Abstract
Vav is a hematopoietic cell–specific guanine nucleotide exchange factor (GEF) whose activation is mediated by receptor engagement. The relationship of Vav localization to its function is presently unclear. We found that Vav redistributes to the plasma membrane in response to Fc∈ receptor I (Fc∈RI) engagement. The redistribution of Vav was mediated by its Src homology 2 (SH2) domain and required Syk activity. The Fc∈RI and Vav were found to colocalize and were recruited to glycosphingolipid-enriched microdomains (GEMs). The scaffold protein, linker for activation of T cells (LAT), and Rac1 (a target of Vav activity) were constitutively present in GEMs. Expression of an SH2 domain–containing COOH-terminal fragment of Vav inhibited Vav phosphorylation and movement to the GEMs but had no effect on the tyrosine phosphorylation of the adaptor protein, SLP-76 (SH2 domain–containing leukocyte protein of 76 kD), and LAT. However, assembly of the multiprotein complex containing these proteins was inhibited. In addition, Fc∈RI-dependent activation of c-Jun NH2-terminal kinase 1 (JNK1) was also inhibited. Thus, Vav localization to the plasma membrane is mediated by its SH2 domain and may serve to regulate downstream effectors like JNK1.
Keywords: Vav, Fc∈ receptor I, mast cell, glycosphingolipid-enriched microdomains, plasma membrane
Introduction
Understanding how receptors communicate with downstream effectors poses a significant challenge in biology. Recent studies have begun to elucidate some of the workings, suggesting that receptor-induced formation of macromolecular signaling complexes, mediated by molecular scaffolds and adaptors 1 2, can link receptors to multiple signaling pathways. However, where and how receptors engage these macromolecular complexes are still unresolved questions. The recognition that plasma membrane proteins and lipids can differentially partition, forming discrete microdomains that are enriched in particular types of proteins and lipids, has prompted investigative efforts to determine if such domains are critical components of receptor-mediated cellular signaling 3 4 5. Glycosphingolipid-enriched microdomains (GEMs) constitute one such membrane domain that concentrates sphingolipids, cholesterol, glycophosphatidylinositol-linked proteins, and numerous signaling molecules 4 5. The concentration of diverse signaling molecules in these domains suggests their importance in multiple signaling pathways.
Recently, Ag receptors (including Fc∈RI) and costimulatory molecules have been shown to be constitutively present or to partition to these domains upon their engagement 6 7 8. Furthermore, recent work suggests that Vav can be found complexed with linker for activation of T cells (LAT), a scaffolding protein that partially partitions to GEMs 9. Thus, GEMs are reasonable candidates as sites for receptor–downstream effector coupling. In a prior study, we found that Fc∈RI could be coimmunoprecipitated with the hematopoietic cell–specific protein, Vav, after receptor aggregation 10. Because Fc∈RI engagement results in the movement of a considerable fraction of these receptors into GEMs 6, perhaps GEMs might be sites of interaction between Fc∈RI and Vav and thus Vav localization to GEMs a required step for its function.
Tyrosine phosphorylation of Vav is important for its GDP/GTP exchange activity that activates Rho family GTPases like Rac1, which function(s) in the regulation of cellular architechture 11 12. The guanine nucleotide exchange factor (GEF) activity of Vav is encoded by the Dbl homology (DH) domain, whose function has also been demonstrated to contribute to c-Jun NH2-terminal kinase (JNK) activation 11 13. In addition to tyrosine phosphorylation, products of phosphatidylinositol 3-kinase activation can also contribute to the activation of Vav by binding to the Vav pleckstrin homology (PH) domain, thus increasing its activity 14. Among other structural domains of Vav are two Src homology 3 (SH3) domains and an SH2 domain 15 that can interact with a diverse array of proteins 15 16 and thus contribute to Vav function. For example, the Vav SH2 domain interacts with tyrosine kinases Syk and ZAP-70 17 18 and adaptor molecules such as SH2 domain–containing leukocyte protein of 76 kD (SLP-76 [19]) and Cbl 20. Another adaptor molecule, Grb2, binds to the NH2-terminal SH3 domain of Vav 21 22. In addition, the Vav COOH-terminal SH3 domain binds the heterogeneous ribonuclear proteins (hnRNPs) K and C 23 24, the nuclear protein Ku-70 25, and the cytoskeletal protein zyxin 26. Given the diversity of proteins with which Vav can interact, enormous potential exists for the modulation of its localization in response to cellular stimuli.
To investigate the localization of Vav and its functional consequences, we tagged Vav with green fluorescent protein (GFP) and followed its distribution in the RBL-2H3 mast cell line before and after Fc∈RI engagement. Phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAMs 27) of the Fc∈RI by Lyn, after Fc∈RI engagement, results in the recruitment and activation of Syk by its interaction with Fc∈RIγ ITAM 28. Because Vav phosphorylation is downstream of Syk activation 29 30, we also investigated the importance of Syk to Vav localization. Furthermore, as a measure of whether the intracellular localization of Vav is important to its function, we assessed the activation of JNK1, as Fc∈RI-dependent JNK1 activation is mediated by Vav 31. Our findings show that phosphorylation and recruitment of Vav to the plasma membrane is an important step in Vav function in mast cells.
Materials and Methods
Antibodies, Reagents, and Cell Cultures.
Mouse mAbs to Vav, Rac1, LAT, phosphotyrosine (4G10), Grb2, SLP-76, and GFP were purchased from Upstate Biotechnology, Transduction Laboratories, and Clontech. A rabbit polyclonal antibody to LAT was a gift of Lawrence Samelson (National Cancer Institute, National Institutes of Health, Bethesda, MD). A rabbit polyclonal antibody to Vav and a goat polyclonal antibody to JNK1 were from Santa Cruz Biotechnology. Fluorescently labeled secondary antibodies (Cy-5 or fluorescein conjugated) were purchased from Jackson ImmunoResearch Labs. Mouse mAb to Fc∈RI β chain has been described 32, and a mouse monoclonal to Syk was a gift from Petr Draber (Institute of Molecular Genetics, Prague, Czech Republic). Anti-dinitrophenyl (DNP) specific murine IgE was purified as described previously 33. DNP-human serum albumin (HSA) was from Sigma Chemical Co. Mounting medium (PermaFluor® aqueous mounting medium) was from Immunon. Enzymes used in this study were purchased from Life Technologies, Inc., and New England Biolabs. Piceatannol was purchased from Calbiochem. The rat basophilic leukemia cell line (RBL-2H3), RBL-2H3 TB1A2 (Syk−), RBL-2H3 3A1 (Syk+), and RBL-2H3 3BA12 (kinase-dead Syk+) were cultured as described previously 30. Baby hamster kidney (BHK) cells were cultured as described by the American Type Culture Collection.
DNA Constructs, Recombinant Semliki Forest Virus, and Infection.
The Semliki Forest virus (SFV) expression system, pSFV1 and helper pSFV2, was purchased from Life Technologies, Inc. All Vav constructs were generated from the previously described rat Vav 13. The pSFV1-GFP expression vector and Vav-GFP were generated as described 34. Vav (R694K) was PCR amplified from plasmid SRαVav and cloned into pSFV1-GFP to generate the pSFV1-VavSH2(R694K)-GFP as stated above. The calponin homology (CH) domain–deleted, DH domain–deleted, PH domain–deleted, and SH2 domain–deleted Vav constructs were generated from pZeoSV-Vav plasmid by overlapping extension PCR with primers to selectively delete the CH domain (from M1 to I126), DH domain (from K194 to I405), PH domain (from R402 to N505), and SH2 domain (from W669 to L758). The COOH-terminal region of Vav encoding the SH3-SH2-SH3 domains (Vav-C), starting at amino acid I567 to C845, was also PCR amplified. Vav-C encoding the R694K mutation was also PCR amplified from SRαVav. Full-length rat Syk was PCR amplified from plasmid SRαSyk. PCR reactions used the proof-reading Pfu DNA polymerase (Stratagene). Fidelity was confirmed by direct sequencing.
Recombinant SFV generation and infection of RBL-2H3 cells with the recombinant viruses were as described 34. Infectivity of cells with Vav ranged from 75 to 90%.
Confocal Microscopy.
RBL-2H3 cells were grown on acid-washed no. 1 coverslips in a 24-well plate as a subconfluent monolayer culture for 16 h. After infection, cells were incubated for an additional 4–6 h and sensitized with DNP-specific IgE 34. Stimulation with varying concentrations of DNP-HSA was for 3 min or as indicated. Coverslips were washed with PBS and fixed with 3% paraformaldehyde in PBS. Fixed cells were either directly analyzed or permeabilized with 0.5% Triton X-100 for 5 min at room temperature for immunostaining with primary antibodies to Fc∈RI β chain, Grb2, LAT, Rac1, or Syk followed by a fluorescent secondary antibody (Cy-5–labeled goat anti–mouse or anti–rabbit). Coverslips were air dried and mounted on glass slides using PermaFluor® aqueous mounting medium. Slides were examined on a Zeiss LSM 410 confocal microscope using a 63× Plan Apochromat objective (N.A. 1.4). GFP fluorescence was visualized with 488-nm krypton/argon laser excitation and a 515–540-nm band pass emission filter. Cy-5 fluorescence was simultaneously viewed using 633-nm helium/neon excitation and a 670–810-nm band pass emission filter. Brightness and contrast settings for acquired images were adjusted to maximize dynamic range and prevent detector saturation.
Isolation of GEMs, Immunoprecipitations, Western Blots, and In-Gel Kinase Assays.
GEMs were isolated from transfected 125I-IgE–labeled RBL-2H3 cells as described previously 35, except that 0.1% Triton X-100 was used for cell solubilization. Fractions were collected, and aliquots were counted for 125I-IgE distribution, then diluted 1:1 with lysis buffer 35 and subjected sequentially to Vav, LAT, or Fc∈RI immunoprecipitation or to precipitation of all proteins with 20% trichloroacetic acid (TCA). For immunoprecipitations, lysis buffer (which for solubilization of GEMs included 0.5% β-octyl glucoside) was added for 30 min at 4°C. Lysates were incubated with protein A or protein G Sepharose–bound antibodies to SLP-76, Vav, JNK1, Syk, Grb2, or LAT overnight at 4°C as indicated. SDS-solubilized proteins were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with 4G10 antibody. For TCA precipitates, recovered pellets were washed once in ice-cold acetone, dried, and resuspended in 50 μl of 1% SDS in 50 mM Tricine and 50 μl of twofold-concentrated SDS sample buffer. Proteins were resolved and probed with antibodies to Grb2, LAT, Lyn, paxillin, Rac1, SLP-76, Syk, and Vav as indicated. In-gel kinase assays for JNK1 activation were done as described 36. In brief, transfected cells were lysed as described 34, and soluble lysates were incubated with goat anti-JNK1. Immunoprecipitates were resolved by SDS-PAGE gels containing glutathione S-transferase (GST)-ATF2 (300 μg/ml) as the substrate for JNK1. The resolved proteins in the gel were denatured, and the kinase reaction was carried out in kinase buffer 36.
Results
Vav Redistributes to the Plasma Membrane after Fc∈RI Engagement.
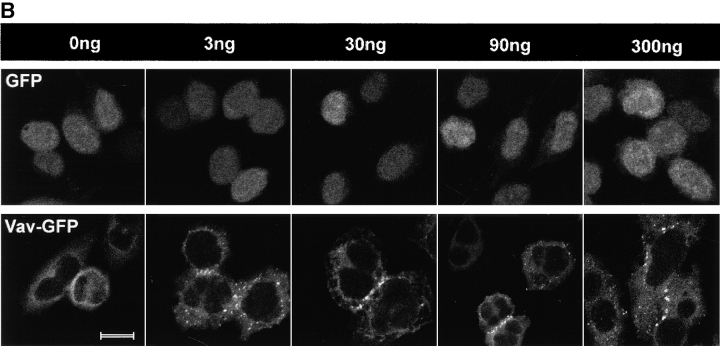
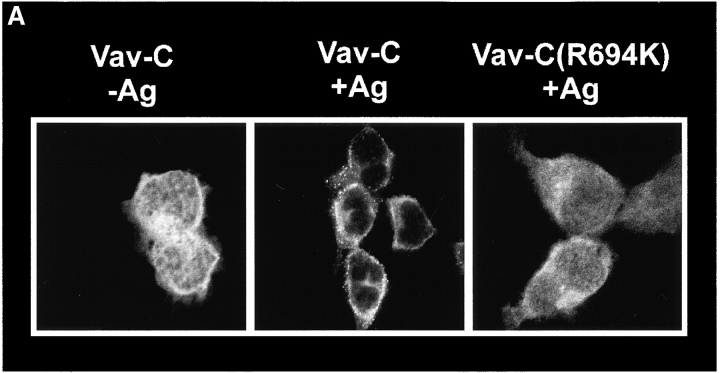
RBL-2H3 cells expressing either GFP or Vav-GFP were analyzed for the distribution of Vav. As shown in Fig. 1 A, GFP was evenly distributed throughout the cell and was included in the nucleus. No apparent association of GFP with the plasma membrane was evident ( Fig. 1 A). In contrast, the addition of Vav to GFP caused a dramatic change in the distribution of GFP, with little inclusion of Vav-GFP in the nucleus. This suggested that Vav is primarily localized to the cytosol and sequesters the fused GFP in the cytosol. To verify that the addition of GFP to Vav did not affect its localization, we determined the distribution of endogenous Vav by immunofluorescence and found it to be essentially identical ( Fig. 1 A). A consistently higher background was found for the immunofluorescence staining with antibody to Vav, and thus we focused on Vav-GFP redistribution.
Figure 1.
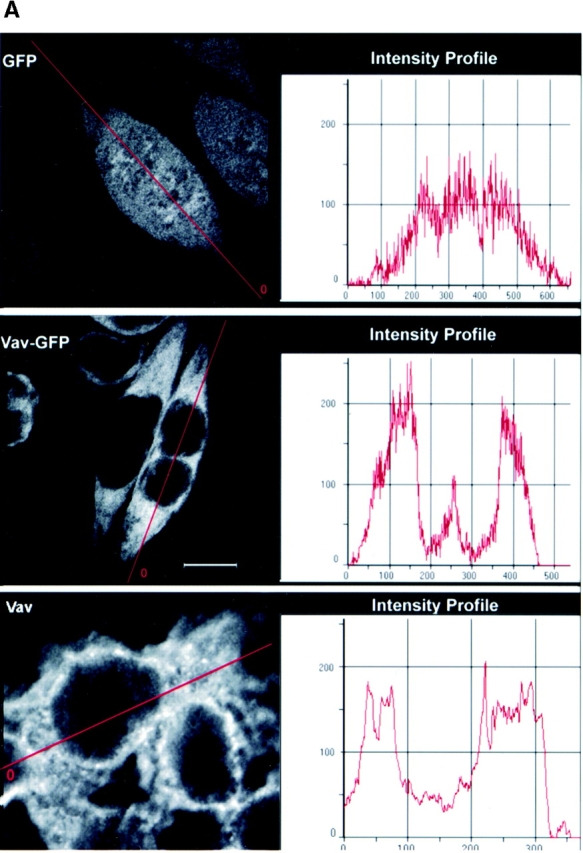
Distribution of Vav in RBL-2H3 mast cells. (A) Cells were transfected with GFP, Vav-GFP, or analyzed for endogenous Vav. Nontransfected or 4 h posttransfected cells were fixed (nontransfected cells were immunostained for endogenous Vav) and examined by confocal microscopy. A fluorescence intensity (arbitrary units) profile shows the cytosolic localization of Vav, Vav-GFP, and the uniform distribution of GFP. (B) 4 h posttransfected GFP or Vav-GFP expressing cells sensitized with IgE were stimulated or not (0 ng) with 3, 30 , 90, or 300 ng of Ag (DNP-HSA) for 3 min at 37°C. Cells were fixed and examined by confocal laser microscopy. Bars, 10 μm.
Fc∈RI engagement resulted in the redistribution of a significant fraction of Vav to the plasma membrane as a submembranous aggregate at the indicated Ag concentrations ( Fig. 1 B). In contrast, no change in the distribution of GFP was observed in response to Fc∈RI engagement, although the cells treated with 300 ng Ag showed increased spreading or flattening ( Fig. 1 B). Two distinct features of Vav overexpression and its relationship to Fc∈RI engagement were observed. First, Vav overexpression resulted in the spreading or flattening of the cells ( Fig. 1 B), and this was further enhanced by Fc∈RI engagement. Second, the redistribution of Vav was not uniform and seemed to be most prominent at cell–cell contacts where one might expect larger aggregates of Fc∈RI to form, provided that receptors on contacting cells bound the same Ag ( Fig. 1 B). In kinetic experiments, Vav moved to the plasma membrane within 1 min and persisted in the plasma membrane even at 27 min after receptor engagement.
Vav Colocalization with Fc∈RI Is Dependent on an Intact SH2 Domain.
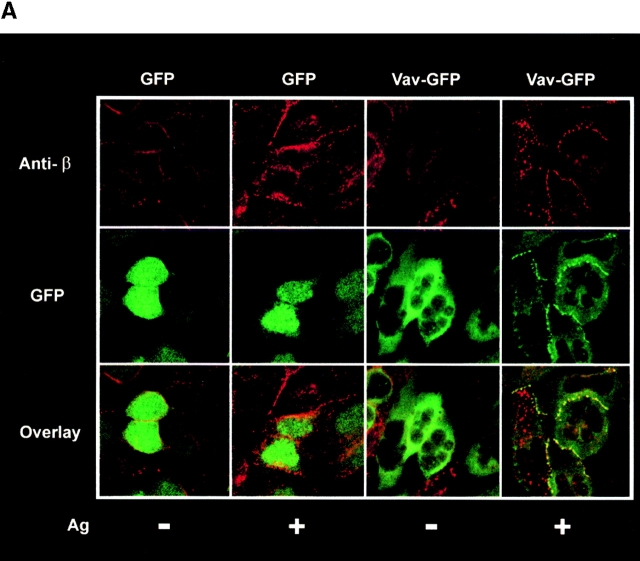
We previously reported the specific coimmunoprecipitation of Fc∈RI with Vav in response to Fc∈RI engagement 10. We revisited this issue to explore whether the observed aggregates of Vav represent the fraction of Vav that might colocalize with Fc∈RI. Fc∈RI was evenly distributed in unstimulated control (GFP) and Vav (Vav-GFP) transfected cells ( Fig. 2 A, Anti-β). After Fc∈RI engagement, microaggregates of Fc∈RI were formed at the plasma membrane. This is in agreement with earlier reports of Fc∈RI clustering after stimulation, as visualized with FITC- or PE-labeled IgE 37 38 39. In adherent cells, these Fc∈RI microaggregates appeared to focalize to the cell–cell contact points after Fc∈RI engagement ( Fig. 2 A). Vav and Fc∈RI aggregates showed an identical distribution, with a significant fraction of Fc∈RI codistributing with Vav in the stimulated adherent ( Fig. 2 A) and in nonadherent cells (data not shown). Colocalization of a considerable fraction of Fc∈RI aggregates (>30%) with plasma membrane–localized Vav aggregates was observed ( Fig. 2 A, Overlay).
Figure 2.
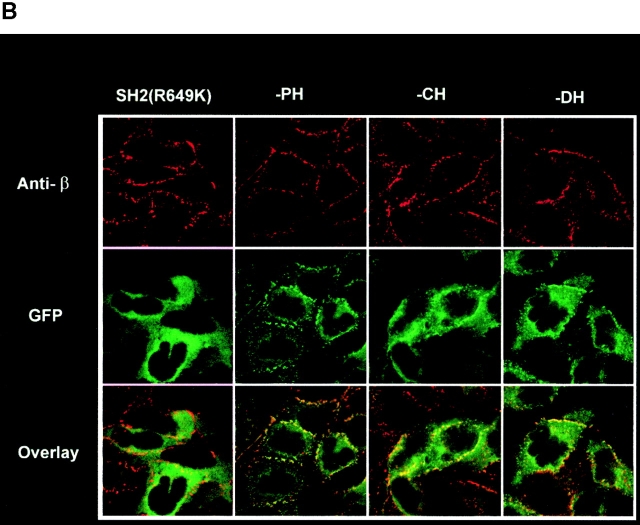
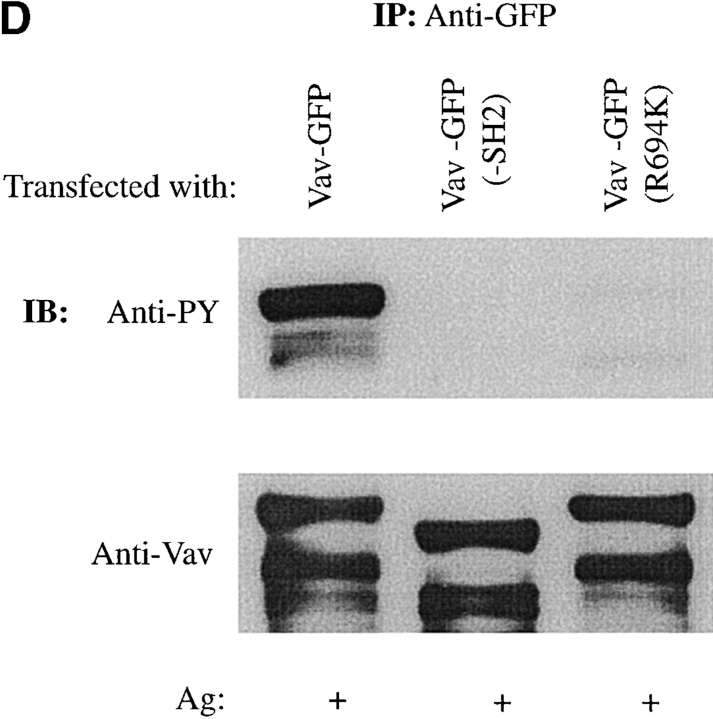
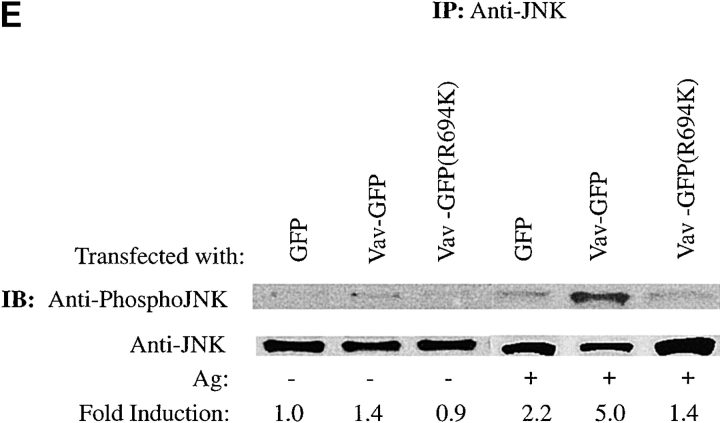
Vav phosphorylation, JNK1 activation, and Fc∈RI colocalization are dependent on the Vav SH2 domain. (A) Vav colocalizes with Fc∈RI. RBL-2H3 cells transfected with GFP or Vav-GFP were sensitized with IgE and stimulated with 30 ng of Ag (Ag+) or not (Ag−) for 3 min. Cells were fixed, permeabilized, and incubated with mouse anti-Fc∈RI β chain followed by Cy-5–conjugated goat anti–mouse. Overlay shows colocalization (yellow) where the green and red fluorescence overlap. (B) Mutants of Vav-GFP were analyzed for distribution after Ag stimulation as above. R694K, a Vav SH2 domain mutant, a PH domain–deleted mutant (−PH), a DH domain–deleted mutant (−DH), and a CH domain–deleted mutant (−CH) were analyzed as above for plasma membrane localization and colocalization with Fc∈RI. (C) The SH2 domain of Vav is required for colocalization with Fc∈RI. Fluorescence pixel intensity (arbitrary units) profile of Fc∈RI (red line) and Vav (green line) from the plasma membrane of Fc∈RI-stimulated cells transfected with Vav-GFP or Vav-GFP (R694K). (D) Tyrosine phosphorylation of Vav requires its SH2 domain. RBL-2H3 cells were transfected with Vav-GFP, Vav-GFP (R694K), or Vav-GFP (−SH2 domain). 4 h posttransfection cells were stimulated with 300 ng/ml of Ag for 3 min, lysed, and incubated with anti-GFP. SDS-PAGE–resolved proteins were transferred for immunoblotting (IB). Blots were first probed with antiphosphotyrosine (Anti-PY), then stripped and reprobed with anti-Vav. (E) An intact Vav SH2 domain is needed for JNK1 activation. RBL-2H3 cells were transfected with GFP, Vav-GFP, or Vav-GFP (R694K). Cells were stimulated (Ag+) or not (Ag−) for 8 min as in D, and incubated with anti-JNK1. Immunoblots (IB) were first probed with antiphospho-JNK1, then stripped and reprobed with anti-JNK1. Quantitation of immunoblots was by densitometry. Fold induction is the mean of three experiments where values obtained were normalized to the immunoprecipitated protein. SD were <10%.
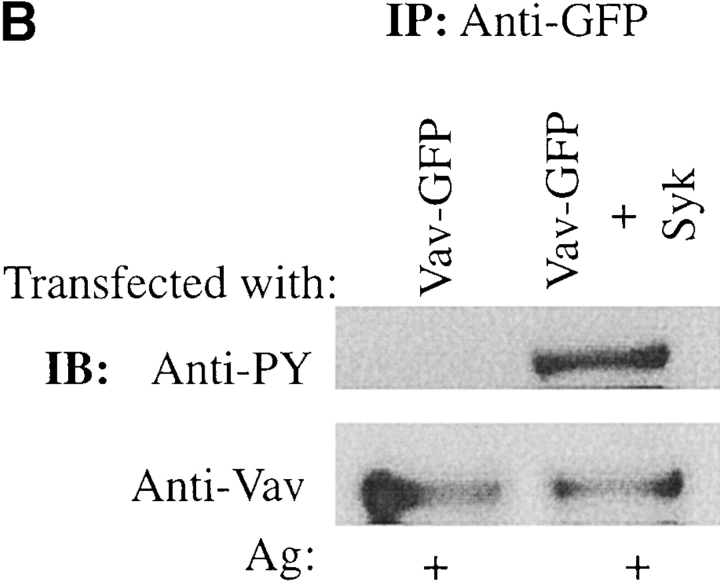
We previously found that Vav did not directly interact with the Fc∈RIγ ITAMs. Nevertheless, as Vav is known to be phosphorylated by and to interact with Syk 17 29 30, we investigated which structural domain(s) of Vav might be important for its colocalization with Fc∈RI. Fig. 2 B shows that deletion of the CH, DH, and PH domains had little effect on Vav redistribution and colocalization with Fc∈RI. Only the SH2 domain was found to be important for redistribution of Vav to the plasma membrane and for its colocalization with Fc∈RI. Deletion of the SH2 domain (data not shown) or mutation of R694K in the SH2 domain of Vav completely ablated the plasma membrane redistribution of Vav and thus the colocalization with Fc∈RI ( Fig. 2 B). As shown in Fig. 2 C, a fluorescence intensity profile of Fc∈RI-stimulated cells expressing Vav-GFP showed almost complete fluorescence overlap with Fc∈RI compared with cells expressing the R694K mutation of Vav. In addition, the deletion or mutation of the Vav SH2 domain also resulted in the loss of Vav tyrosine phosphorylation ( Fig. 2 D) while no significant effect on the phosphorylation of the CH, DH, or PH domain–deleted constructs was observed (data not shown). Thus, one might conclude that plasma membrane localization is required for Vav phosphorylation. Alternatively, the Vav SH2 domain–mediated interaction with Syk or ZAP-70 17 40 may be required for Vav phosphorylation, or both possibilities may contribute to Vav phosphorylation.
The SH2 Domain of Vav Contributes to JNK1 Activation.
Kinetic analysis of JNK1 activation in RBL cells revealed that maximal enzymatic activity was at 8 min after Fc∈RI engagement (our unpublished results). Fc∈RI engagement (8 min) of cells overexpressing wild-type Vav enhanced the activation of JNK1 ( Fig. 2 E). We investigated the importance of the SH2 domain for Vav function by studying whether the Vav mutant (R694K) was capable of enhancing JNK1 activation. Mutant Vav (R694K) did not enhance the JNK1 activation, unlike the wild-type Vav ( Fig. 2 E). In addition, more prolonged (16 min) Ag stimulation of cells expressing Vav (R694K) did not provide any enhancement of JNK1 activity (data not shown). The JNK1 response for the mutant Vav (R694K) was similar to that observed for the control GFP transfectant, suggesting that this mutation did not exhibit a dominant negative phenotype. Additionally, as we reported elsewhere 13, the deletion of the Vav DH domain also resulted in reduced JNK1 activation and mimicked the expression of an inactive Rac1 (N17). Deletion of the CH or PH domains showed no or little effect, respectively, on JNK1 activation. Thus, because ablation of the GEF activity (DH−) of a plasma membrane localized Vav ( Fig. 2 B) or inhibition of redistribution of wild-type Vav to the plasma membrane resulted in a loss of enhanced JNK activity, we conclude that the movement of Vav to the plasma membrane is important to its role in JNK1 activation.
Syk Activity Is Required for Vav Redistribution.
Inhibition of Syk activity or a deficiency of Syk in mast cells results in the loss of Vav phosphorylation 30 41. To assess if Vav redistribution was dependent on Syk activity or on the presence of Syk, we used several approaches. Vav was overexpressed in the TB1A2 cells, a Syk− RBL-2H3 cell line 30. In the absence or presence of Fc∈RI engagement, Vav failed to redistribute to the plasma membrane and form aggregates ( Fig. 3 A). Furthermore, analogous to the mutation of the Vav SH2 domain, the endogenous and overexpressed Vav was not phosphorylated in these cells ( Fig. 3 B, and data not shown). Cotransfection of Syk and Vav in the TB1A2 cells led to the reconstitution of plasma membrane–localized Vav, its tyrosine phosphorylation, and its colocalization with Fc∈RI after the latter's engagement ( Fig. 3a and Fig. b). As summarized in Table , Vav aggregates were also observed after Fc∈RI engagement in a TB1A2-derived clone stably reconstituted with Syk (clone 3A1). In contrast, the TB1A2-derived clone reconstituted with a catalytically inactive Syk (clone 3BA12) or RBL cells pretreated with piceatannol, a Syk-selective inhibitor, failed to show Vav redistribution (Table ), clearly demonstrating the requirement for Syk activity in the tyrosine phosphorylation of Vav and its redistribution to the plasma membrane.
Figure 3.
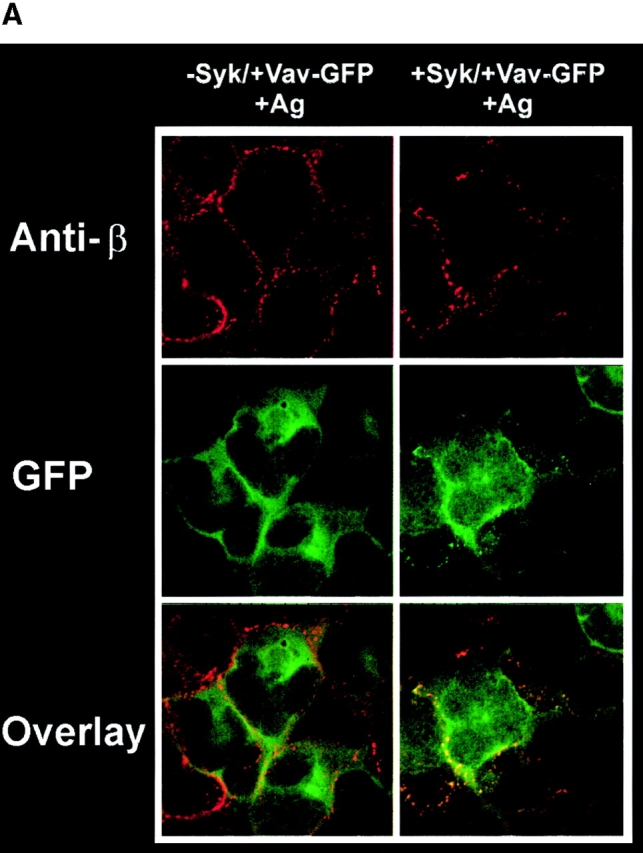
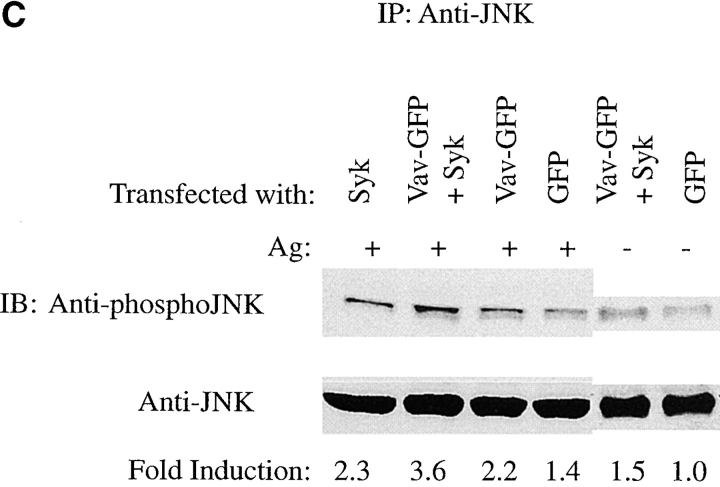
Vav phosphorylation, membrane localization, and JNK1 activation depends on Syk activity. (A) Plasma membrane localization of Vav requires Syk activity. Syk-deficient cells (−Syk) were transfected with Vav-GFP (−Syk/+Vav-GFP) or with both Vav-GFP and Syk (+Syk/+Vav-GFP). Transfected cells were stimulated for 3 min with 30 ng of Ag (+Ag). Fixed and permeabilized cells were incubated with mouse antibody to Fc∈RI β chain followed by Cy-5–conjugated goat anti–mouse. (B) Vav tyrosine phosphorylation requires Syk. Syk-deficient cells were transfected with Vav-GFP or with both Vav-GFP and Syk. Fc∈RI-stimulated (Ag+) cells were lysed and incubated with anti-GFP. Proteins were resolved and transferred for immunoblots (IB) and probed with antiphosphotyrosine (Anti-PY), then stripped and reprobed with anti-Vav. (C) Syk and Vav synergize to activate JNK1. Syk-deficient cells were transfected with either GFP, Syk, Vav-GFP, or both Vav-GFP and Syk. Transfected cells were stimulated (Ag+) or not (Ag−) with 300 ng/ml of Ag for 8 min, lysed, and incubated with antibody to JNK1. Resolved proteins were first immunoblotted with anti-phosphoJNK1, then stripped and reprobed with anti-JNK1. Quantitation of immunoblots (IB) was by densitometry. Fold induction is the mean of two experiments where values obtained were normalized to the immunoprecipitated protein. SD were <7%.
Table 1.
Vav Plasma Membrane Localization Is Syk Dependent
| Cell line (Transfectant or treatment) | Vav plasma membrane localization | |
|---|---|---|
| Ag: − | + | |
| RBL-2H3 | − | ++++ |
| RBL-2H3 | ||
| (Piceatannol) | − | − |
| TB1A2 | ||
| (Syk− cells) | − | − |
| TB1A2 | ||
| (Syk+, transient transfection) | − | +++ |
| 3A1 (TB1A2 derived) | ||
| (Stable wild-type Syk) | − | ++ |
| 3BA12 (TB1A2 derived) | ||
| (Stable catalytically inactive Syk) | − | − |
We also found that Syk and Vav synergized in the activation of JNK1 in Syk-deficient cells. Expression of Vav alone in these cells, followed by Fc∈RI engagement, caused a 2.2-fold enhancement in JNK1 activation compared with the control nonstimulated GFP transfectant ( Fig. 3 C). Expression of Syk alone also caused a 2.3-fold enhancement in JNK1 activity. However, a 3.6-fold increase in JNK1 activation was seen when Vav was coexpressed with Syk. As shown below, the activation of JNK1 required the plasma membrane localization of Vav.
We investigated whether Syk mediates the interaction of Vav with the plasma membrane. The following evidence suggested that Vav aggregate formation is not directly mediated by Vav interaction with Syk. First, we did not detect colocalization of Vav with Syk under conditions, described previously 39, where Syk is maximally localized to the plasma membrane (data not shown). Second, other adaptor proteins that could facilitate Vav interaction with the plasma membrane were localized with Vav aggregates (see Fig. 4d and Fig. e, and Fig. 5 A). Third, preliminary experiments in Fc∈RI- and Lyn-transfected Chinese hamster ovary (CHO) cells 42 showed no redistribution of transfected Vav to the plasma membrane under conditions where Syk was membrane localized and both Syk and Vav were tyrosine phosphorylated (data not shown). Although we cannot formally exclude a plasma membrane–localized interaction of Syk and Vav, the preponderance of evidence suggests a different mechanism for Vav plasma membrane association.
Figure 4.
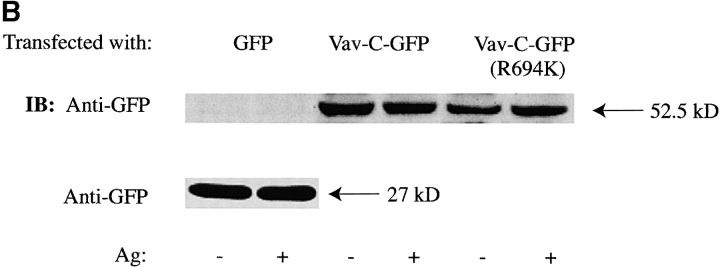
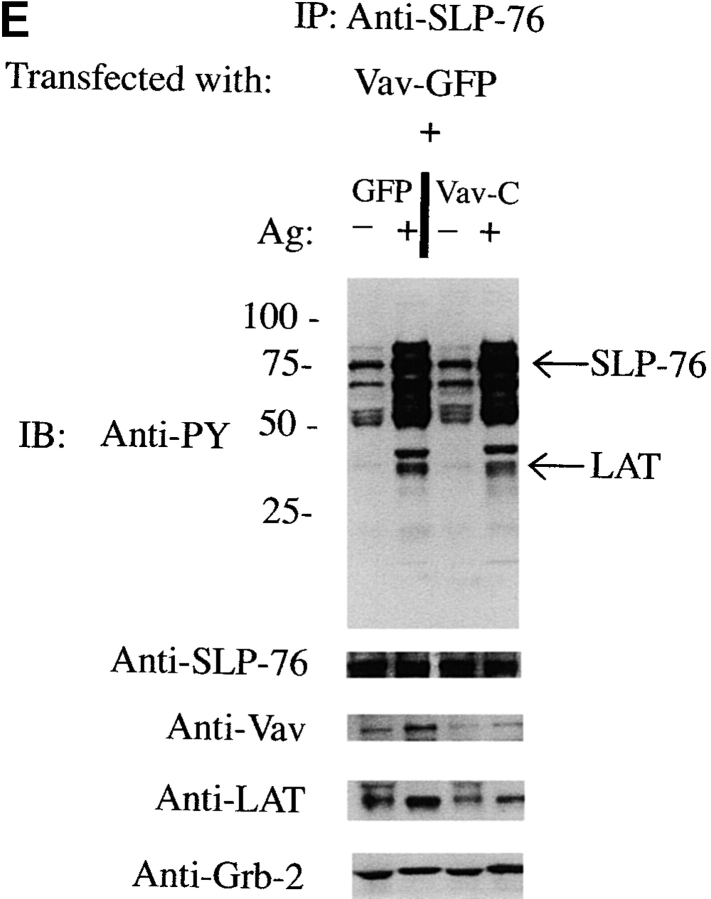
Vav-C inhibits Vav phosphorylation and association with but not phosphorylation of Syk, SLP-76, and LAT. (A) Vav-C (SH3-SH2-SH3) is membrane localized upon Fc∈RI engagement. RBL-2H3 cells were transfected with Vav-C-GFP or an SH2 domain–mutated Vav-C (R694K)-GFP. Cells were stimulated with 30 ng/ml of Ag for 3 min (+Ag) or left untreated (−Ag) and examined by confocal laser microscopy. (B) Expression levels of GFP alone, Vav-C, or Vav-C (R694K) tagged with GFP. Cells transfected as in A were lysed, and 5 × 104 cell equivalents per lane of protein were resolved by SDS-PAGE and transferred for immunoblotting (IB). For immunoprecipitation experiments, 1.0–2.0 × 107 cells were used. Expression levels were determined by probing with an antibody to GFP (shown) or in some cases with a mouse mAb to Vav. (C) Vav-C inhibits phosphorylation of Vav and its association with Syk but not Syk phosphorylation. RBL-2H3 cells transfected with Vav, Vav-C, and Syk or Vav, Vav-C (R694K), and Syk were stimulated as in the legend to Fig. 2 D, lysed, and incubated with antibody to Syk. Recovered proteins were immunoblotted (IB) with antiphosphotyrosine (Anti-PY), then stripped and reprobed sequentially with anti-Vav and anti-Syk. (D) Nontransfected RBL-2H3 cells were stimulated (Ag+) or not (Ag−) as in the legend to Fig. 2 D. Cells were lysed as described in Materials and Methods, and LAT or Vav was immunoprecipitated. Proteins were resolved and immunoblotted with antibody to LAT, SLP-76, and Vav. (E) Vav-C inhibits the association of Vav and LAT with SLP-76 without affecting the SLP-76 phosphorylation. RBL-2H3 cells were transfected with Vav and GFP or Vav and Vav-C-GFP. Cells were stimulated (Ag+) or not (Ag−) as in the legend to Fig. 2 D, then lysed and incubated with antibody to SLP-76. Immunoblots were first probed with antiphosphotyrosine (Anti-PY), then stripped and reprobed with anti–SLP-76, anti-Vav, anti-LAT, and anti-Grb2. (F) Vav-C has no effect on the tyrosine phosphorylation of LAT. Experiments were done as in E, but cell lysates were incubated with antibody to LAT (Anti-LAT). Immunoblots were probed with antiphosphotyrosine and subsequently with anti-LAT.
Figure 5.
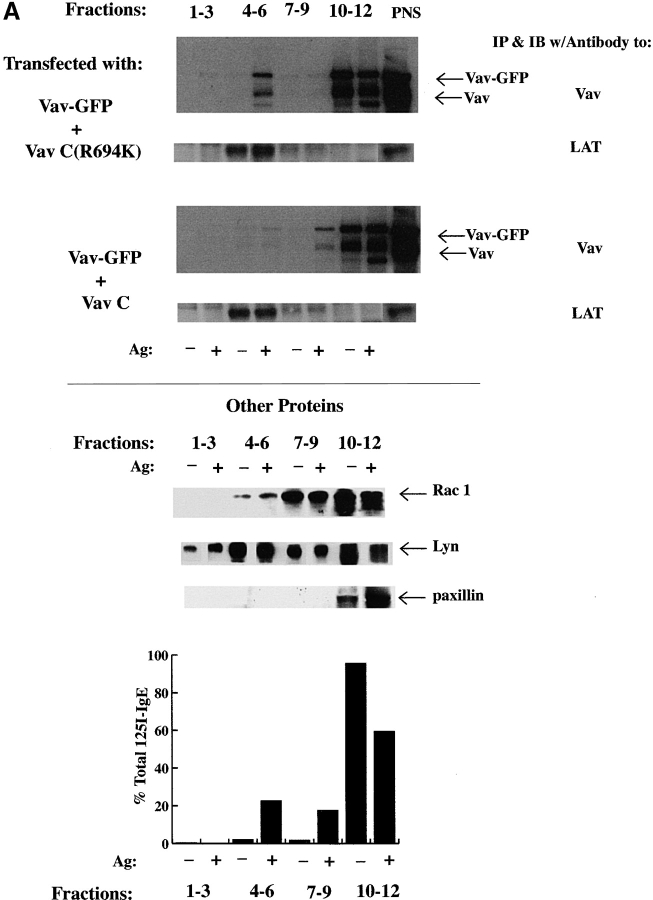
LAT, Rac 1, Fc∈RI, and Vav are constitutively present or recruited to GEMs: Vav localization to GEMs and JNK1 activation are inhibited by Vav-C expression. (A) RBL-2H3 cells were transfected with Vav-GFP and Vav-C or Vav-GFP and Vav-C (R694K). Cells were sensitized with 125I-IgE and stimulated (Ag+) or not (Ag−) as in the legend to Fig. 2 D. GEMs were isolated as described (reference 35), and Vav or LAT was immunoprecipitated from pooled fractions or proteins concentrated by TCA precipitation. Proteins were immunoblotted with anti-Vav, anti-LAT, anti-Rac1, or anti-Lyn. Numbers indicate pooled fractions (GEMs are found in fractions 4–6). The equivalent fractions from nonstimulated (Ag−) and Fc∈RI-stimulated (Ag+) cells are shown next to each other. PNS, postnuclear supernatant. Lyn was used as a positive control for the GEM fraction (reference 65), whereas the absence of paxillin in GEMs was a negative control (reference 48). Fc∈RI distribution before and after stimulation is shown as a percentage of the total 125I-IgE recovered in the pooled fractions. (B) Vav-C inhibits the Fc∈RI-mediated activation of endogenous JNK1. RBL-2H3 cells were transfected with GFP or Vav-C-GFP. Cells were stimulated as in the legend to Fig. 2 E (Ag+) or not (Ag−). Cells were lysed, and endogenous JNK1 and Vav were immunoprecipitated. Vav blots were first probed with antiphosphotyrosine (Anti-PY) and then with anti-Vav. For direct quantitation of activity, in-gel kinase assays were performed with JNK1 immunoprecipitates as described (reference 36). Quantitation was by Molecular Storm® imaging (Molecular Dynamics). One representative of four experiments using either GFP or Vav-C (R694K)-GFP as negative controls is shown. No difference was observed with either negative control.
Vav, LAT, Rac1, and Fc∈RI Are Found in GEMs.
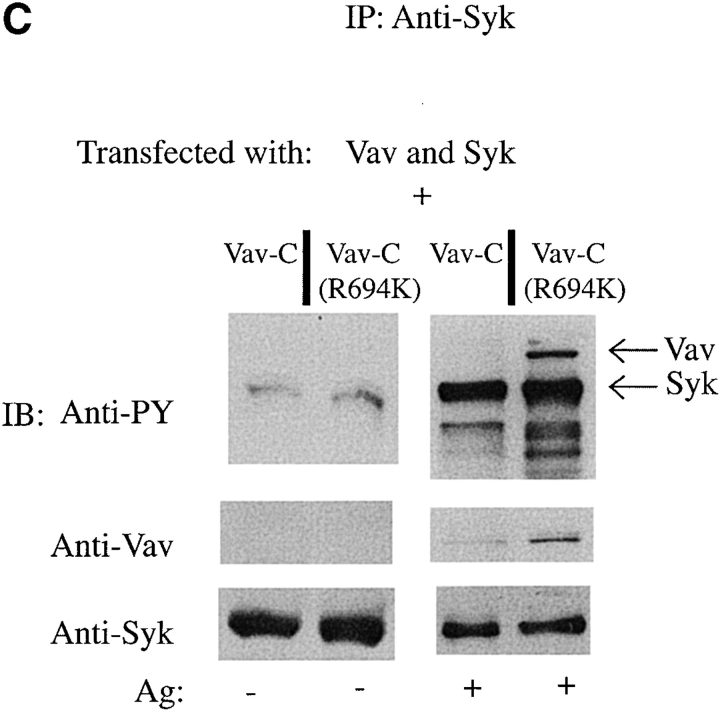
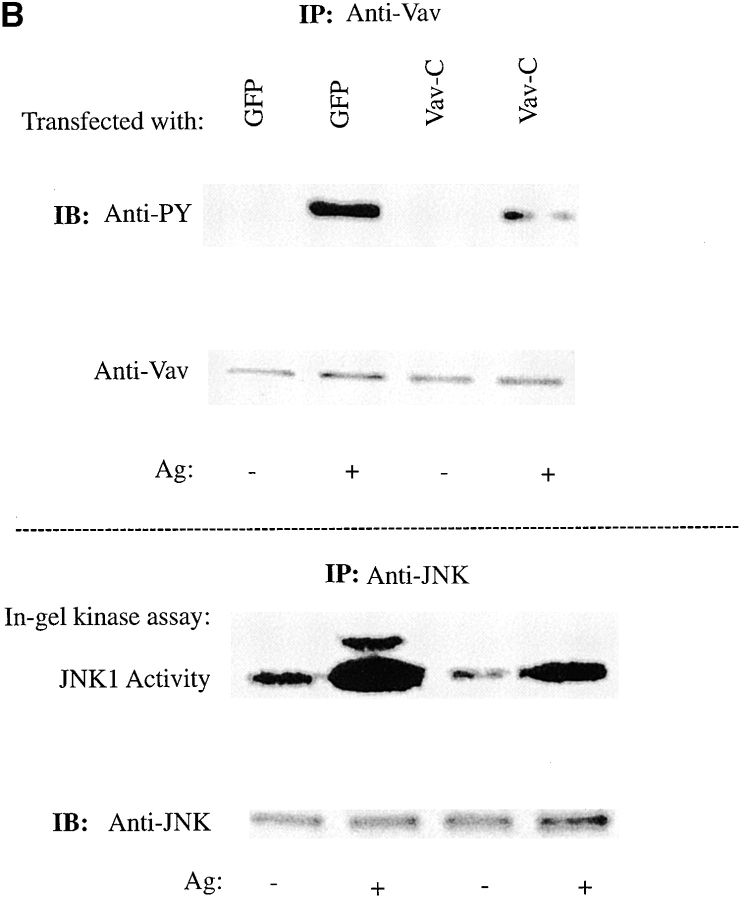
Biochemical studies demonstrated the constitutive association of Vav with Grb2 10 21 22 and the receptor-dependent association of Vav with SLP-76 19 43 44 and LAT 45. LAT is a palmitoylated protein that partitions to GEMs and can form a complex with SLP-76 and Vav 9 46. To establish a link of communication, it was possible that Vav and Fc∈RI might be found in GEMs after Fc∈RI engagement. Because the SH2 domain of Vav is critical for plasma membrane localization, we reasoned that the SH3-SH2-SH3 domains of Vav (Vav-C), which were previously described to inhibit Vav activity 19, might compete with endogenous Vav for plasma membrane localization. To test this hypothesis, we investigated whether Vav-C would localize to the plasma membrane by expressing both Vav-C and Vav-C (R694K) tagged with GFP and subsequently engaging the Fc∈RI. Vav-C was redistributed to the plasma membrane, whereas the Vav-C (R694K) mutant failed to membrane localize ( Fig. 4 A). The expression of GFP, Vav-C, or Vav-C (R694K) was found to be similar ( Fig. 4 B). In addition, Vav-C was tyrosine phosphorylated as described 19, whereas Vav-C (R694K) was only weakly phosphorylated (data not shown). This suggested that Vav-C might act as a competitive inhibitor of Vav phosphorylation, and led us to explore its effect on the phosphorylation of wild-type Vav. Fig. 4 C shows that Vav-C inhibited the tyrosine phosphorylation and association of Vav with Syk, whereas Vav-C (R694K) did not. In addition, although in the experiment shown there is a minor effect on Syk phosphorylation, multiple experiments suggested that Vav-C expression had no effect on Syk phosphorylation before or after Fc∈RI engagement. Therefore, Vav-C competes with Vav as a substrate for Syk and can localize to the plasma membrane as a competitor for endogenous Vav.
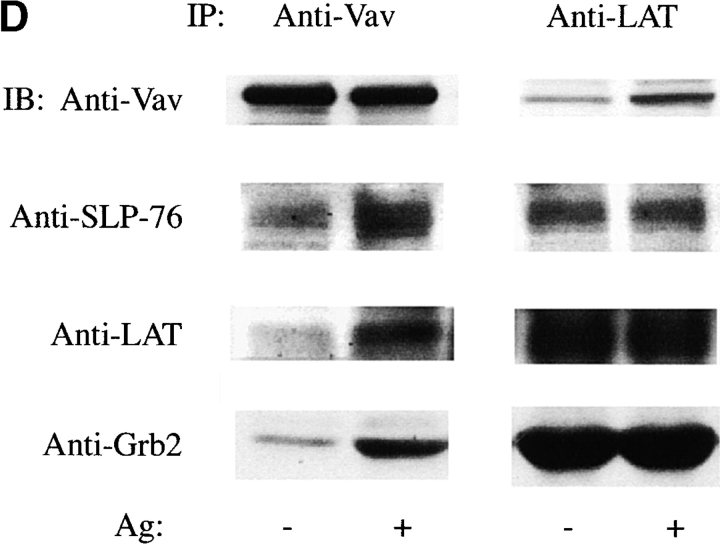
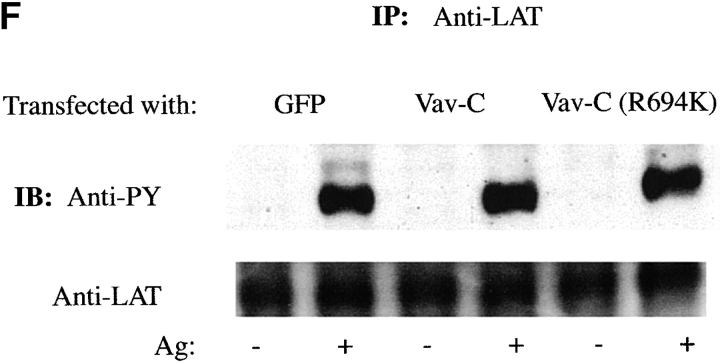
We established that immunoprecipitation of endogenous Vav or LAT resulted in the coimmunoprecipitation of endogenous LAT, Grb2, SLP-76, or Vav ( Fig. 4 D). We used the expression of Vav-C to analyze whether the competitor had any effect on formation of a multiprotein complex of Vav with the SLP-76 and LAT. SLP-76 was immunoprecipitated from Fc∈RI-stimulated or -nonstimulated cells that were transiently transfected with Vav tagged with GFP (to enhance the formation of a complex with SLP-76) in the presence of the Vav-C inhibitor also tagged with GFP, or of GFP alone. SLP-76 immunoprecipitates from the stimulated cells that were expressing Vav-C showed marked inhibition of Vav coimmunoprecipitation with SLP-76 ( Fig. 4 E). Moreover, an inhibition of LAT association with SLP-76 was also observed, although this was not as dramatic as the inhibition of Vav and SLP-76 interaction ( Fig. 4 E). The association of Grb2 with SLP-76 was not inhibited ( Fig. 4 E). As determined by immunoprecipitating each individual protein, no effect on the tyrosine phosphorylation of Syk, SLP-76, or LAT ( Fig. 4c, Fig. e, and Fig. f) was observed by expression of Vav-C or the controls, Vav-C (R694K) and GFP. Therefore, Vav-C specifically inhibits Vav phosphorylation and its association with SLP-76 and LAT.
In RBL-2H3 cells, a significant fraction (as much as 50%) of the Fc∈RI can be found in GEMs after extensive aggregation of this receptor 6. Given that LAT is also found in these domains 9, we now asked whether Vav might also be recruited to these membrane domains in response to Fc∈RI engagement. Furthermore, if the LAT/SLP-76/Vav complex formation takes place in the GEMs, we should effectively compete the recruitment of Vav in the GEMs by overexpression of Vav-C. Fig. 5 A shows that Vav and Fc∈RI are recruited in the LAT-containing GEMs (fractions 4–6) after Fc∈RI engagement. Fc∈RI recruitment to GEMs was primarily monitored by the irreversible binding of radiolabeled IgE ( 47; see graph, Fig. 5 A) and ranged from 15 to 50% with a mean of 20%. However, we could also detect the presence of phosphorylated Fc∈RI β chain and the GEM-localized Lyn 6, but not paxillin (a GEM-excluded protein 48), when tested by immunoblotting (data not shown, and Fig. 5 A). The presence of Vav in the GEMs was effectively reduced by the overexpression of Vav-C but not by the overexpression of the control Vav-C (R694K). In five experiments, inhibition of Vav localization to GEMs by Vav-C ranged from 35 to 100%. Both exogenously and endogenously expressed Vav were tyrosine phosphorylated and localized to the GEMs, and the localization was inhibited by Vav-C expression ( Fig. 5 A). No inhibition was observed by expression of Vav-C (R694K) ( Fig. 5 A). In addition, we also found that Rac1, the target of Vav GEF activity, was constitutively present in GEMs and its presence in GEMs was slightly increased by Fc∈RI engagement ( Fig. 5 A). This is consistent with a recent study 49 demonstrating that Rac1 is constitutively present in caveolae and is further recruited to these membrane domains in response to platelet-derived growth factor. Thus, engagement of Fc∈RI actively recruits phosphorylated Vav and Fc∈RI into LAT- and Rac1-containing GEMs, suggesting that the presence of Vav in these domains may be functionally important.
Vav Activity Is Required for JNK1 Activation.
To address the functional importance of Vav, we tested whether the expression of Vav-C would have an effect on endogenous Vav activity and explored the possible correlation with the formation of a Vav-containing GEM-residing complex. Because a Vav-dependent activation of JNK1, which mimics Rac1 activation of JNK1, had been demonstrated 13 31 50, we assayed for the activation of JNK1 in cells expressing Vav-C. We chose to look at JNK1 activity, rather than its phosphorylation, to obtain a more direct quantitative measure of any effect. As shown in Fig. 5 B, expression of Vav-C inhibited the tyrosine phosphorylation of endogenous Vav and decreased the Fc∈RI-induced JNK1 activation by 81 ± 17%. Some reduction of the basal levels of JNK1 activity in these cells was also observed. No effect was observed by expression of either GFP or Vav-C (R694K) as controls. Thus, the expression of Vav-C, which inhibits Vav phosphorylation and movement to GEMs, also results in inhibition of Fc∈RI-mediated JNK1 activation ( Fig. 5 B) in the absence of an effect on Syk activity or on the phosphorylation of SLP-76 and LAT ( Fig. 4c, Fig. e, and Fig. f). We previously demonstrated 13 that the constitutively active Rac1 (V12) and the inactive Rac1 (N17) (which function at the plasma membrane [12, 49]) mimic or inhibit, respectively, Vav-induced JNK1 activation. Thus, collectively, our results suggest that phosphorylation of Vav and/or formation of a Vav-containing plasma membrane–localized multiprotein complex may be required for the Vav-dependent activation of JNK1 after Fc∈RI engagement.
Discussion
This study demonstrates that Vav moves to the plasma membrane upon Fc∈RI engagement ( Fig. 6). In addition, we found that most of the membrane-localized Vav and a portion of the Fc∈RI partition to LAT- and Rac1-containing GEMs ( Fig. 5 A), suggesting that these specialized membrane domains represent sites where receptors engage downstream effectors. Because Vav forms a complex with SLP-76 and LAT 9 19 44 45, our findings promote the view that Vav functions as part of a multiprotein signaling complex that is engaged by the Fc∈RI in the GEMs ( Fig. 6).
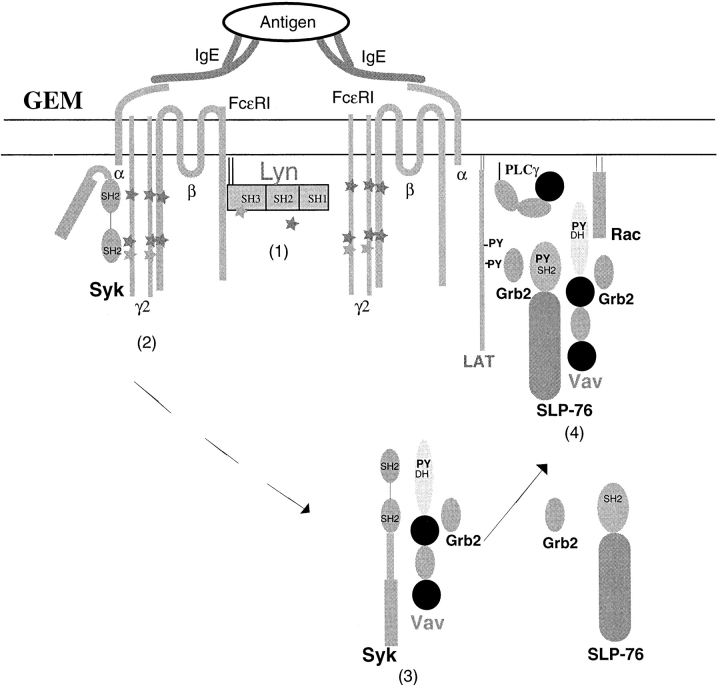
Figure 6.
Model of Fc∈RI communication with Vav. Fc∈RI engagement results in Lyn phosphorylation of the ITAMs of the receptors and recruitment of receptors into GEMs 1. Which step occurs first is presently unclear. Syk is recruited to the phosphorylated ITAMs of Fc∈RI and is activated 2. Syk may target Vav for phosphorylation in the membrane or cytosol. Our data show the presence of phosphorylated Vav in both the cytosol and membrane, suggesting the possibility that phosphorylation may take place in the cytosol before membrane localization 3. Phosphorylated Vav can associate with the adaptor protein, SLP-76, which can mediate Vav movement to the GEMs because of SLP-76 binding to the scaffold protein, LAT, which is found in GEMs. Recruitment of Vav into these domains allows Vav to target Rac1 and activate it 4. Full activation of JNK1 via the Fc∈RI requires Vav activity.
The movement of Vav to the plasma membrane is dependent on Syk activity and the SH2 domain of Vav. Interestingly, we did not observe a loss in plasma membrane localization by deletion of the PH domain of Vav ( Fig. 2 B), a domain thought to be important for membrane targeting and Vav function 14 51. Thus, only the SH2 domain was found to mediate association with the plasma membrane components. The importance of this domain to the activation of Vav is underscored by the inability to phosphorylate the Vav (R694K) mutant in Syk-containing cells ( Fig. 2 D) and the inhibition of Vav phosphorylation by Vav-C, which also inhibits Vav binding to Syk in the absence of an effect on Syk phosphorylation ( Fig. 4 C). Mutations of both Y342 and Y346 of rat Syk or the equivalent tyrosines on ZAP-70 have been demonstrated to inhibit Vav interaction 17 18. These mutations also inhibit the tyrosine phosphorylation of Vav (Yamashita, T., personal communication). Thus, the use of the above Syk mutant would not allow us to distinguish whether Syk activity or Syk interaction is necessary for Vav redistribution. At present, our data suggest that the association of Vav with the plasma membrane is not mediated by Syk. This conclusion is best supported by our preliminary results in Fc∈RI-transfected CHO cells 42 in which phosphorylation of receptor Syk and Vav did not result in Vav plasma membrane localization (under conditions where Syk was membrane localized). These results also suggest that Vav phosphorylation is not the sole requirement for plasma membrane localization. However, we cannot formally exclude the possibility that Syk might mediate some association of Vav with the plasma membrane.
Vav also interacts with adaptor proteins like SLP-76 that mediate binding to plasma membrane–localized scaffolding proteins like LAT 46 and thus could provide plasma membrane localization of Vav. Because a significant fraction of phosphorylated LAT is localized to GEMs ( 9; and Fig. 5 A), communication between the Vav-containing molecular complex and Fc∈RI may likely occur in GEMs. Preliminary support for this hypothesis is provided by the similar kinetics of Fc∈RI and Vav localization to GEMs ( 6 52; and this study) and also by the observation that coexpression of Vav-C, which inhibits Fc∈RI-mediated JNK1 activation ( Fig. 5 B), also effectively inhibited the presence of endogenous and exogenous Vav in GEMs ( Fig. 5 A). In addition, the previous finding 13 that a DH domain–deleted Vav is incapable of activating JNK1 although it localizes to the plasma membrane ( Fig. 2b) underscores the importance of functional Vav in the plasma membrane. Thus, collectively, the findings promote the view that recruitment of Vav to GEMs may be an important step that couples the Fc∈RI 6 to a macromolecular complex that regulates JNK1 activation 2 53 54 55.
Although not all of the cellular Vav moves to the plasma membrane, of the fraction localized in the plasma membrane almost all could be found to colocalize with the Fc∈RI (see Fig. 2a and Fig. c). In contrast, ∼30% of receptors were found to colocalize with Vav. This may reflect the possibility that only activated receptors, or alternatively only receptors that become active, can associate with Vav. Our data are consistent with a possible site of communication for Fc∈RI and Vav being in GEMs, because the numbers obtained for colocalization correspond nicely with the fraction of Fc∈RI found in our study to be recruited to GEMs (∼20%). In addition, these numbers are also consistent with the best estimates of the number of Fc∈RI molecules (25%) that have the potential to associate with the activating Lyn kinase in RBL-2H3 cells 56. Thus, our findings support the notion that the fraction of Fc∈RI found in GEMs is active 6 and can link to downstream effectors like Vav. Notably, although phosphorylation of the Fc∈RI is not required for GEM localization 6, we found that receptors in the GEMs showed at least a 13-fold higher level of Fc∈RI β chain phosphorylation compared with the remaining receptors after normalization for protein content (data not shown). This is consistent with the studies of Field and colleagues 6 and suggests that the GEMs may function both as a site of receptor phosphorylation 6 and a site where receptors engage other signaling molecules 8.
Among the numerous proteins localized to GEMs, LAT has been clearly demonstrated to play an important functional role in TCR signaling 45 46. A recent study demonstrated that the costimulatory effects of CD28 on TCR-dependent activation are mediated by the CD28-dependent clustering of GEMs to the TCR 57. Thus, sustained T cell activation and/or sensitivity of TCR engagement is seemingly dependent on the coclustering of the TCR/CD28 in GEMs and is likely to be dependent on communication with LAT 45 46 57. In mast cells, a phosphoprotein of the approximate molecular mass of LAT was first described by Turner and colleagues as an Fc∈RI-activated link to Grb2-Sos 58. This protein was recently identified in RBL-2H3 mast cells as LAT 46. This scaffold protein is important in assembling a macromolecular signaling complex that includes at least phospholipase Cγ1, SLP-76, Vav, Grb2, and Cbl 45 46. We now demonstrate that in mast cells LAT, Rac1, and Vav are found in the GEMs. The Fc∈RI is also found in GEMs 6, and specific inhibition of Vav phosphorylation, which also inhibits its localization to GEMs, has a significant downregulatory effect on Fc∈RI-mediated JNK1 activation. Although we cannot formally exclude the possibility that another fraction (other than the plasma membrane–localized fraction) of Vav is responsible for JNK1 activation, it is likely that a mechanism similar to that required for activation of Ras on the plasma membrane 59 may also function to activate Rac. In the present model ( Fig. 6), activated Vav (a Rac1 GEF) is sequestered on the plasma membrane by the LAT–SLP-76 complex where it activates Rac1.
Recent studies 55 60 61 on T cells derived from Vav-null mice reported differences in mitogen-activated protein kinase (MAPK) activation. Costello and colleagues 61 demonstrated that in T cells from these mice, extracellular signal–regulated kinase (ERK) activation was impaired. In contrast, the studies of Fischer et al. 60 and Holsinger et al. 55 found no effect on ERK or JNK activation. However, we 13 and others 31 50 have observed Vav-mediated JNK activation in various cell types. The apparent discrepancy may be explained by cell type differences (i.e., mast cells versus T cells), redundancy of signaling pathways, or as proposed by Costello et al. 61, differences in the knockout targeting strategy that could result in low levels of Vav expression. Nevertheless, because we demonstrate an effect on JNK1 activation by the deletion of the Vav SH2 domain and by expression of Vav-C, and found that Syk and Vav synergize to enhance JNK1 activation, we propose that Vav significantly contributes to Fc∈RI-mediated JNK1 activation. Moreover, this hypothesis is also supported by our recent findings that the inactive mutants Rac1 (N17) or JNK (APF) inhibit Vav-dependent JNK1 activation in the RBL-2H3 mast cell line 13.
A recent study 43 demonstrated that mutation of SLP-76 (Y112F, Y128F, Y145F) or mutation of the Vav SH2 domain abrogated both TCR-dependent cytoskeletal rearrangement and p21-activated kinase (PAK) activation. This is consistent with our findings that the SH2 domain of Vav is important for JNK activation, since PAK functions upstream of JNK activation in mast cells 62 but downstream of the Vav target, Rac1 13 50. We also observed that the expression of Vav-C, which localizes to the plasma membrane upon Fc∈RI stimulation and inhibits Vav phosphorylation ( Fig. 4 A and 5 B), resulted in the inhibition of Vav association with SLP-76 and partial inhibition of LAT interaction with SLP-76 without affecting the tyrosine phosphorylation of LAT and SLP-76 ( Fig. 4e and Fig. f). Since Vav, LAT, and SLP-76 can participate in a complex, and along with Rac1, are localized in GEMs, we propose that this multiprotein signaling complex is important to Vav function, including its ability to activate JNK1. In SLP-76–deficient T cells 63, phosphorylation of the calcium response modulator, phospholipase Cγ1, was decreased; thus, it is also possible that a partial loss of the SLP-76 and LAT interactions in the absence of Vav may explain the defective calcium response of Vav-null cells 64. This possibility is currently being explored. The model proposed in Fig. 6 provides a possible mechanism for Vav contribution to cytoskeleton rearrangement, calcium signals, and kinase activation. By complexing with a LAT-organized multiprotein signaling complex 45, Vav activity and protein interactions may contribute to activation of Rac 11 13 50, Ras 10 15 45, and calcium signals 45 55 60 64, thus participating in cytoskeletal rearrangement, MAPK activation, and gene expression 13 19.
Acknowledgments
We thank Drs. Petr Draber and Lawrence E. Samelson for providing reagents. We also acknowledge the excellent technical assistance of Ms. Sandra Odom.
Footnotes
Abbreviations used in this paper: CH, calponin homology; DH, Dbl homology; DNP, dinitrophenyl; GEF, guanine nucleotide exchange factor; GEM, glycosphingolipid-enriched microdomain; GFP, green fluorescent protein; HSA, human serum albumin; ITAM, immunoreceptor tyrosine-based activation motif; JNK, c-Jun NH2-terminal kinase; LAT, linker for activation of T cells; PH, pleckstrin homology; SH, Src homology; SFV, Semliki Forest virus; SLP-76, SH2 domain–containing leukocyte protein of 76 kD; TCA, trichloroacetic acid.
References
- Rudd C.E. Adaptors and molecular scaffolds in immune cell signaling. Cell. 1999;96:5–8 . doi: 10.1016/s0092-8674(00)80953-8. [DOI] [PubMed] [Google Scholar]
- Cantrell D. The real LAT steps forward. Trends Cell Biol. 1998;8:180–182 . doi: 10.1016/s0962-8924(98)01264-1. [DOI] [PubMed] [Google Scholar]
- Lisanti M.P., Scherer P.E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y.H., Cook R.F., Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich sourceimplications for human disease. J. Cell Biol. 1994;126:111–126 . doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572 . doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Schlegel A., Volonte D., Engelman J.A., Galbiati F., Mehta P., Zhang X.L., Scherer P.E., Lisanti M.P. Crowded little cavesstructure and function of caveolae. Cell Signal. 1998;10:457–463 . doi: 10.1016/s0898-6568(98)00007-2. [DOI] [PubMed] [Google Scholar]
- Field K.A., Holowka D., Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J. Biol. Chem. 1997;272:4276–4280 . doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- Montixi C., Langlet C., Bernard A.M., Thimonier J., Dubois C., Wurbel M.A., Chauvin J.P., Pierres M., He H.T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5334–5348 . doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732 . doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylationits essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246 . doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Song J.S., Gomez J., Stancato L.F., Rivera J. Association of a p95 Vav-containing signaling complex with the Fc∈RI gamma chain in the RBL-2H3 mast cell line. Evidence for a constitutive in vivo association of Vav with Grb2, Raf-1, and ERK2 in an active complex. J. Biol. Chem. 1996;271:26962–26970 . doi: 10.1074/jbc.271.43.26962. [DOI] [PubMed] [Google Scholar]
- Crespo P., Schuebel K.E., Ostrom A.A., Gutkind J.S., Bustelo X.R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172 . doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62 . doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Song J.S., Haleem-Smith H., Arudchandran R., Gomez J., Scott P.M., Mill J.F., Tan T.-H., Rivera J. Tyrosine phosphorylation of Vav stimulates IL-6 production in mast cells by a Rac/JNK-dependent pathway. J. Immunol. 1999;163:802–810 . [PubMed] [Google Scholar]
- Han J., Luby-Phelps K., Das B., Shu X., Xia Y., Mosteller R.D., Krishna U.M., Falck J.R., White M.A., Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560 . doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Collins T.L., Deckert M., Altman A. Views on Vav. Immunol. Today. 1997;18:221–225 . doi: 10.1016/s0167-5699(97)01037-2. [DOI] [PubMed] [Google Scholar]
- Ramos-Morales F., Druker B.J., Fischer S. Vav binds to several SH2/SH3 containing proteins in activated lymphocytes. Oncogene. 1994;9:1917–1923 . [PubMed] [Google Scholar]
- Deckert M., Tartare-Deckert S., Couture C., Mustelin T., Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604 . doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhao Q., Kurosaki T., Weiss A. The Vav binding site (Y315) in ZAP-70 is critical for antigen receptor–mediated signal transduction. J. Exp. Med. 1997;185:1877–1882 . doi: 10.1084/jem.185.10.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Motto D.G., Koretzky G.A., Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602 . doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- Marengere L.E., Mirtsos C., Kozieradzki I., Veillette A., Mak T.W., Penninger J.M. Proto-oncoprotein Vav interacts with c-Cbl in activated thymocytes and peripheral T cells. J. Immunol. 1997;159:70–76 . [PubMed] [Google Scholar]
- Ye Z.S., Baltimore D. Binding of Vav to Grb2 through dimerization of Src homology 3 domains. Proc. Natl. Acad. Sci. USA. 1994;91:12629–12633 . doi: 10.1073/pnas.91.26.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Morales F., Romero F., Schweighoffer F., Bismuth G., Camonis J., Tortolero M., Fischer S. The proline-rich region of Vav binds to Grb2 and Grb3-3. Oncogene. 1995;11:1665–1669 . [PubMed] [Google Scholar]
- Hobert O., Jallal B., Schlessinger J., Ullrich A. Novel signaling pathway suggested by SH3 domain-mediated p95vav/heterogeneous ribonucleoprotein K interaction. J. Biol. Chem. 1994;269:20225–20228 . [PubMed] [Google Scholar]
- Romero F., Germani A., Puvion E., Camonis J., Varin-Blank N., Gisselbrecht S., Fischer S. Vav binding to heterogeneous nuclear ribonucleoprotein (hnRNP) C. Evidence for Vav-hnRNP interactions in an RNA-dependent manner. J. Biol. Chem. 1998;273:5923–5931 . doi: 10.1074/jbc.273.10.5923. [DOI] [PubMed] [Google Scholar]
- Romero F., Dargemont C., Pozo F., Reeves W.H., Camonis J., Gisselbrecht S., Fischer S. p95vav associates with the nuclear protein Ku-70. Mol. Cell. Biol. 1996;16:37–44 . doi: 10.1128/mcb.16.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., Schilling J.W., Beckerle M.C., Ullrich A., Jallal B. SH3 domain-dependent interaction of the proto-oncogene product Vav with the focal contact protein zyxin. Oncogene. 1996;12:1577–1581 . [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384 . doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Benhamou M., Ryba N.J., Kihara H., Nishikata H., Siraganian R.P. Protein-tyrosine kinase p72syk in high affinity IgE receptor signaling. Identification as a component of pp72 and association with the receptor gamma chain after receptor aggregation. J. Biol. Chem. 1993;268:23318–23324 . [PubMed] [Google Scholar]
- Costello P.S., Turner M., Walters A.E., Cunningham C.N., Bauer P.H., Downward J., Tybulewicz V.L. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605 . [PubMed] [Google Scholar]
- Zhang J., Berenstein E.H., Evans R.L., Siraganian R.P. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor–mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J. Exp. Med. 1996;184:71–79 . doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto H., Salem P., Robbins K.C., Bustelo X.R., Gutkind J.S. Tyrosine phosphorylation of the vav proto-oncogene product links Fc∈RI to the Rac1-JNK pathway. J. Biol. Chem. 1997;272:10751–10755 . doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- Rivera J., Kinet J.P., Kim J., Pucillo C., Metzger H. Studies with a monoclonal antibody to the β subunit of the receptor with high affinity for immunoglobulin E. Mol. Immunol. 1988;25:647–661 . doi: 10.1016/0161-5890(88)90100-9. [DOI] [PubMed] [Google Scholar]
- Holowka D., Metzger H. Further characterization of the β-component of the receptor for immunoglobulin E. Mol. Immunol. 1982;19:219–227 . doi: 10.1016/0161-5890(82)90334-0. [DOI] [PubMed] [Google Scholar]
- Arudchandran R., Brown M.J., Song J.S., Wank S.A., Haleem-Smith H., Rivera J. Polyethylene glycol-mediated infection of non-permissive mammalian cells with semliki forest virusapplication to signal transduction studies. J. Immunol. Methods. 1999;222:197–208 . doi: 10.1016/s0022-1759(98)00161-6. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Rose J.K. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J. Cell Biol. 1996;135:1515–1523 . doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M.C., Qiu W.R., Wang X., Meyer C.F., Tan T.H. Human HPK1, a novel human hematopoietic progenitor kinase that activates the JNK/SAPK kinase cascade. Genes Dev. 1996;10:2251–2264 . doi: 10.1101/gad.10.18.2251. [DOI] [PubMed] [Google Scholar]
- Furuichi K., Rivera J., Triche T., Isersky C. The fate of IgE bound to rat basophilic leukemia cells. IV. Functional association between the receptors for IgE. J. Immunol. 1985;134:1766–1773 . [PubMed] [Google Scholar]
- Seagrave J.C., Deanin G.G., Martin J.C., Davis B.H., Oliver J.M. DNP-phycobiliproteins, fluorescent antigens to study dynamic properties of antigen-IgE-receptor complexes on RBL-2H3 rat mast cells. Cytometry. 1987;8:287–295 . doi: 10.1002/cyto.990080309. [DOI] [PubMed] [Google Scholar]
- Stauffer T.P., Meyer T. Compartmentalized IgE receptor–mediated signal transduction in living cells. J. Cell Biol. 1997;139:1447–1454 . doi: 10.1083/jcb.139.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S., Sutherland M., Packham G., Yi T., Weiss A. The protein tyrosine kinase ZAP-70 can associate with the SH2 domain of proto-Vav. J. Biol. Chem. 1994;269:32579–32585 . [PubMed] [Google Scholar]
- Oliver J.M., Burg D.L., Wilson B.S., McLaughlin J.L., Geahlen R.L. Inhibition of mast cell Fc∈R1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J. Biol. Chem. 1994;269:29697–29703 . [PubMed] [Google Scholar]
- Vonakis B.M., Chen H., Haleem-Smith H., Metzger H. The unique domain as the site on Lyn kinase for its constitutive association with the high affinity receptor for IgE. J. Biol. Chem. 1997;272:24072–24080 . doi: 10.1074/jbc.272.38.24072. [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J., Pappu R., Bu J.Y., Mayer B., Chernoff J., Straus D., Chan A.C. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616 . doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- Tuosto L., Michel F., Acuto O. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J. Exp. Med. 1996;184:1161–1166 . doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco T.S., Kadlecek T., Zhang W., Samelson L.E., Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–626 . doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LATthe ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92 . doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr., Metzger H. The interaction of IgE with rat basophilic leukemia cells. II. Quantitative aspects of the binding reaction. J. Exp. Med. 1974;140:1676–1695 . doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart E.J., Ying Y.-S., Mineo C., Anderson R.G.W. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. USA. 1995;92:10104–10108 . doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaely P.A., Mineo C., Ying Y.-S., Anderson R.G.W. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J. Biol. Chem. 1999;274:21430–21436 . doi: 10.1074/jbc.274.30.21430. [DOI] [PubMed] [Google Scholar]
- Crespo P., Bustelo X.R., Aaronson D.S., Coso O.A., Lopez-Barahona M., Barbacid M., Gutkind J.S. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene. 1996;13:455–460 . [PubMed] [Google Scholar]
- Zheng Y., Zangrill D., Cerione R.A., Eva A. The pleckstrin homology domain mediates transformation by oncogenic Dbl through specific intracellular targeting. J. Biol. Chem. 1996;271:19017–19020 . doi: 10.1074/jbc.271.32.19017. [DOI] [PubMed] [Google Scholar]
- Field K.A., Holowka D., Baird B. Structural aspects of the association of Fc∈RI with detergent-resistant membranes. J. Biol. Chem. 1999;274:1753–1758 . doi: 10.1074/jbc.274.3.1753. [DOI] [PubMed] [Google Scholar]
- Hu P., Margolis B., Schlessinger J. Vava potential link between tyrosine kinases and ras-like GTPases in hematopoietic cell signaling. Bioessays. 1993;15:179–183 . doi: 10.1002/bies.950150306. [DOI] [PubMed] [Google Scholar]
- Fischer K.D., Tedford K., Penninger J.M. Vav links antigen-receptor signaling to the actin cytoskeleton. Semin. Immunol. 1998;10:317–327 . doi: 10.1006/smim.1998.0124. [DOI] [PubMed] [Google Scholar]
- Holsinger L.J., Graef I.A., Swat W., Chi T., Bautista D.M., Davidson L., Lewis R.S., Alt F.W., Crabtree G.R. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 1998;8:563–572 . doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Mao S.Y., Metzger H. Aggregation of the high-affinity IgE receptor and enhanced activity of p53/56lyn protein-tyrosine kinase. Proc. Natl. Acad. Sci. USA. 1994;91:11251–11255 . doi: 10.1073/pnas.91.23.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682 . doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Turner H., Reif K., Rivera J., Cantrell D.A. Regulation of the adapter molecule Grb2 by the Fc∈R1 in the mast cell line RBL2H3. J. Biol. Chem. 1995;270:9500–9506 . doi: 10.1074/jbc.270.16.9500. [DOI] [PubMed] [Google Scholar]
- Campbell S.L., Khosravi-Far R., Rossman K.L., Clark G.J., Der C.J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413 . doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Fischer K.D., Kong Y.Y., Nishina H., Tedford K., Marengere L.E., Kozieradzki I., Sasaki T., Starr M., Chan G., Gardener S. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 1998;8:554–562 . doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- Costello P.S., Walters A.E., Mee P.J., Turner M., Reynolds L.F., Prisco A., Sarner N., Zamoyska R., Tybulewicz V.L.J. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proc. Natl. Acad. Sci. USA. 1999;96:3035–3040 . doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Hartman S.E., Holland P.M., Cooper J.A., Kawakami T. Multiple signaling pathways for the activation of JNK in mast cellsinvolvement of Bruton's tyrosine kinase, protein kinase C, and JNK kinases, SEK1 and MKK7. J. Immunol. 1998;161:1795–1802 . [PubMed] [Google Scholar]
- Yablonski D., Kuhne M.R., Kadlecek T., Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-γ1 in an SLP-76-deficient T cell. Science. 1998;281:413–416 . doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- Turner M., Mee P.J., Walters A.E., Quinn M.E., Mellor A.L., Zamoyska R., Tybulewicz V.L. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460 . doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- Field K.A., Holowka D., Baird B. Fc∈RI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc. Natl. Acad. Sci. USA. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]