HHV8. Previously, it had been suggested that MC148, analogous to vMIP-II, could also be a broad-spectrum antagonist 32. However, we report here that MC148 binds very selectively to CCR8 and blocks the function of only this receptor without affecting the function of any other chemokine receptor tested. Thus, the viral protein can be a very useful immunological tool due to its high selectivity for this particular receptor. Furthermore, the selective targeting of CCR8 by the virus also indicates that this receptor could be an interesting drug target.
Materials and Methods
Chemokines.
I-309 and murine lymphotactin were purchased from R & D Systems, Inc., and thymus-expressed chemokine was purchased from PeproTech, Inc. IL-8 and MIP-1α were expressed in Escherichia coli and purified in our facilities. Fractalkine, liver-activated and -regulated chemokine, MCP-1, MCP-3, RANTES (regulated upon activation, normal T cell expressed and secreted), and vMIP-II were provided by Tim Wells (Ares-Serono, S.A. Geneva, Switzerland). Macrophage-derived chemokine and secondary lymphoid tissue chemokine were provided by Thomas Schall (ChemoCentryx, San Carlos, CA). I-TAC (IFN-inducible T cell α chemoattractant) was provided by Kuldeep Neote (Pfizer, Inc., Groton, CT). Stromal cell–derived factor (SDF)-1α was provided by Michael Luther (Glaxo-Wellcome, Research Triangle Park, NC). B cell–attracting chemokine 1 was provided by Bernhard Moser (Thedor-Kocher Institute, Bern, Switzerland).
Stable Cell Lines.
The Chinese hamster ovary (CHO) cells stably expressing CCR1, CCR2, CCR3, CCR5, CXCR1, CXCR2, and CXCR4, as well as human embryonic kidney (HEK)293 cells stably expressing CCR6 and CCR8, were provided by Tim Wells (Serono, Geneva, Switzerland). L12 cells stably expressing CCR4, XCR1, and CX3CR1 were a gift from Osamu Yoshie (Kinki University, Osaka, Japan). Kuldeep Neote (Pfizer, Inc., Groton, CT) provided 300.19 cells expressing CXCR3, and Bernhard Moser (Thedor-Kocher Institute, Bern, Switzerland) provided 300.19 cells expressing CXCR5. Gabriel Marquez (Autonomous University, Madrid, Spain) provided HEK293 cells stably expressing CCR9. L1.2 cell lines expressing CCR7 and CCR8 were established at ICOS Corp. (Johnny Stine).
Isolation of PBLs and PMNs.
To purify PBLs, whole blood from healthy donors was diluted 1:1 in PBS and centrifuged on Histopaque® (Sigma Chemical Co.). PBMCs were collected from the interface and were washed twice in PBS. The cells were further purified by negative selection with anti-CD14 magnetic beads (Miltenyi Biotec) to remove monocytes. PMNs were isolated by centrifugation on a 7.4% Ficoll, 15.5% Hypaque solution (Sigma Chemical Co.). The PMN layer was collected, and the cells were washed twice in PBS before resuspension in chemotaxis buffer.
Cloning of the Gene.
A biopsy was taken from an MCV element from a 9-yr-old child, and a QIAamp tissue kit (QIAGEN Inc.) was used to extract total DNA. Based on the nucleotide sequence deposited in EMBL/GenBank/DDBJ (available under accession no. U60315), the MC148R gene was amplified by PCR. The full-length coding sequence was inserted in the pTEJ vector, which uses the Ubiquitin UbC promotor 33. Nucleotide sequence analysis was performed on an ABI 377 sequence system (Perkin-Elmer Corp.).
Production of Recombinant Protein.
COS-7 cells were transiently transfected by a calcium phosphate precipitate method with addition of chloroquine. Serum-free medium was collected 24, 48, and 72 h after transfection. The medium was centrifuged at 1,500 g for 20 min, and the supernatant was adjusted to pH 4.5 and filtered through 0.22-μm filters (Nalgene). The medium was diluted with water 1:1 to decrease ionic strength and loaded on cation exchange SP-Sepharose columns (Pharmacia Biotech). The columns were washed with 50 mM sodium acetate buffer, pH 4.5, and the protein was eluted with 2 M NaCl in the same buffer. The eluate was made 0.2% in TFA, filtered, and loaded on a C8 column (Vydac) for reverse-phase HPLC, from which the protein was eluted with 0.1% TFA in water on a gradient of CH3CN. The elution position of the recombinant MC148 protein as well as the purity was identified with mass spectroscopy and NH2-terminal sequence analysis on an ABI 494 protein sequencer (Perkin-Elmer Corp.). The identity of each subsequent batch was identified with mass spectroscopy. The yield was ∼4–6 μg/175 cm2 flask per harvest.
Cell Binding.
Whole cell binding (0.8–2.5 × 105 cells per well) was performed at 4°C for 3 h in 0.5 ml of 25 mM Hepes buffer containing 1 mM CaCl2 and 5 mM MgCl2, at pH 7.2, supplemented with 0.5% BSA on transiently transfected COS-7 cells. The incubation was stopped by washing four times with 0.5 ml of ice cold binding buffer made 0.5 mM in NaCl. Cell-associated radioactivity was determined after extraction of the cells with 8 M urea in 3 M acetic acid supplemented with 1% NP-40. Nonspecific binding, determined in the presence of the relevant chemokine peptide (0.1 μM), was subtracted. The following radioactively labeled peptides were from Amersham: 125I–MIP-1α (IM285), 125I–MCP-1 (IM280), 125I–RANTES (IM288), 125I–I-309 (IM313), and 125I–IL-8 (IM249). 125I–metSDF-1α, 125I–fractalkine, and 125I–MC148R were prepared in our facilities, the first two by oxidative iodination and the latter by Bolton-Hunter iodination before HPLC purification.
Calcium Fluorimetry.
Cells stably transfected with various chemokine receptors were loaded with Fura-2AM (Molecular Probes, Inc.) in a Krebs ringer buffer, pH 7.4 (CHO and HEK293 stable transfectants) or RPMI with 1% FCS (L12 and 300.19 stable transfectants) for 20–30 min. Aliquots were made of 106 cells, and each aliquot was pelleted and resuspended in 500 μl of Krebs ringer buffer or RPMI (depending on cell type) with 10 mM EGTA. Fluorescence was measured on a Jobin Yvon FlouroMax-2 (Jobin Yvon Spex) as the ratio of emission at 490 nm when excited at 340 and 380 nm, respectively.
In Vitro Chemotaxis.
Chemotaxis was measured using 24 transwells polycarbonate membranes (3 μm; Corning Costar Corp.). Chemokines were diluted in 0.6 ml chemotaxis buffer (RPMI containing 0.5% BSA) and added to the lower chemotaxis chamber. 106 PMNs, PBLs, or L1.2/CCR8 cells or 0.75 × 106 THP-1 cells were resuspended in 0.1 ml chemotaxis buffer and added to the top chamber insert. Chemotaxis plates were then incubated for 30 min (PMN) or 4 h (PBL, THP-1, or L1.2/CCR8) at 37°C in a 5% CO2-humidified incubator. After the incubation, the cells from the bottom well were collected and counted by FACS® (Becton Dickinson).
Results
Cloning and Expression of the MC148R Gene.
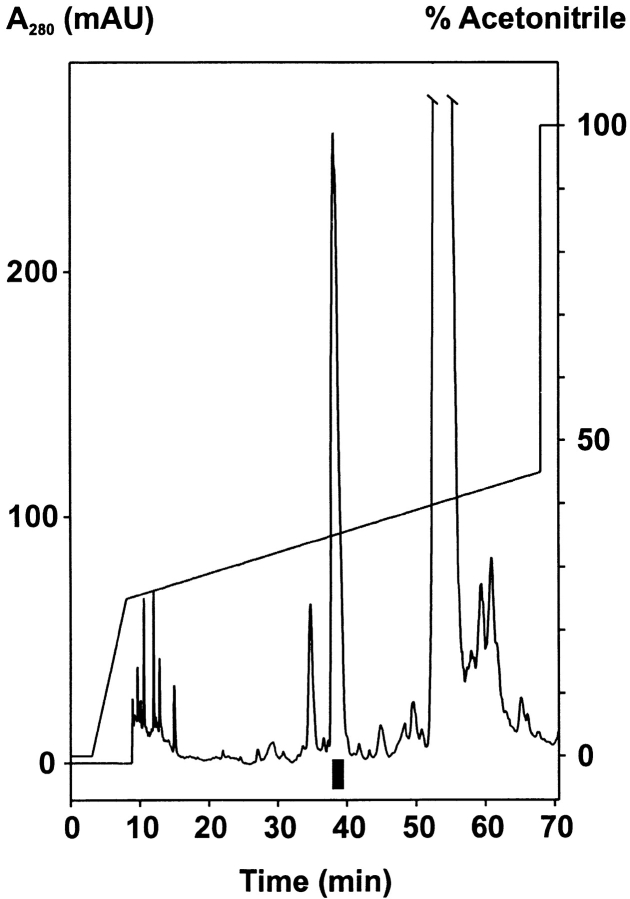
The MC148R gene was cloned from a skin lesion of a patient, and sequence analysis showed that it was identical to the gene from the MCV subtype 1 (deposited in EMBL/GenBank/DDBJ under accession no. U60315). COS-7 cells were used for production of the MC148 chemokine protein, which was collected in conditioned, serum-free medium, subjected to cation exchange chromatography followed by HPLC purification. Production of the viral chemokine in the eukaryotic cells should secure that the protein product corresponds to the chemokine normally secreted from cells infected by the virus. The conditioned medium contained a major protein peak eluting at 35% acetonitrile that corresponded to the processed, secreted MC148 protein ( Fig. 1). Mass spectroscopy and NH2-terminal sequence analysis revealed a single protein with a molecular mass of 8,984 daltons and a cleavage site for the secretory signal peptide between residues 24 and 25 of the precursor protein ( Fig. 2). The HPLC-purified MC148 protein was used for receptor activation studies, in chemotaxis assays, and, after being radioactively labeled with 125I and HPLC purified, in receptor binding studies.
Figure 1.
Purification of recombinant MC148 expressed in COS cells. The elution profile of MC148 (black box) on reverse-phase HPLC is shown. mAU, milliabsorbance units.
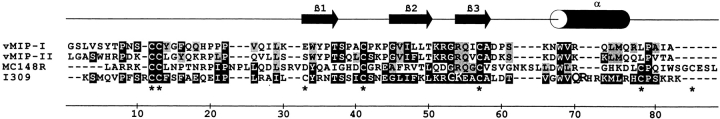
Figure 2.
Alignment of known CCR8 ligands to MC148. vMIP-II, vMIP-I, MC148, and I-309 were aligned using CLUSTALW 1.7. Identical amino acids are shown white on black, whereas similar amino acids are shown white on gray. The line above the alignment shows the secondary structure of vMIP-II as determined by NMR 51. *Cysteine residues.
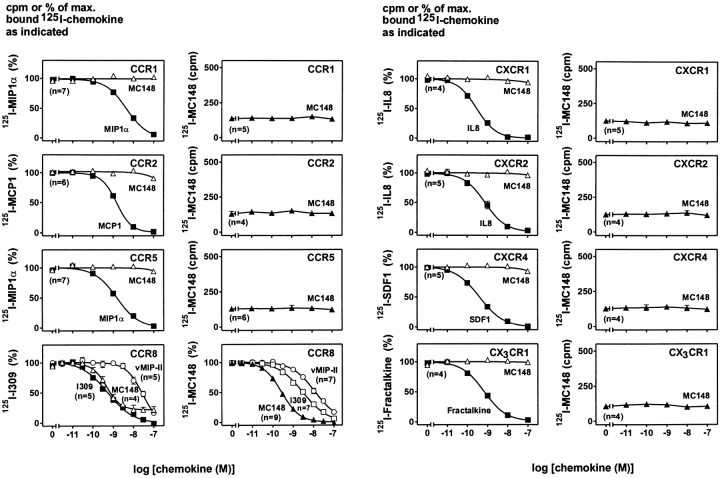
Receptor Binding Studies.
To characterize the binding profile of MC148, we probed a panel of human chemokine receptors in both heterologous and homologous binding experiments. MC148 was not able to compete for binding against the radioactively labeled human chemokines used as radioligands for CCR1, CCR2, CCR5, CXCR1, CXCR2, CXCR4, and CX3CR1 ( Fig. 3). However, MC148 did displace 125I–I-309 from CCR8 transiently expressed in COS-7 cells with subnanomolar affinity (IC50 = 0.47 nM; 95% CI; 0.33–0.67 nM). The affinity of the MC148 protein corresponds to that of the endogenous CCR8 ligand I-309 (IC50 = 0.52 nM), but interestingly, ∼20% of the bound 125I–I-309 could apparently not be displaced by the viral protein ( Fig. 3). vMIP-II from HHV8 also bound to CCR8, albeit with a somewhat lower affinity (IC50 = 27.8 nM; Fig. 3).
Figure 3.
Heterologous and homologous competition binding experiments with recombinant MC148 with various chemokine receptors. Heterologous competition binding curves for MC148 are shown to the left; human chemokine corresponding to the radioactively labeled human chemokine used (▪), MC148 (▵), and vMIP-II (○). Homologous binding curves for MC148 are shown to the right; MC148 (▴), I-309 (□), and vMIP-II (○). For every homologous binding experiment with 125I–MC148, a homologous control curve was made with the human chemokine known to bind the receptor to make sure that the receptor was expressed on the surface of the cell.
Heterologous competition binding experiments can sometimes be misleading, as a high-affinity ligand may not always be able to compete for binding against another high-affinity ligand 34. Direct binding experiments using 125I–MC148 confirmed the high-affinity binding of the viral protein to human CCR8 (IC50 = 0.27 nM; 95% CI; 0.24–0.30 nM) and demonstrated the suitability of the radioactive peptide as a radioligand in receptor binding experiments. Nevertheless, 125I–MC148 was unable to bind specifically to any other human chemokine receptor tested (CCR1, CCR2, CCR5, CXCR1, CXCR2, CXCR4, and CX3CR1; Fig. 3). In all these cases, binding experiments were performed in parallel with the appropriate radioactive, endogenous chemokine to confirm suitable receptor expression ( Fig. 3). Thus, both heterologous and homologous binding experiments indicate that the MC148 chemokine from MCV is a selective CCR8 ligand.
Calcium Mobilization Studies.
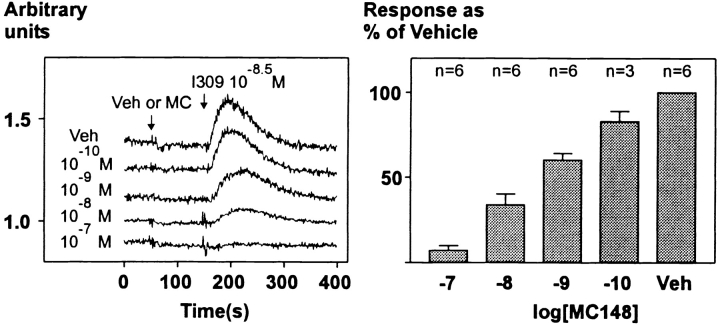
Calcium mobilization was studied in 16 cell lines individually expressing 16 different human chemokine receptors. As shown in Fig. 4, in each cell line the appropriate, endogenous human chemokine was able to rapidly increase intracellular calcium concentrations. In contrast, MC148 given at 10−7 M concentration had no effect on calcium mobilization in any of the cell lines ( Fig. 4). More importantly, pretreatment with 10−7 M MC148 did not diminish the response to the appropriate endogenous chemokine elicited through any of the tested human chemokine receptors, except for CCR8 ( Fig. 4). In the HEK293 cells expressing CCR8, pretreatment with MC148 in a dose-dependent manner and with an IC50 of 7.1 nM blocked the calcium response to I-309 ( Fig. 5). Despite the observation that MC148 in binding experiments was unable to compete for binding against 20% of the bound 125I–I-309, the viral chemokine totally blocked the calcium response elicited by I-309 through CCR8.
Figure 4.

Effect of recombinant MC148 and vMIP-II on calcium mobilization on a panel of cell lines stably transfected with humane chemokine receptors. 10−7 M MC148, vMIP-II, or vehicle was added to the cells at 50 s, followed by a submaximal dose of an appropriate endogenous human chemokine at 150 s. The height of the response with the endogenous ligand was measured, and the heights in the experiments with MC148 and vMIP-II were expressed as percent of the height in the experiment with the vehicle. A representative example of each experiment is shown to the left, whereas the box diagram to the right shows the average inhibition with SEM as indicated. Asterisks (*) indicate statistically significant inhibition (P < 0.05). The chemokine concentrations used were: CCR1, 10−10 M RANTES; CCR2, 10−8 M MCP-1; CCR3, 10−9 M MCP-3; CCR4, 10−9 M macrophage-derived chemokine; CCR5, 10−9 M RANTES; CCR6, 10−8 M liver-activated and -regulated chemokine; CCR7, 10−9 M secondary lymphoid tissue chemokine; CCR8, 10−8.5 M I-309; CCR9, 10−7 M thymus-expressed chemokine; CXCR1, 10−9 M IL-8; CXCR2, 10−9 M IL-8; CXCR3, 10−10 M I-TAC; CXCR4, 10−10 M SDF-1α; CXCR5, 10−10 M B cell–attracting chemokine 1; XCR1, 10−7 M murine lymphotactin; and CX3CR1, 10−9 M fractalkine.
Figure 5.
Dose–response curve of recombinant MC148 on I-309–induced calcium mobilization in stably CCR8-transfected HEK293 cells. 10−7, 10−8, 10−9, and 10−10 M MC148 or vehicle was added to the cells at 50 s, followed by 10−8.5 M I-309 at 150 s. The height of the response with I-309 was measured, and the heights in the experiments with the different concentrations of MC148 were expressed as percent of the height in the experiment with the vehicle. A representative example is shown to the left; the box diagram to the right shows the average inhibition with SEM as indicated.
In contrast to MC148, vMIP-II acted as a broad-spectrum antagonist, as it inhibited the receptor-mediated calcium mobilization in 10 out of 16 human chemokine receptors tested. As previously reported, vMIP-II fully blocked the ligand-induced calcium mobilization through CCR1, CCR2, CCR5, and CXCR4 and partially blocked the CCR3-mediated response, whereas the CXCR1-mediated calcium flux was unaffected 27. Interestingly, the antagonistic repertoire of vMIP-II was here extended to also cover the two minor families of chemokines, fractalkine and lymphotactin, as it totally blocked the calcium responses induced by these chemokines through CX3CR1 and XCR1, respectively ( Fig. 4). Finally, it was found that vMIP-II could partially block CCR4, CCR8, and CXCR3. In contrast, vMIP-II had no effect on the calcium responses elicited by the appropriate endogenous chemokine through CCR6, CCR7, CCR9, CXCR2, and CXCR5.
Thus, as studied in cells individually expressing the various cloned receptors and in respect of signal transduction through calcium mobilization, vMIP-II from HHV8 truly is a broad-spectrum chemokine antagonist covering receptors for all four families of chemokines, whereas MC148 from MCV acts as a highly selective CCR8 antagonist.
Effect on Chemotaxis.
The potential chemotactic activity of MC148 was tested on freshly isolated and purified neutrophils, PBLs, and THP-1 cells, as well as on L1.2 cells transfected with CCR8. When administered alone, MC148 was not able to chemoattract any of these cell types. MC148 was also unable to inhibit SDF-1–induced chemotactic response of PBLs ( Fig. 6), conceivably mediated through CXCR4, which is in agreement with the lack of effect of MC148 on the SDF-1–induced calcium mobilization. MC148 did not affect the IL-8–induced chemotactic response of neutrophils or the MCP-1–induced response of THP-1 cells, which is in agreement with the lack of effect of MC148 on cloned CXCR2 and CCR2, respectively. However, as expected based on the effect on signal transduction, MC148 did block the I-309–induced chemotactic response on L1.2 cells transfected with CCR8 ( Fig. 6). It is noteworthy that MC148 inhibited CCR8-mediated chemotaxis in a concentration similar to the concentration of I-309 used to chemoattract the cells. Furthermore, we tested the chemotactic activity of vMIP-II on CCR8-transfected L12 cells. vMIP-II was unable to chemoattract any of the cell types tested; it did block the I-309–mediated chemotaxis, but at concentrations ∼100-fold higher than the effective concentration of I-309.
Figure 6.
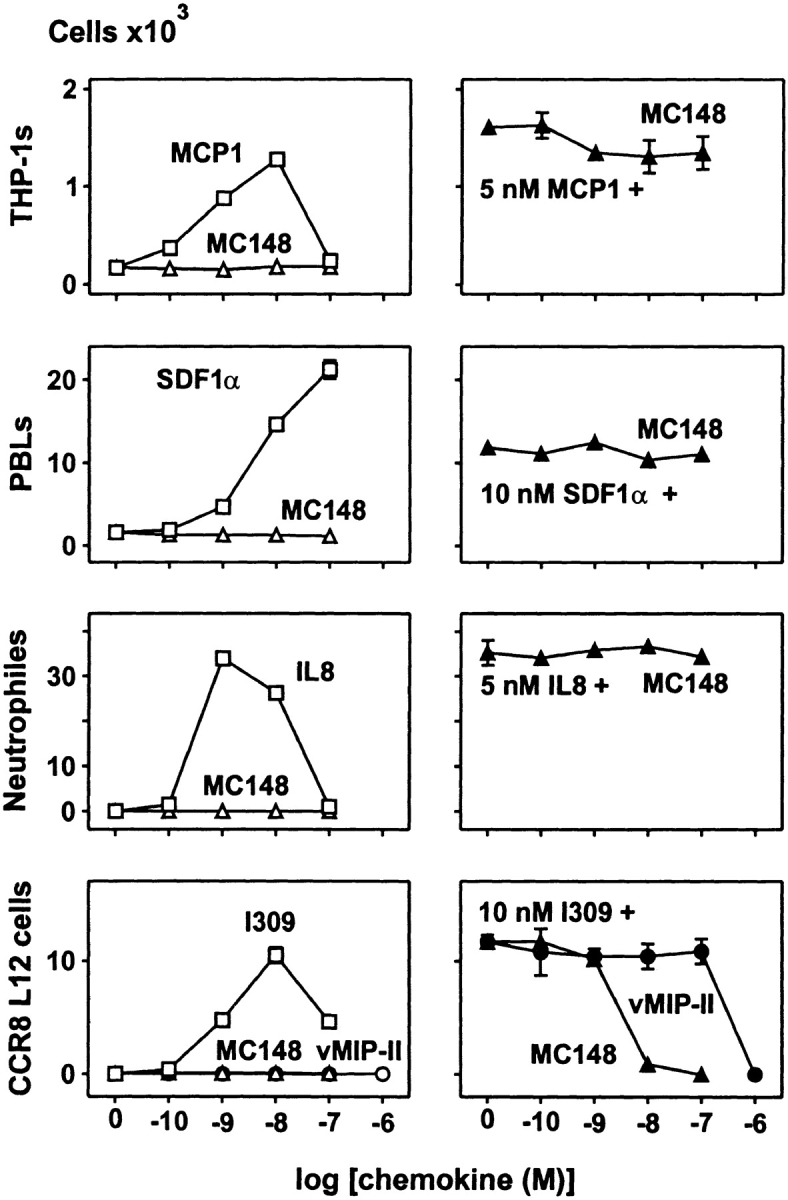
Effect of MC148 on chemotactic activity on THP-1 cells (monocytic cell line), PBLs, neutrophils, and CCR8-transfected L12 cells. To the left, dose–response curves for MC148 (▵). Dose–response curves (□) for MCP-1, SDF-1α, IL-8, and I-309 are shown for comparison. The bottom panel includes a dose–response curve for vMIP-II (○). To the right, dose–response curves for inhibition by MC148 (▴) of chemotaxis induced by 5 nM MCP-1 on THP-1 cells, 10 nM SDF-1α on PBLs, 10 nM IL-8 on neutrophils, and 10 nM I-309 on CCR8-transfected L12 cells. The bottom panel includes a dose–response curve for inhibition by vMIP-II (•). One representative assay out of three is shown for THP-1 cells, out of four for PBLs, out of three for neutrophils, and out of five for CCR8-transfected L12 cells.
Discussion
Through a systematic analysis using 16 cloned and categorized human chemokine receptors, we find here that the MC148 chemokine from MCV is a highly specific ligand for CCR8 and that it specifically blocks the binding and action of the endogenous ligand I-309 on this receptor. In contrast, another virally encoded chemokine, vMIP-II from HHV8, is found to be an even more broad-spectrum chemokine antagonist than originally described 27, as it bound and blocked 10 out of 16 chemokine receptors tested, covering all four classes of chemokine receptors. Thus, the two viruses, MCV and HHV8, have apparently exploited two different avenues to interfere with the chemokine branch of the immune system of their host. The fact that MCV specifically addresses CCR8 is interesting and points to this receptor as playing a key role in our immune system. This also suggests that CCR8 could be a useful drug target as well.
MC148 Is a Surprisingly Selective Chemokine Antagonist.
MC148 was discovered only recently as an ORF during the sequencing of the genome of MCV and, based on its deduced amino acid sequence, it was then suggested that it might function as a chemokine antagonist 4. This notion was supported by the short NH2-terminal extension of the virally encoded chemokine and the observation that several endogenous CC chemokines become antagonists by modification or shortening of their NH2 termini 35 36 37 38. It is interesting that the two extra cysteine residues of MC148, which very likely could form a third intramolecular disulfide bridge, are found in the COOH-terminal end of the molecule. However, the possible structural and functional consequences of these two cysteine residues are still unclear and remain to be determined experimentally. When the MC148 protein was subsequently expressed and tested, it was found to act, as expected, as a chemokine antagonist 32 39. However, in contrast to the findings in our study, MC148 was in the first reports described as a broad-spectrum chemokine antagonist based on its apparent ability to block the chemotactic response of several types of chemokines 32. Nevertheless, very little data was presented in these preliminary reports on the effect of MC148 on individual, cloned chemokine receptors except for CCR8. We cannot explain why it was originally found that the MC148 protein could inhibit the chemotaxis induced by various chemokines, as the protein product apparently was identical, although the protein in our study was produced by expression in COS-7 cells from an artificial eukaryotic expression plasmid and in the previous study was produced in HeLa/BS-C-1 cells after infection with recombinant vaccinia virus 32. Nevertheless, it should be noted that in both studies it was found that MC148 acts potently as an antagonist on CCR8. We would like to emphasize that the selective action of MC148 on CCR8 in our report is supported by the parallel characterization of vMIP-II as a truly broad-spectrum antagonist. Moreover, the double HPLC-purified, radioactively labeled MC148 protein also bound with high affinity only to CCR8.
Why Does MCV Specifically Block CCR8?
Unfortunately, this important question cannot be answered well in this report due to the limited information available concerning both CCR8 as such and its specific ligand, I-309. CCR8 has been found on the surfaces of monocytes 40, and I-309 has been to shown to be a chemoattractant for monocytes 41, whereas Th2 cells have been shown to upregulate CCR8 transcripts as well as respond with an intracellular I-309–mediated calcium flux upon activation 42 43. Furthermore, I-309 has been shown to be produced by activated T cells 44 and IL-1–stimulated monocytes 45, as well as by an activated human mast cell line. MCV lesions are characterized by an absence of inflammatory cell infiltrate 1 2 as well as the absence of Langerhans cells, the APCs of the epidermis 2 3. Monocytes have been shown to be precursors of Langerhans cells 47 as well as antigen-presenting dendritic cells 48. Thus, it could be speculated that MCV has optimized MC148 to block CCR8-mediated recruitment of monocytes, resulting in a lack of APCs that creates a stalemate between the virus and the host in which the host cannot eliminate the virus and the virus cannot disseminate. It should also be noted that MCV does code for proteins other than MC148, which also could be involved in interfering with the host immune response 4.
The question posed above can in fact be turned around. The fact that the virus has taken the effort to carefully optimize the MC148 chemokine to be such a selective, high-affinity antagonist for CCR8 indicates that this as yet poorly characterized receptor probably plays an important role in our immune system. Importantly, with MC148, the virus at the same time has provided a highly useful pharmacological tool for delineating the function of CCR8 and its endogenous ligand, I-309.
vMIP-II Is an Even More Broad-Spectrum Chemokine Antagonist than Expected.
Originally, we found that vMIP-II could block CCR1, CCR2, CCR5, and the CMV-encoded US28, as well as the CXC chemokine receptor CXCR4 27. Subsequently, vMIP-II has also been reported to block CX3CR1 and CCR8, as well as the HHV8-encoded chemokine receptor ORF-74 22 28 30 49. Here we find that vMIP-II also can antagonize CCR4, CXCR3, and XCR1. Thus, vMIP-II is able to bind and block receptors for all known classes of chemokines: XCR, CX3CR, CCR, and CXCR. Endogenous chemokines, in contrast to many other chemical messengers, are often able to bind to more than one receptor type. However, even the most “promiscuous” endogenous chemokines do not cross-react with receptors outside of their own class. For example, RANTES binds to CCR1, CCR3, and CCR5, and IL-8 binds to CXCR1 and CXCR2. In contrast, HHV8 has during its efficient evolution and in vivo selection been able to optimize vMIP-II to exploit not only one but all four classes of chemokine receptors. The receptors not targeted by vMIP-II appear to be the neutrophil-activating ones, that is CXCR1 and CXCR2, and those with housekeeping functions, CCR7, CCR9, CXCR5, and CCR6.
However, the function of vMIP-II may be dependent on the cellular setting, as it has been reported under certain circumstances to be able to act as a chemoattractant for certain cells, i.e., eosinophils—conceivably acting through CCR3—and even for CCR8-transfected Jurkat cells 31 49 50. It should be noted that in both cases these are receptors for which vMIP-II does not appear to be a particularly high-affinity ligand ( Fig. 4). Nevertheless, the function of vMIP-II as a general chemokine antagonist has been supported by an in vivo study, where vMIP-II was used in a rat kidney inflammatory disease model. Here, vMIP-II given intravenously was able to act as a potent and efficient immunosuppressive agent 28. It will be interesting to see if vMIP-II and especially the poxvirus-encoded, selective CCR8 antagonist MC148 can function as immunosuppressive agents in other disease models.
Acknowledgments
We thank Ulrik Gether for use of the spectroflurometer.
This study was supported by grants from the Danish Medical Research council. H.R. Lüttichau was supported by the Danish AIDS Foundation, the Foundation of Else and Mogens Wedell-Wedellsborg, a Max and Anna Friedmanns grant for prevention of diseases, the Beckett-Foundation, the Foundation of Konsul Ehrenfried Owesen and Wife, the Foundation of President Jacob Madsen and Wife Olga Madsen, the Research Foundation of Løvens Chemical Factory, the Foundation of Dagmar Marshall, the Foundation of President E. Danielsen and Wife, the Foundation of Agnes and Poul Friss, and the Foundation of Bjørn Aastrup.
Footnotes
Abbreviations used in this paper: HEK, human embryonic kidney; HHV, human herpesvirus; MCP, monocyte chemoattractant protein; MCV, molluscum contagiosum virus; ORF, open reading frame; RANTES, regulated upon activation, normal T cell expressed and secreted; SDF, stromal cell–derived factor; vMIP, viral macrophage inflammatory protein.
References
- Viac J., Chardonnet Y. Immunocompetent cells and epithelial cell modifications in molluscum contagiosum. J. Cutan. Pathol. 1990;17:202–205 . doi: 10.1111/j.1600-0560.1990.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Heng M.C., Steuer M.E., Levy A., McMahon S., Richman M., Allen S.G., Blackhart B. Lack of host cellular immune response in eruptive molluscum contagiosum. Am. J. Dermatopathol. 1989;11:248–254 . doi: 10.1097/00000372-198906000-00009. [DOI] [PubMed] [Google Scholar]
- Bhawan J., Dayal Y., Bhan A.K. Langerhans cells in molluscum contagiosum, verruca vulgaris, plantar wart, and condyloma acuminatum. J. Am. Acad. Dermatol. 1986;15:645–649 . doi: 10.1016/s0190-9622(86)70219-3. [DOI] [PubMed] [Google Scholar]
- Senkevich T.G., Bugert J.J., Sisler J.R., Koonin E.V., Darai G., Moss B. Genome sequence of a human tumorigenic poxvirusprediction of specific host response-evasion genes. Science. 1996;273:813–816 . doi: 10.1126/science.273.5276.813. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568 . doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Rollins B.J. Chemokines. Blood. 1997;90:909–928 . [PubMed] [Google Scholar]
- Zlotnik A., Morales J., Hedrick J.A. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 1999;19:1–47 . [PubMed] [Google Scholar]
- McFadden G., Kelvin D. New strategies for chemokine inhibition and modulation. Biochem. Pharmacol. 1997;54:1271–1280 . doi: 10.1016/s0006-2952(97)00182-2. [DOI] [PubMed] [Google Scholar]
- Dairaghi D.J., Greaves D.R., Schall T.J. Abduction of chemokine elements by herpesviruses. Semin. Virol. 1998;8:377–385 . [Google Scholar]
- Wells T.N.C., Schwartz T.W. Plagiarism of the host immune systemlessons about chemokine immunology from viruses. Curr. Opin. Biotechnol. 1997;8:741–748 . doi: 10.1016/s0958-1669(97)80129-2. [DOI] [PubMed] [Google Scholar]
- Ahuja S.K., Murphy P.M. Viral mimicry of chemokines and chemokine receptors. In: Herbert C.A., editor. Chemokines in Disease. Humana Press Inc; Totowa, NJ : 1999. pp. 235–251. [Google Scholar]
- Stine J.T., Chantry D., Gray P.W. Virally encoded chemokines and chemokine receptorsgenetic embezzlement of host DNA. In: Mantovani A., editor. Chemokines. Karger AG; Basel, Switzerland : 1999. pp. 161–180. [DOI] [PubMed] [Google Scholar]
- Gao J.-L., Murphy P.M. Human cytomegalovirus open reading frame US28 encodes a functional B chemokine receptor. J. Biol. Chem. 1994;269:28539–28542 . [PubMed] [Google Scholar]
- Chee M.S., Satchwell S.C., Preddie E., Weston K.M., Barrell B.G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990;344:774–777 . doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Gompels U.A., Nicholas J., Lawrence G., Jones M., Thomson B.J., Martin M.E.D., Efstathiou S., Craxton M., Macaulay H.A. The DNA sequence of human herpes virus-6structure, coding content, and genome evolution. Virology. 1995;209:29–51 . doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- Isegawa Y., Ping Z., Nakano K., Sugimoto N., Yamanishi K. Human herpesvirus 6 open reading frame U12 encodes a functional B-chemokine receptor. J. Virol. 1998;72:6104–6112 . doi: 10.1128/jvi.72.7.6104-6112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J. Virol. 1996;70:5975–5989 . doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis L., Geras-Raaka E., Varma A., Gershengorn M.C., Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350 . doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- Birkenbach M., Josefsen K., Yalamanchili R., Lenoir G., Kieff E. Epstein-Barr virus-induced genesfirst lymphocyte-specific G protein-coupled peptide receptors. J. Virol. 1993;67:2209–2220 . doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Utsunomiya Y., Yasukawa M., Yanagisawa K., Fujita S. Induction of G protein-coupled peptide receptor EBI 1 by human herpesvirus 6 and 7 infection in CD4+ T cells. J. Virol. 1994;68:5326–5329 . doi: 10.1128/jvi.68.8.5326-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais C., Santomasso B., Coso O., Arvanitakis L., Raaka E.G., Gutkind J.S., Asch A.S., Cesarman E., Gershengorn M.C., Mesri E.A. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89 . doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Rosenkilde M.M., Kledal T.N., Bräuner-Osborne H., Schwartz T.W. Agonist and inverse agonist for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J. Biol. Chem. 1999;274:956–961 . doi: 10.1074/jbc.274.2.956. [DOI] [PubMed] [Google Scholar]
- Kledal T.N., Rosenkilde M.M., Schwartz T.W. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett. 1998;441:209–214 . doi: 10.1016/s0014-5793(98)01551-8. [DOI] [PubMed] [Google Scholar]
- Cha T.-A., Tom E., Kemble G.W., Duke G.M., Mocarski E.S., Spaete R.R. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 1996;70:78–83 . doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold M.E.T., Dairaghi D.J., Duke G.M., Saederup N., Mocarski E.S., Kemble G.W., Schall T.J. Cytomegalovirus encodes a potent alpha chemokine. Proc. Natl. Acad. Sci. USA. 1999;96:9839–9844 . doi: 10.1073/pnas.96.17.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou P., Isegawa Y., Nakano K., Haque M., Horiguchi Y., Yamanishi K. Human herpesvirus 6 open reading frame U83 encodes a functional chemokine. J. Virol. 1999;73:5926–5933 . doi: 10.1128/jvi.73.7.5926-5933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kledal T.N., Rosenkilde M.M., Coulin F., Simmons G., Johnsen A.H., Alouani S., Power C.A., Luttichau H.R., Gerstoft J., Clapham P.R. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science. 1997;277:1656–1659 . doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- Chen S., Bacon K.B., Li L., Garcia G.E., Xia Y., Lo D., Thompson D.A., Siani M.A., Yamamoto T., Harrison J.K. In vivo inhibition of CC and CX3C chemokine–induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J. Exp. Med. 1998;188:193–198 . doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M.J., Garlisi C.G., Xiao H., Shan L., Hedrick J.A. The Kaposi's sarcoma–related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 1999;189:1993–1998 . doi: 10.1084/jem.189.12.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairaghi D.J., Fan R.A., McMaster B.E., Hanley M.R., Schall T.J. HHV8-encoded vMIP-1 selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J. Biol. Chem. 1999;274:21569–21574 . doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- Boshoff C., Endo Y., Collins P.D., Takeuchi Y., Reeves J.D., Schweickart V.L., Siani M.A., Sasaki T., Williams T.J., Gray P.W. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294 . doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- Damon I., Murphy P.M., Moss B. Broad spectrum chemokine antagonistic activity of a human poxvirus chemokine homolog. Proc. Natl. Acad. Sci. USA. 1998;95:6403–6407 . doi: 10.1073/pnas.95.11.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T.E., Scholler M.S., Tolstroy S., Schwartz T.W. Biosynthesis of peptide precursors and protease inhibitors using new constitutive and inducible eukaryotic expression vectors. FEBS Lett. 1990;267:289–294 . doi: 10.1016/0014-5793(90)80947-h. [DOI] [PubMed] [Google Scholar]
- Maggi C.A., Schwartz T.W. The dual nature of the tachykinin NK1 receptor. Trends Pharmacol. Sci. 1997;18:351–356 . doi: 10.1016/s0165-6147(97)01107-3. [DOI] [PubMed] [Google Scholar]
- Moser B., Dewald B., Barella L., Schumacher C., Baggiolini M., Clark-Lewis I. Interleukin-8 antagonists generated by N-terminal modification. J. Biol. Chem. 1993;268:7125–7128 . [PubMed] [Google Scholar]
- Zhang Y.J., Rutledge B.J., Rollins B.J. Structure/activity analysis of human monocyte chemoattractant protein-1 (MCP-1) by mutagenesis. J. Biol. Chem. 1994;269:15918–15924 . [PubMed] [Google Scholar]
- Gong J.H., Clark-Lewis I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J. Exp. Med. 1995;181:631–640 . doi: 10.1084/jem.181.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Clapham P.R., Picard L., Offord R.E., Rosenkilde M.M., Schwartz T.W., Buser R., Wells T.N.C., Proudfoot A.E.I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279 . doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- Krathwohl M.D., Hromas R., Brown D.R., Broxmeyer H.E., Fife K.H. Functional characterization of the C-C-chemokine-like molecules encoded by molluscum contagiosum virus types 1 and 2. Proc. Natl. Acad. Sci. USA. 1997;94:9875–9880 . doi: 10.1073/pnas.94.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R., Hesselgesser J., Zhou Y., Faulds D., Halks-Miller M., Harvey S., Taub D., Samson M., Parmentier M., Rucker J. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J. Biol. Chem. 1998;273:386–391 . doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- Miller M.D., Krangel M.S. The human cytokine I-309 is a monocyte chemoattractant. Proc. Natl. Acad. Sci. USA. 1992;89:2950–2954 . doi: 10.1073/pnas.89.7.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio D., Iellem A., Bonecchi R., Mazzeo D., Sozzani S., Mantovani A., Sinigaglia F. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J. Immunol. 1998;161:5111–5115 . [PubMed] [Google Scholar]
- Zingoni A., Soto H., Hedrick J.A., Stoppacciaro A., Storlazzi J.A., Sinigaglia F., D'Ambrosio D., O'Garra A., Robinson D., Rocchi M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J. Immunol. 1998;161:547–551 . [PubMed] [Google Scholar]
- Miller M.D., Hata S., De Waal Malefyt R., Krangel M.S. A novel polypeptide secreted by activated human T lymphocytes. J. Immunol. 1989;143:2907–2916 . [PubMed] [Google Scholar]
- Selvan R.S., Zhou L.-J., Krangel M.S. Regulation of I-309 gene expression human monocytes by endogenous interleukin-1. Eur. J. Immunol. 1997;27:687–694 . doi: 10.1002/eji.1830270317. [DOI] [PubMed] [Google Scholar]
- Selvan R.S., Butterfield J.H., Krangel M.S. Expression of multiple chemokine genes by a human mast cell leukemia. J. Biol. Chem. 1994;269:13893–13898 . [PubMed] [Google Scholar]
- Geissmann F., Prost C., Monnet J.-P., Dy M., Brousse N., Hermine O. Transforming growth factor B1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic langerhans cells. J. Exp. Med. 1998;187:961–966 . doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph G.J., Beaulieu S., Lebecque S., Steinman R.M., Mullers W.A. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483 . doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- Geras-Raaka E., Varma A., Clark-Lewis I., Gershengorn M.C. Kaposi's sarcoma-associated herpesvirus (KSHV) chemokine vMIP-II and human SDF-1α inhibit signaling by KSHV G protein-coupled receptor. Biochem. Biophys. Res. Commun. 1998;253:725–727 . doi: 10.1006/bbrc.1998.9557. [DOI] [PubMed] [Google Scholar]
- Sozzani S., Luini W., Bianchi G., Allavena P., Wells T.N.C., Napolitano M., Bernardini G., Vecchi A., D'Ambrosio D., Mazzeo D. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood. 1998;92:4036–4039 . [PubMed] [Google Scholar]
- LiWang A.C., Cao J.J., Zheng H., Lu Z., Peiper S.C., LiWang P.J. Dynamics study on the anti-human immunodeficiency virus chemokine viral macrophage-inflammatory protein-II (VMIP-II) reveals a fully monomeric protein. Biochemistry. 1999;38:442–453. doi: 10.1021/bi9812726. [DOI] [PubMed] [Google Scholar]