Abstract
A major problem in the study of chlamydial genital infections in animal models has been the use of varied doses of chlamydiae for infection in different laboratories. It is clearly desirable to use a dose which approximates that of natural sexual infection, but that dose to date has not been determined because of the inability of researchers to quantify chlamydiae in semen. Fortunately, sexual transmission of chlamydiae has been described for the guinea pig model of infection with the chlamydial agent of guinea pig inclusion conjunctivitis (GPIC). In this study, we undertook to determine the approximate infection dose in actual sexual transmission by comparing the kinetics of infection in female guinea pigs acquired via sexual contact to those of genital infections induced artificially with known quantities of chlamydiae. Groups of guinea pigs were infected intravaginally with 104, 103, 102, and 101 inclusion-forming units (IFU) of GPIC, and the kinetics of the infection were determined. Infection with 102 IFU produced infections with lower peak levels than those in animals receiving 104 or 103 IFU. Seventy percent of animals receiving 102 IFU became infected, while 100 and 79% of animals receiving 104 and 103 IFU, respectively, became infected. Animals receiving 102 IFU also had a longer incubation period. Of 19 animals that mated with infected males, 63.2% became infected, with an infection course which was not significantly different than that of the 102-IFU-infected group. The data suggest that female guinea pigs received approximately 102 IFU by sexual transmission. Of interest was the observation that the guinea pigs infected by sexual transmission shed organisms for a significantly shorter time period than that of any group that was artificially infected. This result suggests that there may be factors associated with semen which passively transfer antimicrobial activity to the female or enhance the innate host response in the female. Immunization of females with an inactivated vaccine was also found to elicit a protective immune response against sexual challenge, demonstrating that the model can be used in the evaluation of possible vaccine candidates and/or methodologies. There is currently no other animal model available for any sexually transmitted disease in which the disease or the ability to prevent the disease may be studied in animals infected by the natural means.
It is fortunate that for the study of chlamydial genital infections, several excellent animal models exist in which the basic aspects of the host response and pathogenesis of infection can be studied. However, a major problem in the use of animal models for the study of human infections has always been the determination of a realistic infection dose. This has been especially true in the study of animal models for chlamydial genital infections, simply because there is no accurate information available as to the natural infection dose delivered during sexual intercourse. Thus, investigators have used doses based on arbitrary decisions. Ideally, one should use a range of doses, but this is often difficult for logistical and financial reasons. Nevertheless, it is quite obvious that the experimental data can be profoundly affected by altering the infecting or challenge doses, making it difficult to evaluate the resulting information in the context of actual clinical infection. For instance, using the ocular model of guinea pigs infected with the chlamydial agent of guinea pig inclusion conjunctivitis (GPIC), Ahmad and Schachter (1) demonstrated major differences in the courses of clinical disease after primary and challenge infections with doses ranging from 101 to 105 50% egg-lethal doses.
While genital tract infection models have been developed with primates (15), mice (2), marmosets (8), rabbits (16), and guinea pigs (9), sexual transmission has been demonstrated only for guinea pigs infected with GPIC. Barron first reported that male guinea pigs could be infected intraurethrally with GPIC and that, when they were housed with female guinea pigs, they transmitted the infection sexually (10). Interestingly, pregnant female guinea pigs could also transmit the infection to their offspring during parturition, producing conjunctivitis in the newborns, analogous to the human situation (9). Although Barron was able to show sexual transmission in guinea pigs, it was not possible to determine when the animals became infected or what the infecting dose from the male was.
In this laboratory, we have always infected guinea pigs and mice with high doses in order to guarantee infection and provide a strong antigenic stimulus. Other groups have used low infection doses in mice under the assumption that this dose may be more realistic. With regard to the evaluation of vaccine candidates for the animal model, it is particularly important that a dose closely approximating the natural infecting dose be used in the studies to accurately judge the protective capacity of the vaccine. Since sexual transmission has been demonstrated for the GPIC-guinea pig model, we undertook in this study to (i) develop a reliable and predictable model of sexual transmission, (ii) attempt to define the approximate infecting dose from a male guinea pig during sexual intercourse, and (iii) determine the effect of a “natural” challenge dose on the protective capacity of an inactivated chlamydial elementary body vaccine.
MATERIALS AND METHODS
Experimental animals.
Male and female Hartley strain guinea pigs, weighing approximately 450 to 500 g, were purchased from Simonsen Laboratories (Gilroy, Calif.). All guinea pigs were housed singly in cages covered with a fiberglass filter in a room with a 12-h-light-12-h-dark cycle and were provided food and water ad libitum. During mating, a single male and female were housed together in large breeding cages but were subsequently moved back to individual cages.
Female guinea pigs were infected intravaginally with various doses of chlamydiae suspended in 25 μl of sucrose-phosphate-glutamate buffer by insertion of a micropipette tip approximately 3 cm into the vagina so the suspension was deposited against the cervix. Male guinea pigs were anesthetized with methoxyflurane, and 25 μl of a GPIC suspension containing 107 inclusion-forming units (IFU) of GPIC was placed on the tip of the urethra with the external meatus retracted. Because of the anatomy, it is impossible to know exactly how many organisms entered the urethra, but it must be assumed that a large portion did not actually come in contact with the urethral epithelium.
In the dose-response and immunization experiments, animals not infected sexually were inoculated intravaginally on the same day. Thus, the stage of their estrous cycles was random in comparison to that of sexually infected animals, which, by definition, were in estrus. We have never observed a difference in the infection kinetics associated with the time of the estrous cycle when animals were infected, although we have seen significant differences in the appearances of upper genital tract pathologies (20).
Culture and isolation of chlamydiae.
The agent of GPIC, a member of the species Chlamydia psittaci, was originally obtained from the late Edward Murray and has been continuously passaged in our laboratory. For infection purposes, GPIC is maintained in McCoy cells by standard techniques and aliquots of stock are frozen at −70°C in sucrose-phosphate-glutamate buffer. Chlamydiae were cultured and quantified from swabs according to standard procedures.
For isolation of chlamydiae from female animals, a sterile Dacroswab was inserted in the vagina until it came in contact with the cervix, and the swab was rotated against the cervix, removed, and placed in sucrose-phosphate buffer (23) containing 5% fetal calf serum, 50 μg of gentamicin per ml, 100 μg of vancomycin per ml, and 2.5 μg of amphotericin B (Fungizone) per ml. The swabs were frozen at −70°C until needed. Swabs were collected from male guinea pigs anesthetized with methoxyflurane. The urethra was exposed, and a Dacroswab was inserted approximately 2 cm into the urethra, rotated, removed, and placed in sucrose-phosphate buffer.
Sexual transmission.
In order to establish a model in which the time of sexual transmission could be reliably determined, we took advantage of a unique aspect of the female guinea pig reproductive system. When animals are not in estrus, a membrane which covers the vagina is produced; however, as soon as the animal enters estrus, this membrane disappears and the female is receptive to copulation. Within 3 days following the onset of estrus, the membrane regenerates. Guinea pigs have an estrous cycle of approximately 15 to 17 days and are in estrus for only about 8 h.
Therefore, all female guinea pigs were monitored daily to determine the presence or absence of the vaginal membrane and each individual cycle was recorded. Females were monitored through a least two estrous cycles or until the time that the opening of the vaginal membrane could be predicted to a 3-day period (1 day with an error margin of plus or minus 1 day). Four to six days prior to a given female entering estrus, a male guinea pig was infected intraurethrally (21) and housed with the female in a large breeder cage. A swab was collected from the male to verify infection, and all males used in this study were found to be isolation positive. Monitoring for the opening of the vaginal membrane was continued until it was observed. The date when the vaginal membrane opened was considered to be the day that copulation had taken place. Beginning 3 days later, cervical swabs were collected from the female every 3 days until the resulting infection had resolved or we were convinced by repeated cultures that the animal remained uninfected. Animals were routinely monitored for at least 30 days. The male was removed 1 week after the date of copulation, and the female was moved to a single cage. Following resolution of the infection, the female was monitored to determine whether she had become pregnant. Pregnancy and/or infection was considered confirmation that the animals had copulated.
Immunization protocol.
Guinea pigs were immunized subcutaneously with 100 μg of UV-inactivated GPIC elementary bodies (18) in phosphate-buffered saline emulsified to a 1:1 dilution in Freund's complete adjuvant (Sigma, St. Louis, Mo.). Two and 4 weeks later, the animals were given a booster injection with a similar preparation emulsified in Freund's incomplete adjuvant (Sigma). This regimen has been shown to elicit a significant protective response against genital challenge (18).
RESULTS
Determination of ID50.
No data on the quantification of chlamydiae from semen have been published because of the difficulties in isolating organisms associated with the nature of the composition of semen and its viscosity. Moreover, studies describing the collection of guinea pig semen have indicated that it coagulates within minutes of collection (6), making culture by standard techniques impossible. Since it is not possible to directly determine the number of organisms which is transmitted to a female guinea pig during copulation, we decided to approximate the dosage on the basis of infection curves generated by artificial inoculation with different amounts of chlamydiae. Thus, a dose-response experiment in which separate groups of guinea pigs were infected intravaginally with various doses of chlamydiae, ranging from 100 to 104 IFU, was first performed. The experiment was repeated with the 102- to 103-IFU doses, and the data were pooled. Based on the rate of infection for the different doses (Table 1), a 50% infective dose (ID50) of 48 IFU was obtained.
TABLE 1.
Effect of infectious dose on infectivity and incubation perioda
| Infection rate and dose (IFU) | No. of animals | % of animals infected | Mean length of incubation period (days) | Mean length of active shedding (days) |
|---|---|---|---|---|
| Artificial | ||||
| 104 | 5 | 100 | 3.6 (0.04) | 16.8 (0.001) |
| 103 | 14 | 79 | 4.9 | 17.7 (0.0005) |
| 102 | 10 | 70 | 9.5 | 16.3 (0.005) |
| 101 | 5 | 40 | 9.0 | 15.0 (NA) |
| 100 | 5 | 0 | ||
| Sexual contact | 19 | 62.2 | 8.3 | 9.9 |
Values in parentheses are P values obtained by one-tailed t test versus results for the sexual-contact control group. NA, not applicable (too few values).
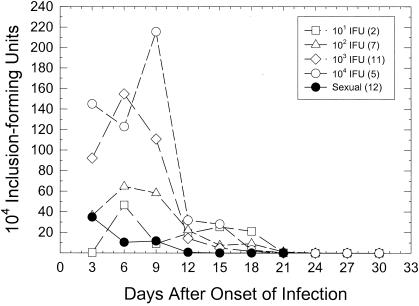
When the mean day of onset of infection was determined, there was a marked delay in the onset of infection between doses of 103 and 102 or 101 IFU. In general, for the animals in all groups which did become infected, there was no significant difference in the numbers of days that the organisms were shed. The average length of infection ranged from 15 days for 101 IFU to 17.7 days for 103 IFU. When the kinetics of the infection with the different inoculants were determined, differences between the higher doses (104 and 103 IFU) and the lower doses (102 and 101 IFU) were seen. In Fig. 1, the mean course of infection for only those animals which became infected is presented. The curves were all shifted to the left so that the first day of positive isolation represented the day of onset of infection in order to facilitate comparisons of kinetics among groups. The kinetics of the infection curves from animals inoculated with 102 IFU were significantly different than those of the curves of animals inoculated with 104 IFU (P < 0.027; two-factor [days and group] analysis of variance [ANOVA] with repeated measures), but there was no difference between the 102- and 103-IFU groups. The 101-IFU group had too few animals for a statistical analysis to be performed, but the mean level of infection of the two infected animals in that group was similar to that seen in the group infected with 102 IFU. In particular, the peak levels of infection were considerably lower with the 102- and 101-IFU inoculants.
FIG. 1.
Kinetics of genital infection in female guinea pigs infected intravaginally with various doses of chlamydiae or by sexual transmission. In order to facilitate statistical comparison of infection curves for different animals and different groups, the curves were normalized by reporting the data in relation to when infection was first detected; i.e., the first day of positive isolation was arbitrarily called day 3 of infection. The numbers in parentheses are the numbers of animals which became infected in each group. The average numbers of IFU are plotted. Animals that did not become infected were not included.
Sexual transmission.
To determine the approximate infection dose in actual sexual transmission, a total of 19 female guinea pigs were mated with infected males as described in Materials and Methods. Urethral swabs were collected from all males 6 days after infection to confirm that they were infected. The median number of IFU from the male urethra was 1.0 × 105, with a range of 4.4 × 103 to 1.4 × 106. Of 19 female guinea pigs mated with infected males, 12 (63.2%) of the animals became infected (Table 1), demonstrating that sexual transmission of chlamydiae was accomplished. Of the 7 animals which did not become infected, 4 became pregnant, indicating that copulation had taken place; therefore, there were only 3 animals of the 19 total (15.8%) for which copulation could not be confirmed.
The average length of the incubation period for the sexually infected guinea pigs was 8.3 days. This was significantly different from the length for the group receiving 104 IFU (P < 0.04, one-tailed t test) but not from those for the other groups. When the infection kinetics were compared (Fig. 1) by a two-factor (days and group) ANOVA with repeated measures, the infection curves of animals infected by sexual transmission were significantly different than the curves of guinea pigs artificially inoculated with either 104 IFU (P < 0.001) or 103 IFU (P < 0.01) but not 102 IFU. Only the animals which became infected in each group were compared. In general, the peak levels of infection were lower in the 102-IFU, 101-IFU, and sexual-transmission groups than the peak levels in the 104- and 103-IFU groups. The infection kinetics, with respect to the peak level of infection for this sexual-transmission group, were similar to those seen for the 102- and 101-IFU groups but were significantly different (P < 0.05, one-tailed t test) from those of the groups infected with 103 and 104 IFU. Based on the comparison of the infection kinetics, the length of the incubation period, and the percentage of animals becoming infected upon challenge, one can conclude that the female guinea pigs were receiving approximately 102 IFU by sexual transmission at this point in the males' infections.
Of major interest was the observation that the period of time that GPIC could be isolated from the genital tract was significantly shorter in the group which acquired their infection by sexual transmission (Table 1). The average length of shedding of organisms in this group was 9.9 days, whereas the minimum mean length of time of shedding for the groups receiving the inoculant artificially was 15 days. These data were highly significant when they were assessed by a one-tailed t test.
We also determined the percentage of infected animals that became pregnant versus the percentage of those that were not infected following sexual contact that became pregnant. There was no observed difference in the pregnancy rates of the uninfected animals (57%) and the infected guinea pigs (50%), suggesting that, at least in animals infected at the time of fertilization, chlamydial infection had no effect on conception or early pregnancy.
Effect of immunization upon challenge by sexual transmission.
An important potential application of a model for sexual transmission is to evaluate the protective capacity of vaccines. In order to determine whether protection against a sexual challenge could be induced with a vaccine, female guinea pigs were immunized with a preparation of UV-inactivated elementary bodies of GPIC. A group of 10 females was immunized as indicated in Materials and Methods. Beginning a minimum of 2 weeks after the last booster inoculation, infected male guinea pigs were grouped singly with individual females as described above. This experiment was being performed at the same time as the experiment characterizing the course of infection by sexual transmission described above; thus, the animals described above served as unimmunized controls. Because of the variation associated with the estrous cycles of the individual guinea pigs, immunized animals could not be challenged at exactly the same time. Thus, the range of time following the last booster when the females were challenged extended from 46 to 143 days.
For a comparison of the results of the above-described experiments to those of artificial challenge of immunized guinea pigs with known numbers of IFU, we performed an experiment in which guinea pigs were inoculated intravaginally with either 103, 102, or 101 IFU 2 weeks after the last booster injection. The experiment was repeated with just 103 and 102 IFU. All of the data from the two artificial-challenge experiments and the sexual-transmission challenge experiment are shown in Fig. 1 for comparison.
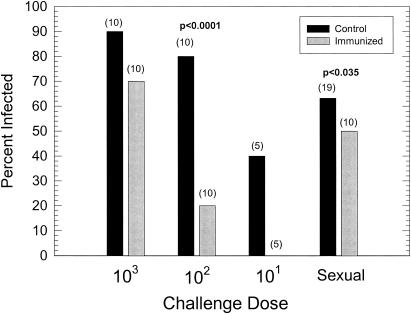
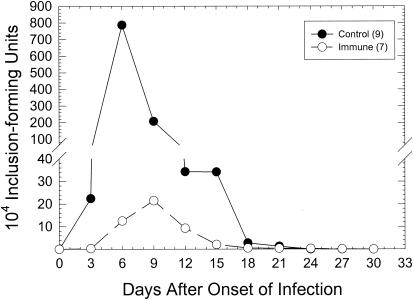
When the percentage of animals becoming infected upon artificial challenge was determined, guinea pigs receiving 102 IFU demonstrated a high level (P < 0.0001 by chi-square analysis) of sterile immunity; i.e., no infectious organisms could be isolated from cervical swabs at any of the times tested (Fig. 2). Because only two of five control animals inoculated with 101 organisms became infected, we could not determine statistical differences for that group. Although only a low level of sterilizing immunity was seen in the group challenged with 103 organisms (70% positive for infection in the immunized group versus 90% in the unimmunized group), the peak level of infection was significantly reduced by immunization (P < 0.001; two-factor [days and group] ANOVA with repeated measures) (Fig. 3). However, the length of the infection remained unchanged. The length of infection in unimmunized animals was 19.7 days, compared to 17.6 days in infected immunized animals. This difference was not statistically different. This finding was very similar to what we had seen before in animals challenged with higher doses of chlamydiae (18).
FIG. 2.
Effect of immunization with UV-inactivated elementary bodies upon artificial-challenge infection with various doses of chlamydiae in comparison to the effect after sexual transmission. The numbers in parentheses are the total numbers of guinea pigs per group. The P value represents the level of significance according to a chi-square analysis. The absence of a P value indicates that the differences between the groups were not significant.
FIG. 3.
Course of infection in immunized guinea pigs challenged with 103 IFU. The numbers in parentheses are the total numbers of guinea pigs per group. Only guinea pigs which were isolation positive are included in this figure.
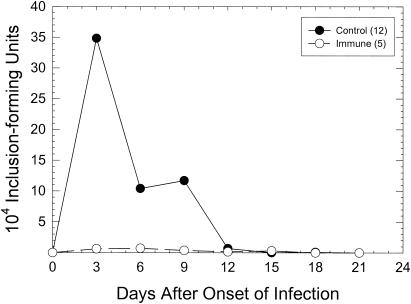
When immunized female guinea pigs were challenged by sexual transmission, a significant difference (P < 0.035; chi-square analysis) in the percentages of animals becoming infected was noted (Fig. 2). Interestingly, even though the apparent infectious dose in sexual transmission is approximately 102 IFU, the level of sterile immunity was considerably lower. The peak levels of the resultant infections were also much lower than those of the control group, although statistical significance by a two-way ANOVA with repeated measures was not attained (Fig. 4). As in the artificial infection with 103 IFU, there was no significant difference in the lengths of the periods during which chlamydiae could be isolated between the unimmunized (9.8 days) and immunized (10.8 days) groups. It should be noted that, similar to the abbreviated infections seen in control animals infected sexually, the infections in immunized animals challenged sexually were also significantly shorter than those of the immunized animals challenged artificially with 103 IFU (P < 0.04, t test). These data suggest that the sexual-transmission model may be used to demonstrate a protective response in the evaluation of possible vaccine candidates.
FIG. 4.
Course of infection in immunized guinea pigs challenged by sexual transmission. The numbers in parentheses are the total numbers of guinea pigs per group. Only results for guinea pigs which were isolation positive are included in this figure.
DISCUSSION
Over the years, animal models for chlamydial genital infections have provided a tremendous amount of information regarding the natural course of genital infection with chlamydiae, the pathological response, and the immune response. Fortunately, there are several excellent models which can be used to address a variety of questions on the disease process in chlamydial genital infections. However, all of the models to this point have utilized infections by artificial means, i.e., intravaginal inoculation of chlamydiae from cell culture or earlier, yolk sac preparations. A major point of discussion over the years has concerned the number of organisms which should be used in artificial infection to mimic most closely the natural infection acquired by sexual intercourse. To date, there are no data published on the natural-infection dose in animals and, more importantly, in humans.
The GPIC genital infection model is the only experimental animal model for any sexually transmitted disease for which sexual transmission of infection has actually been demonstrated (10). It is made even more significant because the reproductive physiology of the guinea pig, in contrast to that of true rodents, resembles very closely that of the human (26). Guinea pigs have a 15- to 17-day estrous cycle, in contrast to the 5-day cycle of rodents, and are excellent models for human reproductive physiology. As we have previously reported, the pathological response in guinea pigs to GPIC genital infection is remarkably similar to that of humans in virtually all pathological parameters (19). We have also observed that, as in humans, GPIC genital infections are exacerbated by treatment with physiologic levels of estrogen (13) and with oral contraceptives (11). While we had previously found no effect on the course of infection by treatment with progesterone when high infection doses were used (12), we have recently observed that if one challenges animals receiving medroxyprogesterone acetate (Depo-Provera) with a natural-infection dose, the infection is increased in intensity (R. G. Rank, unpublished data).
Ultimately, in order to use the guinea pig model for the assessment of potential vaccine candidates, it was critical that we knew the actual infectious dose in infections acquired by sexual contact. Because the quantification of chlamydiae from urethral swabs of the male would not give the number of IFU contained in guinea pig semen during copulation, and because direct culture of guinea pig semen was impractical, we elected to gain an approximation of the infecting dose by comparing the course of the resulting infections in females to the course of those resulting from artificial intravaginal inoculation with known amounts of chlamydiae. Fortunately, the reproductive physiology and anatomy of the guinea pig are such that one can easily map the estrous cycle of individual animals and accurately predict when the female will be in estrus, the only time at which she will mate. The infection kinetics and percentage of animals that became infected were similar to those of animals artificially infected with 102 IFU. Obviously, there is likely to be a great deal of variability, especially related to the stage of the infection in the male when copulation takes place. In general, the male guinea pigs in this study were in the early part of their infection cycle (14). If the males had been at the peak of their infections, more organisms may have been transferred to the females, or conversely, if the infection was subsiding, the dose would have been considerably lower. It is difficult to know the minimal infectious dose for female guinea pigs by sexual transmission, although the ID50 for GPIC in the genital tracts of female guinea pigs was found to be 48 IFU. The infection rate for the animals infected sexually was 63.2%, so 100 IFU would be a reasonable approximation. In any case, our data suggest that, at least for the guinea pig, to model genital disease, an infectious dose of 102 to 103 IFU should be used. Note that Cotter et al. (5) observed an ID50 for mice with the mouse pneumonitis agent of 19 to 48 IFU as well. Moreover, in studies of human male volunteers with Neisseria gonorrhoeae, the infectious dose was found to be in the 102- to 103-IFU range (24).
We considered the possibility that infection may not have been via sexual contact but may have occurred through grooming or close contact in the cage. Fifty percent of the infected animals did become pregnant, which definitely indicates sexual contact. If genital infection was via grooming, we would expect to see ocular infections in the animals as well, since GPIC is by nature an ocular pathogen. We monitored the females for ocular infection, and none of the 29 females in the study developed ocular infections; thus, it is highly unlikely that transmission occurred without sexual contact.
While 102 IFU is suggested as the infectious dose for guinea pigs, there is no way to extrapolate to humans or other animal models. There are no published studies quantifying the number of chlamydiae in semen and few studies determining the number of IFU in the male genital tract. Geisler et al. (7) did analyze the association between various signs and symptoms in the male to the number of IFU in a 100-μl well. They reported that the number of IFU was in the 101-to-102 range. With this number of organisms, even considering a larger volume transmitted to the female, it is unlikely that very high numbers of organisms are in the infectious inoculum. It is interesting that in the male guinea pig, there is inflammation in the urethral epithelium but not overt exudate as one sees in human males with urethritis (21). Moreover, if one considers that frequent urine flow provides a periodic flushing action, then any organisms transmitted must be newly released from infected cells. The majority of human males infected with Chlamydia are asymptomatic, with no apparent exudate (22), so the guinea pig data support the concept that just a few organisms can elicit an infection.
Perhaps the most important and novel finding in our study was the observation that infections acquired sexually, in contrast to infections acquired by artificial intravaginal inoculation with any dose tested, have a significantly shorter course of shedding of detectable organisms. This finding was quite consistent, even when infection courses in immunized animals challenged artificially and sexually were compared. While it is certainly not clear at this point what the reasons are for the abbreviated infection, the most likely possibility is that there are factors associated with semen that inhibit chlamydial growth. One of the antimicrobial factors which may have an effect on early chlamydial infection is the group of polypeptides known as defensins. Defensins are 3- to 6-kDa cationic peptides containing six disulfide-paired cysteines which make up 5 to 15% of the total protein content of neutrophils and have been found to have activity against a number of bacterial and viral pathogens. Defensins have been found to be present in various parts of the male reproductive tract, including the semen of rats, mice, and humans (4). Moreover, two different guinea pig defensins derived from neutrophils have been described previously (25, 27). It is possible that defensins in semen deposited in the female genital tract kill some of the chlamydiae before they can infect their target cells and/or remain in the genital tract and interfere with subsequent generations of chlamydiae. It is also conceivable that cytokines and chemokines produced in the male as a result of an ongoing infection are being passively transferred and act to enhance or hasten the inflammatory response in the female.
Whatever the mechanism, these data indicate that artificial inoculation of the genital tract in animal models may not accurately mimic all of the factors involved in natural infection and that one must take into consideration the possible role of passively transferred factors from the male. Even the transfer of factors from the male vary depending on the stage of the infection course in the male. Clearly, there is great variability in the course of chlamydial genital infection from individual to individual, and this variability may result from factors beyond those associated with the host response in the female.
Finally, a major role for animal models is in the evaluation of vaccine candidates. We have previously demonstrated that the GPIC-guinea pig model resembles human female genital infection very closely with regard to the pathological response (19). Moreover, we have reported that immunity to intravaginal-challenge infection could be elicited by immunization with an inactivated whole-organism vaccine or by purified outer membrane protein 2 (3, 18). While immunization with UV-inactivated GPIC did not prevent infection in 50% of the animals, the resultant infections were much less severe. Whether infection ascending to the upper genital tract was prevented remains to be determined in future experiments. It was apparent from guinea pigs artificially challenged with GPIC that animals with low challenge doses, i.e., 102 and 101 IFU, were more likely to have solid immunity in which no organisms could be isolated. That the infection rate in animals challenged by sexual transmission was somewhat higher suggests that they may have received actual challenge doses of 102 to 103 IFU by sexual transmission. Alternatively, it is entirely possible that the viscous nature of semen blocked the attachment of vaginal or cervical antibodies to the organism, effectively increasing the infection rate compared to that of animals inoculated with organisms suspended in sucrose-phosphate-glutamate buffer. Antibody is essential for immunity to reinfection in the guinea pig model (17). This finding demonstrates the real value of a sexual-transmission model in that there may be complicating factors associated with the natural means of infection which cannot be identified in the artificial inoculation of organisms suspended in a buffer. Of interest was the observation that the resultant infections in immunized animals infected sexually were also significantly abbreviated compared to those of animals challenged artificially with similar doses. This finding again suggests some contribution of antimicrobial or immunity factors from the male.
The ability to reliably and predictably accomplish sexual transmission in the guinea pig model is a unique opportunity to evaluate possible vaccine candidates or strategies for a natural infection, especially since there is no other animal model for chlamydial infection or, for that matter, any other sexually transmitted diseases in which sexual transmission has been demonstrated. In addition, the model provides an excellent opportunity to evaluate the effect of immunizing the male on his ability to infect an immunized or unimmunized female. We have previously reported that an inactivated whole-organism vaccine could induce a marked reduction in the level of a challenge infection in the male (14). This finding suggests that an immunized male may transmit fewer organisms and, consequently, maximize the protective capability of the vaccine in a female. With the guinea pig model, this strategy as well as other strategies can finally be tested.
Acknowledgments
This study was supported by Public Health Service grant AI23044 from the National Institutes of Health.
Editor: F. C. Fang
REFERENCES
- 1.Ahmad, A., C. R. Dawson, C. Yoneda, B. Togni, and J. Schachter. 1977. Resistance to reinfection with a chlamydial agent (guinea pig inclusion conjunctivitis). Investig. Ophthalmol. Vis. Sci. 16:549-553. [PubMed] [Google Scholar]
- 2.Barron, A. L., H. J. White, R. G. Rank, B. L. Soloff, and E. B. Moses. 1981. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J. Infect. Dis. 143:63-66. [DOI] [PubMed] [Google Scholar]
- 3.Batteiger, B. E., R. G. Rank, P. M. Bavoil, and L. S. F. Soderberg. 1993. Partial protection against reinfection by immunization of guinea pigs with isolated outer-membrane proteins of the chlamydial agent of guinea pig inclusion conjunctivitis. J. Gen. Microbiol. 139:2965-2972. [DOI] [PubMed] [Google Scholar]
- 4.Com, E., F. Bourgeon, B. Evrard, T. Ganz, D. Colleu, B. Jegou, and C. Pineau. 2003. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol. Reprod. 68:95-104. [DOI] [PubMed] [Google Scholar]
- 5.Cotter, T. W., G. S. Miranpuri, K. H. Ramsey, C. E. Poulsen, and G. I. Byrne. 1997. Reactivation of chlamydial genital tract infection in mice. Infect. Immun. 65:2067-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freund, M. 1969. Interrelationships among the characteristics of guinea-pig semen collected by electro-ejaculation. J. Reprod. Fertil. 19:393-405. [DOI] [PubMed] [Google Scholar]
- 7.Geisler, W. M., R. J. Suchland, W. L. Whittington, and W. E. Stamm. 2001. Quantitative culture of Chlamydia trachomatis: relationship of inclusion-forming units produced in culture to clinical manifestations and acute inflammation in urogenital disease. J. Infect. Dis. 184:1350-1354. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, A. P., C. M. Hetherington, M. F. Osborn, B. J. Thomas, and D. Taylor-Robinson. 1980. Experimental infection of the marmoset genital tract with Chlamydia trachomatis. Br. J. Exp. Pathol. 61:291-295. [PMC free article] [PubMed] [Google Scholar]
- 9.Mount, D. T., P. E. Bigazzi, and A. L. Barron. 1972. Infection of genital tract and transmission of ocular infection to newborns by the agent of guinea pig inclusion conjunctivitis. Infect. Immun. 5:921-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mount, D. T., P. E. Bigazzi, and A. L. Barron. 1973. Experimental genital infection of male guinea pigs with the agent of guinea pig inclusion conjunctivitis and transmission to females. Infect. Immun. 8:925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasley, J. N., R. G. Rank, and A. L. Barron. 1986. Effects of oral contraceptive agents on chlamydial genital infection in female guinea pigs, p. 392-395. In D. Oriel, G. Ridgway, J. Schachter, D. Taylor-Robinson, and M. Ward (ed.), Chlamydial infections. Cambridge University Press, London, United Kingdom.
- 12.Pasley, J. N., R. G. Rank, A. J. Hough, C. Cohen, and A. L. Barron. 1985. Absence of progesterone effects on chlamydial genital infection in female guinea pigs. Sex. Transm. Dis. 12:155-158. [DOI] [PubMed] [Google Scholar]
- 13.Pasley, J. N., R. G. Rank, A. J. Hough, Jr., C. Cohen, and A. L. Barron. 1985. Effects of various doses of estradiol on chlamydial genital infection in ovariectomized guinea pigs. Sex. Transm. Dis. 12:8-13. [DOI] [PubMed] [Google Scholar]
- 14.Patterson, T. L., and R. G. Rank. 1996. Immunity to reinfection and immunization of male guinea pigs against urethral infection with the agent of guinea pig inclusion conjunctivitis. Sex. Transm. Dis. 23:145-150. [DOI] [PubMed] [Google Scholar]
- 15.Patton, D. L., S. A. Halbert, C. C. Kuo, S. P. Wang, and K. K. Holmes. 1983. Host response to primary Chlamydia trachomatis infection of the fallopian tube in pig-tailed monkeys. Fertil. Steril. 40:829-840. [PubMed] [Google Scholar]
- 16.Patton, D. L., S. A. Halbert, and S.-P. Wang. 1982. Experimental salpingitis in rabbits provoked by Chlamydia trachomatis. Fertil. Steril. 37:691-700. [PubMed] [Google Scholar]
- 17.Rank, R. G., and A. L. Barron. 1983. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect. Immun. 39:463-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rank, R. G., B. E. Batteiger, and L. S. F. Soderberg. 1990. Immunization against chlamydial genital infection in guinea pigs with UV-inactivated and viable chlamydiae administered by different routes. Infect. Immun. 58:2599-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rank, R. G., and M. M. Sanders. 1992. Pathogenesis of endometritis and salpingitis in a guinea pig model of chlamydial genital infection. Am. J. Pathol. 140:927-936. [PMC free article] [PubMed] [Google Scholar]
- 20.Rank, R. G., M. M. Sanders, and A. T. Kidd. 1993. Influence of the estrous cycle on the development of upper genital tract pathology as a result of chlamydial infection in the guinea pig model of pelvic inflammatory disease. Am. J. Pathol. 142:1291-1296. [PMC free article] [PubMed] [Google Scholar]
- 21.Rank, R. G., H. J. White, B. L. Soloff, and A. L. Barron. 1981. Cystitis associated with chlamydial infection of the genital tract in male guinea pigs. Sex. Transm. Dis. 8:203-210. [DOI] [PubMed] [Google Scholar]
- 22.Robinson, A. J., G. L. Ridgway, J. M. Zelin, E. Allason-Jones, and P. Williams. 1994. Chlamydia trachomatis in heterosexual men and women: prevalence, symptoms, and sexual behaviour, p. 25-27. In J. Orfila, G. I. Byrne, M. A. Chernesky, J. T. Grayston, R. B. Jones, G. L. Ridgway, P. Saikku, J. Schachter, W. E. Stamm, and R. S. Stephens (ed.), Chlamydial infections. Societa Editrice Esculapio, Bologna, Italy.
- 23.Schachter, J., and H. D. Caldwell. 1980. Chlamydiae. Annu. Rev. Microbiol. 34:285-309. [DOI] [PubMed] [Google Scholar]
- 24.Schneider, H., A. S. Cross, R. A. Kuschner, D. N. Taylor, J. C. Sadoff, J. W. Boslego, and C. D. Deal. 1995. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J. Infect. Dis. 172:180-185. [DOI] [PubMed] [Google Scholar]
- 25.Selsted, M. E., and S. S. Harwig. 1987. Purification, primary structure, and antimicrobial activities of a guinea pig neutrophil defensin. Infect. Immun. 55:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sisk, D. B. 1976. Physiology, p. 63-98. In J. E. Wagner and P. J. Manning (ed.), The biology of the guinea pig. Academic Press, Inc., New York, N.Y.
- 27.Yamashita, T., and K. Saito. 1989. Purification, primary structure, and biological activity of guinea pig neutrophil cationic peptides. Infect. Immun. 57:2405-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]