Abstract
Infections with gram-positive bacteria are a major cause of morbidity and mortality in humans. Opsonin-dependent phagocytosis plays a major role in protection against and recovery from gram-positive infections. Inborn and acquired defects in opsonin generation and/or recognition by phagocytes are associated with an increased susceptibility to bacterial infections. In contrast, the physiological significance of opsonin-independent phagocytosis is unknown. Type I and II class A scavenger receptors (SR-AI/II) recognize a variety of polyanions including bacterial cell wall products such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA), suggesting a role for SR-AI/II in innate immunity to bacterial infections. Here, we show that SR-AI/II–deficient mice (MSR-A−/−) are more susceptible to intraperitoneal infection with a prototypic gram-positive pathogen, Staphylococcus aureus, than MSR-A+/+ control mice. MSR-A−/− mice display an impaired ability to clear bacteria from the site of infection despite normal killing of S. aureus by neutrophils and die as a result of disseminated infection. Opsonin-independent phagocytosis of gram-positive bacteria by MSR-A−/− macrophages is significantly decreased although their phagocytic machinery is intact. Peritoneal macrophages from control mice phagocytose a variety of gram-positive bacteria in an SR-AI/II–dependent manner. Our findings demonstrate that SR-AI/II mediate opsonin-independent phagocytosis of gram-positive bacteria, and provide the first evidence that opsonin-independent phagocytosis plays a critical role in host defense against bacterial infections in vivo.
Keywords: scavenger receptor, macrophage, phagocytosis, gram-positive bacteria, Staphylococcus aureus
Introduction
Phagocytosis of microorganisms is a key element in host defense against bacterial infections 1. Two principal mechanisms of phagocytosis have been described, opsonin-dependent phagocytosis 2 and opsonin-independent phagocytosis (for a review, see reference 3). In opsonin-dependent phagocytosis, immunoglobulin or complement molecules bind to microorganisms, thereby promoting ingestion via Fcγ or complement receptors on phagocytic leukocytes 2. In contrast, in opsonin-independent phagocytosis, ligands on the surfaces of microorganisms are directly recognized by receptors on the plasma membranes of phagocytes 3.
Several lines of evidence link defects in opsonin-dependent phagocytosis to an increased susceptibility to infection. First, immunoglobulin- or complement-deficient animals 4 5 6 and humans 7 exhibit an increased incidence of bacterial infections. Second, administration of antibodies against bacterial capsular antigens 8 9 10, or immunization against bacterial capsular antigens 11, protects against infections with bacteria expressing these antigens. Third, Fcγ receptor polymorphisms in humans 12 13 are associated with increased susceptibility to infection.
Much less is known about the physiological roles of opsonin-independent phagocytosis in host defense against bacterial infections. Types I and II class A macrophage scavenger receptors (SR-AI/II) are homotrimeric membrane proteins 14 of mononuclear phagocytes that mediate phagocytosis of apoptotic thymocytes 15, endocytosis of modified lipoproteins 16, and adhesion of macrophages to surfaces coated with serum proteins 17, glucose-modified basement membrane proteins 18, and β-amyloid fibrils 19. The demonstration that macrophage scavenger receptors bind bacterial cell wall components such as LPS from gram-negative bacteria 20 or lipoteichoic acid (LTA) from gram-positive bacteria 21, and that SR-AI/II–deficient mice (MSR-A−/−) exhibit increased susceptibility to infection with Listeria monocytogenes 22 and to LPS-mediated shock 23 suggested that these receptors function in the absence of serum opsonins in host defense against bacterial infections.
We hypothesized that SR-AI/II mediate opsonin-independent phagocytosis of bacteria via a direct interaction of SR-AI/II with bacterial cell wall products such as LTA, leading to clearance of bacteria from sites of infection. To test this hypothesis, we chose Staphylococcus aureus, a prototypical gram-positive microorganism and an important cause of life-threatening bacterial infections in humans 24 25 26. Since SR-AI/II are expressed mainly on mononuclear phagocytes, and these cells are the first line of antimicrobial defense in the peritoneal cavity 27 28 29, we compared the susceptibility of MSR-A−/− and MSR-A+/+ mice to intraperitoneal challenge with two strains of S. aureus. Here, we show that intraperitoneal injection of S. aureus Cowan strain I leads to overwhelming infection and death in MSR-A−/− mice at a significantly lower dose than in wild-type mice. The impaired ability of MSR-A−/− mice to clear S. aureus from the peritoneal cavity is associated with a marked decrease in the ability of their peritoneal macrophages to phagocytose nonopsonized S. aureus as well as several other gram-positive bacteria. This is the first direct evidence that opsonin-independent phagocytosis of bacteria is a critical determinant of host survival in bacterial infection.
Materials and Methods
Mice and Bacteria.
SR-AI/II knockout mice (MSR-A−/−) are described in detail elsewhere 22. MSR-A−/− or BALB/c control mice (The Jackson Laboratory) were kept in a germ-free barrier facility with free access to autoclaved water and irradiated Purina-Pico mouse diet (W.F. Fisher & Son, Inc.). S. aureus (Wood strain, 10832), S. aureus (Cowan I strain, 12598), S. aureus (Smith diffuse strain, 13709), Streptococcus agalactiae (12386), Streptococcus pyogenes (10403), L. monocytogenes (43251), and Enterococcus hirae (9790) were from the American Type Culture Collection. S. aureus (strain DB) was a gift from Dr. A. Cheung, Rockefeller University, New York, NY. Heat-inactivated, BODIPY® fluorophore–labeled S. aureus (Wood strain) and zymosan particles were from Molecular Probes.
Infection Assay.
S. aureus (Cowan I) was grown overnight in Brain Heart broth (Difco) in a bacterial shaker at 37°C. On the day of an experiment, bacteria were washed three times in phosphate-buffered saline (PD) without Ca2+/Mg2+ and spectrophotometrically adjusted to OD ≈ 2.0 (108 CFU/ml). The number of viable bacteria was confirmed by plating serial dilutions on Brain Heart agar plates and counting bacterial colonies after overnight incubation at 37°C. For each experiment, four MSR-A−/− or four MSR-A+/+ control mice (4–6 wk of age, 20–25 g) were injected intraperitoneally with 1 ml PD containing 2 × 107–109 CFU of the indicated microorganism and observed for 72 h. Moribund animals were killed with CO2. At various time points after injection, mice were killed and blood and peritoneal fluid were harvested and assayed for the presence of viable bacteria by plating serial dilutions on agar plates. Blood was obtained by cardiac puncture. Three to six experiments of this type were performed, as indicated in the figure legends.
Analysis of Peritoneal Cells of Mice Inoculated Intraperitoneally with S. aureus.
The total number of white blood cells in the peritoneal fluid was determined using a hemocytometer. Leukocytes were typed by evaluating a Wright's stained smear of peritoneal fluid.
Killing of S. aureus by Peritoneal Neutrophils In Vitro.
Neutrophil bactericidal activity was assayed in a modified tumble assay 30. In brief, MSR-A+/+ control mice or MSR-A−/− mice were inoculated intraperitoneally with 1 ml of 2% sodium caseinate (Sigma Chemical Co.) 31, and the resulting neutrophil-rich exudate was harvested 6 h later by lavage as described above. The exudate cells (75% neutrophils) were washed, counted, and suspended at a concentration of 2.6 × 106 exudate cells/ml in PBS (with Ca2+ and Mg2+) containing 0.1% human serum albumin and 5 mM glucose. S. aureus (Cowan I) was grown overnight, washed, suspended at a concentration of 2 × 105 CFU/ml in PBS containing 0.9 mM Ca2+ and 0.5 mM Mg2+, 5 mM glucose, 0.1% human serum albumin, and 20% mouse serum (as a source of complement; Sigma Chemical Co.), and incubated for 10 min at 37°C to opsonize the bacteria. 250 μl aliquots of this bacterial suspension were mixed with 250 μl of the neutrophil suspension, and the mixture was incubated at 37°C for 1 h on a rotary shaker. The mixture was then diluted sixfold in sterile distilled water, incubated for 5 min to lyse the neutrophils, and the number of CFU of S. aureus was determined by plating serial dilutions on agar plates. Data are reported as percent reduction in CFU of S. aureus incubated with neutrophils, and were calculated as 1 − no. of CFU recovered at 1 h/no. of CFU in the inoculum at time 0 (i.e., 1 × 105 CFU). In the absence of neutrophils, the number of CFU of S. aureus increased by 1.5-fold over 1 h. No S. aureus killing occurred when neutrophils and bacteria were incubated in medium containing mouse serum lacking complement activity.
Fluorescent Labeling of Bacteria.
Bacteria were labeled with BODIPY® FL (Molecular Probes) according to the manufacturer's specifications. Bacteria were grown overnight in 5–10 ml Brain Heart broth, washed twice, and resuspended in 0.2 ml buffer (0.1 M NaHCO3, 125 mM NaCl). 100 μg BODIPY® FL was added slowly, and bacteria were incubated under constant stirring in the dark at room temperature for 1 h. The reaction was stopped by dropwise addition of 1.5 M hydroxylamine (Sigma Chemical Co.). Bacteria were washed and resuspended in PD at OD = 2.0 (≈108 CFU/ml). Bacterial viability was checked by plating serial dilutions and was typically >80%.
Phagocytosis of Bacteria by Macrophages In Vitro.
Thioglycollate broth–elicited peritoneal macrophages (TMφ) were harvested by irrigating the peritoneal cavity of mice with cold PD 4 d after intraperitoneal injection of 2 ml aged thioglycollate broth (Sigma Chemical Co.). TMφ were plated in 96-well plates at 105 cells/well in 100 μl RPMI 1640/10% fetal calf serum and incubated overnight. TMφ were washed once with PD, and labeled bacteria were added at ≈100 CFU/macrophage for 30 min at 37°C. Phagocytosis was stopped by addition of cold PD. Fluorescence of extracellular bacteria was quenched by incubation with PD containing 1.0 mg/ml trypan blue for 20 min at room temperature as described 32 33. Intracellular fluorescence of phagocytosed bacteria was measured at λ = 485 nm (excitation) and λ = 530 (emission), using a fluorescence plate reader (Cytofluor II; PerSeptive Biosystems). To determine background fluorescence from noningested extracellular bacteria, TMφ were incubated with 1.0 μM cytochalasin D (Sigma Chemical Co.) to inhibit phagocytosis, and fluorescence was measured after trypan blue quenching 32 33 34. Phagocytosis is expressed as the ratio of fluorescence of phagocytosed bacteria to fluorescence of adherent but uningested bacteria: a value of 1 is equal to background fluorescence; values >1.0 indicate the presence of intracellular (phagocytosed) bacteria. Results were confirmed by fluorescence microscopy. In some experiments, TMφ were preincubated for 30 min (at room temperature) with the antibodies 2F8 (Serotec) or EM-34.1 (Sigma Chemical Co.), or 500 μg/ml of one of the following SR-AI/II ligands: polyinosinic acid, polyguanylic acid, fucoidan, or LTA, or the control reagent polycytidylic acid in the presence of a ribonuclease inhibitor (all from Sigma Chemical Co.).
Results
Decreased Clearance of S. aureus by MSR-A−/− Mice.
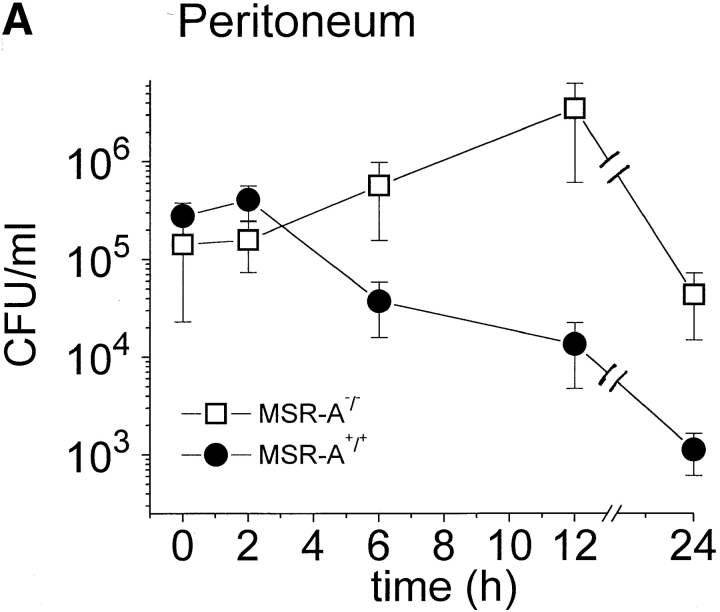
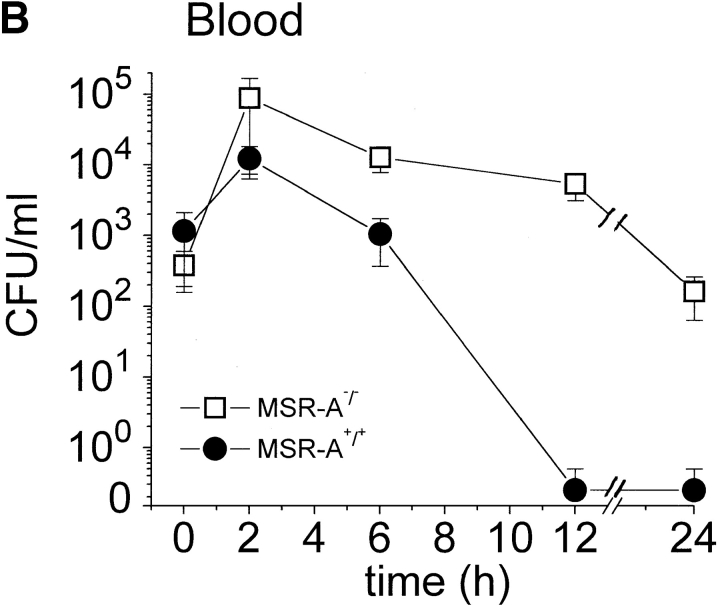
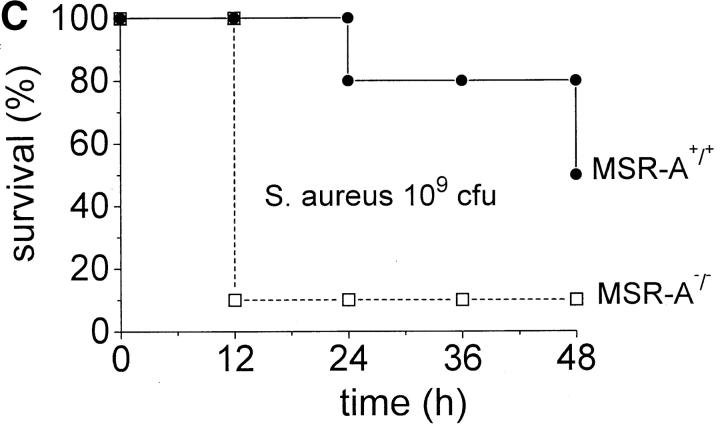
MSR-A+/+ and MSR-A−/− mice were injected intraperitoneally with 107 CFU S. aureus Cowan I, and viable bacteria were quantified in blood and peritoneal lavage samples at the indicated time points. The number of viable bacteria recovered from the peritoneum of MSR-A+/+ mice decreased to ∼0.01% of the inoculum and to <0.3% of the number of bacteria recovered from the peritoneum immediately after infection (i.e., from 4.0 × 105 to 1.1 × 103 CFU/mouse) within 24 h ( Fig. 1 A). No bacteria were detected in the blood of MSR-A+/+ mice by 12 h ( Fig. 1 B). In contrast, the number of viable bacteria in the peritoneum of MSR-A−/− mice was 20% of the inoculum at 24 h ( Fig. 1 A) and increased 200-fold in the blood at 12 h compared with 5 min after inoculation ( Fig. 1 B).
Figure 1.
Clearance of S. aureus by MSR-A−/− and MSR-A+/+ mice. 107 CFU S. aureus (Cowan I) were injected into the peritoneal cavity of four MSR-A−/− and four MSR-A+/+ mice. (A) At the indicated times, the peritoneum was lavaged and its content of viable bacteria was determined by plating serial dilutions on agar plates. (B) Viable bacteria in the blood were determined after collecting blood via cardiac puncture of killed animals and subsequent plating on agar plates at the indicated times. Data represent the mean of three experiments.
Decreased Survival of MSR-A−/− Mice after Infection with S. aureus.
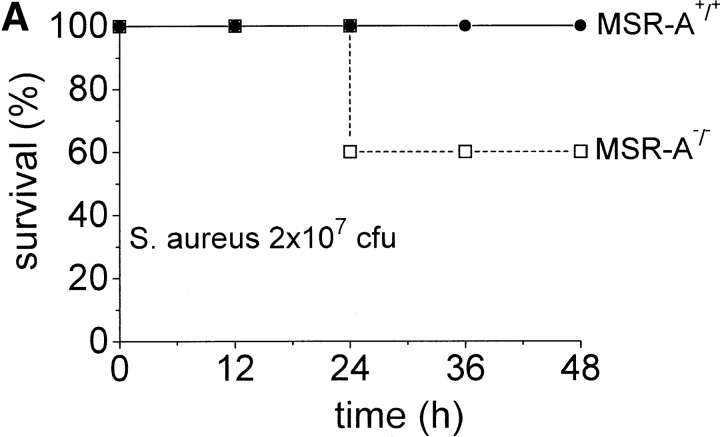
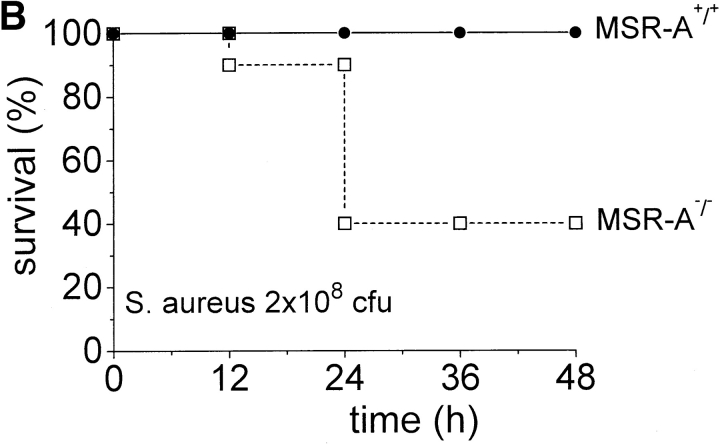
Impaired ability to eliminate S. aureus may lead to increased mortality from disseminated infection. To test this, we injected MSR-A−/− and MSR-A+/+ mice with increasing numbers of S. aureus. All control mice survived intraperitoneal infection with 2 × 107 and 2 × 108 CFU of S. aureus, whereas 40 and 60% of MSR-A−/− mice became moribund within 24 h ( Fig. 2a and Fig. b) after inoculation with 2 × 107 and 2 × 108 CFU of S. aureus, respectively. 20% of control mice became moribund within 24 h of infection with 109 CFU/mouse ( Fig. 2 C). In contrast, 90% of MSR-A−/− mice became moribund within 12 h of infection with 109 CFU of S. aureus ( Fig. 2 C). No further deaths were observed in either group after 48 h. Thus, targeted disruption of the SR-AI/II gene significantly increased the susceptibility of mice to infection with S. aureus.
Figure 2.
Mortality of MSR-A−/− and MSR-A+/+ mice after S. aureus challenge. Four MSR-A−/− and four MSR-A+/+ mice were injected intraperitoneally with 1 ml of buffer containing S. aureus (Cowan I): (A) 2 × 107 CFU, (B) 2 × 108 CFU, (C) 109 CFU. Mice were observed for signs of systemic infection, and moribund animals were killed. Data represent the mean of six experiments.
Normal Recruitment of Inflammatory Cells by MSR-A−/− Mice after Infection with S. aureus.
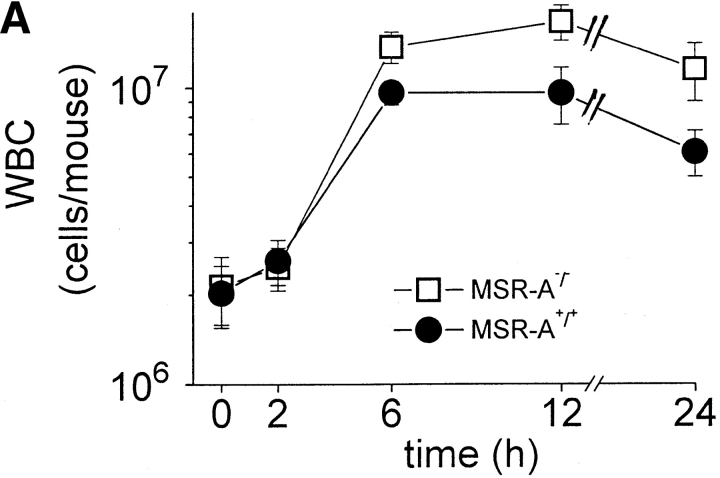
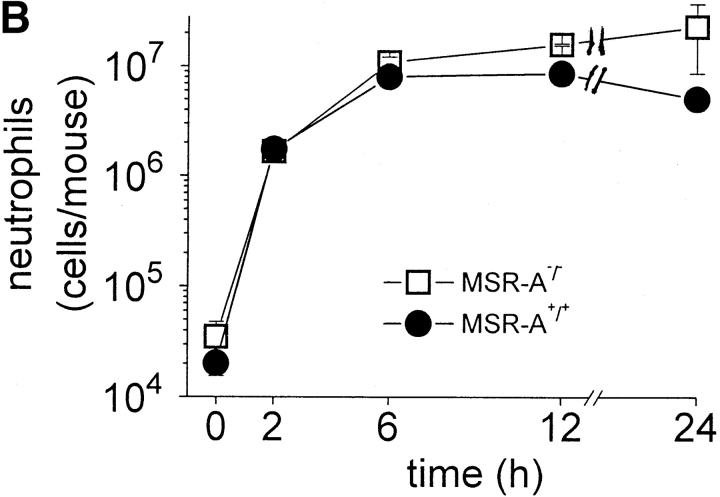
Atherosclerotic lesions in MSR-A−/− mice contain significantly fewer macrophages compared with lesions in MSR-A+/+ mice 22. To examine whether the increased susceptibility of MSR-A−/− mice to S. aureus reflected a similar defect in recruitment of macrophages or other leukocytes to the site of infection, peritoneal cells from MSR-A−/− and MSR-A+/+ mice were harvested at various times after intraperitoneal injection of S. aureus. Before infection, the peritoneal cavities of MSR-A−/− and MSR-A+/+ mice contained similar numbers of resident peritoneal cells, >99% of which were mononuclear leukocytes, and the majority of which were mononuclear phagocytes (not shown). As described previously 27, intraperitoneal injection of S. aureus induced a marked influx of leukocytes, >99% of which were neutrophils ( Fig. 3). There were no significant differences between MSR-A−/− and MSR-A+/+ mice in either the number or types of leukocytes that were recovered from the peritoneal cavity after intraperitoneal injection of S. aureus ( Fig. 3). Thus, the increased susceptibility of MSR-A−/− mice to S. aureus infection cannot be explained by lack of recruitment of neutrophils or mononuclear phagocytes to the peritoneal cavity.
Figure 3.
Recruitment of peritoneal leukocytes. Four MSR-A−/− mice and four MSR-A+/+ mice were injected intraperitoneally with 107 CFU of S. aureus (Cowan I). At the indicated times, mice were killed and their peritoneal cavities were irrigated with cold buffer. (A) The total number of leukocytes (WBC) in peritoneal lavage fluids of each mouse was determined microscopically. (B) The total number of neutrophils in the peritoneal lavage fluid was calculated after the percentage of neutrophils was determined microscopically by Wright's stain. Data represent the mean of three experiments.
SR-AI/II Mediate Opsonin-independent Phagocytosis of Gram-positive Bacteria.
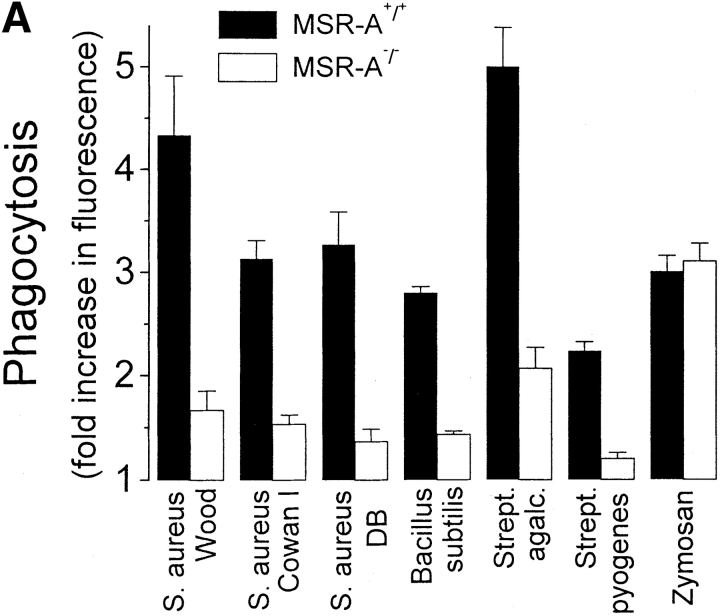
SR-AI/II bind LPS from gram-negative bacteria 20, LTA and gram-positive bacteria 21, and possibly Mycobacterium tuberculosis 35 to macrophages. We hypothesized that SR-AI/II play an important role in host defense against intraperitoneal infection with S. aureus by mediating opsonin-independent phagocytosis of this bacterium. To test this, we compared phagocytosis of four strains of S. aureus, as well as of three other gram-positive microorganisms, by TMφ from MSR-A−/− and MSR-A+/+ mice. All gram-positive bacteria, with the exception of an encapsulated S. aureus strain (Smith diffuse), were efficiently ingested by TMφ from MSR-A+/+ mice in the absence of added opsonins ( Fig. 4 A). TMφ from MSR-A−/− mice phagocytosed 72–83% fewer bacteria than TMφ from MSR-A+/+ mice ( Fig. 4 A). In contrast, zymosan, a particle whose uptake is mediated by mannose and β-glucan receptors 36, was phagocytosed with equal efficiency by TMφ from MSR-A−/− and MSR-A+/+ mice, indicating that macrophages from MSR-A−/− mice possess intact phagocytic machinery.
Figure 4.
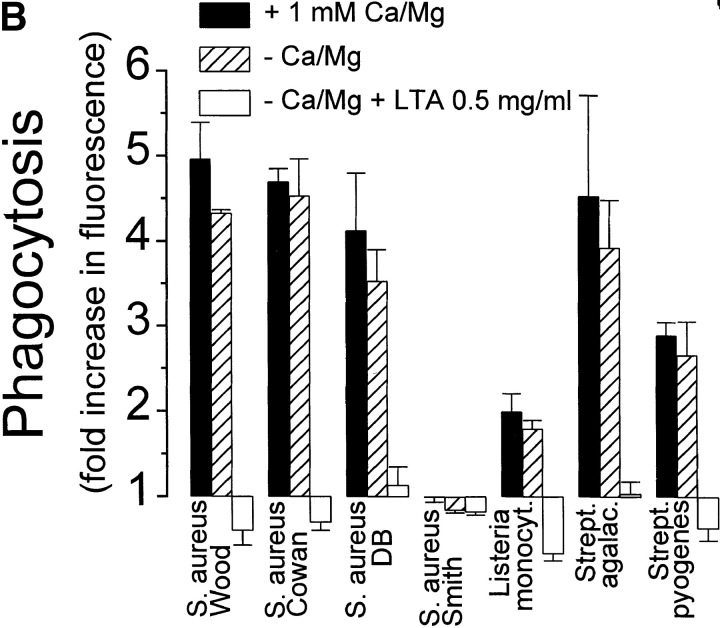
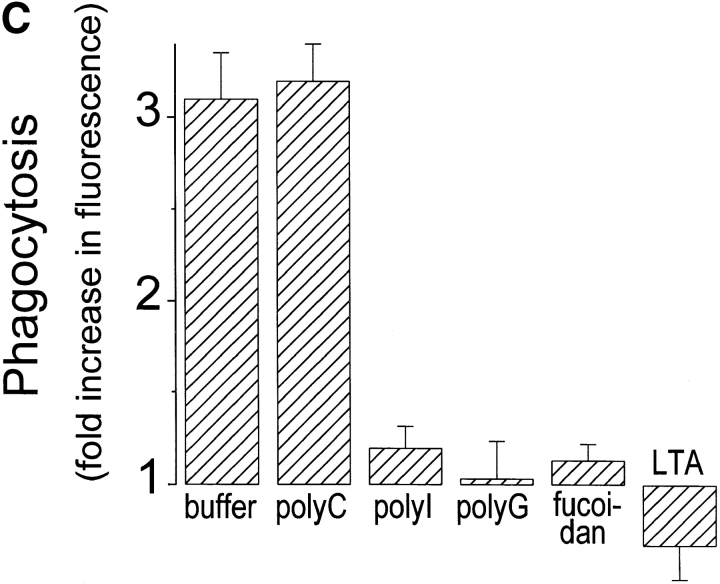
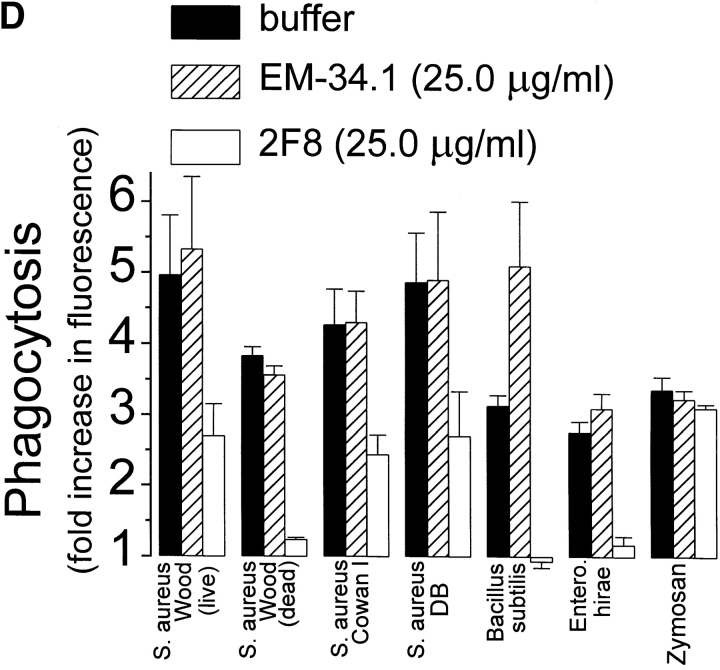
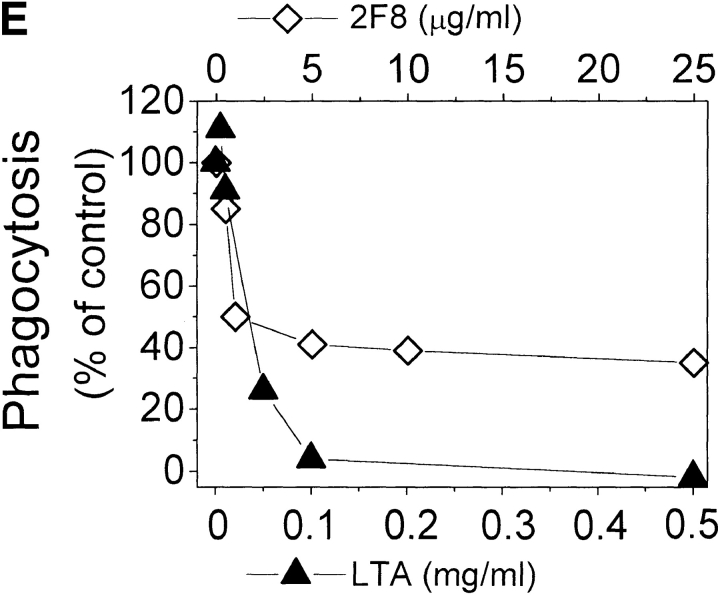
Phagocytosis of gram-positive bacteria by TMφ from MSR-A−/− and MSR-A+/+ mice. Macrophages were prepared, and phagocytosis was measured as described in Materials and Methods. (A) Phagocytosis of various fluorescently labeled gram-positive bacteria and of zymosan particles by TMφ from MSR-A−/− or MSR-A+/+ mice. (B) Phagocytosis of various fluorophore-labeled live gram-positive bacteria in serum-free buffer, with or without Ca2+, Mg2+, or LTA, by TMφ from MSR-A+/+ mice. (C) Phagocytosis of S. aureus (Cowan I) by TMφ from MSR-A+/+ mice in the absence or presence of various SR-AI/II ligands: polyguanylic acid (polyG, 0.5 mg/ml), polyinosinic acid (polyI, 0.5 mg/ml), fucoidan (0.5 mg/ml), LTA (1.0 mg/ml), or polycytidylic acid (polyC, 0.5 mg/ml) in the presence of an RNase inhibitor (10 U/ml). (D) Phagocytosis of various gram-positive bacteria and of zymosan particles by TMφ from MSR-A+/+ mice in the presence of the anti–SR-AI/II antibody 2F8, or an isotype-matched control antibody (EM-34.1). (E) Phagocytosis of S. aureus (Cowan I) by TMφ from MSR-A+/+ mice in the presence of various concentrations of 2F8 or LTA. All data represent the mean of six to eight experiments.
Macrophages have been shown to secrete sufficient complement to opsonize zymosan for phagocytosis 37. To test the possibility that complement receptors or other membrane integrins played a role in phagocytosis of gram-positive bacteria, we compared phagocytosis of gram-positive bacteria by incubating TMφ from MSR-A−/− and MSR-A+/+ mice in medium with and without Ca2+ and Mg2+. Ca2+ and Mg2+ are required for complement activation and for most integrin-mediated functions, but not for interactions of SR-AI/II with their ligands 14 17 18 19. TMφ from MSR-A−/− and MSR-A+/+ mice ingested 62–95% as many organisms in Ca2+/Mg2+-free medium ( Fig. 4 B) as in Ca2+/Mg2+-containing medium, demonstrating that the phagocytosis of these bacteria was both complement independent and integrin independent.
LTA, a major cell wall component of gram-positive bacteria 38 and a known ligand of SR-AI/II 21, inhibited opsonin-independent phagocytosis of S. aureus and other gram-positive bacteria ( Fig. 4c and Fig. e), suggesting that soluble LTA was competing with LTA on the surfaces of these bacteria for receptors on the macrophage plasma membrane. In addition to LTA, phagocytosis of S. aureus was inhibited by other SR-AI/II ligands (polyinosinic acid, polyguanylic acid, fucoidan), whereas a control reagent (polycytidylic acid) that does not block SR-AI/II receptors had no effect ( Fig. 4 C). Furthermore, 2F8, an mAb that specifically recognizes murine SR-AI/II 17 and that has been shown to block SR-AI/II–mediated phagocytosis of apoptotic thymocytes 15, inhibited phagocytosis of three different strains of S. aureus and of E. hirae and B. subtilis by 56–95% ( Fig. 4 D). An isotype-matched control antibody (EM-34.1) had no effect ( Fig. 4 D). In contrast, phagocytosis of unopsonized zymosan was unaffected by 2F8 ( Fig. 4 C). Both 2F8 and LTA inhibited S. aureus phagocytosis by TMφ from wild-type mice in a dose-dependent manner ( Fig. 4 E). Interestingly, LTA inhibited S. aureus phagocytosis to a greater extent than 2F8 ( Fig. 4 E). This was similar to a previous report that 2F8 incompletely blocks phagocytosis of apoptotic thymocytes 15. It may reflect the inability of 2F8 to fully mask the binding site(s) for S. aureus in the collagenous domain of SR-AI/II, and/or the participation of other scavenger receptors, such as MARCO 39 or CD36 40, in phagocytosis of gram-positive bacteria. Regardless of the reasons for the incomplete inhibition of binding and ingestion of gram-positive bacteria by 2F8, it is likely that LTA on the surface of these bacteria interacts directly with SR-AI/II 21, and that this interaction is an essential first step in initiating opsonin-independent phagocytosis of unencapsulated gram-positive bacteria.
Killing of S. aureus (Cowan I) by Neutrophils from MSR-A−/− and MSR-A+/+ Mice.
Approximately equal numbers of neutrophils were elicited after intraperitoneal injection of S. aureus (Cowan I) into the peritoneal cavities of MSR-A−/− and MSR-A+/+ mice ( Fig. 3 B). However, ∼12-fold more viable bacteria (4 × 105 vs. 3 × 104) were recovered from the peritoneal cavity of MSR-A−/− than MSR-A+/+ mice ( Fig. 1 A). Although neutrophils do not express SR-AI/II 41, it was possible that genetic disruption of the class A scavenger receptor impaired in some unknown manner the ability of neutrophils from MSR-A−/− mice to phagocytose and kill bacteria. To exclude this possibility, we compared killing of S. aureus (Cowan I) by peritoneal exudate neutrophils from MSR-A−/− and MSR-A+/+ mice. S. aureus (Cowan I) bacteria were killed equally by neutrophils from MSR-A−/− and MSR-A+/+ mice (Table ). These results are in agreement with previous findings that macrophages are the first line of defense against S. aureus infection in the mouse peritoneal cavity 27 28.
Table 1.
Killing of S. aureus (Cowan I) by Peritoneal Exudate Neutrophils from MSR-A−/− and MSR-A+/+ Mice
| PMN/S. aureus ratio | MSR-A+/+ | MSR-A−/− |
|---|---|---|
| % | % | |
| 6:1 | 55 ± 7 | 51 ± 6 |
Killing of S. aureus (Cowan I) by neutrophils was determined in a tumble assay as described in Materials and Methods. Data represent mean percent killing ± SEM for three experiments.
Ineffective Phagocytosis of an Encapsulated S. aureus Strain (Smith Diffuse).
The data presented thus far indicate that S. aureus strain Cowan I is phagocytosed by macrophages by an opsonin-independent mechanism that involves interactions of ligands on the surfaces of these bacteria, presumably LTA, with SR-AI/II. They suggest that SR-AI/II–mediated opsonin-independent phagocytosis of S. aureus strain Cowan I by macrophages in the peritoneum of MSR-A+/+ mice permits these mice to resist intraperitoneal infection with a dose of this bacterium that is lethal for MSR-A−/− mice. If this interpretation is correct, then MSR-A−/− and MSR-A+/+ mice should be equally susceptible to infection with an encapsulated S. aureus strain, such as Smith diffuse, which is not phagocytosed in the absence of opsonins by TMφ from either MSR-A−/− or MSR-A+/+ mice ( Fig. 4 A). Indeed, MSR-A−/− and MSR-A+/+ mice were equally susceptible to intraperitoneal challenge with S. aureus Smith diffuse (Table ), confirming that the increased susceptibility of MSR-A−/− mice to infection with S. aureus Cowan I is related to the inability of macrophages lacking SR-AI/II to phagocytose S. aureus Cowan I in an opsonin-independent manner, and not to other immune defects.
Table 2.
Mortality of MSR-A+/+ and MSR-A−/− Mice after Injection with Encapsulated S. aureus
| S. aureus (Smith diffuse) | MSR-A+/+ | MSR-A−/− |
|---|---|---|
| CFU/mouse | ||
| 5 × 107 | 6/8 (75%) | 7/8 (87.5%) |
| 5 × 108 | 8/8 (100%) | 7/8 (87.5%) |
MSR-A+/+ and MSR-A−/− mice were infected intraperitoneally by injection of S. aureus Smith diffuse (5 × 107 and 5 × 108 CFU). Mice were observed for signs of systemic infections, and sick animals were killed. Data are presented as number of dead mice per total number of mice.
Discussion
The inability of peritoneal macrophages from MSR−/− mice to phagocytose S. aureus (Cowan I), the impaired ability of MSR-A−/− mice to clear S. aureus from their peritoneal cavities, and the increased susceptibility of MSR−/− versus MSR+/+ mice to lethal intraperitoneal challenge with S. aureus provide the first direct evidence that macrophage SR-AI/II play an essential role in host defense against S. aureus and other gram-positive microorganisms by promoting opsonin-independent phagocytosis of these bacteria.
The majority of S. aureus infections in humans are caused by microencapsulated or unencapsulated strains similar to the Cowan I strain used in the present studies 42. Like other gram-positive bacteria, they express LTA on the surfaces of their cell walls. LTA interacts directly with SR-AI/II on mononuclear phagocytes 21, leading to the opsonin-independent phagocytosis of these bacteria ( Fig. 4a Fig. b Fig. c Fig. d Fig. e). This may explain why shortly after intraperitoneal infection a much larger percentage of S. aureus Cowan I was cleared by resident peritoneal macrophages of MSR+/+ than of MSR−/− mice ( Fig. 1).
In humans, introduction of dialysis fluid into the peritoneal cavity reduces the concentration of antibodies and complement to a level insufficient to opsonize bacteria for phagocytosis 43. In mice, intraperitoneal inoculation of 1 ml of buffered saline containing S. aureus dilutes enormously the very small volume of fluid that coats the peritoneal cavity and is likely to have a similar inhibitory effect on the efficiency of opsonization. However, the inflammatory response initiated by peritoneal inoculation of these bacteria promotes the influx of neutrophils, monocytes ( Fig. 3), and plasma proteins (e.g., complement, antibodies). We envision that this led to opsonization of S. aureus Cowan I and clearance of these bacteria from the peritoneum of MSR−/− mice challenged with sublethal doses of S. aureus Cowan I ( Fig. 1). Similarly, it is likely that bacteria that entered the bloodstream of sublethally challenged MSR−/− mice ( Fig. 1) were opsonized by antibodies and complement and were cleared by Fcγ- and complement receptor–dependent phagocytosis. This is consistent with our observation that even in the absence of opsonins, phagocytosis of gram-positive bacteria by MSR+/+ macrophages is somewhat more efficient in the presence of divalent cations than in their absence ( Fig. 4 B). Divalent cations are required both for the activation of complement, and for binding of complement-coated bacteria by integrins such as CD11b/CD18 (complement receptor 3). CD11b/CD18 plays an important role in phagocytosis of other bacterial pathogens (for a review, see reference 3). We suggest that these opsonin-dependent mechanisms account for the ability of MSR−/− mice challenged intraperitoneally with sublethal numbers of S. aureus Cowan I to clear these bacteria from the peritoneum and blood.
As indicated above, LTA on the surface of unencapsulated S. aureus (Cowan I strain) is probably responsible for the phagocytosis of these bacteria by MSR+/+ macrophages ( Fig. 4). In contrast, capsular polysaccharides mask the LTA of Smith strain S. aureus 44 45. These capsular polysaccharides are not ligands for SR-AI/II on mononuclear phagocytes. Presumably this is the reason why the encapsulated Smith strain of S. aureus was not phagocytosed in the absence of opsonins by MSR+/+ macrophages ( Fig. 4 A).
Virtually all adult humans express antibodies to S. aureus cell wall constituents. However, antibodies against S. aureus cell wall constituents do not promote phagocytosis of encapsulated S. aureus strains by neutrophils and monocytes. This is because the capsular polysaccharides of S. aureus mask antibodies and complement deposited on the S. aureus cell wall 45 46 47, thereby preventing the interaction of these opsonins with Fc and complement receptors on neutrophils and mononuclear phagocytes. This is probably the reason why the LD50 of the encapsulated Smith strain of S. aureus is substantially lower than that of the unencapsulated Cowan I strain, and why MSR−/− and MSR+/+ mice were equally susceptible to lethal infection with the encapsulated Smith strain. The report by Karakawa et al. 48 that specific anticapsular antibodies are required to promote phagocytosis of encapsulated S. aureus, and Cohn's 29 observation that antibodies directed against the capsular polysaccharides of Smith strain S. aureus protect against intraperitoneal challenge with this bacterium are consistent with this interpretation.
Staphylococcal species from the normal skin flora are the most frequent causative agents of bacterial peritonitis 49, a significant cause of morbidity and mortality in patients undergoing peritoneal dialysis 50. Carrozi and colleagues 51 52 have shown that intraperitoneal administration of IgG reduces the incidence of bacterial peritonitis in some but not all peritoneal dialysis patients. Peritoneal macrophages of dialysis patients unresponsive to intraperitoneal IgG administration exhibited deficient Fc receptor activity, and this deficit was partially reversed by IFN-γ administration 53. The findings presented here suggest that cytokines such as M-CSF 54 or pharmacologic agents that increase SR-AI/II expression on mononuclear phagocytes may help reduce the incidence of bacterial peritonitis, especially with unencapsulated gram-positive bacteria, in peritoneal dialysis patients.
MSR−/− mice are more susceptible to lethal infection with S. aureus (Cowan I) ( Fig. 2), L. monocytogenes, HSV-1 22, and to the lethal effects of LPS 23 than MSR+/+ mice. Our observations suggest that SR-AI/II exert their protective effect against S. aureus infection by promoting the clearance of these bacteria by macrophages ( Fig. 1 and Fig. 4). SR-AI/II may also protect against the lethal effects of LPS by promoting endocytosis of LPS, which would minimize LPS–CD14 interaction and thereby diminish synthesis and secretion of TNF-α. However, L. monocytogenes and HSV-1 are intracellular pathogens, and neither organism requires SR-AI/II to enter or grow within host cells. Thus, increased uptake of L. monocytogenes or HSV-1 does not explain the beneficial effect of macrophage SR-AI/II in host defense against infections with these intracellular pathogens.
SR-AI/II mediate phagocytosis of apoptotic thymocytes 15. Phagocytosis of apoptotic Mycobacterium avium–infected macrophages by fresh uninfected macrophages leads to the killing of M. avium contained within the apoptotic macrophages 55. It is possible that SR-AI/II play a similar role in clearing apoptotic Listeria- or HSV-1–infected cells. Two findings are consistent with this suggestion. First, both Listeria 56 and HSV-1 57 induce apoptosis of the cells they infect. Second, mice whose monocytes have been inhibited by antibodies that block CR3 (CD11b/CD18 58) or whose macrophages have been depleted die when infected with doses of HSV-1 59 or L. monocytogenes 60 that are tolerated by normal mice. We suggest that SR-AI/II participate in controlling infections with these intracellular pathogens by promoting the phagocytosis and subsequent degradation of infected apoptotic cells and the pathogens they contain.
Acknowledgments
This study was supported by a grant from the Arthur N. Saydman Trust Fund for Research in Septicemia in honor of Dr. Harold C. Neu (to C.A. Thomas), a Research Fellowship from the Lucille P. Markey Charitable Trust (to C.A. Thomas), and grant AI20516 from the National Institutes of Health (to S.C. Silverstein).
Footnotes
Abbreviations used in this paper: LTA, lipoteichoic acid; MSR-A−/− mice, macrophage scavenger receptor–deficient mice; MSR-A+/+ mice, wild-type mice; SR-AI/II, type I and/or II class A macrophage scavenger receptors; TMφ, thioglycollate broth–elicited peritoneal macrophage(s).
J. El Khoury's present address is Infectious Disease Unit, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St., Boston, MA 02129.
References
- Silverstein S.C., Steinberg T.H. Host defense against bacterial and fungal infections. In: Davis B.D., Dulbecco R., Elsen H.N., Ginsberg H.S., editors. Microbiology. J.B. Lippincott Company; Philadelphia : 1989. pp. 485–505. [Google Scholar]
- Wright S.D., Silverstein S.C. Overviewthe function of receptors in phagocytosis Weir D.M. Cellular Immunology 1986. 41 Blackwell Scientific Publications; Oxford/London/Edinburgh/Boston/Palo Alto/Melbourne: 1–41.14. [Google Scholar]
- Ofek I., Keisari J., Sharon N. Nonopsonic phagocytosis of microorganisms. Annu. Rev. Microbiol. 1995;49:239–276 . doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- Wessels M.R., Butko P., Ma M., Warren H.B., Lage A.L., Carroll M.C. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. 1995;USA. 92:11490–11494 . doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodeus A.P., Zhou X., Maurer M., Galli S.J., Carroll M.C. Impaired mast cell-dependent immunity in complement C3-deficient mice. Nature. 1997;390:172–175 . doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- Fischer M.B., Prodeus A.P., Nicholson-Weller A., Ma M., Murrow J., Reid R.R., Warren H.B., Lage A.L., Moore F.D., Jr., Carroll M.C. Increased susceptibility to endotoxin shock in complement C3- and C4-deficient mice is corrected by C1 inhibitor replacement. J. Immunol. 1997;159:976–982 . [PubMed] [Google Scholar]
- Buckley R.H. Immunodeficiency diseases. JAMA (J. Am. Med. Assoc.). 1992;268:2797–2806 . [PubMed] [Google Scholar]
- von Behring E., Kitasato S. Ueber das ZustandeKommen der Diphterie Immunität und der Tetanus-Immunität bei Thieren. Dtsch. Med. Wochenschr. 1890;16:113–114 . [PubMed] [Google Scholar]
- Bull C.G. A method of serum treatment of pneumococcic septicemia in rabbits. J. Exp. Med. 1915;22:466–475 . doi: 10.1084/jem.22.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D.E., Claflin J.L., Schroer K., Forman C. Mouse IgG3 antibodies are highly protective against infection with Streptococcus pneumoniae . Nature. 1981;294:88–90 . doi: 10.1038/294088a0. [DOI] [PubMed] [Google Scholar]
- Shapiro E.D., Clemens J.D. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Ann. Intern. Med. 1984;101:325–330 . doi: 10.7326/0003-4819-101-3-325. [DOI] [PubMed] [Google Scholar]
- Sanders L.A.M., van de Winkel J.G.J., Rijkers G.T., Voorhorst-Ogink M.M., de Haas M., Capel P.J.A., Zegers B.J.M. Fcγ receptor IIa (CD32) heterogeneity in patients with recurrent bacterial respiratory tract infections. J. Infect. Dis. 1994;170:854–861 . doi: 10.1093/infdis/170.4.854. [DOI] [PubMed] [Google Scholar]
- Platonov A.E., Kuijper E.J., Vershinina I.V., Shipulin G.A., Westerdaal N., Fijen C.A., van de Winkel J.G. Meningococcal disease and polymorphism of FcγRIIa (CD32) in late complement component-deficient individuals. Clin. Exp. Immunol. 1998;111:97–101 . doi: 10.1046/j.1365-2249.1998.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M., Herz J. Structures and functions of multiligand lipoprotein receptorsmacrophage scavenger receptors and LDL receptor-related protein (LRP) Annu. Rev. Biochem. 1994;63:601–637 . doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Platt N., Suzuki H., Kurihara Y., Kodama T., Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. 1996;USA. 93:12456–12460 . doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.L., Ho Y.K., Basu S.K., Brown M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. 1979;USA. 76:333–337 . doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser I., Hughes D., Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364:343–346 . doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- El Khoury J., Thomas C.A., Loike J.D., Cao L., Silverstein S.C. Macrophages adhere to glucose-modified collagen type IV via their scavenger receptors. J. Biol. Chem. 1994;269:10197–10200 . [PubMed] [Google Scholar]
- El Khoury J., Hickman S.E., Thomas C.A., Cao L., Silverstein S.C., Loike J.D. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils and secretion of reactive oxygen species. Nature. 1996;382:716–719 . doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Hampton R.Y., Golenbock D.T., Penman M., Krieger M., Raetz C.R.H. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344 . doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Dunne D.W., Resnick D., Greenberg J., Krieger M., Joiner K.A. The type I macrophage scavenger receptor binds gram-positive bacteria and recognizes lipoteichoic acid. Proc. Natl. Acad. Sci. 1994;USA. 91:1863–1867 . doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Kurihara Y., Takeya M., Kamada N., Kataoka M., Jishage K., Ueda O., Sakaguchi H., Higasi T., Suzuki T. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296 . doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- Haworth R., Platt N., Keshav S., Hughes D., Darley E., Suzuki H., Kurihara Y., Kodama T., Gordon S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J. Exp. Med. 1997;186:1431–1439 . doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanson D.C. Staphylococcal infections in hospital. Brit. J. Hosp. Med. 1986;35:312–320 . [PubMed] [Google Scholar]
- Brun-Buisson C., Doyon F., Carlet J. Bacteremia and severe sepsis in adultsa multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia-Sepsis Study Group. Am. J. Resp. Crit. Care Med. 1996;154:617–624 . doi: 10.1164/ajrccm.154.3.8810595. [DOI] [PubMed] [Google Scholar]
- Sands K.E., Bates D.W., Lanken P.N., Graman P.S., Hibberd P.L., Kahn K.L., Panzer R., Orav E.J., Snydman D.R., Black E. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. JAMA (J. Am. Med. Assoc.). 1997;278:234–240 . [PubMed] [Google Scholar]
- Cohn Z.A. Determinants of infection in the peritoneal cavity. I. Response to and fate of Staphylococcus aureus and Staphylococcus albus in the mouse. Yale J. Biol. Med. 1962;35:12–28 . [PMC free article] [PubMed] [Google Scholar]
- Cohn Z.A. Determinants of infection in the peritoneal cavity. II. Factors influencing the fate of Staphylococcus aureus in the mouse. Yale J. Biol. Med. 1962;35:29–47 . [PMC free article] [PubMed] [Google Scholar]
- Cohn Z.A. Determinants of infection in the peritoneal cavity. III. The action of selected inhibitors on the fate of Staphylococcus aureus in the mouse. Yale J. Biol. Med. 1962;35:48–61 . [PMC free article] [PubMed] [Google Scholar]
- Horwitz M.A., Silverstein S.C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J. Clin. Invest. 1980;65:82–94 . doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., McEwen J.G., Stevens D.A. Fungicidal activity of murine inflammatory polymorphonuclear neutrophilscomparison with murine peripheral blood PMN. Clin. Exp. Immunol. 1986;66:681–690 . [PMC free article] [PubMed] [Google Scholar]
- Wan C.P., Park C.S., Lau B.H.S. A rapid and simple microfluorometric phagocytosis assay. J. Immunol. Methods. 1993;162:1–7 . doi: 10.1016/0022-1759(93)90400-2. [DOI] [PubMed] [Google Scholar]
- Loike J.D., Silverstein S.C. A fluorescence quenching technique using trypan blue to differentiate between ingested glutaraldehyde-fixed red blood cells in phagocytosing murine macrophages. J. Immunol. Methods. 1983;57:373–379 . doi: 10.1016/0022-1759(83)90097-2. [DOI] [PubMed] [Google Scholar]
- Sahlin S., Hed J., Rundquist I. Differentiation between attached and ingested immune complexes by a fluorescence quenching cytofluorimetric assay. J. Immunol. Methods. 1983;60:115–124 . doi: 10.1016/0022-1759(83)90340-x. [DOI] [PubMed] [Google Scholar]
- Zimmerli S., Edwards S., Ernst J.D. Selective receptor blockade during phagocytosis does not alter survival and growth of Mycobacterium tuberculosis in human macrophages. Am. J. Respir. Cell Mol. Biol. 1996;15:760–770 . doi: 10.1165/ajrcmb.15.6.8969271. [DOI] [PubMed] [Google Scholar]
- Giaimis J., Lombard Y., Finteneau P., Muller C.D., Levy R., Makaya-Kumba M., Lazdins J., Poindron P. Both mannose and beta-glucan receptors are involved in phagocytosis of unopsonized, heat killed Saccharomyces cerevisiae by murine macrophages. J. Leukoc. Biol. 1993;54:564–571 . doi: 10.1002/jlb.54.6.564. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R., Sim R.B., Gordon S. Local opsonization by secreted macrophage components. Role of receptors for complement in uptake of zymosan. J. Exp. Med. 1984;159:244–260 . doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A.J., Knox K.W. Lipoteichoic acidsa new class of bacterial antigen. Science. 1975;187:1161–1167 . doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Elomaa O., Kangas M., Sahlberg C., Tuukkanen J., Sormunen R., Liakka A., Thesleff I., Kraal G., Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609 . doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- Endemann G., Stanton L.W., Madden K.S., Bryant C.M., Tyler-White R., Protter A.A. CD36 is a receptor for oxidized LDL. J. Biol. Chem. 1993;268:11811–11816 . [PubMed] [Google Scholar]
- Hughes D.A., Frase I.P., Gordon S. Murine macrophage scavenger receptorin vivo expression and function for macrophage adhesion in lymphoid and non-lymphoid organs. Eur. J. Immunol. 1995;25:466–473 . doi: 10.1002/eji.1830250224. [DOI] [PubMed] [Google Scholar]
- West T.E., West M.E., Mylotte J.M. Antiserum agar method for identification of Smith-type exopolysaccharides in clinical isolates of S. aureus . J. Clin. Microbiol. 1985;21:490–492 . doi: 10.1128/jcm.21.4.490-492.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H.A., Keane W.F., Hoidal J.R., Freiberg M.R., Peterson P.K. Peritoneal macrophages and opsoninsantibacterial defense in patients undergoing chronic peritoneal dialysis. J. Infect. Dis. 1983;147:1018–1029 . doi: 10.1093/infdis/147.6.1018. [DOI] [PubMed] [Google Scholar]
- Melly M.A., Duke L.J., Deng-Fong L., Hash J.H. Biological properties of the encapsulated Staphylococcus aureus M. Infect. Immun. 1974;10:389–397 . doi: 10.1128/iai.10.2.389-397.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizono T., Umeda A., Amako K. Distribution of capsular material on the cell wall surface of strain Smith diffuse of S. aureus . J. Bacteriol. 1991;173:4333–4340 . doi: 10.1128/jb.173.14.4333-4340.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P.K., Wilkinson B.J., Kim Y., Schmeling D., Quie P.G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 1978;19:943–949 . doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B.J., Peterson P.K., Quie P.G. Cryptic peptidoglycan and the antiphagocytic effect of the Staphylococcus aureus capsulemodel for the antiphagocytic effect of bacterial cell surface polymers. Infect. Immun. 1979;23:502–508 . doi: 10.1128/iai.23.2.502-508.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W.W., Sutton A., Schneerson R., Karpas A., Vann W.F. Capsular antibodies induce type-specific phagocytosis of encapsulated S. aureus by human polymorphonuclear leukocytes. Infect. Immun. 1988;56:1090–1095 . doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.D., Coles G.A. Gram-positive infections related to CAPD J. Antimicrob. Chemother 27Suppl. B1991. 31 35 [DOI] [PubMed] [Google Scholar]
- Saklayen M.G. CAPD peritonitis. Incidence, pathogens, diagnosis, and management. Med. Clin. North Am. 1990;74:997–1010 . doi: 10.1016/s0025-7125(16)30532-6. [DOI] [PubMed] [Google Scholar]
- Carrozi S., Nasini M.G., Kunkl A., Cantarella S., Lamperi S. Response of CAPD patients with a high incidence of peritonitis to intraperitoneal immunoglobulin therapy. ASAIO (Am. Soc. Artif. Intern. Organs) Trans. 1988;34:635–639 . [PubMed] [Google Scholar]
- Carrozi S., Lamperi S. Peritonitis prevention in CAPD Clin. Nephrol. 30Suppl. 11988. S45 S48 [PubMed] [Google Scholar]
- Lamperi S., Carozzi S. Interferon-γ (IFN-γ) as in vitro enhancing factor of peritoneal macrophage defective bactericidal activity during continuous ambulatory peritoneal dialysis (CAPD) Am. J. Kidney Dis. 1988;11:225–230 . doi: 10.1016/s0272-6386(88)80154-9. [DOI] [PubMed] [Google Scholar]
- deVilliers W.J., Fraser I.P., Hughes D.A., Doyle A.G., Gordon S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J. Exp. Med. 1994;180:705–709 . doi: 10.1084/jem.180.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratazzi C., Arbeit R.D., Carini C., Remold H.G. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J. Immunol. 1997;158:4320–4327 . [PubMed] [Google Scholar]
- Rogers H.W., Callery M.P., Deck B., Unanue E.R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J. Immunol. 1996;156:679–684 . [PubMed] [Google Scholar]
- Galvan V., Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. 1998;USA. 95:3931–3936 . doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S., North R.J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor on myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J. Exp. Med. 1989;170:27–37 . doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie H., Koyama H., Kubo A., Fukuda K., Aita T., Koike A., Yoshimura T., Yoshida J., Shiga J., Hill T. Herpes simplex virus hepatitis in macrophage-depleted micethe role of massive, apoptotic cell death in pathogenesis. J. Gen. Virol. 1998;79:1225–1231 . doi: 10.1099/0022-1317-79-5-1225. [DOI] [PubMed] [Google Scholar]
- Pinto A.J., Stewart D., van Rooijen N., Morahan P.S. Selective depletion of liver and splenic macrophages using liposomes encapsulating the drug dichloromethylene diphosphateeffects on antimicrobial resistance. J. Leukoc. Biol. 1991;49:579–586. doi: 10.1002/jlb.49.6.579. [DOI] [PubMed] [Google Scholar]