Abstract
Skin cancer incidence is highest in white-skinned people. Within this group, skin types I/II (sun sensitive/tan poorly) are at greater risk than skin types III/IV (sun tolerant/tan well). Studies in mice demonstrate that ultraviolet radiation (UVR)-induced suppression of cell-mediated immune function plays an important role in the development of skin cancer and induces a susceptibility to infectious disease. A similar role is suspected in humans, but we lack quantitative human data to make risk assessments of ambient solar exposure on human health. This study demonstrates that ambient levels of solar UVR, typically experienced within 1 h of exposure to noonday summer sunlight, can suppress contact hypersensitivity (CHS) responses in healthy white-skinned humans in vivo (n = 93). There was a linear relationship between increase in erythema and suppression of CHS (P < 0.001), and a moderate sunburn (two minimal erythema doses [2 MED]) was sufficient to suppress CHS in all volunteers by 93%. However, a single suberythemal exposure of either 0.25 or 0.5 MED suppressed CHS responses by 50 and 80%, respectively, in skin types I/II, whereas 1 MED only suppressed CHS by 40% in skin types III/IV. The two- to threefold greater sensitivity of skin types I/II for a given level of sunburn may play a role in their greater sensitivity to skin cancer.
Keywords: human, immunosuppression, contact hypersensitivity, seasons, sunscreen
Introduction
Solar ultraviolet radiation (UVR; ∼295–400 nm) is a major environmental carcinogen and the principle cause of skin cancer, the most common cancer worldwide. The incidence of all types of skin cancer has increased steadily over the past few decades, and this is widely attributed to changes in lifestyle that have resulted in increased exposure to ambient UVR 1. Further increases in UVR exposure are expected as a result of stratospheric ozone depletion 2.
UVR exposure also results in sunburn (erythema), skin aging, and immune suppression. The latter plays an important role in the development of skin cancer in mice, and a similar role is suspected in humans 3. Furthermore, a positive correlation between the incidence of non-Hodgkin's lymphoma and ambient UVR, along with an elevated risk of non-Hodgkin's lymphoma in skin cancer patients 4, suggests a possible role for UVR in noncutaneous malignancies.
Exposure to UVR also increases the incidence and/or severity of infections in mice challenged with bacterial, fungal, viral, or parasitic agents 5 and therefore has important implications for susceptibility to infectious diseases and vaccine effectiveness in humans. UVR suppresses cell-mediated immune (Th1) responses, leaving humoral (Th2) responses intact 6. Given that Th1 and Th2 responses are mutually antagonistic, UVR may have the potential to suppress or exacerbate the pathogenesis of several immunopathological conditions, including some hypersensitivity states that are dominated by a Th1 (e.g., organ-specific autoimmune disorders) or Th2 (e.g., atopic allergy) immune response 7. The United Nations Environment Programme has expressed concerns about the potential health effects that may result from increased exposure to UVR 8 but states that there are currently no quantitative data on UVR-induced immunosuppression in humans to make assessments of the risks of ambient exposure on human health.
The aim of this study in white-skinned people was to determine the UVR dose–response relationship for suppression of cell-mediated immune function in vivo using single UVR exposures at levels typical of those that might be experienced in UK summer sunlight. We also investigated the relationship between UVR-induced erythema and immunosuppression in different skin types to determine if protection against erythema, e.g., by sunscreens, is indicative of protection against immunosuppression. As it is well established that different genetic backgrounds affect susceptibility to skin cancer in humans, we also investigated whether susceptibility to UVR-induced immunosuppression is skin type dependent.
Materials and Methods
Volunteers.
93 healthy, white-skinned Caucasian volunteers (18–35 yr old) were recruited from staff at Guy's and St. Thomas' Hospitals, London. The study was approved by the local ethical committee, and volunteers gave informed consent to participate. Skin type was assessed by interview and erythema assessment. 93 volunteers completed the study; 62 volunteers were skin type I/II (sun sensitive/tan poorly), 44 females and 18 males. The remaining 31 volunteers were skin type III/IV (sun tolerant/tan readily), 23 females and 8 males. Exclusion criteria were medication other than the oral contraceptive pill, previous exposure of buttock skin (test site) to sunlamps or sunlight, a history of atopy, or previous exposure to the contact allergen 2,4-dinitrochlorobenzene (DNCB; Sigma-Aldrich Co. Ltd.). Pregnant or lactating females were also excluded.
UVR Source and Dosimetry.
Solar-simulated radiation (SSR) was generated by a 1-kW xenon arc solar simulator (Oriel Corp.) giving an even field of irradiance (290–400 nm) of ∼15 mW/cm2 on the skin surface when 11 cm from the source. Irradiance was routinely determined with a wide band thermopile radiometer (Medical Physics) calibrated against a DM150 double monochromator Bentham spectroradiometer (Bentham Instruments, Ltd.). The spectral output of the solar simulator, normalized at 320 nm, compared with the solar spectrum of noon summer sunlight (London, 51° N) has previously been reported 9.
Irradiation Protocol.
The minimal dose of SSR required to induce a just visibly perceptible erythema at 24 h (minimal erythema dose [MED]) was determined on the buttock skin of each volunteer by a geometric series of six exposure doses with increments of 2. Volunteers were randomly assigned to different SSR treatment groups and received a single SSR exposure on a 5 × 5 cm2 site on the buttock of 0, 0.25, 0.5, 1.0, 2.0, or 3.0 MED. Each dose group contained eight skin type I/II and eight skin type III/IV volunteers, except for 0.25 and 3 MED, which were not given for skin types III/IV. Quantitative measurements of erythema (arbitrary units) were made in triplicate both before and 24 h after SSR exposure using a reflectance meter (Diastron). For each individual, the increase in erythema was calculated by subtracting the mean background reading from adjacent nonirradiated skin.
Sensitization.
Volunteers were sensitized on buttock skin with DNCB 24 h after irradiation. This was via the irradiated site using a petrolatum-backed 12-mm filter disk, soaked in 50 μl of 0.0625% DNCB in ethanol (31.2 μg/50 μl). The filter paper disc was mounted inside a 12-mm aluminum Finn chamber (Biodiagnostics Ltd.) and taped in place for 48 h. Two control groups (skin type I/II) were sensitized with ethanol only to determine the nonspecific irritant effects of DNCB challenge. Sites were either unirradiated (n = 8) or received a single 3 MED SSR exposure (n = 6) 24 h before application of ethanol.
Elicitation of Contact Hypersensitivity Response.
3 wk after sensitization, volunteers were challenged on the normally UVR-protected upper inner arm. 8-mm filter paper discs were placed in 8-mm Finn chambers and soaked with 20 μl of hapten solutions of various strengths. Five patches were placed on the test site; one was soaked in ethanol only, and four were soaked in incremental doses of DNCB (3.125, 6.25, 12.5, and 25.0 μg/20 μl). The elicitation sites were marked on the arm with a surgical skin marker. The patch was taped in place for 48 h. At hour 48, the patch was removed and the skin was allowed to recover for a period of 1 h. 49 and 72 h after challenge, elicitation sites were quantified as outlined below.
Quantification of Contact Hypersensitivity Responses.
Full details have already been published 10. In brief, the dermal thickness of each elicitation site was quantified using a high frequency 20 MHz ultrasound scanner (Quality Medical Instruments Ltd.). Ultrasound images of each site were recorded immediately before and 49 and 72 h after challenge, because the time course for the maximum elicitation of the contact hypersensitivity (CHS) response varies from person to person. The time point at which the greatest response was measured was used to calculate the percentage increase in dermal thickness as follows:
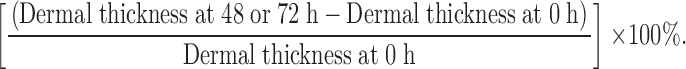 |
The percentage increase in dermal thickness for each elicitation site was plotted versus DNCB challenge dose (x-axis), and the dose–response relationship was determined using linear regression analysis. The CHS response of a given individual is represented by the slope of the linear regression line. The steeper the slope, the stronger the response.
Calculation of UVR-induced Immunosuppression.
The slope of the elicitation response for each of the SSR-treated individuals was then used in the formula below to calculate percentage suppression of CHS:
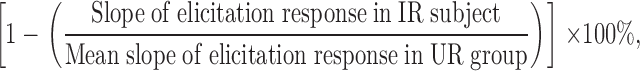 |
where IR = irradiated and UR = unirradiated.
Statistical Analyses.
Erythema was analyzed using multiple regression analysis on the measured erythema response, and interaction terms were used to allow for different responses by different skin types while omitting the constant to force the lines through zero. Immune response (CHS) was analyzed using interval regression on the log10 of the slope, taking zero values below 0.02 (slightly below the lowest real observed value). The multiple regression models fitted were adjusted for seasonal differences. Observations were placed in five groups according to the month of the year in which they were made: April/May, July/August, October, or December/January. Comparisons were made with June (the month with the highest response). Standard multiple regression techniques were used to compare erythema or CHS responses between these time periods and to make adjustments for skin types and SSR dose. Data was analyzed, on an individual volunteer basis, with Stata statistics/data analysis software (Stata Corp.). Robust SEs were used to correct for nonnormality and unequal variances 11.
Results
UVR-induced Erythema.
Exposure to 2 or 3 MED of SSR induced a vivid but nonblistering sunburn in all volunteers regardless of skin type. Tanning (assessed at 7–14 d) was not visually detectable in volunteers with skin type I, but exposure to 2 and 3 MED induced a very light tan in skin type II volunteers and a medium tan in volunteers with skin types III and IV.
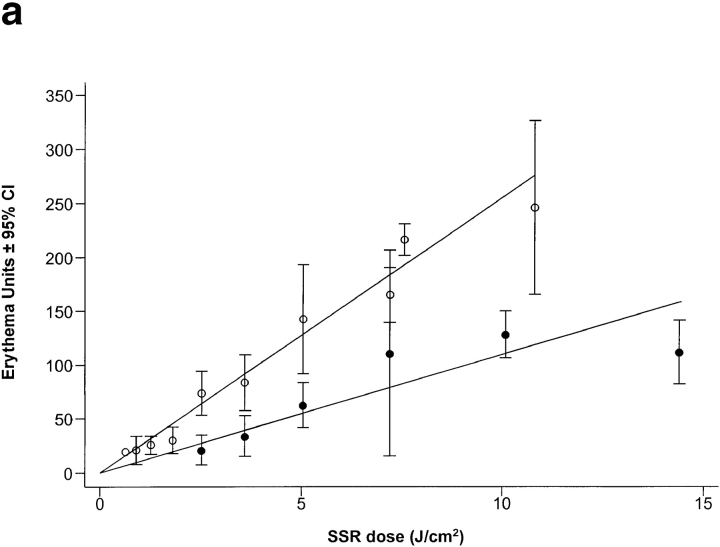
Exposure to UVR induced a dose-dependent increase in erythema in all skin types (Fig. 1 a), but for every 1 J.cm−2 of SSR, the erythema response of skin types I/II was significantly greater than for skin types III/IV by 14.5 arbitrary erythema units (P < 0.0001; 95% CI 9.3–19.7). The mean physical SSR dose required to induce a MED in skin types I/II was about twofold lower than for skin types III/IV: 3.2 and 5.5 J.cm−2, respectively (P < 0.001).
Figure 1.
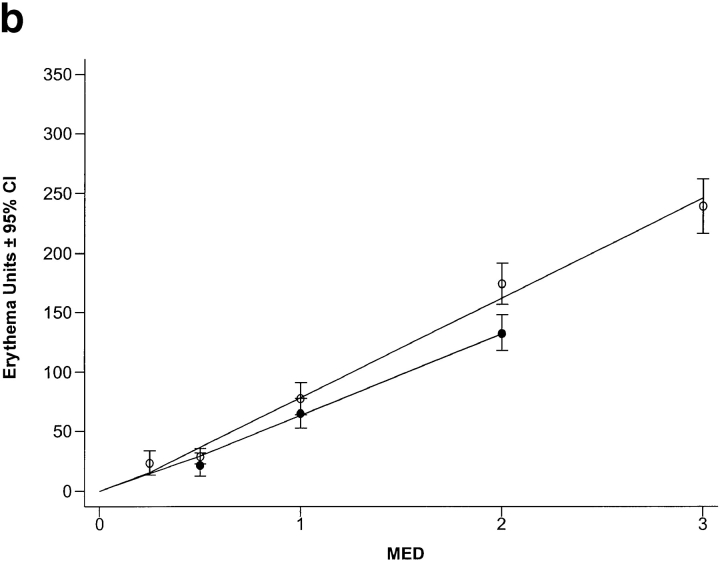
SSR dose–response curves for erythema. Skin types I/II (○) were more sensitive to sunburn/erythema than skin types III/IV (•). The dose of UVR required to induce a just visible reddening of the skin (MED) was 3.2 and 5.5 J.cm−2, respectively (a). If the same data are expressed as multiples of MED rather than physical dose, the two dose–response curves are comparable, but skin types I/II still have a small (below the visual threshold) but significantly higher erythema response than skin types III/IV (P < 0.002) (b).
When the erythema responses were expressed in terms of biological dose (i.e., MED; Fig. 1 b), the two dose–response curves were almost superimposable. However, skin types I/II had a small but significantly greater erythema response to each MED challenge (16.0 erythema units; P < 0.004; 95% CI 5.2–26.6) compared with skin types III/IV, but this was below the visual detection limit (∼50 erythema units).
CHS Responses.
Unirradiated volunteers: All unirradiated volunteers, regardless of skin type, were successfully sensitized and developed a dose-dependent CHS response to all four incremental challenge doses of DNCB. Control volunteers who were sensitized with ethanol alone, with or without prior exposure to 3 MED SSR, did not develop a CHS response to any of the four incremental challenge doses of DNCB.
Skin Types I/II Are More Sensitive to UVR-induced Immunosuppression than Skin Types III/IV.
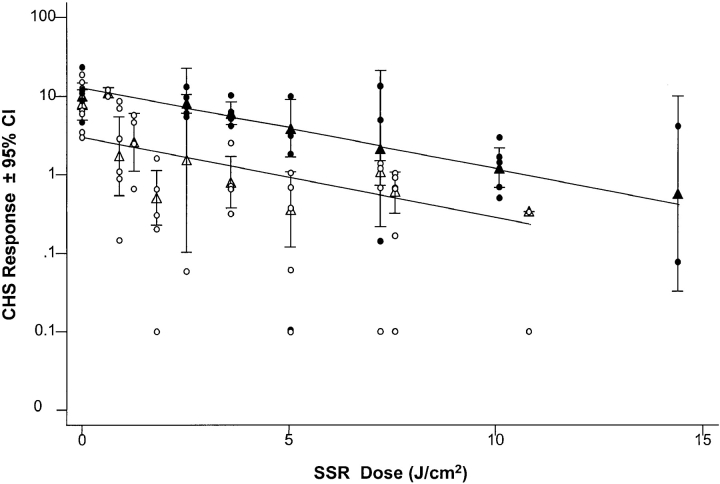
The relationship between suppression of CHS and SSR exposure was assessed using three different measures of exposure: (a) physical dose (J.cm−2) and biological dose expressed as (b) MED multiples and (c) erythema units. However the data were expressed, exposure to SSR induced a dose-dependent suppression of CHS in both skin type groups. Skin types I/II were 5.3 times more sensitive (95% CI 2.9–9.6, P < 0.001) than types III/IV when the CHS responses were plotted against SSR dose (Fig. 2). The use of biological indicators of SSR exposure reduced this difference, but the CHS response of skin type I/II was still significantly lower than that of skin type III/IV (P < 0.01) by a factor of 2.8 (95% CI 1.4–5.6) throughout the MED dose range studied (data not shown). To determine if this difference was due to the higher erythema responses of skin type I/II per MED challenge, the CHS responses were compared with dose expressed as erythema units (assessed just before sensitization). Reanalysis confirmed that the CHS responses of skin types I/II were still significantly lower than skin types III/IV (P < 0.03) by a factor of 2.2 (95% CI 1.07–4.6).
Figure 2.
Skin types I/II are more susceptible to UVR-induced immunosuppression than skin types III/IV. Analysis of individual (○, skin type I/II; •, skin type III/IV) and mean (▵, skin type I/II; ▴, skin type III/IV) slopes for CHS response shows that SSR exposure induced a highly significant dose-dependent suppression of cell-mediated immunity in all skin types (P < 0.001). CHS responses of skin types I/II were 5.3-fold lower than those of skin types III/IV throughout the dose range studied (P < 0.001; 95% CI 2.9–9.6).
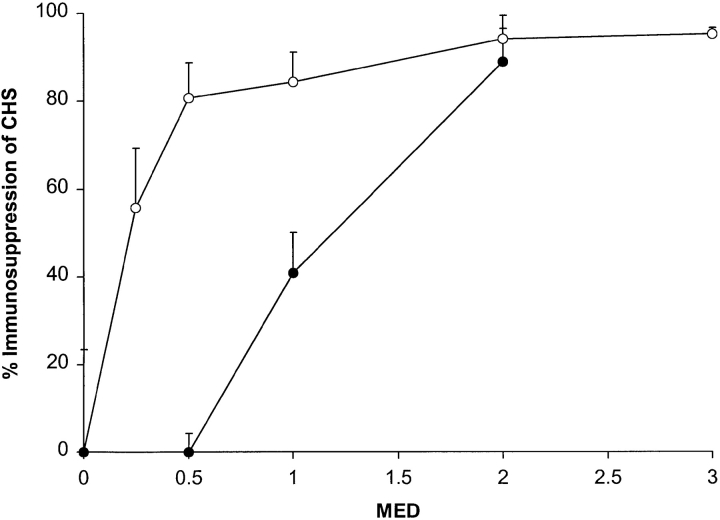
The percentage of immunosuppression was calculated from the CHS data, as outlined in Materials and Methods, to compare the dose–response curves for immunosuppression with erythema (Fig. 3). A moderate but vivid sunburn (2 MED) completely suppressed CHS (93%) in all volunteers regardless of skin type, but skin types I/II were more sensitive to suberythemal exposure.
Figure 3.
Sunburn is not a useful indicator of immunosuppression in skin types I/II. Skin types I/II (○) were suppressed with doses of SSR that were below the visual threshold for erythema (1 MED). In contrast, skin types III/IV (•) were suppressed with erythemogenic doses of SSR. All skin types were suppressed with a moderate sunburn (2 MED).
Seasonal Variation.
As the study took 14 mo to complete, we investigated the influence of season on CHS and quantitative erythema responses. Both responses varied with season, regardless of skin type or SSR challenge. Erythema responses were highest in June and lower than June levels from July to October by 15 erythema units (P < 0.01). CHS responses were also highest in June compared with other months. Between July and October, CHS responses were 50% of those seen in June (CI 39–64%, P < 0.0001). Between December and April, CHS responses were 29% of the June levels (CI 10–83%, P = 0.021). Fig. 1 and Fig. 2 are adjusted for seasonal variation.
Discussion
Skin type, which is defined by an individual's sensitivity to sunburn and ability to tan, is a major risk factor for skin cancer in white-skinned populations. Thus, Celtic populations (skin types I/II) are at greater risk than Mediterranean populations (skin types III/IV) 1. In this study, we have assessed whether sun-sensitive skin types I/II, who tan poorly, and sun-tolerant skin types III/IV, who tan well, might also differ in their susceptibility to UVR-induced immunosuppression. To eliminate any possible confounding effects of prior UVR exposure, we exposed our volunteers to SSR on a small area of previously unexposed buttock skin, where constitutive levels of pigmentation were similar.
Susceptibility to immunosuppression was clearly skin type dependent. Skin types I/II were more readily suppressed than skin types III/IV, requiring a fivefold lower physical SSR dose than skin types III/IV to produce an equivalent amount of immunosuppression (Fig. 2). A twofold difference was still observed when the two groups were challenged with an equivalent erythemally effective dose of SSR. This sensitivity of skin types I/II to SSR-induced immunosuppression was striking. A single exposure to 0.25 MED suppressed CHS by 50%, and 0.5 MED suppressed immune function by 80%. In comparison, skin types III/IV were only suppressed with erythemal UVR exposures (1 MED and above; Fig. 3).
Release of soluble mediators from UVR-exposed skin, in particular TNF-α and IL-10, plays an important role in the induction of immunosuppression in mice 3 6. We have recently shown that the in vivo release of TNF-α and IL-10 in human skin is significantly greater in skin types I/II than skin types III/IV after the same physical dose of UVR or an equivalent MED challenge 12. We propose that this differential release of mediators may be the basis for the difference in susceptibility to immunosuppression in the two skin type groups. Given that UVR-induced immunosuppression plays an important role in the development of skin cancers in mice 3, it is likely that the greater sensitivity to UVR-induced immunosuppression in skin types I/II may contribute to their increased skin cancer risk. However, we must stress that despite a difference in sensitivity to immunosuppression, a vivid but nonblistering sunburn (2 MED) was sufficient to suppress immune function in all skin types by 93%. Therefore, it is important that all white-skinned Caucasians take steps to reduce exposure to sunlight and avoid sunburn to reduce their risk of skin cancer.
Previous studies in white-skinned people, using a more aggressive irradiation protocol delivered over several days, have suggested that only 40% (12/32) of the normal population is susceptible to UVR-induced immunosuppression 13. Similar results, with the same irradiation protocol, were observed in sun-tolerant brown/black-skinned people, suggesting no relationship between the erythemal response and immunosuppression 14. To date, no immunological or genetic basis for the UVR-resistant and UVR-susceptible human phenotypes has been found 15 16. Similar studies by the same group identified UVR-resistant and -susceptible strains of mice 17, but this finding was refuted by other workers, who showed that all mouse strains were susceptible to UVR-induced immunosuppression when a lower dose of hapten was used to sensitize the mice 18. Other workers have also shown that the level of UVR-induced immunosuppression is dependent on the dose of sensitizer applied to the skin 19 and that there was less suppression of CHS when high doses of sensitizer were used. The dose of sensitizer used in our study was 64-fold lower than that used by previous investigators 13 14 and is probably a more sensitive way of detecting UVR-induced immunosuppression.
The ability of low to moderate doses of UVR to alter the CHS response suggests a possible role for solar exposure in the regulation of normal cutaneous immune function. Not only did low-dose UVR suppress local CHS responses, but our previous studies showed that 3 MED SSR suppressed CHS responses by 93% (12/12 volunteers) when sensitizer was applied to irradiated skin and also suppressed CHS responses by the same amount (10/12 volunteers) when sensitizer was applied at a distant site 24 h after irradiation 10. The ability of UVR to suppress CHS responses systemically suggested that our results may have been influenced by variations of ambient solar exposure over the 14-mo study period. We investigated this possibility and found that, in contrast to what may have been predicted, CHS responses were actually higher in the summer than in the winter, even though in the UK daily ambient UVR is ∼40-fold higher in summer than in winter. Furthermore, a similar seasonal trend was seen for erythema. The reasons for this seasonal variation are unknown, but evidence is accumulating that seasonal influences on the neuroendocrine network play a role in systemic modulation of immune responses and cytokine release 20 21. We stress, however, that our data clearly show that the immunosuppressive effects of acute UVR exposure in an experimental situation are dominant over any seasonal enhancement of immune function.
Our data show that equivalent erythema responses in different skin types result in different levels of immunosuppression. Consequently, the erythema response is not a useful indicator of immunosuppression, especially in skin types I/II, in which immunosuppression is seen with suberythemal exposure. Sunscreens are widely advocated to reduce the risk of skin cancer, but there have been concerns about their ability to protect against immunosuppression 22. Significant suppression of CHS by suberythemal exposure in skin types I/II indicates that prevention of erythema by sunscreens does not necessarily protect against immunosuppression. For example, a sunscreen of sun protection factor (SPF) 10 reduces the UVR reaching the skin to 1/10th. 3 MED is readily achievable by skin types I/II in the UK after 1–2-h exposure to summer sunlight, allowing an SPF 10 user to receive 0.3 MED (3 MED/10). Under these conditions, there will be protection against erythema but not immunosuppression, but shorter exposure times might result in protection from both endpoints. The real impact of UVR exposure may be greater than predicted by our studies. We irradiated a small area of skin, but mouse studies indicate that a given UVR dose per unit area is more immunosuppressive when larger areas of skin are irradiated 19, such as with sunbathing. These data indicate the importance of public health campaigns stressing that sunscreens should be used as a part of an overall strategy to reduce UVR exposure, which includes wearing protective clothing and the avoidance of sun around noon, when levels of UVR are highest.
The study of the immunosuppressive effects of UVR has so far focused on skin cancer and infectious disease. Our study also implies that vaccinations would be less effective if given in the summer or after holidays in the sun. However, as vaccines are usually given intradermally rather than epicutaneously, as was the antigen used in our study, further work is needed to clarify this point. Finally, given the potency of UVR to modulate immune function in humans, our data suggest that the effects of UVR on allergic and autoimmune diseases should be explored. Further work is also needed to extend our knowledge about the possible effects of UVR-induced immunosuppression and susceptibility of people of different ethnic backgrounds to these diseases.
Acknowledgments
This project was funded by the UK Department of Health (contract no. 121/6379).
References
- Armstrong B.K., Kricker A. Skin cancer. Dermatol. Clin. 1995;13:583–594 . [PubMed] [Google Scholar]
- Madronich S., McKenzie R.L., Björn L.O., Caldwell M.M. Environmental effects of ozone depletion1998 assessment. Changes in biologically active ultraviolet radiation reaching the Earth's surface. J. Photochem. Photobiol. B. 1998;46:5–19. doi: 10.1016/s1011-1344(98)00182-1. [DOI] [PubMed] [Google Scholar]
- Nishigori C., Yarosh D.B., Donawho C., Kripke M.L. The immune system in ultraviolet carcinogenesis. J. Investig. Dermatol. Symp. Proc. 1996;1:143–146. [PubMed] [Google Scholar]
- McMichael A.J., Giles G.G. Have increases in solar ultraviolet exposure contributed to the rise in incidence of non-Hodgkin's lymphoma? Br. J. Cancer. 1996;73:945–950. doi: 10.1038/bjc.1996.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevan A., Brown E., Kripke M.L. UVR and infectious diseases. In: Krutmann J., Elmets C.A., editors. Photoimmunology. Blackwell Science, Inc.; Cambridge, MA: 1995. pp. 153–163. [Google Scholar]
- Ullrich S.E. Does exposure to UVR radiation induce a shift to a Th-2-like immune reaction? Photochem. Photobiol. 1996;64:254–258. doi: 10.1111/j.1751-1097.1996.tb02454.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., Sad S. The expanding universe of T-cell subsetsTh1, Th2 and more. Immunol. Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Longstreth J., de Gruijl F.R., Kripke M.L., Abseck S., Arnold F., Slapper H.I., Velders G., Takizawa Y., van der Leun J.C. Environmental effects of ozone depletion1998 assessment. Health risks. J. Photochem. Photobiol. B. 1998;46:20–39. doi: 10.1016/s1011-1344(98)00183-3. [DOI] [PubMed] [Google Scholar]
- Young A.R., Chadwick C.A., Harrison G.I., Hawk J.L., Nikaido O., Potten C.S. The in situ repair kinetics of epidermal thymine dimers and 6-4 photoproducts in human skin types I and II. J. Invest. Dermatol. 1996;106:1307–1313. doi: 10.1111/1523-1747.ep12349031. [DOI] [PubMed] [Google Scholar]
- Kelly D.A., Walker S.L., McGregor J.M., Young A.R. A single exposure of solar simulated radiation suppresses contact hypersensitivity responses both locally and systemically in humansquantitative studies with high-frequency ultrasound. J. Photochem. Photobiol. B. 1998;44:130–142. doi: 10.1016/S1011-1344(98)00136-5. [DOI] [PubMed] [Google Scholar]
- Amemiya T. Regression analysis when the dependent variable is truncated normal. Econometrica. 1973;41:997–1016. [Google Scholar]
- Walker S.L., Tsang W., Chadwick C., Sheehan J., Barr R.M., Potten C.S., Young A.R. DNA damage, cytokine release and alloantigen presentation in vivo and their relationship to skin type in humans exposed to solar simulated ultraviolet radiation Photochem. Photobiol. 67 1998. 71(Abstr.) [Google Scholar]
- Yoshikawa T., Rae V., Bruins-Slot W., van den Berg J.W., Taylor J.R., Streilein J.W. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J. Invest. Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- Vermeer M., Schmieder G.J., Yoshikawa T., van den Berg J.-W., Metzman M.S., Taylor J.R., Streilein J.W. Effects of ultraviolet B light on cutaneous immune responses of humans with deeply pigmented skin. J. Invest. Dermatol. 1991;97:729–734. doi: 10.1111/1523-1747.ep12484259. [DOI] [PubMed] [Google Scholar]
- Skov L., Hansen H., Dittmar H.C., Barker J.N., Simon J.C., Baadsgaard O. Susceptibility to effects of UVB irradiation on induction of contact sensitivity, relevance of number and function of Langerhans cells and epidermal macrophages. Photochem. Photobiol. 1998;67:714–719. [PubMed] [Google Scholar]
- Allen M.H., Skov L., Barber R., Trembath R., Simon J., Baadsgaard O., Barker J.N. Ultraviolet B suppression of induction of contact sensitivity in human skin is not associated with tumour necrosis factor-alpha-308 or interleukin-10 genetic polymorphisms. Br. J. Dermatol. 1998;139:225–229. doi: 10.1046/j.1365-2133.1998.02358.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Streilein J.W. Genetic basis of the effects of ultraviolet light B on cutaneous immunity. Evidence that polymorphisms at the Tnfa and LPS loci governs susceptibility. Immunogenetics. 1990;32:398–405. doi: 10.1007/BF00241633. [DOI] [PubMed] [Google Scholar]
- Yamawaki M., Katiyar S.K., Anderson C.Y., Tubesing K.A., Mukhtar H., Elmets C.A. Genetic variation in low-dose UVR-induced suppression of contact hypersensitivity and in the skin photocarcinogenesis response. J. Invest. Dermatol. 1997;109:716–721. doi: 10.1111/1523-1747.ep12340683. [DOI] [PubMed] [Google Scholar]
- Miyauchi H., Horio T. Ultraviolet B-induced local suppression of contact hypersensitivity is modulated by ultraviolet irradiation and hapten application. J. Invest. Dermatol. 1995;104:364–369. doi: 10.1111/1523-1747.ep12665832. [DOI] [PubMed] [Google Scholar]
- Nelson R.J., Demas G.E., Klein S.L., Kriegsfeld L.J. The influence of season, photoperiod, and pineal melatonin on immune function. J. Pineal Res. 1995;19:149–165. doi: 10.1111/j.1600-079X.1995.tb00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger T.A., Bhardwaj R.S., Grabbe S., Schwarz T. Regulation of the immune response by epidermal cytokines and neurohormones. J. Dermatol. Sci. 1996;13:5–10. doi: 10.1016/0923-1811(95)00485-8. [DOI] [PubMed] [Google Scholar]
- Wolf P., Kripke M.L. Immune aspects of sunscreens. In: Gasparro F.P., editor. Sunscreen Photobiology. Landes Press; Georgetown, TX: 1997. pp. 99–126. [Google Scholar]