Abstract
Forssman glycolipid (FG), the product of Forssman synthetase (FS), is widely expressed among nonprimate mammalian species. Here, we describe a molecular and genetic relationship between FG expression and Shiga toxin (Stx) susceptibility. We have isolated the FS cDNA from human, canine, and murine cells. Whereas the murine and canine FS genes express a functional enzyme, the human FS cDNA was found to express a protein that lacks FS activity, despite a high degree of sequence identity with the enzymatically active murine and canine FS genes. In order to examine the relationship between FG expression and Stx susceptibility, Vero cells were transfected with the three FS orthologues or a vector control. Complementation with the human FS cDNA had no effect on Stx susceptibility, whereas stable expression of the canine and murine FS resulted in markedly decreased susceptibility to toxin. Among individual cells, an inverse correlation between FG expression and Stx binding was demonstrated. Moreover, only strongly FG-reactive cells were capable of growing in the presence of Stx. These cells were found to have high levels of FG expression and a correspondingly diminished GbO3 content. We conclude that expression of a functionally active FS modifies Stx receptor glycolipids to FG and results in markedly decreased susceptibility to toxin. We speculate that inactivation of the FS gene during primate evolution may account, at least in part, for the marked susceptibility of human cells to Stx.
Shiga toxins (Stx) are highly conserved exotoxins released by serotypes of Escherichia coli (predominantly O157:H7), Shigella, and other pathogenic gram-negative bacteria (23, 28). Human exposure to Stx typically follows ingestion of contaminated foods and results in severe hemorrhagic colitis. In rare cases, systemic toxemia results in damage to other organs, such as the kidneys, brain, and heart. The resulting disease complex, known as hemolytic uremic syndrome (HUS), occurs in up to 10% of children infected with E. coli O157:H7 and is the most common cause of acute renal failure in childhood (26, 32).
Stx follows a complex intracellular pathway in order to kill susceptible cells. After binding to cell surface glycolipids, the toxin is internalized and trafficked in retrograde fashion to the Golgi and endoplasmic reticulum. From the endoplasmic reticulum lumen the toxin must gain access to the cytoplasm, where it enzymatically inactivates rRNA, inhibiting protein synthesis, and ultimately leads to cell death. In cultured cells and in whole tissues, susceptibility to Stx is most strongly correlated with the presence of its predominant receptor, the glycolipid globotriaosylceramide (GbO3) (25, 33). The related glycolipid globoside (GbO4, which differs from GbO3 only by an additional N-acetylgalactosamine residue) also binds some variants of Stx, and its presence confers cells with susceptibility to those variants, particularly the pig edema toxin (Stx2e). Thus, presence of globoseries glycolipid GbO3, and to a lesser extent GbO4, is the most important feature determining whether a cell is susceptible to Stx. These observations have direct clinical relevance in that the tissues damaged most in HUS are those with high levels of GbO3 expression. Moreover, children are more susceptible to HUS than adults at least partially due to their higher levels of kidney GbO3 expression (19, 24).
All mammalian species produce glycolipid GbO3, yet many display markedly less susceptibility to toxin when compared to humans. For example, cattle and sheep are frequently in contact with toxigenic bacteria without consequence. Indeed, between 5 and 80% of animals in individual herds are colonized with Stx-producing E. coli, yet these animals rarely manifest symptoms (3, 4, 17). A corresponding differential in Stx susceptibility exists in vitro, where cell lines derived from human and primate sources are often exquisitely sensitive to Stx while cells from other animal species are generally much less susceptible (25, 35).
We examine here the possibility that Forssman synthetase (FS) expression has an impact on Stx susceptibility. FS modifies receptor-active glycolipids GbO3 and GbO4 to Forssman glycolipid (FG), which does not bind toxin. Whereas many mammals widely express a functional FS enzyme, humans are unable to produce this glycolipid. We have recently demonstrated that mutations in the catalytic domain of the human FS protein render the molecule functionally inactive, accounting for the lack of FG synthesis by human cells (36). Here, we describe cloning of the murine FS cDNA and demonstrate that complementation of primate cells with the canine or murine FS results in markedly reduced susceptibility to Stx. An inverse correlation was seen between FG expression and binding of Stx to individual cells. We demonstrate that exposure of FS-expressing cells to Stx results in selection and maintenance of high-level FG expression and markedly reduces cellular content of Stx receptor glycolipids. These results demonstrate that variability in glycolipid expression among species may have a profound effect on susceptibility to an infectious agent and provide insight into the molecular evolution of glycosyltransferase gene expression.
MATERIALS AND METHODS
Cell lines and antibodies.
Cell lines were maintained in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (Gibco BRL, Grand Island, N.Y.). Vero cells (African green monkey adult kidney cells; ATCC CCL-81; American Type Culture Collection, Bethesda, Md.) were transfected with plasmid pFS-35 (canine FS) (9), pFS-36 (transfected and neomycin-selected wild-type control; created by deleting a 400-bp ApaI fragment from plasmid pFS-35 within the predicted catalytic domain), pHFS (human FS) (36), or pMFS (murine FS) using Lipofectamine reagent (Gibco BRL). Approximately 5 × 105 Vero cells were transfected with a suspension containing 3 μg of each plasmid DNA and 50 μl of Lipofectamine. Forty-eight hours later, neomycin (Gibco BRL) was added to the medium to a concentration of 800 μg/ml, and neomycin-resistant clones were allowed to proliferate. After 10 days, cells transfected with the canine or murine FS were subjected to fluorescence-activated cell sorting (FACS) with monoclonal anti-Forssman antibody (M1/22.25.8). The 5% of cells reacting most strongly with anti-Forssman antibody were selected and further expanded in the presence of neomycin. After passage in the presence of neomycin for 8 weeks, approximately 40% of FS-transfected cells continued to express FG as demonstrated by FACS (see Fig. 3, below). Despite FACS selection for single clones containing FG-positive cells, a large percentage of selected cells became FG negative within several passages. Cells transfected with pFS-36 (wild-type cells) or the human FS were expanded directly in the presence of neomycin.
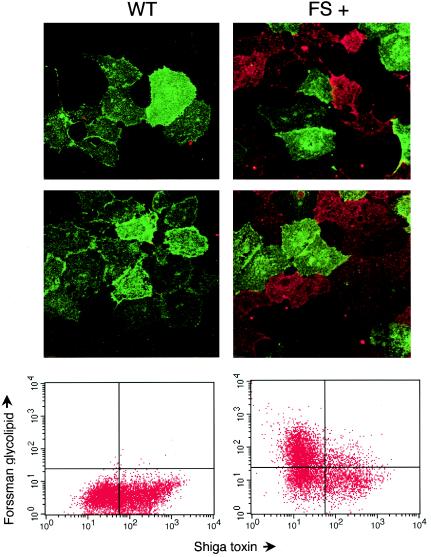
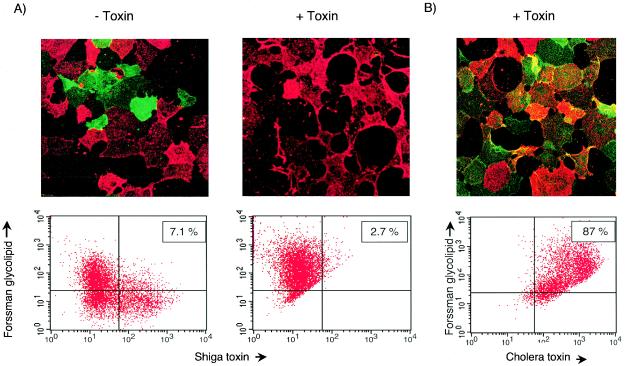
FIG. 3.
Stx binding correlates inversely with FG expression. Wild-type Vero cells or those stably transfected with the canine FS were dual labeled with AlexaFluor 488 StxB (green) or anti-Forssman antibodies and AlexaFluor 568 (red)-conjugated secondary antibody. Labeled cells were visualized by confocal immunofluoroscopy and FACS. For each cell type, two immunofluorescence panels are shown above the FACS profile of the same cells. The FACS data are presented with Stx1 reactivity plotted on the x axis and anti-FG reactivity on the y axis. Binding of both StxB and anti-FG would shift cells to the upper right quadrant, whereas a shift to the upper left or lower right quadrants indicates binding of only anti-FG or StxB, respectively.
Cloning of the murine FS genomic locus and cDNA.
An internal fragment of the murine FS gene was amplified from mouse genomic DNA with degenerate primers FS-26 (5′-TTCRTCCAGYMCTTCCTGGAGTC-3′, where R = A/G, Y = C/T, M = A/C) and FS-28 (5′-CAGAGGTACTCRGGGGACAGC3′), based on conserved regions within the canine (9) and human (36) FS sequences and known to be within a single exon of the human FS gene. A PCR product of 530 bp was obtained, subcloned, and sequenced. The insert was radiolabeled and used as a probe to screen an SvJ mouse genomic library cloned into λ-Fix (Stratagene). One million plaques were screened, revealing three hybridizing clones. These were further purified until two individual strongly hybridizing plaques were isolated. The insert in one (λ-2-2-3) was found to contain the entire coding region, spanning seven exons, over approximately 9 kb. In order to identify the start codon and transcriptional start site, 5′ rapid amplification of cDNA ends (5′-RACE) was performed. Primers MFS-7 (5′-CCCCACCATAATAGAAGTCCC-3′) and AP-1 (Clontech) were used in the first-round PCR using primer-anchored mouse lung cDNA as template (Clontech). A second round of PCR utilizing nested primers MFS-8 (5′-CCCCACGGCAAACACSGTGACSCC-3′, where S = G/C) and AP-2 (Clontech) yielded a single product of approximately 535 bp containing approximately 90 bp of 5′-untranslated message and 347 bp of coding region. Using sequence information derived from the 5′-RACE product and the 3′ end of the genomic clone, primers MFS-44(5′-GCCGCCGCCATGACCCGCCCAAGACTGGCCCAGGGCC) andMFS-43 (5′-ATGTTAGGTCCTCAGCCAGTTGGCATTCTTTTTCACTGG-3′), which overlap the start and stop codons, respectively, were used to amplify the full-length mouse FS cDNA from a murine lung cDNA library. The 1.1-kb product was ligated into vector pCR3.1Topo/His/V5 to create plasmid pMFS. The insert was sequenced and compared to the canine and human FS genes as well as the mouse EST database. Transfection of Vero cells with the cloned murine FS cDNA resulted in strong reactivity with anti-FG antibody, indicating that the cloned cDNA was functional (data not shown).
Holotoxin and Stx B-subunit (StxB) purification.
Semipurified Stx1 was prepared from E. coli harboring the genes encoding Shigella dysenteriae Stx1 on plasmid pNAS13 (generously provided by Alison O'Brien), which contains the operon on a 3.4-kb NcoI fragment from pNAS4 (30). Two-liter cultures of these bacteria were grown overnight at 37°C. Periplasmic extraction was performed by osmotic shock (22). The extract was concentrated to 10 ml and then subjected to anion-exchange chromatography on a Q-Sepharose column using a gradient from 20 mM Tris (pH 7.5) to 20 mM Tris (pH 7.0)-1 M NaCl. A peak containing Stx1 eluted at 0.15 mS/cm. These fractions were pooled and further purified by gel filtration using a Superdex 75 HR 10/30 column equilibrated with phosphate-buffered saline (PBS). Stx1 eluted at approximately 6 ml, as determined by reactivity with anti-StxB on dot immunoblotting and cytotoxicity assays. For cytotoxicity assays, highly purified Stx was utilized (List Laboratories).
The gene encoding StxB was amplified by PCR from plasmid pNAS13 and cloned into plasmid pT7-7 to create pT7B5-1. Purification of recombinant StxB from periplasmic extract was performed from a 1-liter culture by growing in Luria-Bertani broth to an optical density at 600 nm of 0.6, after which isopropylthiogalactoside was added to 1 mM and cultures were incubated a further 2 h at 37°C. Periplasmic extraction was performed by osmotic shock (22). The extract was concentrated to 15 ml and then subjected to anion-exchange chromatography on a Q-Sepharose column using a gradient from 20 mM Tris (pH 7.5) to 20 mM Tris (pH 7.0)-1 M NaCl. A single peak containing StxB eluted at 0.44 mS/cm. This fraction was further purified by gel filtration using a Superdex 75 HR 10/30 column equilibrated with appropriate buffer (PBS for radiolabeling; 0.1 M carbonate [pH 9.5] for AlexaFluor 488 conjugation). Pentameric StxB eluted at approximately 14 ml. StxB was radiolabeled with 125I using Iodogen beads (Pierce) to a specific activity of 2 × 105 cpm/pmol. AlexaFluor 488 conjugation of StxB was performed with reagents from Molecular Probes following the manufacturer's instructions.
Cytotoxicity assays.
Equal numbers of wild-type or FS-transfected cells were suspended in 400 μl of replete medium, and 2.5 × 104 cells were aliquoted into 24-well dishes. The following day, increasing dilutions of Stx1 (15,000 to 1.5 ng/ml; List Laboratories) were added. Cells were then exposed to toxin dilutions in complete medium for 48 h in order to maximize toxin effect. Similar results were seen with a 4-h incubation in the presence of toxin, though the degree of protein synthesis inhibition was less profound among all cell types (data not shown). Cell monolayers were then washed two times with PBS and then incubated in Cys-Met-free medium containing [35S]Cys-Met (ICN Biomedicals, Inc., Costa Mesa, Calif.) at 10 μCi/ml. The cells were incubated for 30 min at 37°C, washed six times in PBS, and lysed by the addition of 500 μl of 0.2% sodium dodecyl sulfate in 50 mM Tris (pH 7.5). Trichloroacetic acid (TCA) was added to 10%, and the samples were incubated on ice for 30 min. After a 10-min centrifugation at 14,000 × g, the supernatant was removed and the pellet was washed in 10% TCA and then transferred to a scintillation tube. Incorporation of [35S]Cys-Met was determined by scintillation counting of precipitated lysates.
Confocal immunofluorescence.
Wild-type Vero cells or those transfected with the human, murine, or canine FS cDNA were harvested, and 104 cells were seeded in 500 μl of DMEM containing 10% fetal calf serum in microchamber slides. After allowing cells to attach for 6 h at 37°C, AlexaFluor 488-labeled StxB (prepared as above) or AlexaFluor 488-labeled cholera toxin B-subunit (Molecular Probes) was added at 1 μg/ml. Monoclonal anti-Forssman antibody (M1/22.25.8) was added to all wells at 1:200 dilution, and the cells were allowed to incubate for 30 min at 4°C. Cells were washed once with 1 ml of PBS and then resuspended in 500 μl of DMEM containing 10% fetal calf serum and AlexaFluor 568-conjugated anti-rat immunoglobulin M (IgM; Molecular Probes) at 1:200 and allowed to incubate at 4°C for 60 min. Cells were washed twice in cold DMEM plus 10% fetal calf serum, then once in cold PBS, and then fixed in 50 μl of PBS containing 2% paraformaldehyde (Fisher Scientific, Fair Lawn, N.J.). The slides were washed several times in blocking buffer and then PBS, mounted with Aqua PolyMount (Polysciences, Warrington, Pa.), and visualized by epifluoresence confocal microscopy (Bio-Rad). Image processing was performed with the LaserSharp 1024 software package.
FACS analysis.
The indicated cells were harvested by trypsinization and then labeled with antibody M1/22.25 (anti-Forssman), and either AlexaFluor 488-labeled StxB (prepared as described above) or AlexaFluor 488-labeled cholera toxin B-subunit (Molecular Probes) was added at 1 μg/ml. After rinsing, AlexaFluor 568-conjugated anti-rat IgM was added at a 1:100 dilution in labeling buffer and incubated for 30 min at 4°C. After washing three times with labeling buffer, cells were resuspended in 1% paraformaldehyde in PBS and subjected to FACS analysis.
Stx binding assay.
Wild-type cells or those stably transfected with the canine FS and passaged in the presence of Stx1 (1 μg of semipurified toxin/ml) were resuspended in 200 μl of complete medium containing various amounts of StxB labeled to a specific activity of 2 × 105 cpm/pmol with 125I. After 1 h of incubation at 4°C, cells were centrifuged through 800 μl of ice-cold fetal calf serum at 10,000 × g for 1 min, the supernatant was removed, and cell-associated toxin was quantitated in a gamma counter. In parallel experiments, cells were preincubated in excess unlabeled StxB (2 mM) for 30 min, followed by the addition of 125I-labeled toxin at various concentrations to determine the amount of nonspecific binding at each concentration of labeled toxin.
TLC immuno-overlays and [125I]Stx overlays.
Crude lipid extracts were prepared from 5 × 105 wild-type Vero cells or those transfected with the canine FS and passaged in the presence of Stx. Cells were harvested by trypsinization, pelleted by centrifugation, and then resuspended in a volume of methanol equal to the cell pellet. One volume of chloroform was added, and after vortexing vigorously methanol was added dropwise until organic and aqueous layers resolved into a single phase (approximately one additional volume of methanol). Cellular debris was removed by centrifugation, and the organic supernatant was evaporated under a stream of nitrogen. The lipid residue was resuspended in 60 μl of chloroform-methanol-water (65:35:8), and 20 μl of each was spotted onto a thin-layer chromatography (TLC) plate. After developing in chloroform-methanol-water (65:35:8), the plate was air dried and blocked with 5% bovine serum albumin (BSA) in PBS. Forssman antigen was detected by TLC immunolabelling and enhanced chemiluminescence (ECL) as previously described (8). Briefly, the plate was overlaid with antibody M1/22.25 (1:500 dilution of tissue culture supernatant in 5% BSA in PBS). After incubating for 1 h at room temperature, the plate was rinsed three times in PBS, then overlaid with peroxidase-conjugated goat anti-rat IgM antibodies (1:5,000 in 5% BSA-PBS), and incubated for a further 30 min at room temperature. The plate was then washed seven times for 5 min each with 0.05% Tween 20 in PBS, then immersed for 1 min in ECL reagent (NEN DuPont), and immediately exposed to Kodak XAR film.
For overlay with radiolabeled toxin, a crude lipid extract was prepared from 1.5 × 105 of each of the cell types, and the lipids were separated by TLC as described above. After air drying the plates overnight, the edges were painted with nail polish and the plates were blocked for 1 h in 5% BSA in PBS. Plates were then incubated for 2 h at room temperature with 125I-labeled Stx1 prepared as above (106 cpm diluted into 15 ml of 5% BSA in PBS). Thereafter, plates were washed five times in PBS at room temperature over a 4-h period, covered with Saran wrap, and exposed to film.
RESULTS
Cloning of the murine FS cDNA.
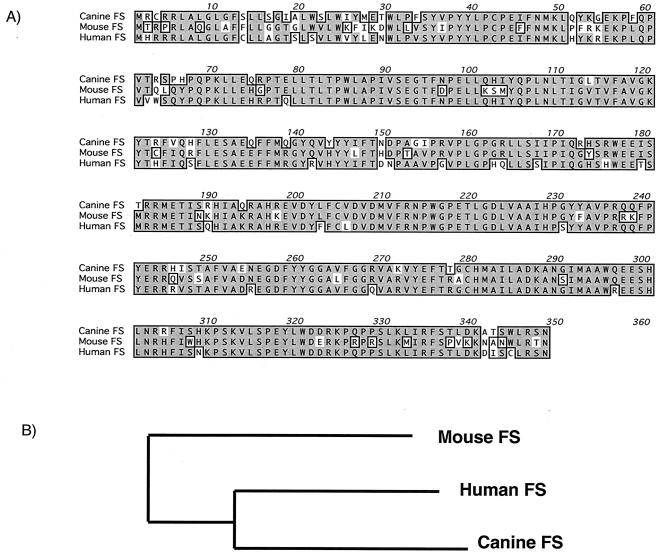
FS modifies GbO4 to FG by adding an N-acetylgalactosamine residue. Our investigators previously described cloning and characterization of the canine (9) and human (36) FS cDNAs. All human tissues were found to express a message encoding full-length FS protein, yet the protein lacked enzymatic activity (36). We demonstrate here that mice also express a functional FS enzyme. Glycolipid extraction and immuno-TLC overlay and RNase protection assays demonstrated that FS is widely expressed in murine tissues (data not shown). A portion of the murine FS cDNA was amplified from mouse genomic DNA using oligonucleotides derived from conserved regions within the human and canine FS genes. Using this fragment of the murine FS as probe, we demonstrated by Northern blot analysis that the FS message was expressed at all stages of development and in all adult tissues examined, with highest expression levels in the lung (data not shown). In order to further elucidate the molecular evolution of the FS gene, we undertook cloning of the murine FS cDNA. The transcriptional start site and start codon were identified by 5′-RACE. The same portion of the murine FS was used as probe to screen a genomic library, yielding one clone containing the entire coding region. Based on the sequence obtained from the RACE product and genomic locus, the entire coding region was amplified from a murine lung cDNA library using flanking primers. The resulting cDNA was found to encode functional FS by transfecting Vero cells and labeling with anti-FG antibodies (data not shown). Sequence analysis demonstrated that overall, the murine, canine, and human FS proteins were 97% identical at the amino acid level. Although highest homology was found between the canine and human FS proteins (Fig. 1), the canine and murine enzymes demonstrated FS activity while the human gene product was devoid of FS activity (36).
FIG. 1.
Sequence comparison of FS orthologues. (A) The deduced peptide sequences of canine, murine, and human FS proteins are aligned. Identical residues are shaded and boxed, while similar residues are lightly shaded. (B) Schematic representation of the relatedness of FS orthologues. The canine, murine, and human FS peptide sequences were compared by using ClustalW, and the output was plotted by unrooted bootstrap analysis.
Vero cells expressing a functional FS demonstrate reduced susceptibility to Stx.
Stx exerts its cytotoxic effect by enzymatic inhibition of the ribosome, resulting in inhibition of protein synthesis and, ultimately, cell death. In order to examine the consequence of FS expression on Stx susceptibility, protein synthesis inhibition assays were performed on Vero cells stably transfected with the three FS homologues or a truncated, nonfunctional version of the canine FS as a wild-type (FS-negative) control. Cells were exposed to various concentrations of toxin, and the percent inhibition of protein synthesis was determined relative to that in cells that did not receive toxin. Whereas wild-type Vero cells or those expressing the human FS were exquisitely susceptible to Stx (greater than 90% inhibition at toxin concentrations as low as 10 pg/ml), those expressing the canine or murine FS cDNA demonstrated only partial inhibition of protein synthesis, even at high toxin concentrations (Fig. 2). Similar results were obtained with human bladder epithelial cells (T-24) transfected with the three FS orthologues (data not shown).
FIG. 2.
Vero cells expressing a functional FS are resistant to Stx. Cells stably transfected with the human, truncated canine (wild-type), murine, or canine FS were incubated with various concentrations of Stx and pulsed with [35S]methionine, and the amount of incorporated radiolabel was determined by TCA precipitation and scintillation counting. Data represent the mean of triplicate samples.
Stx binding correlates inversely with the level of FG expression.
Glycolipid expression levels are known to vary from cell to cell among an asynchronously dividing population (20). We therefore next examined the relationship between levels of FG expression and Stx binding to individual cells. Dual labeling of wild-type Vero cells and those stably transfected with the canine FS was performed with AlexaFluor 488-conjugated StxB and anti-Forssman monoclonal antibody with an AlexaFluor 568-conjugated secondary antibody. Although FS-transfected cells were selected in the presence of high concentrations of neomycin (800 μg/ml) and sorted for the presence of FG, we found within three to four passages that a fraction of cells had lost expression of FG, and this fraction increased with sequential passage. Successive selection of FG-positive cells by FACS was unable to select for stably Forssman-reactive cells, nor was isolation of individual FG-positive clones. In all cases, a portion of selected cells became less Forssman reactive over time. This heterogeneity in FG expression allowed us to examine the relationship between FG expression and binding of Stx to individual cells.
Wild-type cells did not react with anti-Forssman antibodies and, as expected for cells dividing asynchronously (20), bound varying amounts of toxin, as judged by confocal immunofluorescence and FACS (Fig. 3). As demonstrated in the two confocal immunofluorescence images, wild-type cells bound varying amounts of StxB-Alexa 488 and did not bind anti-FG antibodies, as evidenced by the lack of labeling with the Alexa 568-conjugated secondary antibody. These results are presented graphically after FACS analysis and plotting of StxB binding along the x axis and anti-FG binding on the y axis. Wild-type cells are found in the lower two quadrants, reflecting varying amounts of StxB binding. As expected, very few cells are shifted to the upper quadrants, reflecting the lack of anti-FG reactivity. In contrast, within a population of cells expressing the canine FS, there was an inverse relationship between the presence of FG and Stx binding, as demonstrated both by confocal immunofluorescence and FACS (Fig. 3). The two immunofluorescence images demonstrate that binding of StxB and anti-FG tend not to overlap. Cells that were strongly bound by Stx did not express FG, and vice versa. Graphically, this is evident in the FACS profile, where few cells are found in the upper right quadrant (which would indicate binding of both StxB and anti-FG). Instead, there is an inverse correlation between FG expression and StxB reactivity, suggesting that increasing amounts of FG production result in a decreased presence of Stx receptor glycolipids.
Stx exposure selects for high-level FG expression.
Cells stably transfected with the FS cDNA were found to contain varying amounts of FG (Fig. 3). As described previously, in the absence of toxin a tendency toward diminished FG quantity with serial passage was found. We demonstrated above that cells expressing the FS cDNA were heterogeneous in their level of FG expression. Here, we examined the effect of Stx exposure on expression of globoseries glycolipids among cells capable of producing GbO3, GbO4, and FG. Notably, a Stx-resistant Vero cell line was selected by serial passage in toxin, and these cells were found to be deficient in GbO3 synthesis (27). Therefore, in cells expressing FS, resistance to Stx may result either from complete loss of GbO3 expression or by high-level FS expression, with consequent depletion of GbO3 and GbO4 via synthesis of FG. We were therefore interested in whether loss of GbO3 and globoseries glycolipid expression or increased FG would occur in the population of FS-expressing cells upon serial passage in Stx.
In the absence of toxin, a tendency toward diminished FG quantity with serial passage was found. In contrast, cells cultured in the presence of Stx demonstrated uniformly high-level expression of FG over several months of passage (Fig. 4A). These strongly Forssman-positive cells proliferated in toxin concentrations as high as 100 ng of Stx/ml, greater than 10,000-fold above the minimal concentration required to kill FS-negative cells. High-level FG expression correlated with minimal Stx binding (Fig. 4A and 5A, below) but had no effect on binding of cholera toxin B-subunit, which recognizes the unrelated glycolipid GM1 (10, 21) (Fig. 4B). Likewise, internalization and trafficking of cholera toxin B-subunit to the Golgi was unaffected in Stx-selected cells, indicating that FS expression and toxin selection did not affect the retrograde trafficking of another glycolipid-binding toxin (data not shown).
FIG. 4.
Exposure to Stx maintains high-level FG expression. (A) Vero cells stably transfected with the canine FS were passaged serially either in the absence (− toxin) or presence (+ toxin) of Stx and then dual labeled with AlexaFluor 488-conjugated StxB and anti-Forssman antibodies as described above. Cells were imaged by using confocal immunofluoroscopy and FACS. (B) Vero cells stably transfected with the canine FS and passaged in toxin were dual labeled with anti-Forssman antibodies (red) as above and AlexaFluor 488-conjugated cholera toxin B-subunit. Boxed numbers in the FACS profiles indicate the percentage of cells in the upper right quadrant, representing the percentage of cells bound by both anti-FG and labeled toxin (StxB or cholera toxin).
FIG. 5.
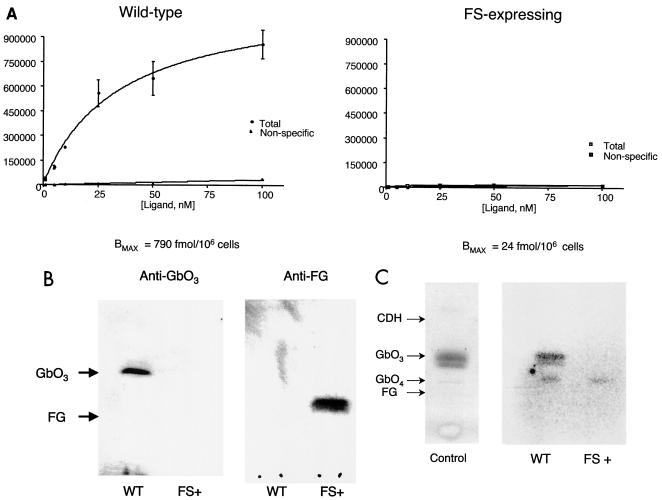
Cells expressing high levels of FG fail to bind Stx due to decreased GbO3 content. (A) Wild-type Vero cells or those transfected with the canine FS and passaged in Stx were incubated with various concentrations of 125I-labeled StxB (total) or excess unlabeled toxin (nonspecific). After washing, the amount of bound [125I]StxB was quantitated. Data represent the mean of duplicate samples. (B) Crude lipid extracts were prepared from wild-type Vero cells (WT) or FS-transfected cells exposed to Stx (FS+). After separation by TLC, GbO3 or FG was detected by monoclonal antibody followed by peroxidase-conjugated secondary antibody and ECL. (C) Glycolipids were extracted from 2 × 105 untransfected (“C”) Vero cells or those transfected with the human FS (WT) or canine FS exposed to Stx (FS+). After separation by TLC, extracts were overlaid with 125I-labeled Stx1. After washing, bound toxin was detected by autoradiography. Migration of glycolipids standards is indicated by the arrows. CDH, ceramide dihexoside.
High-level FG expression correlates with markedly diminished GbO3 content.
As described above, Stx exposure selected for cells expressing high-levels of FG. In order to demonstrate that the mechanism of Stx resistance was depletion of Stx glycolipid receptors GbO3 and GbO4 via their conversion to FG, we first performed Stx binding assays. Wild-type or FS-expressing cells were incubated in increasing concentrations of 125I-labeled StxB in the presence or absence of excess competing unlabeled toxin. While wild-type Vero cells demonstrated maximal binding (Bmax) of 790 fmol of toxin per 106 cells, FS-expressing cells selected in toxin had minimal toxin bound (Fig. 5A).
We next examined wild-type and FS-transfected, toxin-selected cells for the presence of glycolipids GbO3 and FG. Total lipids were extracted and separated by TLC. Glycolipids GbO3 and FG were immunologically detected by TLC overlay (9). As expected, GbO3 but not FG was detected in wild-type cells. In contrast, toxin-selected cells lacked detectable GbO3 and instead demonstrated the presence of FG (Fig. 5B). These results indicate that expression of the FS cDNA is capable of depleting cells of GbO3 via conversion to FG.
Finally, in order to compare binding of Stx to glycolipids of wild-type and FS-transfected cells, total lipid extracts were separated by TLC and the samples were overlaid with radiolabeled Stx1. As expected, Stx1 bound to a band comigrating with GbO3 in control and wild-type Vero cell extracts. Lesser binding was seen to a band comigrating with GbO4. In contrast, in FS+ cell extracts (cells transfected with the canine FS and cultured in the presence of Stx), no binding was seen to GbO3, whereas minimal binding to GbO4 was still detected (Fig. 5C). These results are consistent with the markedly decreased GbO3 levels we demonstrated immunologically in Fig. 5B. Similarly, these findings support the suggestion from whole-cell binding assays that FS+ cells retain very little ability to bind Stx.
DISCUSSION
A crucial first step in the pathogenesis of bacterial and viral infections is adherence of the organism to host cell surfaces (2). In general, microbial adherence demonstrates high specificity for receptors expressed in a species- and tissue-specific manner on the eucaryotic cell surface. As a consequence, microbes usually demonstrate the propensity to bind a limited repertoire of tissues and hosts. Over the last several years it has become evident that this tropism is often a consequence of specific microbial adherence to carbohydrate structures present on glycoproteins or, more commonly, on glycolipids.
We describe here a relationship between FG synthesis and Stx susceptibility. Primate cells, normally exquisitely susceptible to Stx, demonstrated markedly reduced susceptibility upon expression of the FS cDNA. Exposure of a heterogeneous population of cells expressing FS to Stx was found to select for those cells expressing high levels of FG. At the molecular level, these cells were found to lack the ability to bind Stx due to a markedly reduced content of Stx receptor glycolipids. Thus, expression of FS results in diminished susceptibility to Stx by converting toxin receptor glycolipids GbO3 and GbO4 to receptor-inactive FG. We believe that these in vitro conditions mimic an important interplay that occurs in nature between host glycolipid expression and microbial pathogenesis. These results demonstrate that intraspecies differences in glycolipid expression can have marked effects on the susceptibility to an infectious agent. Moreover, these findings provide insight into the molecular evolution of glycosyltransferase gene expression.
Glycolipids consist of a carbohydrate moiety attached to a ceramide backbone. Classification of glycolipids is usually based upon their carbohydrate structure, which varies in length from one sugar residue to six and occasionally more. Variability arises in the oligosaccharide structures through differences in the type of sugar residues incorporated and in the anomeric linkage between sugar residues (α versus β, 1-3 versus 1-4, etc.). Thus, glycolipids may contain a great number of distinct carbohydrate moieties. Each cell type, however, expresses glycolipids that contain a limited repertoire of all possible carbohydrate structures. In mesenchymal tissues of mammalian species, the globoseries glycolipids (those having GbO3 as a core) are the predominant members. As demonstrated herein, modification of globoseries glycolipids to extended forms, such as FG, results in diminished susceptibility to Stx. Aside from apparent differences in susceptibility to infectious agents, the relative costs and benefits of varied glycolipid expression between species is unknown.
There is considerable evidence that glycolipids play important roles in normal cellular biology (1), yet their precise function remains unknown. In many cases it appears that glycolipid function is flexible, given the high degree of variability in glycolipid synthesis among species (7, 34). Like other glycolipids, FG has been proposed to play important roles in cellular biology. FG is expressed in a tissue-specific and developmentally regulated fashion in such diverse mammals as dog, sheep, horse, guinea pig, and mouse. The absence of FG in normal human tissues indicates either that this molecule is dispensable or that the function of FG has been subsumed by another glycolipid during primate evolution. FS expression appears to share many features with the closely related α1,3-galactosyltransferase gene. Like FS, the α1,3-galactosyltransferase gene is conserved among mammalian species, including humans. However, at some point in primate evolution, mutations in the gene rendered the α1,3-galactosyltransferase nonfunctional. Joziasse et al. estimate that the α1,3-galactosyltransferase gene became nonfunctional between 40 and 25 million years ago, based on the observation that Old World monkeys and humans lack functional enzyme while New World primates are capable of synthesizing the α1,3-galactose structure (13, 14). One consequence of the lack of α1,3-galactose synthesis among humans is the natural production of antibodies against the α1,3-galactose linkage. These antibodies have been proposed to be a barrier to infection by enveloped viruses derived from animals that express the α1,3-galactose linkage (31). Thus, loss of α1,3-galactosyltransferase expression may protect humans from infections that are common in other species. Like the human FS gene, the nonfunctional α1,3-galactosyltransferase message was found to be expressed, albeit at low levels, in some human tissues (16). Given the near identity of the human FS with functional canine and murine orthologues, and in analogy to molecular evolution of the related α1,3-galactosyltransferase gene, we propose that the ability to synthesize FG was lost recently in primate evolution.
In mammalian populations, expression of various glycolipid glycosyltransferases ranges from high level to absent between species and even among individuals within a species (e.g., variable histo-blood group antigen expression among humans) (5). A common theme emerging from studies on the molecular evolution of glycosyltransferase expression suggests that selection by infectious microbes or toxins may have had a major influence on the evolution of glycoprotein and glycolipid expression (7, 11, 34). This hypothesis predicts that interaction with infectious agents throughout mammalian evolution may have selected for or against expression of individual glycolipids (7, 16). In the case of FG expression, colonization with toxigenic bacteria may provide selective pressure for maintaining FS expression. Alternatively, modification of GbO3 to other extended-chain glycolipids would be expected to have a similar effect of depleting cells of toxin receptors and to correlate with diminished Stx susceptibility. However, human endothelial cells fail to produce such extended globoseries glycolipids and are unable to synthesize FG, contributing to high levels of GbO3 expression and their marked Stx susceptibility.
Microbes and bacterial toxins typically cause disease in a limited number of host species. A major determinant of such a tropism is the ability of a pathogenic organism or toxin to bind to host cells (2, 8, 12). As is the case with Stx binding, glycolipids are highly represented among cell surface receptors for microbial adhesins and bacterial toxins. Variability in glycolipid expression is expected to affect the tropism for various microbes and toxins for host tissues (6, 11, 15, 18, 29). Expression of a functionally inactive FS in all human tissues suggests that loss of FG synthesis occurred recently in human evolution. The results presented here demonstrate that, were it functional, the human FS would provide a degree of resistance to Stx.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (R01AI47900) and American Heart Association (0050644N). D.H. is a Burroughs Wellcome Foundation Investigator in Microbial Pathogenesis.
E. coli harboring plasmid pNAS13, containing the stx1 operon, was kindly provided by Alison O'Brien, Uniformed Services University of the Health Sciences. Purified Stx1 was kindly provided by Cliff Lingwood.
Editor: A. D. O'Brien
REFERENCES
- 1.Andrews, P. W., E. Nudelman, S. Hakomori, and B. A. Fenderson. 1990. Different patterns of glycolipid antigens are expressed following differentiation of TERA-2 human embryonal carcinoma cells induced by retinoic acid, hexamethylene bisacetamide (HMBA) or bromodeoxyuridine (BUdR). Differentiation 43:131-138. [DOI] [PubMed] [Google Scholar]
- 2.Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J. Infect. Dis. 143:325-345. [DOI] [PubMed] [Google Scholar]
- 3.Besser, T. E., D. D. Hancock, L. C. Pritchett, E. M. McRae, D. H. Rice, and P. I. Tarr. 1997. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 175:726-729. [DOI] [PubMed] [Google Scholar]
- 4.Beutin, L., D. Geier, S. Zimmermann, S. Aleksic, H. A. Gillespie, and T. S. Whittam. 1997. Epidemiological relatedness and clonal types of natural populations of Escherichia coli strains producing Shiga toxins in separate populations of cattle and sheep. Appl. Environ. Microbiol. 63:2175-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartron, J. P., and Y. Colin. 2001. Structural and functional diversity of blood group antigens. Transfus. Clin. Biol. 8:163-199. [DOI] [PubMed] [Google Scholar]
- 6.Dennis, J. W. 1999. Protein glycosylation in development and disease. Bioessays 21:412-421. [DOI] [PubMed] [Google Scholar]
- 7.Gagneux, P., and A. Varki. 1999. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 9:747-755. [DOI] [PubMed] [Google Scholar]
- 8.Haslam, D., T. Borén, P. Falk, D. Ilver, A. Chou, Z. Xu, and S. Normark. 1994. The amino-terminal domain of the P-pilus adhesin determines receptor specificity. Mol. Microbiol. 14:399-410. [DOI] [PubMed] [Google Scholar]
- 9.Haslam, D. B., and J. U. Baenziger. 1996. Expression cloning of Forssman glycolipid synthetase: a novel member of the histo-blood group ABO gene family. Proc. Natl. Acad. Sci. USA 93:10697-10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyningen, S. V. 1974. Cholera toxin: interaction of subunits with ganglioside GM1. Science 183:656-657. [DOI] [PubMed] [Google Scholar]
- 11.Hooper, L. V., and J. I. Gordon. 2001. Glycans as legislators of host-microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology 11:1R-10R. [DOI] [PubMed] [Google Scholar]
- 12.Hultgren, S. J., S. Abraham, M. Caparon, P. Falk, J. W. I. St. Geme, and S. Normark. 1993. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell 73:887-901. [DOI] [PubMed] [Google Scholar]
- 13.Joziasse, D. H., J. H. Shaper, E. W. Jabs, and N. L. Shaper. 1991. Characterization of an alpha 1-3-galactosyltransferase homologue on human chromosome 12 that is organized as a processed pseudogene. J. Biol. Chem. 266:6991-6998. [PubMed] [Google Scholar]
- 14.Joziasse, D. H., J. H. Shaper, D. H. Van den Eijnden, A. J. Van Tunen, and N. L. Shaper. 1989. Bovine alpha 1-3-galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J. Biol. Chem. 264:14290-14297. [PubMed] [Google Scholar]
- 15.Karlsson, K. A. 1998. Meaning and therapeutic potential of microbial recognition of host glycoconjugates. Mol. Microbiol. 29:1-11. [DOI] [PubMed] [Google Scholar]
- 16.Koike, C., J. J. Fung, D. A. Geller, R. Kannagi, T. Libert, P. Luppi, I. Nakashima, J. Profozich, W. Rudert, S. B. Sharma, T. E. Starzl, and M. Trucco. 2002. Molecular basis of evolutionary loss of α1,3-galactosyltransferase gene in higher primates. J. Biol. Chem. 277:10114-10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1996. Escherichia coli O157:H7 in microbial flora of sheep. J. Clin. Microbiol. 34:431-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leffler, H., C. Svanborg-Eden, G. Hansson, G. Larson, K.-A. Karlsson, J. Dahmen, T. Frejd, G. Magnusson, and G. Noori. 1983. Glycolipids as receptors for bacterial adhesion: uropathogenic Escherichia coli, p. 643-644. In M. A. Chester, D. Heinegård, A. Lundblad, and S. Svensson (ed.), Proceedings of the 7th International Symposium on Glycoconjugates. Lund-Ronneby, Lund, Sweden.
- 19.Lingwood, C. A. 1996. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 4:147-153. [DOI] [PubMed] [Google Scholar]
- 20.Majoul, I., T. Schmidt, M. Pomasanova, E. Boutkevich, Y. Kozlov, and H.-D. Söling. 2002. Differential expression of receptors for shiga and cholera toxin is regulated by the cell cycle. J. Cell Sci. 115:817-826. [DOI] [PubMed] [Google Scholar]
- 21.Merritt, E. A., S. Sarfaty, F. van den Akker, C. L’Hoir, J. A. Martial, and W. G. Hol. 1994. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 3:166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neu, H. C., and L. A. Heppel. 1965. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J. Biol. Chem. 240:3685-3692. [PubMed] [Google Scholar]
- 23.O'Brien, A. D., V. L. Tesh, A. Donohue-Rolfe, M. P. Jackson, S. Olsnes, K. Sandvig, A. A. Lindberg, and G. T. Keusch. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180:65-94. [DOI] [PubMed] [Google Scholar]
- 24.Obrig, T. G., C. B. Louise, C. A. Lingwood, B. Boyd, L. Barley-Maloney, and T. O. Daniel. 1993. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268:15484-15488. [PubMed] [Google Scholar]
- 25.Ohmi, K., N. Kiyokawa, T. Takeda, and J. Fujimoto. 1998. Human microvascular endothelial cells are strongly sensitive to Shiga toxins. Biochem. Biophys. Res. Commun. 251:137-141. [DOI] [PubMed] [Google Scholar]
- 26.Pickering, L., T. Obrig, and F. Stapleton. 1994. Hemolytic-uremic syndrome and enterohemorrhagic Escherichia coli. Pediatr. Infect. Dis. J. 13:459-475. [DOI] [PubMed] [Google Scholar]
- 27.Pudymaitis, A., G. Armstrong, and C. A. Lingwood. 1991. Verotoxin-resistant cell clones are deficient in the glycolipid globotriosylceramide: differential basis of phenotype. Arch. Biochem. Biophys. 286:448-452. [DOI] [PubMed] [Google Scholar]
- 28.Sandvig, K. 2001. Shiga toxins. Toxicon 39:1629-1635. [DOI] [PubMed] [Google Scholar]
- 29.Sharon, N. 1996. Carbohydrate-lectin interactions in infectious disease. Adv. Exp. Med. Biol. 408:1-8. [PubMed] [Google Scholar]
- 30.Strockbine, N. A., M. P. Jackson, L. M. Sung, R. K. Holmes, and A. D. O’Brien. 1988. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J. Bacteriol. 170:1116-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi, Y., C. D. Porter, K. M. Strahan, A. F. Preece, K. Gustafsson, F. L. Cosset, R. A. Weiss, and M. K. Collins. 1996. Sensitization of cells and retroviruses to human serum by (alpha 1-3)galactosyltransferase. Nature 379:85-88. [DOI] [PubMed] [Google Scholar]
- 32.Tarr, P. I. 1995. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin. Infect. Dis. 20:1-8. [DOI] [PubMed] [Google Scholar]
- 33.Valdivieso-Garcia, A., D. L. MacLeod, R. C. Clarke, C. L. Gyles, C. Lingwood, B. Boyd, and A. Durette. 1996. Comparative cytotoxicity of purified Shiga-like toxin-IIe on porcine and bovine aortic endothelial and human colonic adenocarcinoma cells. J. Med. Microbiol. 45:331-337. [DOI] [PubMed] [Google Scholar]
- 34.Varki, A. 1993. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3:97-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams, J. M. 1999. A comparison of the effects of verocytotoxin-1 on primary human renal cell cultures. Toxicol. Lett. 105:47-57. [DOI] [PubMed] [Google Scholar]
- 36.Xu, H., T. Storch, M. Yu, S. P. Elliott, and D. B. Haslam. 1999. Characterization of the human Forssman synthetase gene. An evolving association between glycolipid synthesis and host-microbial interactions. J. Biol. Chem. 274:29390-29398. [DOI] [PubMed] [Google Scholar]